Advanced Optical Imaging-Guided Nanotheranostics towards Personalized Cancer Drug Delivery
Abstract
1. Nanotheranostics: Premises and Prospects
1.1. Need for Theranostics
1.2. Role of Nanomaterials in Nanotheranostics
1.3. Nanotheranostics in Cancer Treatment
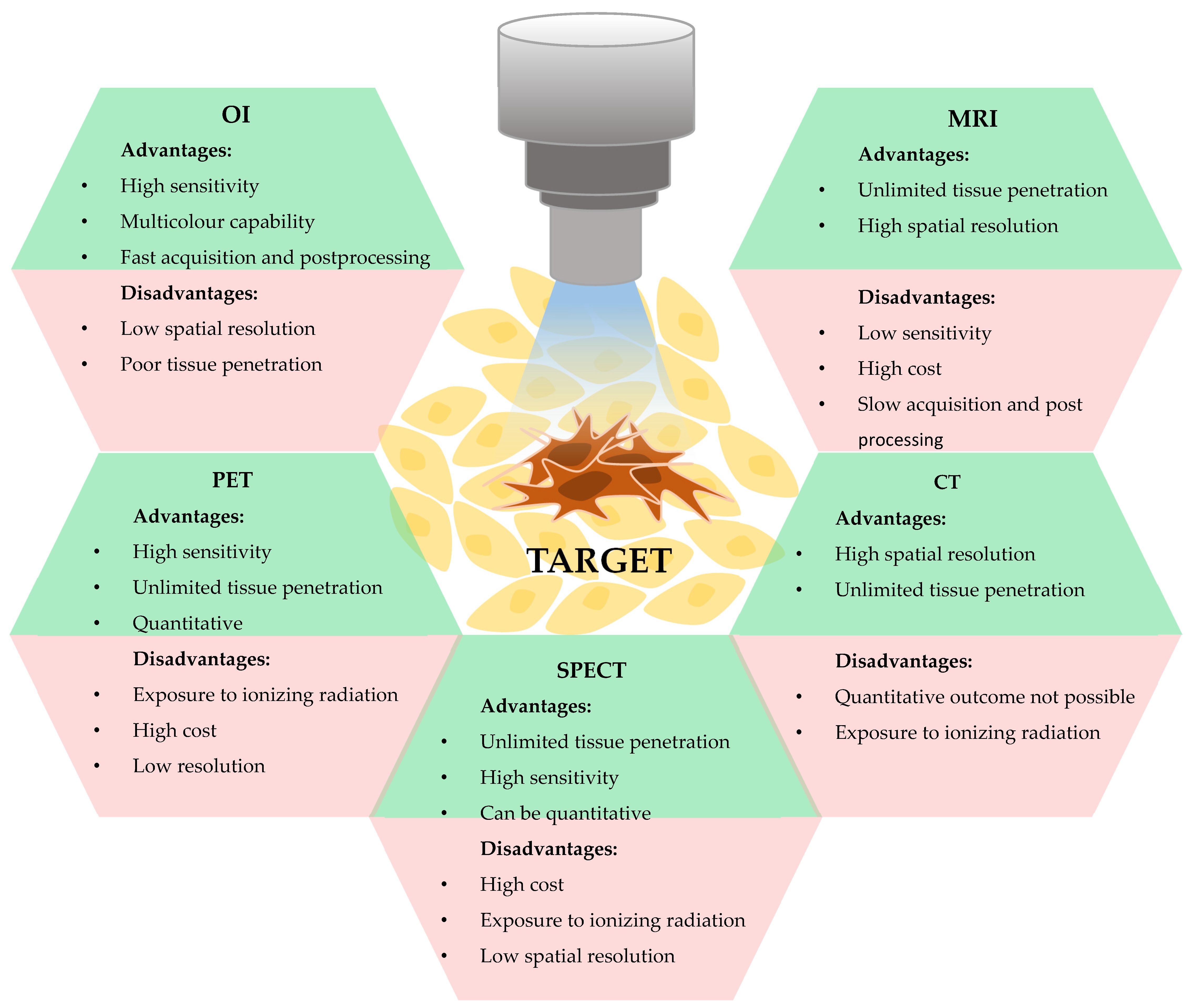
2. Optical Imaging as a Tool for Nanotheranostics
2.1. Nanomaterials as Probes for In Vivo Optical Imaging
2.2. Imaging-Guided Surgery and Drug Delivery
3. Nanotheranostics for Personalized Medicine
3.1. Need for Personalization
3.2. Progress So Far: Nanotheranostics in Clinical Trials
4. Discussion and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
List of Acronyms
| PM | Personalized Medicine |
| MRI | Magnetic Resonance Imaging |
| CT | Computed Tomography |
| PET | Positron Emission Tomography |
| SPECT | Single-Photon Emission Computed Tomography |
| OI | Optical Imaging |
| NPs | Nanoparticles |
| EPR | Enhanced Permeation And Retention |
| PEG | Polyethylene Glycol |
| DOX | Doxorubicin |
| QDs | Quantum Dots |
| NIRF | Near Infra-Red Fluorescence |
| FMT | Fluorescence Molecular Tomography |
| STED | Stimulated Emission Depletion |
| EGFR | Epidermal Growth Factor Receptor |
| NIH | National Institute Of Health |
| 5-ALA | 5-Aminolaevulinic Acid |
| FR-α | Folate Receptor A |
| SPIO | Super Paramagnetic Iron Oxide |
| AI | Artificial Intelligence |
References
- Kelkar, S.S.; Reineke, T.M. Theranostics: Combining Imaging and Therapy. Bioconjug. Chem. 2011, 22, 1879–1903. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.R.; Mukherjee, S.; Das, S.; Patra, C.R.; Iyer, P.K. Multifunctional (3-in-1) cancer theranostics applications of hydroxyquinoline-appended polyfluorene nanoparticles. Chem. Sci. 2017, 8, 7566–7575. [Google Scholar] [CrossRef] [PubMed]
- Theek, B.; Rizzo, L.Y.; Ehling, J.; Kiessling, F.; Lammers, T. The Theranostic Path to Personalized Nanomedicine. Clin. Transl. Imaging 2014, 2, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Burrell, R.A.; McGranahan, N.; Bartek, J.; Swanton, C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature 2013, 501, 338–345. [Google Scholar] [CrossRef]
- Muthu, M.S.; Leong, D.T.; Mei, L.; Feng, S.-S. Nanotheranostics—Application and Further Development of Nanomedicine Strategies for Advanced Theranostics. Theranostics 2014, 4, 660–677. [Google Scholar] [CrossRef]
- Lammers, T.; Rizzo, L.Y.; Storm, G.; Kiessling, F. Personalized nanomedicine. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 4889–4894. [Google Scholar] [CrossRef]
- Cong, Y.; Xiao, H.; Xiong, H.; Wang, Z.; Ding, J.; Li, C.; Chen, X.; Liang, X.-J.; Zhou, D.; Huang, Y. Dual Drug Backboned Shattering Polymeric Theranostic Nanomedicine for Synergistic Eradication of Patient-Derived Lung Cancer. Adv. Mater. 2018, 30, 1706220. [Google Scholar] [CrossRef]
- Ryu, J.H.; Koo, H.; Sun, I.-C.; Yuk, S.H.; Choi, K.; Kim, K.; Kwon, I.C. Tumor-targeting multi-functional nanoparticles for theragnosis: New paradigm for cancer therapy. Adv. Drug Deliv. Rev. 2012, 64, 1447–1458. [Google Scholar] [CrossRef]
- Kim, K.; Kim, J.H.; Park, H.; Kim, Y.-S.; Park, K.; Nam, H.; Lee, S.; Park, J.H.; Park, R.-W.; Kim, I.-S.; et al. Tumor-homing multifunctional nanoparticles for cancer theragnosis: Simultaneous diagnosis, drug delivery, and therapeutic monitoring. J. Control. Release 2010, 146, 219–227. [Google Scholar] [CrossRef]
- Jo, S.D.; Ku, S.H.; Won, Y.-Y.; Kim, S.H.; Kwon, I.C. Targeted Nanotheranostics for Future Personalized Medicine: Recent Progress in Cancer Therapy. Theranostics 2016, 6, 1362–1377. [Google Scholar] [CrossRef]
- Wagner, V.; Dullaart, A.; Bock, A.-K.; Zweck, A. The emerging nanomedicine landscape. Nat. Biotechnol. 2006, 24, 1211. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Lee, S.; Chen, X. Nanoparticle-based theranostic agents. Adv. Drug Deliv. Rev. 2010, 62, 1064–1079. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.Y.; Liu, G.; Lee, S.; Chen, X. Theranostic nanoplatforms for simultaneous cancer imaging and therapy: Current approaches and future perspectives. Nanoscale 2012, 4, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lee, S.; Chen, X. Nanotheranostics for personalized medicine. Expert Rev. Mol. Diagn. 2013, 13, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Yang, N.; Wang, X.; Zhao, Q.; Chen, Q.; Liu, Z.; Cheng, L. Tumor microenvironment-responsive intelligent nanoplatforms for cancer theranostics. Nano Today 2020, 32, 100851. [Google Scholar] [CrossRef]
- Pan, D.; Lanza, G.M.; Wickline, S.A.; Caruthers, S.D. Nanomedicine: Perspective and promises with ligand-directed molecular imaging. Eur. J. Radiol. 2009, 70, 274–285. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, M. Nanoparticle-based theragnostics: Integrating diagnostic and therapeutic potentials in nanomedicine. J. Control. Release Off. J. Control. Release Soc. 2010, 146, 2–5. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Drug Delivery Systems: Entering the Mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef]
- Duncan, R.; Gaspar, R. Nanomedicine(s) under the Microscope. Mol. Pharm. 2011, 8, 2101–2141. [Google Scholar] [CrossRef]
- Ventola, C.L. Progress in Nanomedicine: Approved and Investigational Nanodrugs. Pharm. Ther. 2017, 42, 742–755. [Google Scholar]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release Off. J. Control. Release Soc. 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Dawidczyk, C.M.; Kim, C.; Park, J.H.; Russell, L.M.; Lee, K.H.; Pomper, M.G.; Searson, P.C. State-of-the-Art in Design Rules for Drug Delivery Platforms: Lessons from FDA-approved Nanomedicines. J. Control. Release Off. J. Control. Release Soc. 2014, 187, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [PubMed]
- Bhojani, M.S.; Van Dort, M.; Rehemtulla, A.; Ross, B.D. Targeted Imaging and Therapy of Brain Cancer using Theranostic Nanoparticles. Mol. Pharm. 2010, 7, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Huh, M.S.; Sun, I.-C.; Yuk, S.H.; Choi, K.; Kim, K.; Kwon, I.C. In Vivo Targeted Delivery of Nanoparticles for Theranosis. Acc. Chem. Res. 2011, 44, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Xiao, Y.; Hong, H.; Matson, V.Z.; Javadi, A.; Xu, W.; Yang, Y.; Zhang, Y.; Engle, J.W.; Nickles, R.J.; Cai, W.; et al. Gold Nanorods Conjugated with Doxorubicin and cRGD for Combined Anticancer Drug Delivery and PET Imaging. Theranostics 2012, 2, 757–768. [Google Scholar] [CrossRef]
- He, Y.; Zhang, L.; Song, C.; Zhu, D. Design of multifunctional magnetic iron oxide nanoparticles/mitoxantrone-loaded liposomes for both magnetic resonance imaging and targeted cancer therapy. Int. J. Nanomed. 2014, 9, 4055. [Google Scholar] [CrossRef]
- Arndt-Jovin, D.J.; Kantelhardt, S.R.; Caarls, W.; de Vries, A.H.B.; Giese, A.; Jovin Ast, T.M. Tumor-targeted quantum dots can help surgeons find tumor boundaries. IEEE Trans. Nanobiosci. 2009, 8, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-W.; Hua, M.-Y.; Liu, H.-L.; Tsai, R.-Y.; Chuang, C.-K.; Chu, P.-C.; Wu, P.-Y.; Chang, Y.-H.; Chuang, H.-C.; Yu, K.-J.; et al. Cooperative Dual-Activity Targeted Nanomedicine for Specific and Effective Prostate Cancer Therapy. ACS Nano 2012, 6, 1795–1805. [Google Scholar] [CrossRef] [PubMed]
- Mogoşanu, G.D.; Grumezescu, A.M.; Bejenaru, C.; Bejenaru, L.E. Polymeric protective agents for nanoparticles in drug delivery and targeting. Int. J. Pharm. 2016, 510, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zhou, J.; Zhang, Y.; He, Y.; Jiang, Q.; Yue, D.; Xu, X.; Gu, Z. Highly Stable Fluorinated Nanocarriers with iRGD for Overcoming the Stability Dilemma and Enhancing Tumor Penetration in an Orthotopic Breast Cancer. ACS Appl. Mater. Interfaces 2016, 8, 28468–28479. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.J.Y.; Chan, S.Y.; Goh, Y.-Y.; Luo, Z.; Lau, J.W.; Liu, X. Emerging strategies in developing multifunctional nanomaterials for cancer nanotheranostics. Adv. Drug Deliv. Rev. 2021, 178, 113907. [Google Scholar] [CrossRef]
- Fass, L. Imaging and cancer: A review. Mol. Oncol. 2008, 2, 115–152. [Google Scholar] [CrossRef]
- Frangioni, J.V. New technologies for human cancer imaging. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 4012–4021. [Google Scholar] [CrossRef]
- Janib, S.M.; Moses, A.S.; MacKay, J.A. Imaging and drug delivery using theranostic nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 1052–1063. [Google Scholar] [CrossRef]
- Arms, L.; Smith, D.W.; Flynn, J.; Palmer, W.; Martin, A.; Woldu, A.; Hua, S. Advantages and Limitations of Current Techniques for Analyzing the Biodistribution of Nanoparticles. Front. Pharmacol. 2018, 9, 802. [Google Scholar] [CrossRef]
- Thakare, V.; Tran, V.-L.; Natuzzi, M.; Thomas, E.; Moreau, M.; Romieu, A.; Collin, B.; Courteau, A.; Vrigneaud, J.-M.; Louis, C.; et al. Functionalization of theranostic AGuIX® nanoparticles for PET/MRI/optical imaging. RSC Adv. 2019, 9, 24811–24815. [Google Scholar] [CrossRef]
- Curvers, W.L.; Singh, R.; Song, L.-M.W.-K.; Wolfsen, H.C.; Ragunath, K.; Wang, K.; Wallace, M.B.; Fockens, P.; Bergman, J.J.G.H.M. Endoscopic tri-modal imaging for detection of early neoplasia in Barrett’s oesophagus: A multi-centre feasibility study using high-resolution endoscopy, autofluorescence imaging and narrow band imaging incorporated in one endoscopy system. Gut 2008, 57, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.; MacAulay, C.; le Riche, J.C.; Palcic, B. Detection and localization of early lung cancer by fluorescence bronchoscopy. Cancer 2000, 89, 2468–2473. [Google Scholar] [CrossRef]
- Lane, P.M.; Gilhuly, T.; Whitehead, P.; Zeng, H.; Poh, C.F.; Ng, S.; Williams, P.M.; Zhang, L.; Rosin, M.P.; MacAulay, C.E. Simple device for the direct visualization of oral-cavity tissue fluorescence. J. Biomed. Opt. 2006, 11, 024006. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Follen, M.; Milbourne, A.; Rhodes, H.; Malpica, A.; MacKinnon, N.; MacAulay, C.; Markey, M.K.; Richards-Kortum, R. Automated image analysis of digital colposcopy for the detection of cervical neoplasia. J. Biomed. Opt. 2008, 13, 014029. [Google Scholar] [CrossRef]
- Roblyer, D.; Richards-Kortum, R.; Sokolov, K.; El-Naggar, A.K.; Williams, M.D.; Kurachi, C.; Gillenwater, A.M. Multispectral optical imaging device for in vivo detection of oral neoplasia. J. Biomed. Opt. 2008, 13, 024019. [Google Scholar] [CrossRef]
- Cheng, Y.; Morshed, R.A.; Auffinger, B.; Tobias, A.L.; Lesniak, M.S. Multifunctional nanoparticles for brain tumor imaging and therapy. Adv. Drug Deliv. Rev. 2014, 66, 42–57. [Google Scholar] [CrossRef]
- Hellebust, A.; Richards-Kortum, R. Advances in molecular imaging: Targeted optical contrast agents for cancer diagnostics. Nanomedicine 2012, 7, 429–445. [Google Scholar] [CrossRef]
- Ebert, B.; Sukowski, U.; Grosenick, D.; Wabnitz, H.; Moesta, K.T.; Licha, K.; Becker, A.; Semmler, W.; Schlag, P.M.; Rinneberg, H. Near-infrared fluorescent dyes for enhanced contrast in optical mammography: Phantom experiments. J. Biomed. Opt. 2001, 6, 134–140. [Google Scholar] [CrossRef][Green Version]
- Franceschini, M.A.; Moesta, K.T.; Fantini, S.; Gaida, G.; Gratton, E.; Jess, H.; Mantulin, W.W.; Seeber, M.; Schlag, P.M.; Kaschke, M. Frequency-domain techniques enhance optical mammography: Initial clinical results. Proc. Natl. Acad. Sci. USA 1997, 94, 6468–6473. [Google Scholar] [CrossRef]
- Grosenick, D.; Moesta, K.T.; Wabnitz, H.; Mucke, J.; Stroszczynski, C.; Macdonald, R.; Schlag, P.M.; Rinneberg, H. Time-domain optical mammography: Initial clinical results on detection and characterization of breast tumors. Appl. Opt. 2003, 42, 3170–3186. [Google Scholar] [CrossRef]
- Licha, K.; Riefke, B.; Ntziachristos, V.; Becker, A.; Chance, B.; Semmler, W. Hydrophilic cyanine dyes as contrast agents for near-infrared tumor imaging: Synthesis, photophysical properties and spectroscopic in vivo characterization. Photochem. Photobiol. 2000, 72, 392–398. [Google Scholar] [CrossRef]
- Ntziachristos, V.; Yodh, A.G.; Schnall, M.; Chance, B. Concurrent MRI and diffuse optical tomography of breast after indocyanine green enhancement. Proc. Natl. Acad. Sci. USA 2000, 97, 2767–2772. [Google Scholar] [CrossRef] [PubMed]
- Pifferi, A.; Taroni, P.; Torricelli, A.; Messina, F.; Cubeddu, R.; Danesini, G. Four-wavelength time-resolved optical mammography in the 680–980-nm range. Opt. Lett. 2003, 28, 1138–1140. [Google Scholar] [CrossRef] [PubMed]
- DaCosta, R.S.; Wilson, B.C.; Marcon, N.E. Light-induced fluorescence endoscopy of the gastrointestinal tract. Gastrointest. Endosc. Clin. N. Am. 2000, 10, 37–69. [Google Scholar] [CrossRef]
- Ell, C. Improving endoscopic resolution and sampling: Fluorescence techniques. Gut 2003, 52, iv30–iv33. [Google Scholar] [CrossRef][Green Version]
- Jichlinski, P. New diagnostic strategies in the detection and staging of bladder cancer. Curr. Opin. Urol. 2003, 13, 351–355. [Google Scholar] [CrossRef]
- Li, Q.; He, X.; Wang, Y.; Liu, H.; Xu, D.; Guo, F. Review of spectral imaging technology in biomedical engineering: Achievements and challenges. J. Biomed. Opt. 2013, 18, 100901. [Google Scholar] [CrossRef]
- Lu, G.; Fei, B. Medical hyperspectral imaging: A review. J. Biomed. Opt. 2014, 19, 10901. [Google Scholar] [CrossRef]
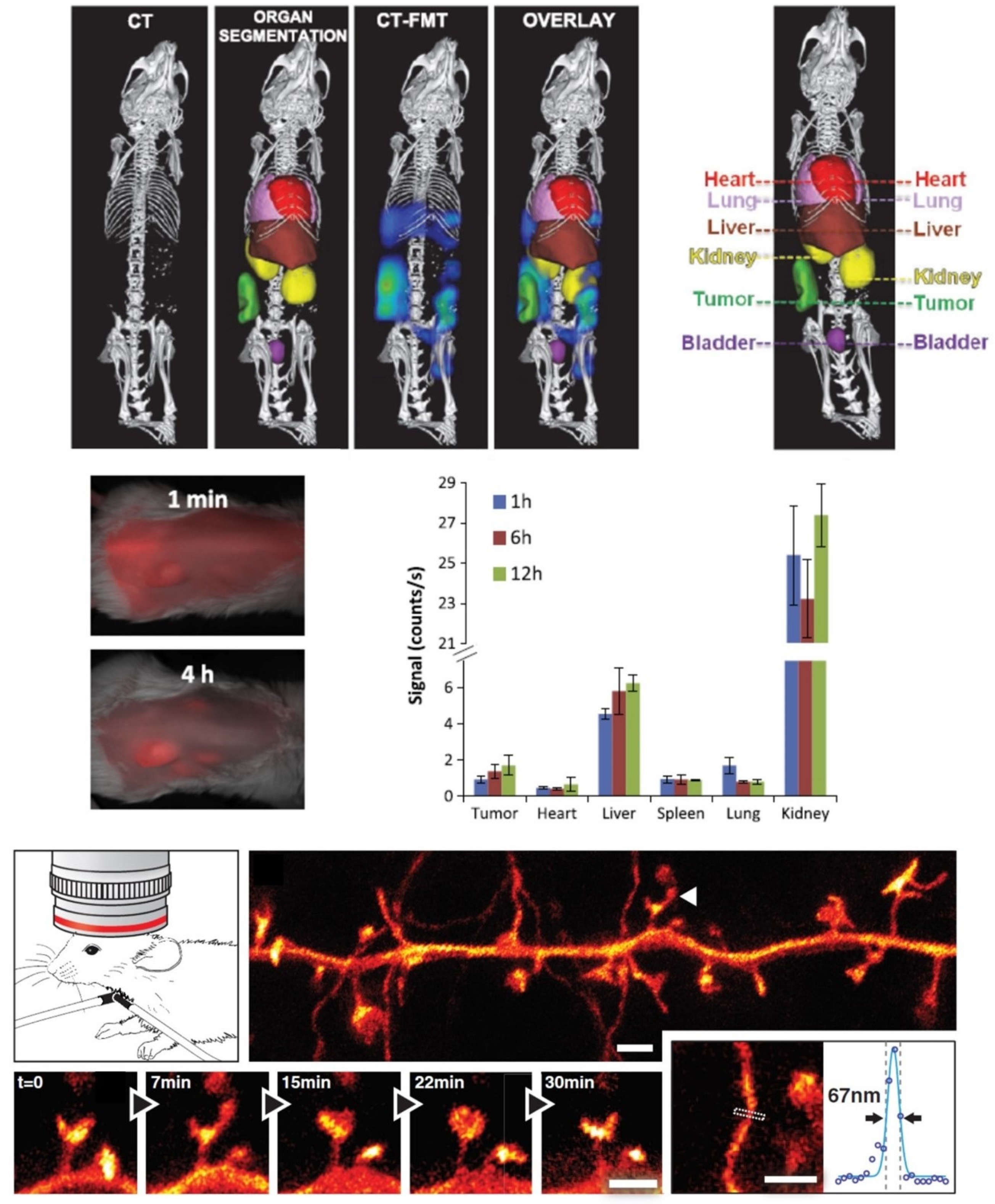
- Ntziachristos, V.; Bremer, C.; Weissleder, R. Fluorescence imaging with near-infrared light: New technological advances that enable in vivo molecular imaging. Eur. Radiol. 2003, 13, 195–208. [Google Scholar] [CrossRef]
- Kunjachan, S.; Ehling, J.; Storm, G.; Kiessling, F.; Lammers, T. Noninvasive Imaging of Nanomedicines and Nanotheranostics: Principles, Progress, and Prospects. Chem. Rev. 2015, 115, 10907–10937. [Google Scholar] [CrossRef]
- Licha, K.; Olbrich, C. Optical imaging in drug discovery and diagnostic applications. Adv. Drug Deliv. Rev. 2005, 57, 1087–1108. [Google Scholar] [CrossRef] [PubMed]
- Kunjachan, S.; Gremse, F.; Theek, B.; Koczera, P.; Pola, R.; Pechar, M.; Etrych, T.; Ulbrich, K.; Storm, G.; Kiessling, F.; et al. Noninvasive Optical Imaging of Nanomedicine Biodistribution. ACS Nano 2013, 7, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Berning, S.; Willig, K.I.; Steffens, H.; Dibaj, P.; Hell, S.W. Nanoscopy in a Living Mouse Brain. Science 2012, 335, 551. [Google Scholar] [CrossRef] [PubMed]
- Steffens, H.; Wegner, W.; Willig, K.I. In vivo STED microscopy: A roadmap to nanoscale imaging in the living mouse. Methods 2019, 174, 42–48. [Google Scholar] [CrossRef]
- Bucci, M.K.; Maity, A.; Janss, A.J.; Belasco, J.B.; Fisher, M.J.; Tochner, Z.A.; Rorke, L.; Sutton, L.N.; Phillips, P.C.; Shu, H.-K.G. Near complete surgical resection predicts a favorable outcome in pediatric patients with nonbrainstem, malignant gliomas: Results from a single center in the magnetic resonance imaging era. Cancer 2004, 101, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Tykocki, T.; Michalik, R.; Bonicki, W.; Nauman, P. Fluorescence-guided resection of primary and recurrent malignant gliomas with 5-aminolevulinic acid. Preliminary results. Neurol. Neurochir. Pol. 2012, 46, 47–51. [Google Scholar] [CrossRef] [PubMed]
- German, C.L.; Gudheti, M.V.; Fleckenstein, A.E.; Jorgensen, E.M. Brain Slice Staining and Preparation for Three-Dimensional Super-Resolution Microscopy. Methods Mol. Biol. 2017, 1663, 153–162. [Google Scholar] [CrossRef]
- Herrmannsdörfer, F.; Flottmann, B.; Nanguneri, S.; Venkataramani, V.; Horstmann, H.; Kuner, T.; Heilemann, M. 3D d STORM Imaging of Fixed Brain Tissue. Methods Mol. Biol. 2017, 1538, 169–184. [Google Scholar] [CrossRef]
- Querol-Vilaseca, M.; Colom-Cadena, M.; Pegueroles, J.; Nuñez-Llaves, R.; Luque-Cabecerans, J.; Muñoz-Llahuna, L.; Andilla, J.; Belbin, O.; Spires-Jones, T.L.; Gelpi, E.; et al. Nanoscale structure of amyloid-β plaques in Alzheimer’s disease. Sci. Rep. 2019, 9, 5181. [Google Scholar] [CrossRef]
- Quesseveur, G.; Fouquier d’Hérouël, A.; Murai, K.K.; Bouvier, D.S. A Specialized Method to Resolve Fine 3D Features of Astrocytes in Nonhuman Primate (Marmoset, Callithrix jacchus) and Human Fixed Brain Samples. Methods Mol. Biol. 2019, 1938, 85–95. [Google Scholar] [CrossRef]
- Hapuarachchige, S.; Artemov, D. Theranostic Pretargeting Drug Delivery and Imaging Platforms in Cancer Precision Medicine. Front. Oncol. 2020, 10, 1131. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-T.; Cao, L.; Luo, P.G.; Lu, F.; Wang, X.; Wang, H.; Meziani, M.J.; Liu, Y.; Qi, G.; Sun, Y.-P. Carbon Dots for Optical Imaging in Vivo. J. Am. Chem. Soc. 2009, 131, 11308–11309. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Yuan, Y.; Chen, J.; Zhang, B.; Li, D.; Zhou, D.; Jing, P.; Xu, G.; Wang, Y.; Holá, K.; et al. In vivo theranostics with near-infrared-emitting carbon dots—Highly efficient photothermal therapy based on passive targeting after intravenous administration. Light Sci. Appl. 2018, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zeng, A.; Liu, Z.; Zheng, C.; Wei, Y.; Yang, P.; Zhang, M.; Yang, F.; Xie, F. Carbon Quantum Dots: In vitro and in vivo Studies on Biocompatibility and Biointeractions for Optical Imaging. Int. J. Nanomed. 2020, 15, 6519–6529. [Google Scholar] [CrossRef]
- Wang, S.; Shao, J.; Li, Z.; Ren, Q.; Yu, X.-F.; Liu, S. Black Phosphorus-Based Multimodal Nanoagent: Showing Targeted Combinatory Therapeutics against Cancer Metastasis. Nano Lett. 2019, 19, 5587–5594. [Google Scholar] [CrossRef]
- Tao, W.; Zhu, X.; Yu, X.; Zeng, X.; Xiao, Q.; Zhang, X.; Ji, X.; Wang, X.; Shi, J.; Zhang, H.; et al. Black Phosphorus Nanosheets as a Robust Delivery Platform for Cancer Theranostics. Adv. Mater. 2017, 29, 1603276. [Google Scholar] [CrossRef]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Nilghaz, A.; Lin, Y.; Xu, J.; Lu, X. Black Phosphorus and its Biomedical Applications. Theranostics 2018, 8, 1005–1026. [Google Scholar] [CrossRef]
- Park, Y.I.; Lee, K.T.; Suh, Y.D.; Hyeon, T. Upconverting nanoparticles: A versatile platform for wide-field two-photon microscopy and multi-modal in vivo imaging. Chem. Soc. Rev. 2015, 44, 1302–1317. [Google Scholar] [CrossRef]
- Del Rosal, B.; Jaque, D. Upconversion nanoparticles for in vivo applications: Limitations and future perspectives. Methods Appl. Fluoresc. 2019, 7, 022001. [Google Scholar] [CrossRef]
- Li, H.; Tan, M.; Wang, X.; Li, F.; Zhang, Y.; Zhao, L.; Yang, C.; Chen, G. Temporal Multiplexed in Vivo Upconversion Imaging. J. Am. Chem. Soc. 2020, 142, 2023–2030. [Google Scholar] [CrossRef]
- Messerli, S.M.; Prabhakar, S.; Tang, Y.; Shah, K.; Cortes, M.L.; Murthy, V.; Weissleder, R.; Breakefield, X.O.; Tung, C.-H. A novel method for imaging apoptosis using a caspase-1 near-infrared fluorescent probe. Neoplasia 2004, 6, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Sheth, R.A.; Upadhyay, R.; Stangenberg, L.; Sheth, R.; Weissleder, R.; Mahmood, U. Improved detection of ovarian cancer metastases by intraoperative quantitative fluorescence protease imaging in a pre-clinical model. Gynecol. Oncol. 2009, 112, 616–622. [Google Scholar] [CrossRef] [PubMed]
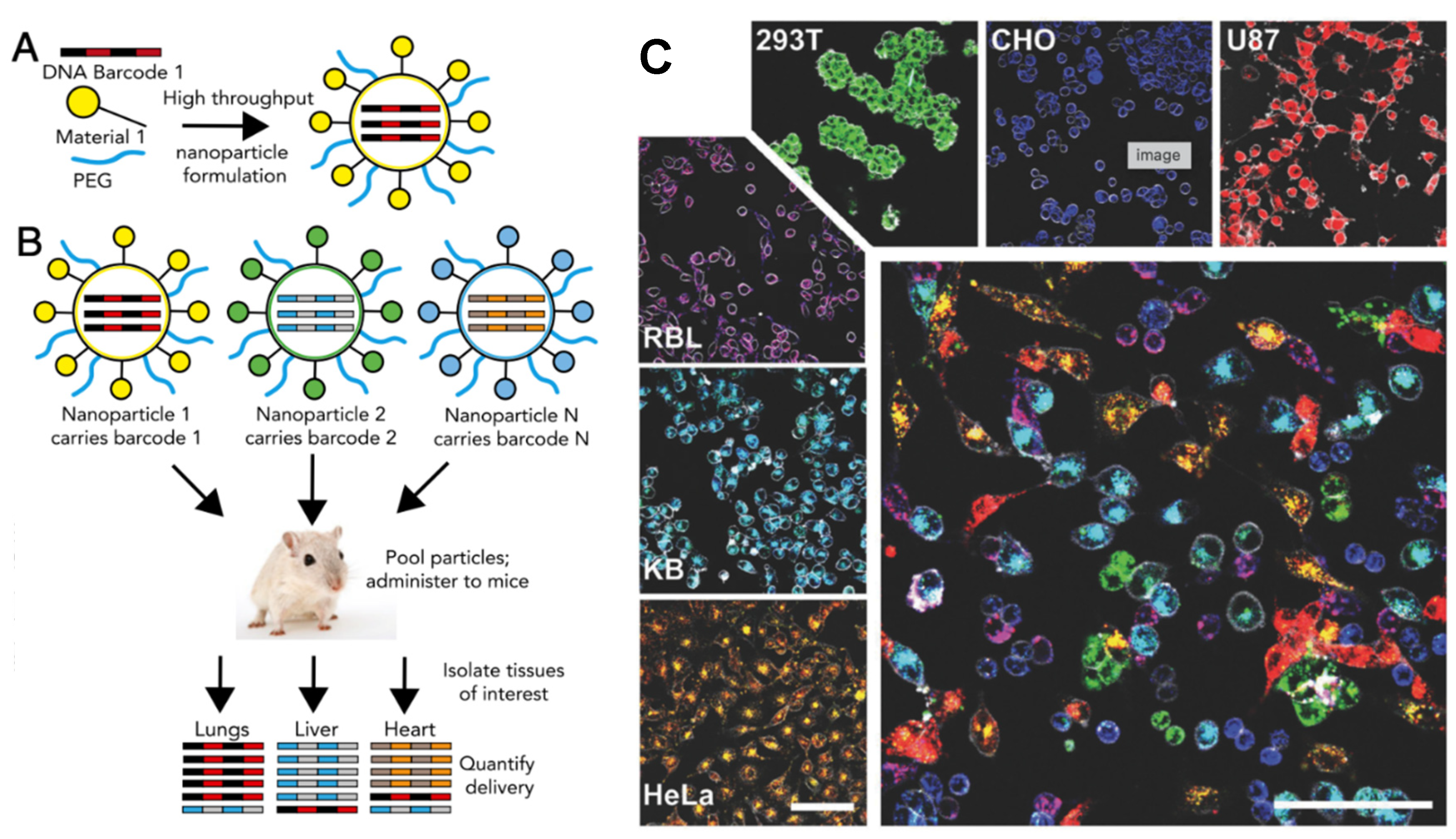
- Andreiuk, B.; Reisch, A.; Lindecker, M.; Follain, G.; Peyriéras, N.; Goetz, J.G.; Klymchenko, A.S. Fluorescent Polymer Nanoparticles for Cell Barcoding In Vitro and In Vivo. Small Weinh. Bergstr. Ger. 2017, 13, 1701582. [Google Scholar] [CrossRef] [PubMed]
- Shikha, S.; Salafi, T.; Cheng, J.; Zhang, Y. Versatile design and synthesis of nano-barcodes. Chem. Soc. Rev. 2017, 46, 7054–7093. [Google Scholar] [CrossRef]
- Dahlman, J.E.; Kauffman, K.J.; Xing, Y.; Shaw, T.E.; Mir, F.F.; Dlott, C.C.; Langer, R.; Anderson, D.G.; Wang, E.T. Barcoded nanoparticles for high throughput in vivo discovery of targeted therapeutics. Proc. Natl. Acad. Sci. USA 2017, 114, 2060–2065. [Google Scholar] [CrossRef] [PubMed]
- Joshi, B.P.; Miller, S.J.; Lee, C.M.; Seibel, E.J.; Wang, T.D. Multispectral endoscopic imaging of colorectal dysplasia in vivo. Gastroenterology 2012, 143, 1435–1437. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Engelbrecht, C.J.; Soper, T.D.; Helmchen, F.; Seibel, E.J. Scanning fiber endoscopy with highly flexible, 1 mm catheterscopes for wide-field, full-color imaging. J. Biophotonics 2010, 3, 385–407. [Google Scholar] [CrossRef]
- Chen, W.; Jarzyna, P.A.; van Tilborg, G.A.F.; Nguyen, V.A.; Cormode, D.P.; Klink, A.; Griffioen, A.W.; Randolph, G.J.; Fisher, E.A.; Mulder, W.J.M.; et al. RGD peptide functionalized and reconstituted high-density lipoprotein nanoparticles as a versatile and multimodal tumor targeting molecular imaging probe. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2010, 24, 1689–1699. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Choi, W.I.; Kim, Y.H.; Tae, G. Highly selective in-vivo imaging of tumor as an inflammation site by ROS detection using hydrocyanine-conjugated, functional nano-carriers. J. Control. Release Off. J. Control. Release Soc. 2011, 156, 398–405. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, F.; Lee, S.; Swierczewska, M.; Kiesewetter, D.O.; Lang, L.; Zhang, G.; Zhu, L.; Gao, H.; Choi, H.S.; et al. Long-term multimodal imaging of tumor draining sentinel lymph nodes using mesoporous silica-based nanoprobes. Biomaterials 2012, 33, 4370–4378. [Google Scholar] [CrossRef]
- Cai, W.; Shin, D.-W.; Chen, K.; Gheysens, O.; Cao, Q.; Wang, S.X.; Gambhir, S.S.; Chen, X. Peptide-labeled near-infrared quantum dots for imaging tumor vasculature in living subjects. Nano Lett. 2006, 6, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Solanki, A.; Memoli, K.A.; Kamei, K.; Kim, H.; Drahl, M.A.; Williams, L.J.; Tseng, H.-R.; Lee, K. Selective inhibition of human brain tumor cells through multifunctional quantum-dot-based siRNA delivery. Angew. Chem. Int. Ed. Engl. 2010, 49, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Hama, Y.; Koyama, Y.; Barrett, T.; Regino, C.A.S.; Urano, Y.; Choyke, P.L. Simultaneous multicolor imaging of five different lymphatic basins using quantum dots. Nano Lett. 2007, 7, 1711–1716. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Olson, E.S.; Nguyen, Q.T.; Roy, M.; Jennings, P.A.; Tsien, R.Y. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc. Natl. Acad. Sci. USA 2004, 101, 17867–17872. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.T.; Olson, E.S.; Aguilera, T.A.; Jiang, T.; Scadeng, M.; Ellies, L.G.; Tsien, R.Y. Surgery with molecular fluorescence imaging using activatable cell-penetrating peptides decreases residual cancer and improves survival. Proc. Natl. Acad. Sci. USA 2010, 107, 4317–4322. [Google Scholar] [CrossRef]
- Olson, E.S.; Jiang, T.; Aguilera, T.A.; Nguyen, Q.T.; Ellies, L.G.; Scadeng, M.; Tsien, R.Y. Activatable cell penetrating peptides linked to nanoparticles as dual probes for in vivo fluorescence and MR imaging of proteases. Proc. Natl. Acad. Sci. USA 2010, 107, 4311–4316. [Google Scholar] [CrossRef]
- Jackson, H.; Muhammad, O.; Daneshvar, H.; Nelms, J.; Popescu, A.; Vogelbaum, M.A.; Bruchez, M.; Toms, S.A. Quantum dots are phagocytized by macrophages and colocalize with experimental gliomas. Neurosurgery 2007, 60, 524–529. [Google Scholar] [CrossRef]
- Wang, J.; Yong, W.H.; Sun, Y.; Vernier, P.T.; Koeffler, H.P.; Gundersen, M.A.; Marcu, L. Receptor-targeted quantum dots: Fluorescent probes for brain tumor diagnosis. J. Biomed. Opt. 2007, 12, 044021. [Google Scholar] [CrossRef]
- Yezhelyev, M.V.; Al-Hajj, A.; Morris, C.; Marcus, A.I.; Liu, T.; Lewis, M.; Cohen, C.; Zrazhevskiy, P.; Simons, J.W.; Rogatko, A.; et al. In Situ Molecular Profiling of Breast Cancer Biomarkers with Multicolor Quantum Dots. Adv. Mater. 2007, 19, 3146–3151. [Google Scholar] [CrossRef]
- McCarthy, J.R. The future of theranostic nanoagents. Nanomedicine 2009, 4, 693–695. [Google Scholar] [CrossRef]
- Ferber, S.; Baabur-Cohen, H.; Blau, R.; Epshtein, Y.; Kisin-Finfer, E.; Redy, O.; Shabat, D.; Satchi-Fainaro, R. Polymeric nanotheranostics for real-time non-invasive optical imaging of breast cancer progression and drug release. Cancer Lett. 2014, 352, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lécuyer, T.; Seguin, J.; Mignet, N.; Scherman, D.; Viana, B.; Richard, C. Imaging and therapeutic applications of persistent luminescence nanomaterials. Adv. Drug Deliv. Rev. 2019, 138, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.; DeGiovanni, P.-J.; Piel, B.; Rai, P. Cancer nanomedicine: A review of recent success in drug delivery. Clin. Transl. Med. 2017, 6, 44. [Google Scholar] [CrossRef]
- Sau, S.; Tatiparti, K.; Alsaab, H.O.; Kashaw, S.K.; Iyer, A.K. A tumor multicomponent targeting chemoimmune drug delivery system for reprograming the tumor microenvironment and personalized cancer therapy. Drug Discov. Today 2018, 23, 1344–1356. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, W.; Zhu, G.; Xie, J.; Chen, X. Rethinking cancer nanotheranostics. Nat. Rev. Mater. 2017, 2, 17024. [Google Scholar] [CrossRef]
- Blau, R.; Krivitsky, A.; Epshtein, Y.; Satchi-Fainaro, R. Are nanotheranostics and nanodiagnostics-guided drug delivery stepping stones towards precision medicine? Drug Resist. Update Rev. Comment. Antimicrob. Anticancer Chemother. 2016, 27, 39–58. [Google Scholar] [CrossRef]
- Beez, T.; Sarikaya-Seiwert, S.; Steiger, H.-J.; Hänggi, D. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of brain tumors in children—A technical report. Acta Neurochir. 2014, 156, 597–604. [Google Scholar] [CrossRef]
- Della Puppa, A.; Rustemi, O.; Gioffrè, G.; Troncon, I.; Lombardi, G.; Rolma, G.; Sergi, M.; Munari, M.; Cecchin, D.; Gardiman, M.P.; et al. Predictive value of intraoperative 5-aminolevulinic acid-induced fluorescence for detecting bone invasion in meningioma surgery. J. Neurosurg. 2014, 120, 840–845. [Google Scholar] [CrossRef]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.-J.; ALA-Glioma Study Group. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Goryaynov, S.A.; Widhalm, G.; Goldberg, M.F.; Chelushkin, D.; Spallone, A.; Chernyshov, K.A.; Ryzhova, M.; Pavlova, G.; Revischin, A.; Shishkina, L.; et al. The Role of 5-ALA in Low-Grade Gliomas and the Influence of Antiepileptic Drugs on Intraoperative Fluorescence. Front. Oncol. 2019, 9, 423. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, G.M.; Themelis, G.; Crane, L.M.A.; Harlaar, N.J.; Pleijhuis, R.G.; Kelder, W.; Sarantopoulos, A.; de Jong, J.S.; Arts, H.J.G.; van der Zee, A.G.J.; et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: First in-human results. Nat. Med. 2011, 17, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Gandon, Y.; Heautot, J.F.; Brunet, F.; Guyader, D.; Deugnier, Y.; Carsin, M. Superparamagnetic iron oxide: Clinical time-response study. Eur. J. Radiol. 1991, 12, 195–200. [Google Scholar] [CrossRef]
- Imai, Y.; Murakami, T.; Yoshida, S.; Nishikawa, M.; Ohsawa, M.; Tokunaga, K.; Murata, M.; Shibata, K.; Zushi, S.; Kurokawa, M.; et al. Superparamagnetic iron oxide–enhanced magnetic resonance images of hepatocellular carcinoma: Correlation with histological grading. Hepatology 2000, 32, 205–212. [Google Scholar] [CrossRef]
- Motoyama, S.; Ishiyama, K.; Maruyama, K.; Narita, K.; Minamiya, Y.; Ogawa, J.-I. Estimating the need for neck lymphadenectomy in submucosal esophageal cancer using superparamagnetic iron oxide-enhanced magnetic resonance imaging: Clinical validation study. World J. Surg. 2012, 36, 83–89. [Google Scholar] [CrossRef]
- Wang, Z.; Qiao, R.; Tang, N.; Lu, Z.; Wang, H.; Zhang, Z.; Xue, X.; Huang, Z.; Zhang, S.; Zhang, G.; et al. Active targeting theranostic iron oxide nanoparticles for MRI and magnetic resonance-guided focused ultrasound ablation of lung cancer. Biomaterials 2017, 127, 25–35. [Google Scholar] [CrossRef]
- Benezra, M.; Penate-Medina, O.; Zanzonico, P.B.; Schaer, D.; Ow, H.; Burns, A.; DeStanchina, E.; Longo, V.; Herz, E.; Iyer, S.; et al. Multimodal silica nanoparticles are effective cancer-targeted probes in a model of human melanoma. J. Clin. Investig. 2011, 121, 2768–2780. [Google Scholar] [CrossRef]
- Bradbury, M.S.; Phillips, E.; Montero, P.H.; Cheal, S.M.; Stambuk, H.; Durack, J.C.; Sofocleous, C.T.; Meester, R.J.C.; Wiesner, U.; Patel, S. Clinically-translated silica nanoparticles as dual-modality cancer-targeted probes for image-guided surgery and interventions. Integr. Biol. Quant. Biosci. Nano Macro 2013, 5, 74–86. [Google Scholar] [CrossRef]
- Phillips, E.; Penate-Medina, O.; Zanzonico, P.B.; Carvajal, R.D.; Mohan, P.; Ye, Y.; Humm, J.; Gönen, M.; Kalaigian, H.; Schöder, H.; et al. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Sci. Transl. Med. 2014, 6, 260ra149. [Google Scholar] [CrossRef]
- Ho, D.; Wang, P.; Kee, T. Artificial intelligence in nanomedicine. Nanoscale Horiz. 2019, 4, 365–377. [Google Scholar] [CrossRef]
| Nanomedicine | Condition | Phase | Clinical Trial Status | Imaging Modality Employed | Clinical Trial Identifier * |
|---|---|---|---|---|---|
| Lyso-thermosensitive liposomal doxorubicin (LTLD, ThermoDox) | Stage IV breast cancer | 1 | Ongoing | Magnetic resonance guided-high intensity focused ultrasound (MR-HIFU) | NCT03749850 |
| Vincristine liposome | CD20+ aggressive B-cell lymphoma | 3 | Recruiting | Fluorodeoxyglucose Positron emission Tomography (FDG-PET) | NCT01478542 |
| Feraheme (SPION) | Pancreatic cancer | 4 | Completed | Ultra-small superparamagnetic iron oxide magnetic resonance imaging (USPIO-MRI) | NCT00920023 |
| Silica NPs | Nodal metastases of neck melanoma, colorectal and breast cancer | 1 and 2 | Recruiting | Real-time OI using fluorescent cRGDY-PEG-Cy5 5-C dots | NCT02106598 |
| 89Zr-nanocolloidal | Colon cancer | 2 and 3 | Completed | PET/CT and intraoperative near infra-red fluorescence (NIRF) imaging | NCT02850783 |
| Implication | Nanotheranostics (Small Molecule) Employed | Specification | Application | References |
|---|---|---|---|---|
| Nanotheranostics with “switchable” properties that can selectively mark specific tissues, such as tumors or inflammation | 5-Aminolaevulinic acid (5-ALA) | 5-ALA, an endogenous precursor of haemoglobin that produces porphyrins (which fluoresce under violet blue light illumination) in some types of malignant brain tissues | Intraoperative fluorescence-guided complete resection of several brain tumors | [108,109,110,111] |
| Nanotheranostics for active targeting | Folate–fluorescein isothiocyanate conjugate. Super paramagnetic iron oxide (SPIO) NPs | Folate receptor α (FR-α) is overexpressed in 90–95% of epithelial ovarian cancers and is therefore a good candidate for active targeting These particles possess excellent biocompatibility and magnetic properties and have been widely used for drug delivery, MRI probes, and tumor thermotherapy | A FR-α-targeted fluorescent agent used for intraoperative fluorescence imaging guided ovarian cancer surgery. SPIO-enhanced MRI mapping of liver cancer, non-invasive imaging of lymph node metastases, active targeting of lung cancer, | [112,113,114,115,116] (p. 2) |
| Ligand targeted nanoparticle drug conjugate (NDC) | Cornell (cRGDY-PEG-Cy5-C) dots conjugated to 124I radioisotope | 124I-cRGDY-PEG-Cy5-C dots are sub-10nm fluorescent core shell silica NP targeted to αV/β3 integrin receptor overexpressed on angiogenic endothelial cells and on various cancer cells | dual-modality (PET–OI) imaging for targeted molecular imaging of integrin-expressing cancers | [117,118,119] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murar, M.; Albertazzi, L.; Pujals, S. Advanced Optical Imaging-Guided Nanotheranostics towards Personalized Cancer Drug Delivery. Nanomaterials 2022, 12, 399. https://doi.org/10.3390/nano12030399
Murar M, Albertazzi L, Pujals S. Advanced Optical Imaging-Guided Nanotheranostics towards Personalized Cancer Drug Delivery. Nanomaterials. 2022; 12(3):399. https://doi.org/10.3390/nano12030399
Chicago/Turabian StyleMurar, Madhura, Lorenzo Albertazzi, and Silvia Pujals. 2022. "Advanced Optical Imaging-Guided Nanotheranostics towards Personalized Cancer Drug Delivery" Nanomaterials 12, no. 3: 399. https://doi.org/10.3390/nano12030399
APA StyleMurar, M., Albertazzi, L., & Pujals, S. (2022). Advanced Optical Imaging-Guided Nanotheranostics towards Personalized Cancer Drug Delivery. Nanomaterials, 12(3), 399. https://doi.org/10.3390/nano12030399







