A Novel Highly Sensitive Electrochemical Nitrite Sensor Based on a AuNPs/CS/Ti3C2 Nanocomposite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents and Materials
2.2. Instruments
2.3. Preparation of MXene and CS/Mxene
2.4. Preparation AuNPs/CS/MXene Modified Glass Carbon Electrodes
2.5. Modified Electrodes Detection Nitrite
3. Results
3.1. Structure and Surface Morphology of AuNPs/CS/MXene
3.2. Electrochemical Characterization of the Sensor
3.3. Electrocatalytic Response of Different Modified GCEs towards Nitrite
3.4. Amperometry
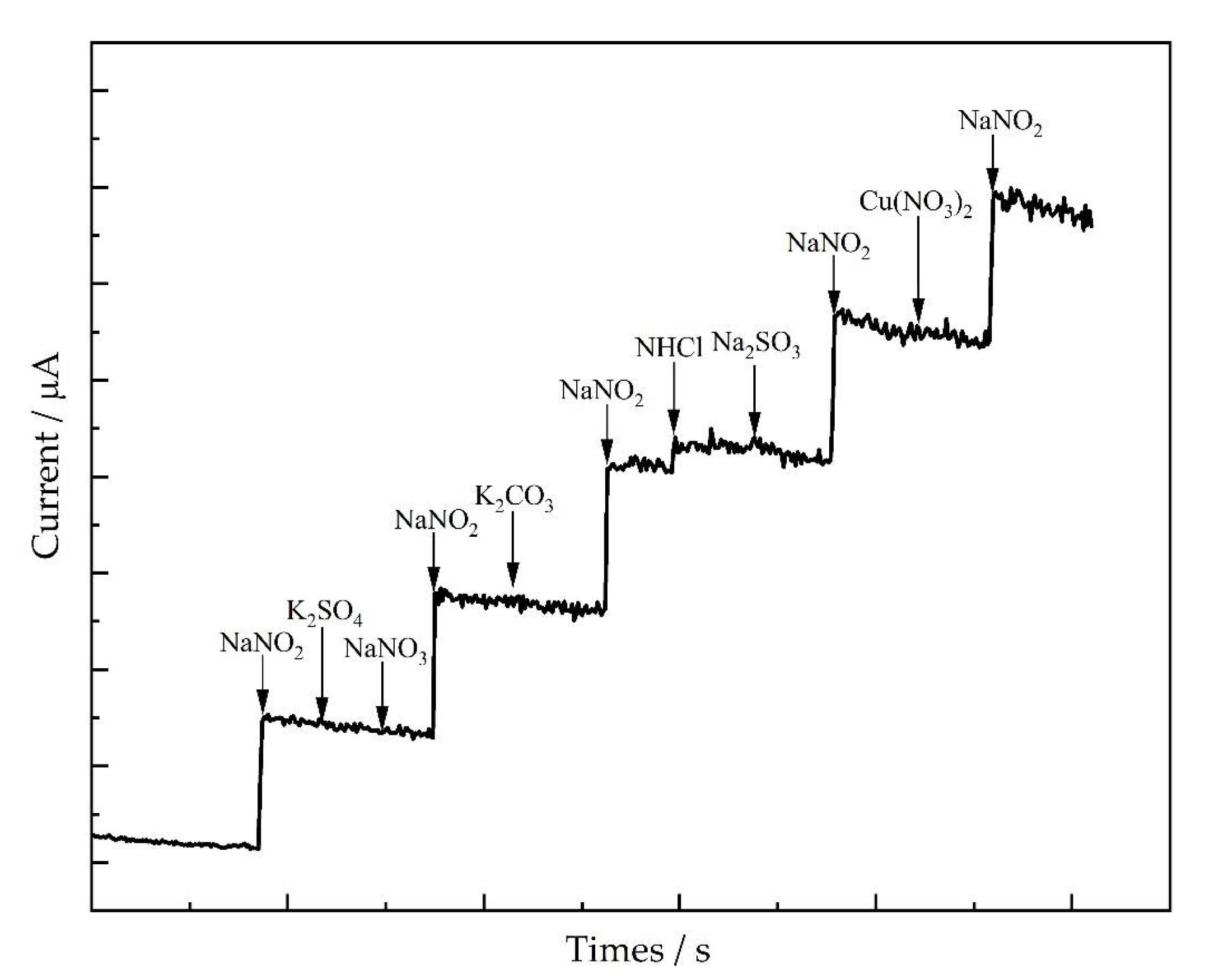
3.5. Selectivity of the Electrode
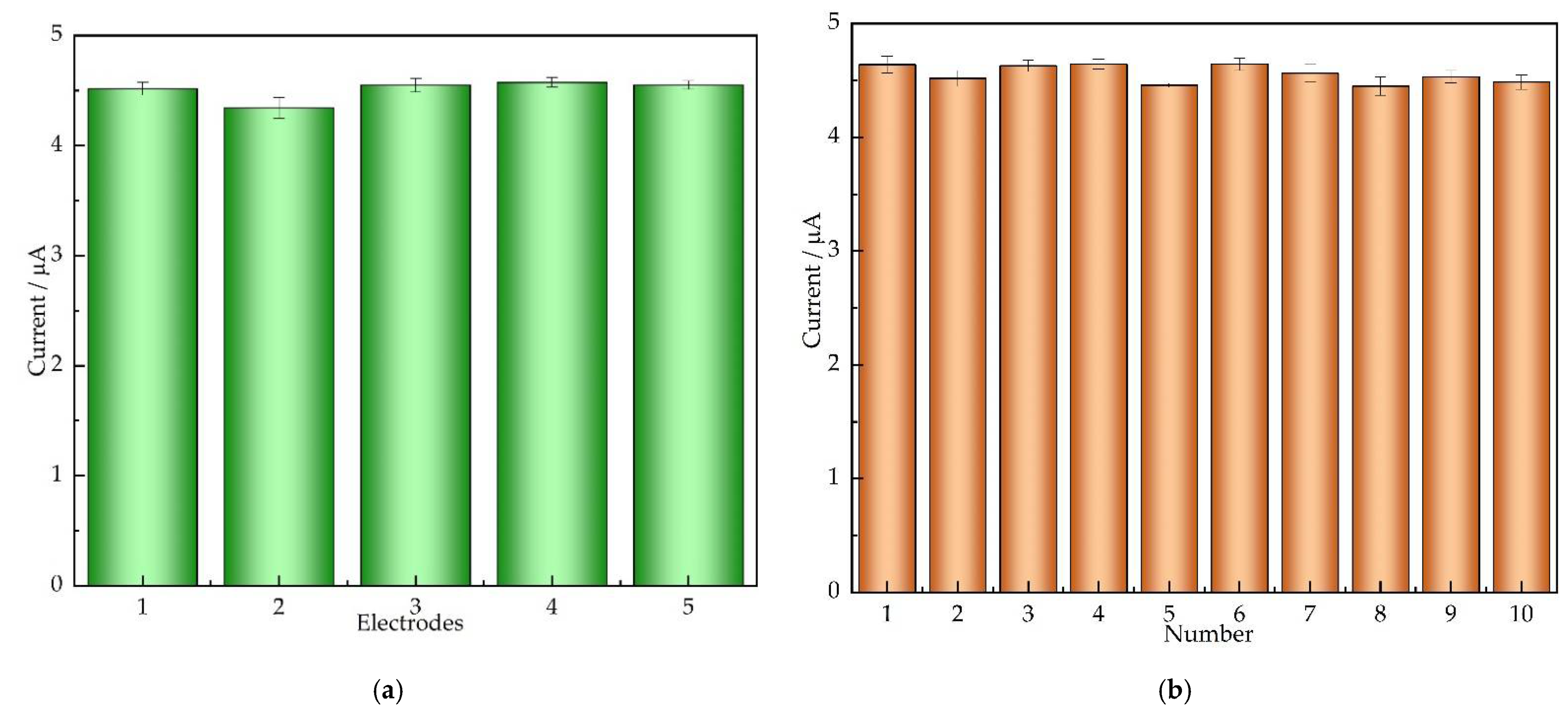
3.6. Repeatability and Stability
3.7. Real Sample Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Han, Y.; Zhang, R.; Dong, C.; Cheng, F.; Guo, Y. Sensitive electrochemical sensor for nitrite ions based on rose-like AuNPs/MoS2/graphene composite. Biosens. Bioelectron. 2019, 142, 111529. [Google Scholar] [CrossRef]
- Li, X.; Ping, J.; Ying, Y. Recent developments in carbon nanomaterial-enabled electrochemical sensors for nitrite detection. Trac-Trend Anal. Chem. 2019, 113, 1–12. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Z.; Wang, X.; Xu, J.; Jia, J. Study on Mechanism Experiments and Evaluation Methods for Water Eutrophication. J. Chem. 2017, 2017, 2036035. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, L.; Liu, Y.; Lin, L.; Lu, R.; Zhu, J.; He, L.; Lu, Z. Methods for the detection and determination of nitrite and nitrate: A review. Talanta 2017, 165, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Brizzolari, A.; Dei Cas, M.; Cialoni, D.; Marroni, A.; Morano, C.; Samaja, M.; Paroni, R.; Rubino, F.M. High-Throughput Griess Assay of Nitrite and Nitrate in Plasma and Red Blood Cells for Human Physiology Studies under Extreme Conditions. Molecules 2021, 26, 4569. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, N.; Jing, M.; Zhang, Y.; Wang, W.; Liu, L.; Xu, Z.; Liu, L.; Li, F.; Wu, N. Monodispersed and spherical silver nanoparticles/graphene nanocomposites from gamma-ray assisted in-situ synthesis for nitrite electrochemical sensing. Electrochim. Acta 2019, 295, 434–443. [Google Scholar] [CrossRef]
- Li, Z.; Li, M.; Wang, C.; Zhou, X.; Li, J.; Li, D. Highly sensitive and selective method for detection of trace amounts of nitrite in aquaculture water by SERRS coupled with diazo reaction. Sens. Actuat. B Chem. 2019, 297, 126757. [Google Scholar] [CrossRef]
- Xu, J.; Shi, Y.; Yang, S.; Yang, J.; Zhang, X.; Xu, L.; Bian, Z.; Xu, Z.; Zhu, B. Highly selective colorimetric fluorescent probe for detecting nitrite in aqueous solution. Microchem. J. 2021, 169, 106342. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, C.; Yue, G.; Yang, Z.; Wang, Y.; Rao, H.; Zhang, W.; Jin, B.; Wang, X. A highly selective chromogenic probe for the detection of nitrite in food samples. Food Chem. 2020, 317, 126361. [Google Scholar] [CrossRef] [PubMed]
- Coviello, D.; Pascale, R.; Ciriello, R.; Salvi, A.M.; Guerrieri, A.; Contursi, M.; Scrano, L.; Bufo, S.A.; Cataldi, T.R.I.; Bianco, G. Validation of an Analytical Method for Nitrite and Nitrate Determination in Meat Foods for Infants by Ion Chromatography with Conductivity Detection. Foods 2020, 9, 1238. [Google Scholar] [CrossRef]
- Thipwimonmas, Y.; Jaidam, J.; Samoson, K.; Khunseeraksa, V.; Phonchai, A.; Thiangchanya, A.; Chang, K.H.; Abdullah, A.F.L.; Limbut, W. A Simple and Rapid Spectrophotometric Method for Nitrite Detection in Small Sample Volumes. Chemosensors 2021, 9, 161. [Google Scholar] [CrossRef]
- Gill, A.; Zajda, J.; Meyerhoff, M.E. Comparison of electrochemical nitric oxide detection methods with chemiluminescence for measuring nitrite concentration in food samples. Anal. Chim. Acta 2019, 1077, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Zhu, H.; Sun, S.; Zhu, Z.; Hao, J.; Lu, S.; Cai, Y.; Zhang, M.; Du, M. Synergistic integration of Au nanoparticles, Co-MOF and MWCNT as biosensors for sensitive detection of low-concentration nitrite. Electrochim. Acta 2021, 365, 137375. [Google Scholar] [CrossRef]
- Zhe, T.; Li, R.; Wang, Q.; Shi, D.; Li, F.; Liu, Y.; Liang, S.; Sun, X.; Cao, Y.; Wang, L. In situ preparation of FeSe nanorods-functionalized carbon cloth for efficient and stable electrochemical detection of nitrite. Sensor. Actuat. B Chem. 2020, 321, 128452. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, J.; Wang, W.; Sun, Y.; Li, P.; Hu, J.; Chen, L.; Gong, W. Synthesis and electrochemical properties of Co3O4-rGO/CNTs composites towards highly sensitive nitrite detection. Appl. Surf. Sci. 2019, 485, 274–282. [Google Scholar] [CrossRef]
- He, B.; Yan, D. Au/ERGO nanoparticles supported on Cu-based metal-organic framework as a novel sensor for sensitive determination of nitrite. Food Control 2019, 103, 70–77. [Google Scholar] [CrossRef]
- Rajaji, U.; Manavalan, S.; Chen, S.; Chinnapaiyan, S.; Chen, T.; Ramalingam, R.J. Facile synthesis and characterization of erbium oxide (Er2O3) nanospheres embellished on reduced graphene oxide nanomatrix for trace-level detection of a hazardous pollutant causing Methemoglobinaemia. Ultrason. Sonochem. 2019, 56, 422–429. [Google Scholar] [CrossRef]
- Muthumariappan, A.; Govindasamy, M.; Chen, S.; Sakthivel, K.; Mani, V. Screen-printed electrode modified with a composite prepared from graphene oxide nanosheets and Mn3O4 microcubes for ultrasensitive determination of nitrite. Microchim. Acta 2017, 184, 3625–3634. [Google Scholar] [CrossRef]
- Mani, V.; Govindasamy, M.; Chen, S.; Chen, T.; Kumar, A.S.; Huang, S. Core-shell heterostructured multiwalled carbon nanotubes@reduced graphene oxide nanoribbons/chitosan, a robust nanobiocomposite for enzymatic biosensing of hydrogen peroxide and nitrite. Sci. Rep. 2017, 7, 11910. [Google Scholar] [CrossRef] [PubMed]
- Gui, J.; Han, L.; Cao, W. Lamellar MXene: A novel 2D nanomaterial for electrochemical sensors. J. Appl. Electrochem. 2021, 51, 1509–1522. [Google Scholar] [CrossRef]
- Shahzad, F.; Zaidi, S.A.; Naqvi, R.A. 2D Transition Metal Carbides (MXene) for Electrochemical Sensing: A Review. Crit. Rev. Anal. Chem. 2020, 50, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, H.; Huang, Y.; Xiong, M.; Wang, F.; Li, C. A label-free electrochemical biosensor for highly sensitive detection of gliotoxin based on DNA nanostructure/MXene nanocomplexes. Biosens. Bioelectron. 2019, 142, 111531. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Duan, C.; Yang, C.; Shen, W.; Wang, F.; Zhu, Z. A novel nitrite biosensor based on the direct electrochemistry of hemoglobin immobilized on MXene-Ti3C2. Sensor. Actuat B Chem. 2015, 218, 60–66. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.; Yang, S.; Shan, J. A novel electrochemical sensor based on TiO2-Ti3C2Tx/CTAB/chitosan composite for the detection of nitrite. Electrochim. Acta 2020, 359, 136938. [Google Scholar] [CrossRef]
- Sui, L.; Zhang, B.; Wang, J.; Cai, A. Polymerization of PEDOT/PSS/Chitosan-Coated Electrodes for Electrochemical Bio-Sensing. Coatings 2017, 7, 96. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Gao, F.; Zhang, X.; Zhang, B.; Li, S.; Hu, Z.; Gao, F. Electrochemical characterization and DNA sensing application of a sphere-like CeO2–ZrO2 and chitosan nanocomposite formed on a gold electrode by one-step electrodeposition. Electrochim. Acta 2012, 62, 250–255. [Google Scholar] [CrossRef]
- Mo, R.; Wang, X.; Yuan, Q.; Yan, X.; Su, T.; Feng, Y.; Lv, L.; Zhou, C.; Hong, P.; Sun, S.; et al. Electrochemical Determination of Nitrite by Au Nanoparticle/Graphene-Chitosan Modified Electrode. Sensors 2018, 18, 1986. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Han, G.; Cai, J.; Wang, X. Au@Carbon quantum Dots-MXene nanocomposite as an electrochemical sensor for sensitive detection of nitrite. J. Colloid Interf. Sci. 2022, 607, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, C.; Xu, X.; Li, Z.; Li, D. One-Step Electrodeposition Synthesized Aunps/Mxene/ERGO for Selectivity Nitrite Sensing. Nanomaterials 2021, 11, 1892. [Google Scholar] [CrossRef]
- Rashed, M.A.; Faisal, M.; Alsaiari, M.; Alsareii, S.A.; Harraz, F.A. MWCNT-Doped Polypyrrole-Carbon Black Modified Glassy Carbon Electrode for Efficient Electrochemical Sensing of Nitrite Ions. Electrocatalysis 2021, 12, 650–666. [Google Scholar] [CrossRef]
- Salagare, S.; Adarakatti, P.S.; Venkataramanappa, Y. Designing and Construction of Carboxyl Functionalised MWCNTs/Co-MOFs-Based Electrochemical Sensor for the Sensitive Detection of Nitrite. Int. J. Environ. Anal. Chem. 2020, 100, 1–20. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Zheng, J. One-pot aqueous method for facile synthesis of platinum-copper bimetallic catalyst based on graphene oxide and its highly enhanced sensing of nitrite. J. Mater. Sci. Mater. Electron. 2020, 31, 13301–13309. [Google Scholar] [CrossRef]
- Rashed, M.A.; Faisal, M.; Harraz, F.A.; Jalalah, M.; Alsaiari, M.; Al-Assiri, M.S. rGO/ZnO/Nafion nanocomposite as highly sensitive and selective amperometric sensor for detecting nitrite ions (NO2−). J. Taiwan Inst. Chem. Eng. 2020, 112, 345–356. [Google Scholar] [CrossRef]
- Ge, Y.; Jamal, R.; Zhang, R.; Zhang, W.; Yu, Z.; Yan, Y.; Liu, Y.; Abdiryim, T. Electrochemical synthesis of multilayered PEDOT/PEDOT-SH/Au nanocomposites for electrochemical sensing of nitrite. Microchim. Acta 2020, 187, 248. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, A.; Ensafi, A.A.; Rezaei, B. Fabrication of Electrochemical Sensor Based on CeO2−SnO2 Nanocomposite Loaded on Pd Support for Determination of Nitrite at Trace Levels. Electroanalysis 2020, 32, 1025–1033. [Google Scholar] [CrossRef]
- Majidi, M.R.; Ghaderi, S. Hydrogen bubble dynamic template fabrication of nanoporous Cu film supported by graphene nanaosheets: A highly sensitive sensor for detection of nitrite. Talanta 2017, 175, 21–29. [Google Scholar] [CrossRef]
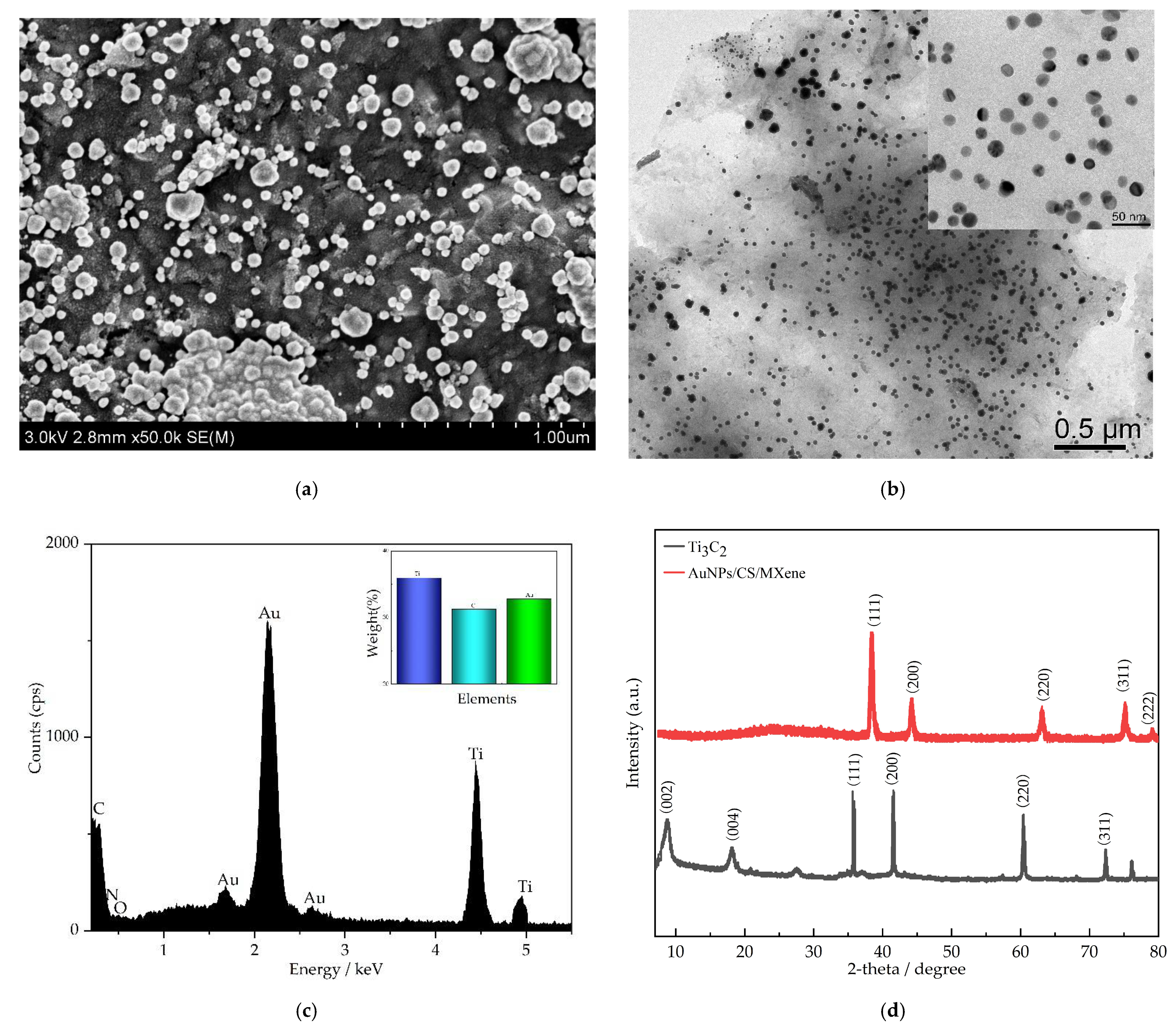
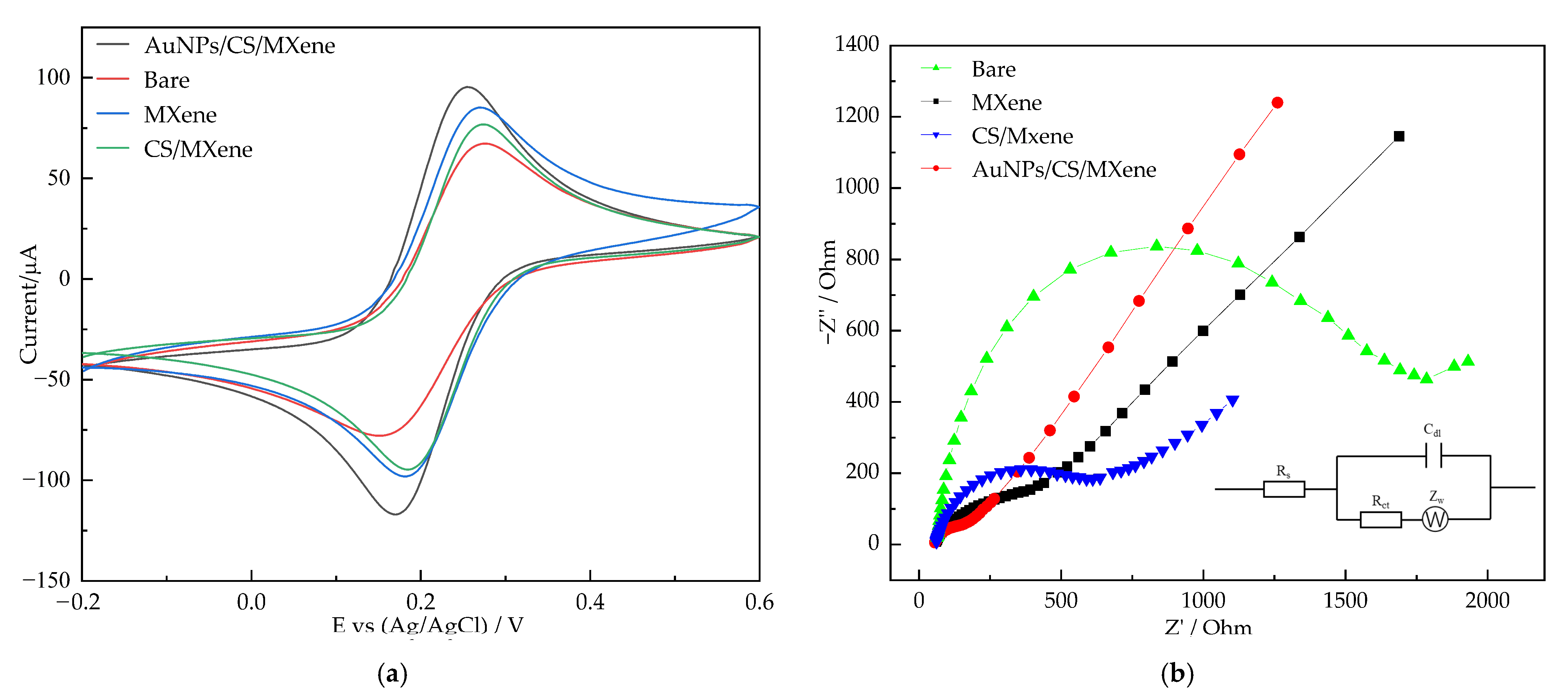

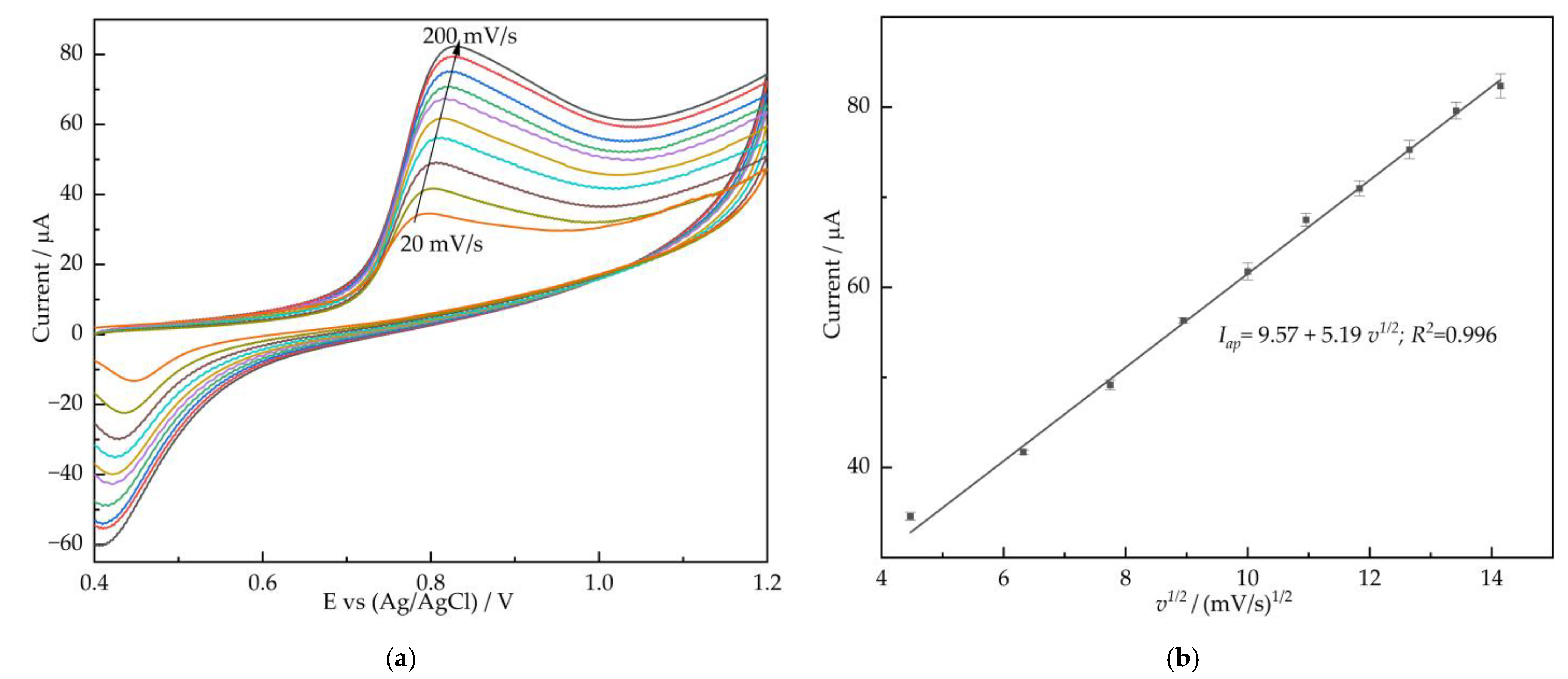
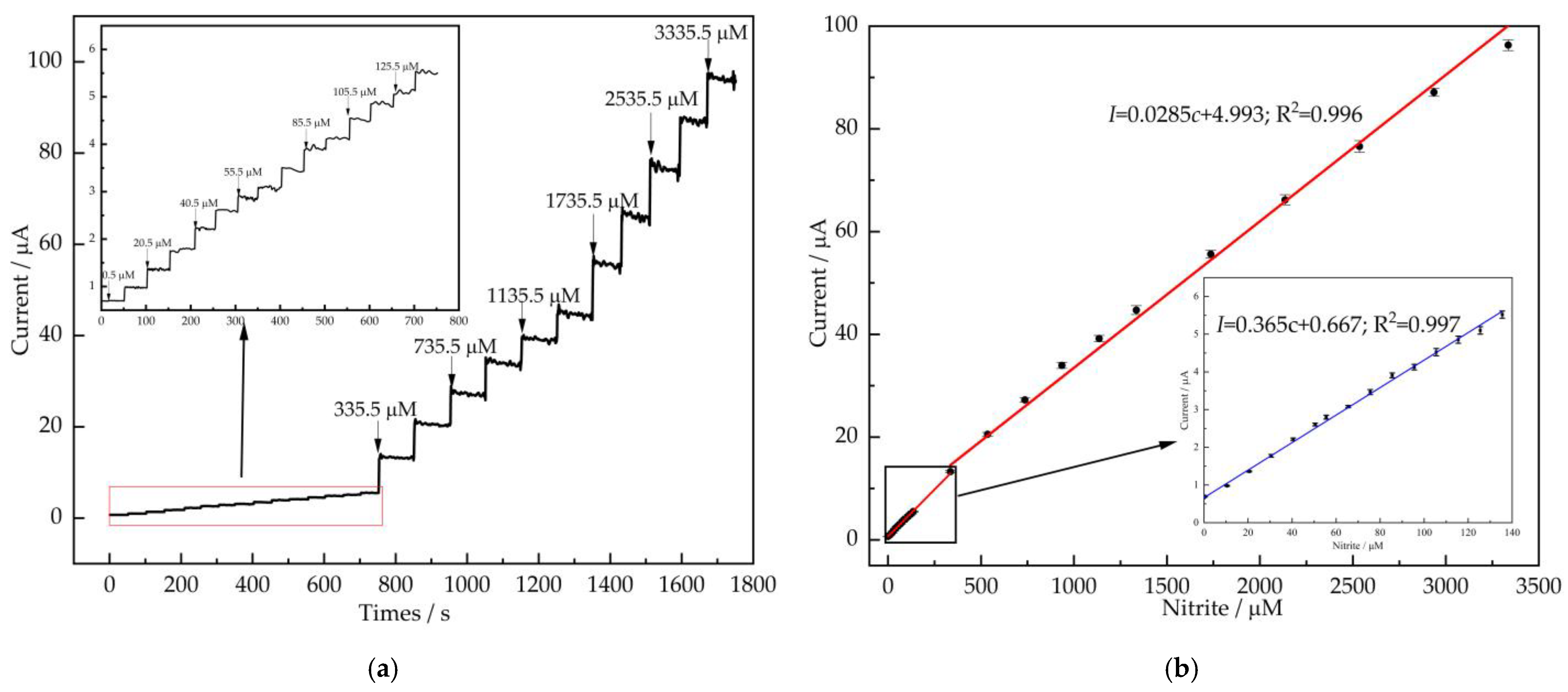
| Electrode Materials | Linear Range (μM) | LOD (μM) | Sensitivity (μA mM−1 cm−2) | Ref. |
|---|---|---|---|---|
| AuNPs/MoS2/GN | 5–5000 | 1 | N/A | [1] |
| MWCNTs/PPy-C | 5–9500 | 3.06 | 117.1 | [30] |
| MWCNTs/Co-MOFs | 80–1160 | 18.8 | 10 | [31] |
| Pt-Cu/GO | 0.2–9000 | 3 | 139.9 | [32] |
| rGO/ZnO/Nafion | 20–520 | 1.36 | 375.4 | [33] |
| PEDOT/PEDOT-SH/Au | 0.15–1000, 1000–16,000 | 0.051 | 301 133 | [34] |
| CeO2-SnO2/Pd | 0.36–2200 | 0.1 | 652.95 | [35] |
| AuNPs/CS/MXene | 0.5–335.5 335.5–3300 | 0.069 | 517.8 403.2 | This work |
| Sample | Added (μM) | This Method (μM) | UV-Vis Method (μM) | RSD (%) | t-Test |
|---|---|---|---|---|---|
| Tap water | 20 | 20.59 | 20.29 | 3.4 | 1.77 |
| River water | 20 | 20.78 | 20.61 | 2.3 | 1.14 |
| Sausage | 20 | 20.59 | 20.83 | 3.5 | 0.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Xu, X.; Wang, C.; Li, Z.; Li, D. A Novel Highly Sensitive Electrochemical Nitrite Sensor Based on a AuNPs/CS/Ti3C2 Nanocomposite. Nanomaterials 2022, 12, 397. https://doi.org/10.3390/nano12030397
Wang T, Xu X, Wang C, Li Z, Li D. A Novel Highly Sensitive Electrochemical Nitrite Sensor Based on a AuNPs/CS/Ti3C2 Nanocomposite. Nanomaterials. 2022; 12(3):397. https://doi.org/10.3390/nano12030397
Chicago/Turabian StyleWang, Tan, Xianbao Xu, Cong Wang, Zhen Li, and Daoliang Li. 2022. "A Novel Highly Sensitive Electrochemical Nitrite Sensor Based on a AuNPs/CS/Ti3C2 Nanocomposite" Nanomaterials 12, no. 3: 397. https://doi.org/10.3390/nano12030397
APA StyleWang, T., Xu, X., Wang, C., Li, Z., & Li, D. (2022). A Novel Highly Sensitive Electrochemical Nitrite Sensor Based on a AuNPs/CS/Ti3C2 Nanocomposite. Nanomaterials, 12(3), 397. https://doi.org/10.3390/nano12030397








