Label-Free Detection of Saxitoxin with Field-Effect Device-Based Biosensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Sensor Fabrication
2.3. LbL Immobilization of PAMAM Dendrimers/Apt and STX
2.4. Electrochemical Measurements
2.5. Characterizations Methods
3. Results and Discussion
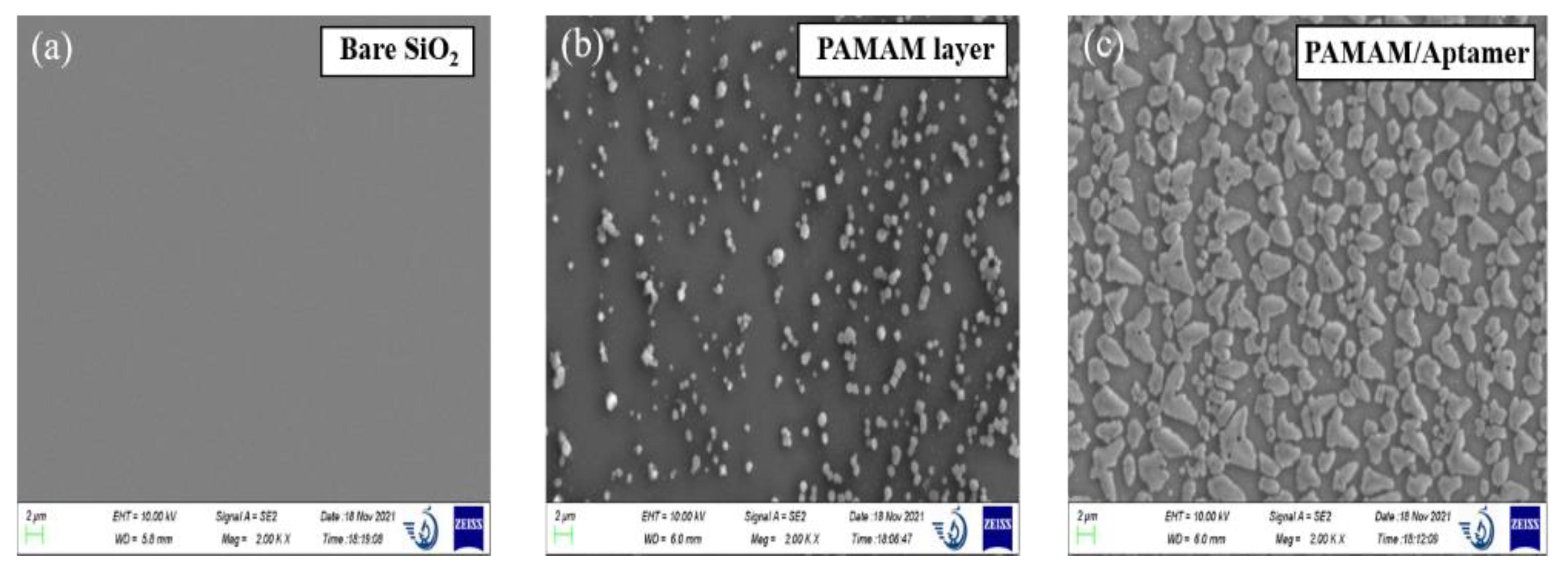
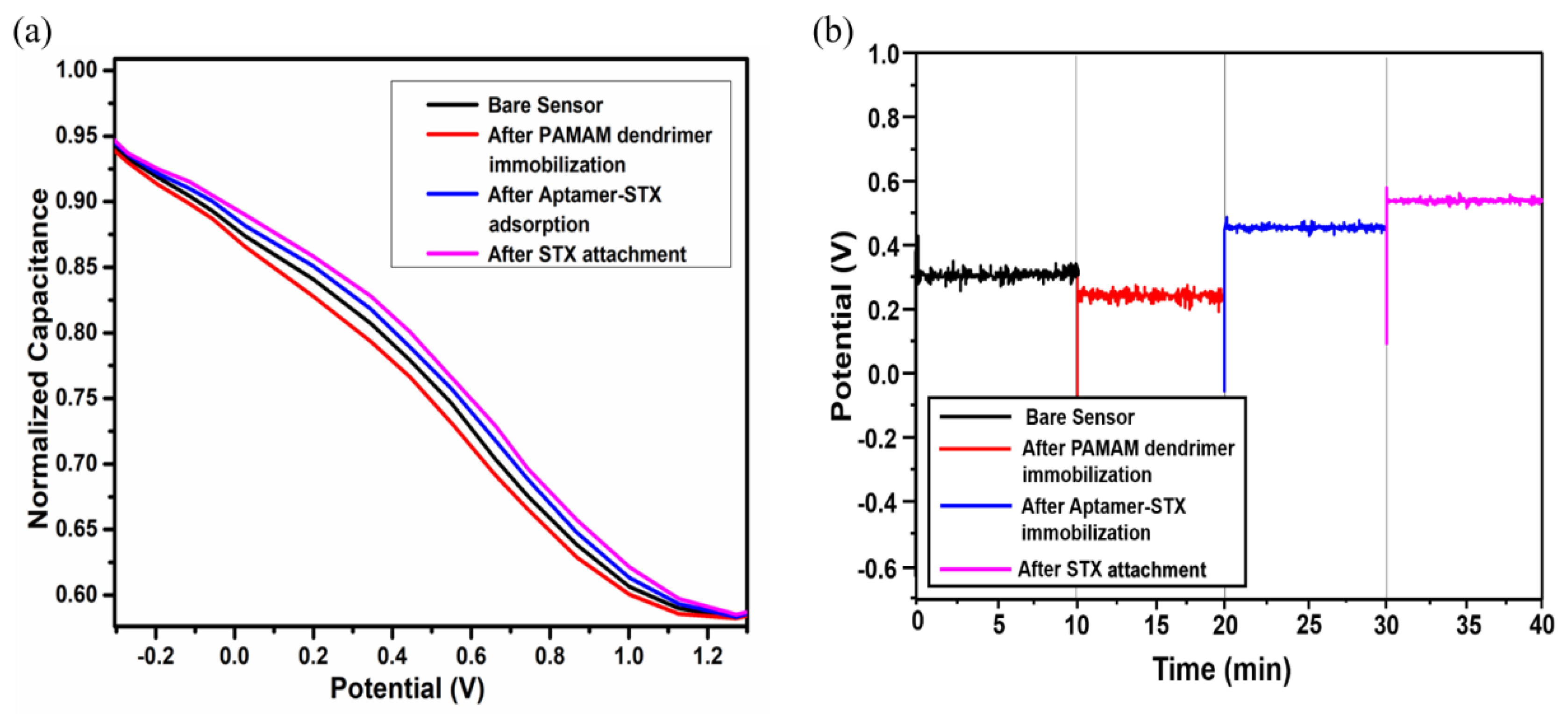
3.1. Characterization of Biosensor Preparation
3.2. Confirmation of Apt Immobilization
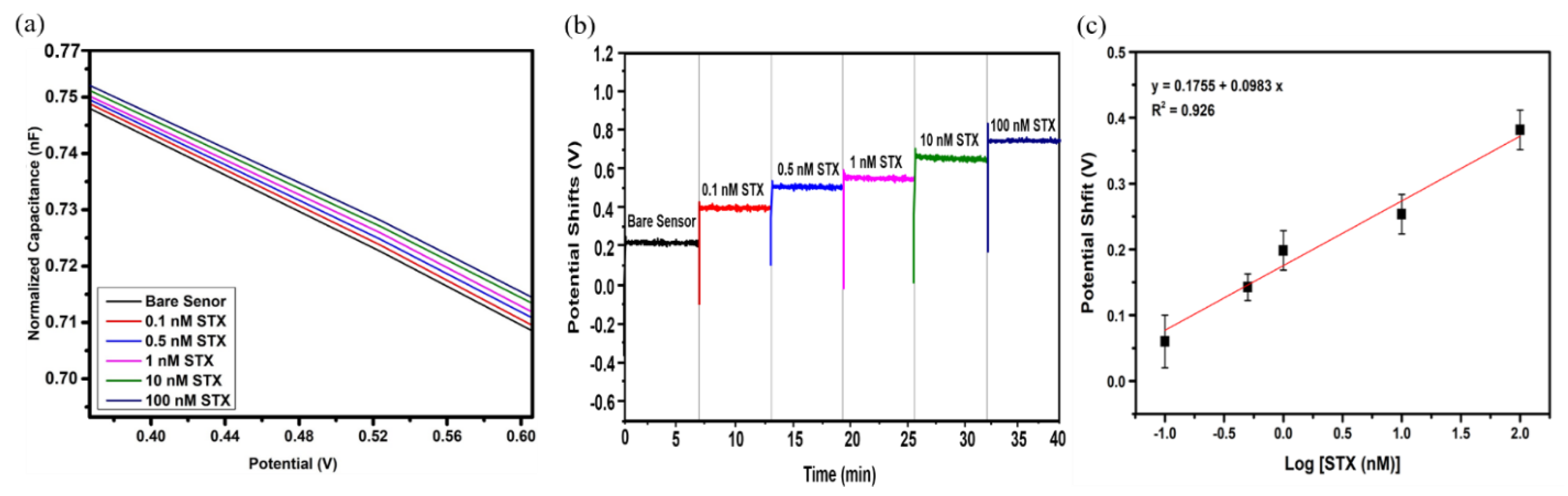
3.3. Real-Time Detection of Saxitoxin
3.4. STX Detection
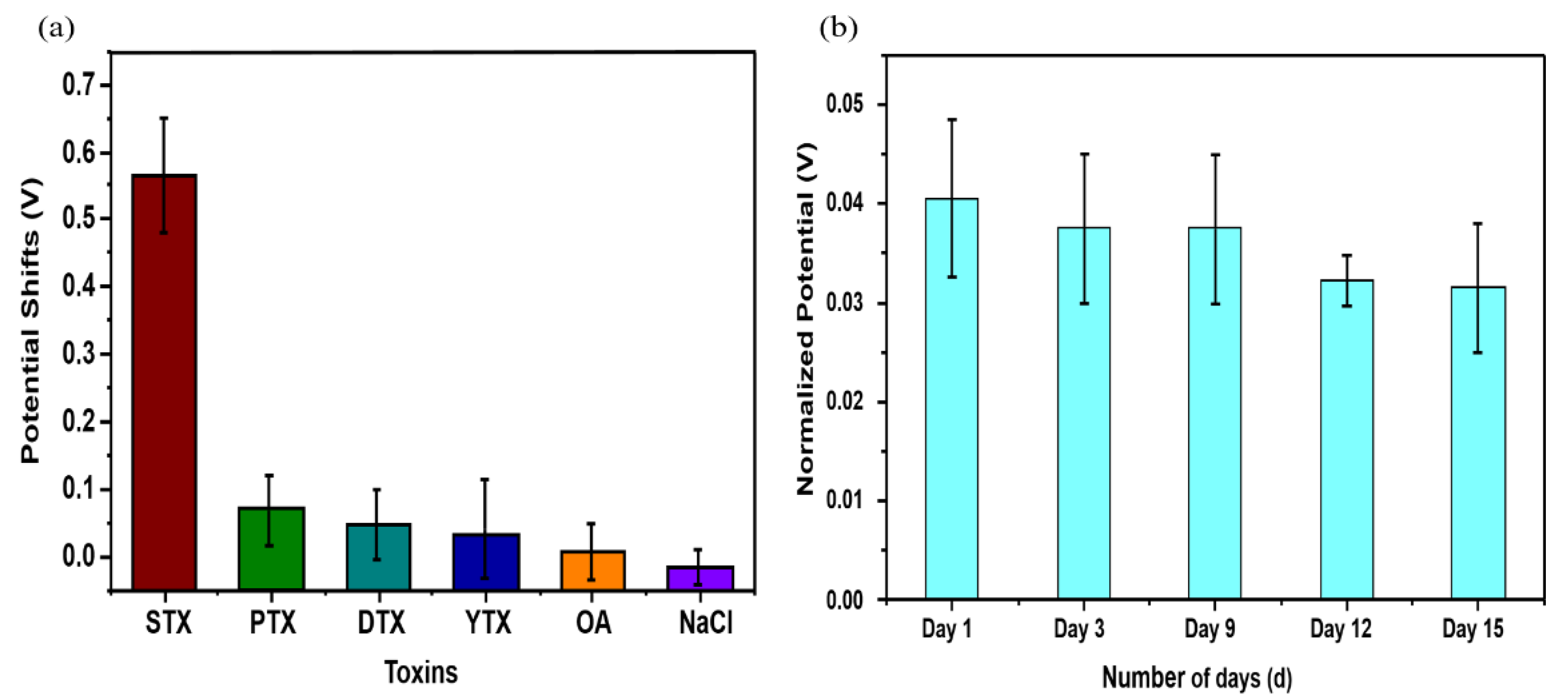
3.5. Sensor Selectivity and Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Shin, C.; Jo, H.; Kim, S.H.; Kang, G.J. Exposure assessment to paralytic shellfish toxins through the shellfish consumption in Korea. Food Res. Int. 2018, 108, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J.; Hoogenboom, R.L.; Hendriksen, P.J.; Bodero, M.; Bovee, T.F.; Rietjens, I.M.; Gerssen, A. Marine biotoxins and associated outbreaks following seafood consumption: Prevention and surveillance in the 21st century. Glob. Food Secur. 2017, 15, 11–21. [Google Scholar] [CrossRef]
- Cusick, K.D.; Sayler, G.S. An overview on the marine neurotoxin, saxitoxin: Genetics, molecular targets, methods of detection and ecological functions. Mar. Drugs 2013, 11, 991–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiese, M.; D’agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic alkaloids: Saxitoxin and its analogs. Mar. Drugs 2010, 8, 2185–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bratakou, S.; Nikoleli, G.-P.; Siontorou, C.G.; Nikolelis, D.P.; Karapetis, S.; Tzamtzis, N. Development of an Electrochemical Biosensor for the Rapid Detection of Saxitoxin Based on Air Stable Lipid Films with Incorporated Anti-STX Using Graphene Electrodes. Electroanalysis 2017, 29, 990–997. [Google Scholar] [CrossRef]
- Hou, L.; Jiang, L.; Song, Y.; Ding, Y.; Zhang, J.; Wu, X.; Tang, D. Amperometric aptasensor for saxitoxin using a gold electrode modified with carbon nanotubes on a self-assembled monolayer, and methylene blue as an electrochemical indicator probe. Microchim. Acta 2016, 183, 1971–1980. [Google Scholar] [CrossRef]
- Gao, S.; Zheng, X.; Wu, J. A biolayer interferometry-based competitive biosensor for rapid and sensitive detection of saxitoxin. Sens. Actuators B Chem. 2017, 246, 169–174. [Google Scholar] [CrossRef]
- Cheng, S.; Zheng, B.; Yao, D.; Kuai, S.; Tian, J.; Liang, H.; Ding, Y. Study of the binding way between saxitoxin and its aptamer and a fluorescent aptasensor for detection of saxitoxin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 204, 180–187. [Google Scholar] [CrossRef]
- Cheng, J.; Pi, S.; Ye, S.; Gao, H.; Yao, L.; Jiang, Z.; Song, Y.; Xi, L. A new simple screening method for the detection of paralytic shellfish poisoning toxins. Chin. J. Oceanol. Limnol. 2012, 30, 786–790. [Google Scholar] [CrossRef]
- Ling, S.; Xiao, S.; Xie, C.; Wang, R.; Zeng, L.; Wang, K.; Zhang, D.; Li, X.; Wang, S. Preparation of Monoclonal Antibody for Brevetoxin 1 and Development of Ic-ELISA and Colloidal Gold Strip to Detect Brevetoxin 1. Toxins 2018, 10, 75. [Google Scholar] [CrossRef] [Green Version]
- Halme, M.; Rapinoja, M.-L.; Karjalainen, M.; Vanninen, P. Verification and quantification of saxitoxin from algal samples using fast and validated hydrophilic interaction liquid chromatography-tandem mass spectrometry method. J. Chromatogr. B 2012, 880, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Krock, B.; Busch, J.A.; Tillmann, U.; García-Camacho, F.; Sánchez-Mirón, A.; Gallardo-Rodríguez, J.J.; López-Rosales, L.; Andree, K.B.; Fernández-Tejedor, M.; Witt, M.; et al. LC-MS/MS Detection of Karlotoxins Reveals New Variants in Strains of the Marine Dinoflagellate Karlodinium veneficum from the Ebro Delta (NW Mediterranean). Mar. Drugs 2017, 15, 391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, W.; Liu, T.; Zhang, W.; Zhu, M.; Liu, Z.; Kong, Y.; Liu, S. Marine Toxins Detection by Biosensors Based on Aptamers. Toxins 2020, 12, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.; Hu, B.; Gao, S.X.; Liu, D.J.; Sun, M.J.; Jiao, B.H.; Wang, L.H. A saxitoxin-binding aptamer with higher affinity and inhibitory activity optimized by rational site-directed mutagenesis and truncation. Toxicon 2015, 101, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Handy, S.M.; Yakes, B.J.; DeGrasse, J.A.; Campbell, K.; Elliott, C.T.; Kanyuck, K.M.; DeGrasse, S.L. First report of the use of a saxitoxin–protein conjugate to develop a DNA aptamer to a small molecule toxin. Toxicon 2013, 61, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Juska, V.B.; Pemble, M.E. A Critical Review of Electrochemical Glucose Sensing: Evolution of Biosensor Platforms Based on Advanced Nanosystems. Sensors 2020, 20, 6013. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, C.; Cai, H.; Hu, N.; Zhou, J.; Wang, P. Cell-Based Biosensors and Their Application in Biomedicine. Chem. Rev. 2014, 114, 6423–6461. [Google Scholar] [CrossRef]
- Kong, H.Y.; Byun, J. Nucleic Acid aptamers: New methods for selection, stabilization, and application in biomedical science. Biomol. Ther. 2013, 21, 423. [Google Scholar] [CrossRef] [Green Version]
- Bazin, I.; Tria, S.A.; Hayat, A.; Marty, J.L. New biorecognition molecules in biosensors for the detection of toxins. Biosens. Bioelectron. 2017, 87, 285–298. [Google Scholar] [CrossRef]
- Zhou, W.; Jimmy Huang, P.-J.; Ding, J.; Liu, J. Aptamer-based biosensors for biomedical diagnostics. Analyst 2014, 139, 2627–2640. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Aguado, J.A.; Penner, G. Determination of Ochratoxin A with a DNA Aptamer. J. Agric. Food Chem. 2008, 56, 10456–10461. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Ng, A.; Siaj, M.; Tavares, A.C.; Zourob, M. Selection and Identification of DNA Aptamers against Okadaic Acid for Biosensing Application. Anal. Chem. 2013, 85, 11794–11801. [Google Scholar] [CrossRef]
- Ng, A.; Chinnappan, R.; Eissa, S.; Liu, H.; Tlili, C.; Zourob, M. Selection, Characterization, and Biosensing Application of High Affinity Congener-Specific Microcystin-Targeting Aptamers. Environ. Sci. Technol. 2012, 46, 10697–10703. [Google Scholar] [CrossRef] [PubMed]
- Newkome, G.R.; Moorefield, C.N.; Vögtle, F.; Vögtle, F.; Vögtle, F.; Chemist, G. Dendrimers and Dendrons: Concepts, Syntheses, Applications; Wiley Online Library: New York, NY, USA, 2001; Volume 623. [Google Scholar]
- Zhang, X.-Q.; Wang, X.-L.; Huang, S.-W.; Zhuo, R.-X.; Liu, Z.-L.; Mao, H.-Q.; Leong, K.W. In vitro gene delivery using polyamidoamine dendrimers with a trimesyl core. Biomacromolecules 2005, 6, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.; Crooks, R.M. Interactions between organized, surface-confined monolayers and vapor-phase probe molecules. 10. Preparation and properties of chemically sensitive dendrimer surfaces. J. Am. Chem. Soc. 1996, 118, 3988–3989. [Google Scholar] [CrossRef]
- Tokuhisa, H.; Crooks, R.M. Interactions between organized, surface-confined monolayers and vapor-phase probe molecules. 12. Two new methods for surface-immobilization and functionalization of chemically sensitive dendrimer surfaces. Langmuir 1997, 13, 5608–5612. [Google Scholar] [CrossRef]
- Bustos, E.; Manríquez, J.; Orozco, G.; Godínez, L.A. Preparation, characterization, and electrocatalytic activity of surface anchored, Prussian Blue containing starburst PAMAM dendrimers on gold electrodes. Langmuir 2005, 21, 3013–3021. [Google Scholar] [CrossRef]
- Ullah, N.; Chen, W.; Noureen, B.; Tian, Y.; Du, L.; Wu, C.; Ma, J. An Electrochemical Ti3C2Tx Aptasensor for Sensitive and Label-Free Detection of Marine Biological Toxins. Sensors 2021, 21, 4938. [Google Scholar] [CrossRef]
- Noureen, B.; Ullah, N.; Tian, Y.; Du, L.; Chen, W.; Wu, C.; Wang, P. An electrochemical PAH-modified aptasensor for the label-free and highly-sensitive detection of saxitoxin. Talanta 2022, 240, 123185. [Google Scholar] [CrossRef]
- Gennady, A.E.; Tibor, H. Layer-by-Layer Polyelectrolyte Assembles Involving DNA as a Platform for DNA Sensors. Curr. Anal. Chem. 2011, 7, 8–34. [Google Scholar]
- Poghossian, A.; Weil, M.; Cherstvy, A.; Schöning, M.J. Electrical monitoring of polyelectrolyte multilayer formation by means of capacitive field-effect devices. Anal. Bioanal. Chem. 2013, 405, 6425–6436. [Google Scholar] [CrossRef] [PubMed]
- Schönhoff, M.; Ball, V.; Bausch, A.R.; Dejugnat, C.; Delorme, N.; Glinel, K.; Klitzing, R.v.; Steitz, R. Hydration and internal properties of polyelectrolyte multilayers. Colloids Surf. A Physicochem. Eng. Asp. 2007, 303, 14–29. [Google Scholar] [CrossRef]
- Matboo, S.A.; Nazari, S.; Niapour, A.; Niri, M.V.; Asgari, E.; Mokhtari, S. Antibacterial effect of TiO2 modified with poly-amidoamine dendrimer–G3 on S. aureus and E. coli in aqueous solutions. Water Sci. Technol. 2022, 85, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.N.; McCormick, M.; Barrett, C.J.; Reven, L.; Spiess, H.W. NMR studies of PAH/PSS polyelectrolyte multilayers adsorbed onto silica. Macromolecules 2004, 37, 4830–4838. [Google Scholar] [CrossRef]
- Bronder, T.S.; Poghossian, A.; Scheja, S.; Wu, C.; Keusgen, M.; Mewes, D.; Schöning, M.J. DNA immobilization and hybridization detection by the intrinsic molecular charge using capacitive field-effect sensors modified with a charged weak polyelectrolyte layer. ACS Appl. Mater. Interfaces 2015, 7, 20068–20075. [Google Scholar] [CrossRef]
- Wu, C.S.; Poghossian, A.; Bronder, T.S.; Schoning, M.J. Sensing of double-stranded DNA molecules by their intrinsic molecular charge using the light-addressable potentiometric sensor. Sens. Actuators B-Chem. 2016, 229, 506–512. [Google Scholar] [CrossRef]
- Chen, F.; Wang, J.; Du, L.; Zhang, X.; Zhang, F.; Chen, W.; Cai, W.; Wu, C.; Wang, P. Functional expression of olfactory receptors using cell-free expression system for biomimetic sensors towards odorant detection. Biosens. Bioelectron. 2019, 130, 382–388. [Google Scholar] [CrossRef]
- Zhu, N.; Gao, H.; Gu, Y.; Xu, Q.; He, P.; Fang, Y. PAMAM dendrimer-enhanced DNA biosensors based on electrochemical impedance spectroscopy. Analyst 2009, 134, 860–866. [Google Scholar] [CrossRef]
- Caglayan, M.O.; Üstündağ, Z. Saxitoxin aptasensor based on attenuated internal reflection ellipsometry for seafood. Toxicon Off. J. Int. Soc. Toxinol. 2020, 187, 255–261. [Google Scholar] [CrossRef]
- Qi, X.; Yan, X.; Zhao, L.; Huang, Y.; Wang, S.; Liang, X. A facile label-free electrochemical aptasensor constructed with nanotetrahedron and aptamer-triplex for sensitive detection of small molecule: Saxitoxin. J. Electroanal. Chem. 2020, 858, 113805. [Google Scholar] [CrossRef]
- Serrano, P.C.; Nunes, G.E.; Avila, L.B.; Reis, C.P.; Gomes, A.; Reis, F.T.; Sartorelli, M.L.; Melegari, S.P.; Matias, W.G.; Bechtold, I.H. Electrochemical impedance biosensor for detection of saxitoxin in aqueous solution. Anal. Bioanal. Chem. 2021, 413, 6393–6399. [Google Scholar] [CrossRef] [PubMed]

| Method | Detection Limit | Linear Range | References |
|---|---|---|---|
| Electrochemical aptasensor | 0.38 nM | 0.9–30 nM | [6] |
| Fluorescent aptasensor | 1.8 ng/mL | 0–24 ng/mL | [8] |
| SE (Spectroscopic Ellipsometry) | 0.11 ng/mL | 0.1–100 ng/mL | [40] |
| AIR-SE (Internal Reflection Spectroscopic Ellipsometry) | 0.01 ng/mL | 0.01–600 ng/mL | [40] |
| Electrochemical aptasensor | 0.92 nM | 1–400 nM | [41] |
| Electrochemical aptasensor | 0.3 µg/L | 0–30 µg/L | [42] |
| Electrochemical aptasensor (PAMAM dendrimers) | 0.09 nM | 0.5–100 nM | This work |
| S.No. | Added Conc. (nM) | Untreated Mussels | Treated Mussels | ||
|---|---|---|---|---|---|
| Found Conc. (nM) | Recovery% | Found Conc. (nM) | Recovery% | ||
| 1 | 0 | *ND | - | - | - |
| 2 | 20 | 21.774 ± 1.061 | 108.8 | 27.865 ± 0.915 | 114.3 |
| 3 | 40 | 41.244 ± 0.575 | 103.1 | 39.736 ± 1.110 | 99.3 |
| 4 | 90 | 89.761 ± 1.135 | 99.7 | 91.317 ± 1.030 | 101.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, N.; Noureen, B.; Tian, Y.; Du, L.; Chen, W.; Wu, C. Label-Free Detection of Saxitoxin with Field-Effect Device-Based Biosensor. Nanomaterials 2022, 12, 1505. https://doi.org/10.3390/nano12091505
Ullah N, Noureen B, Tian Y, Du L, Chen W, Wu C. Label-Free Detection of Saxitoxin with Field-Effect Device-Based Biosensor. Nanomaterials. 2022; 12(9):1505. https://doi.org/10.3390/nano12091505
Chicago/Turabian StyleUllah, Najeeb, Beenish Noureen, Yulan Tian, Liping Du, Wei Chen, and Chunsheng Wu. 2022. "Label-Free Detection of Saxitoxin with Field-Effect Device-Based Biosensor" Nanomaterials 12, no. 9: 1505. https://doi.org/10.3390/nano12091505
APA StyleUllah, N., Noureen, B., Tian, Y., Du, L., Chen, W., & Wu, C. (2022). Label-Free Detection of Saxitoxin with Field-Effect Device-Based Biosensor. Nanomaterials, 12(9), 1505. https://doi.org/10.3390/nano12091505









