Micro/Nanoarchitectonics of 3D Printed Scaffolds with Excellent Biocompatibility Prepared Using Femtosecond Laser Two-Photon Polymerization for Tissue Engineering Applications
Abstract
:1. Introduction
2. Materials and Methods
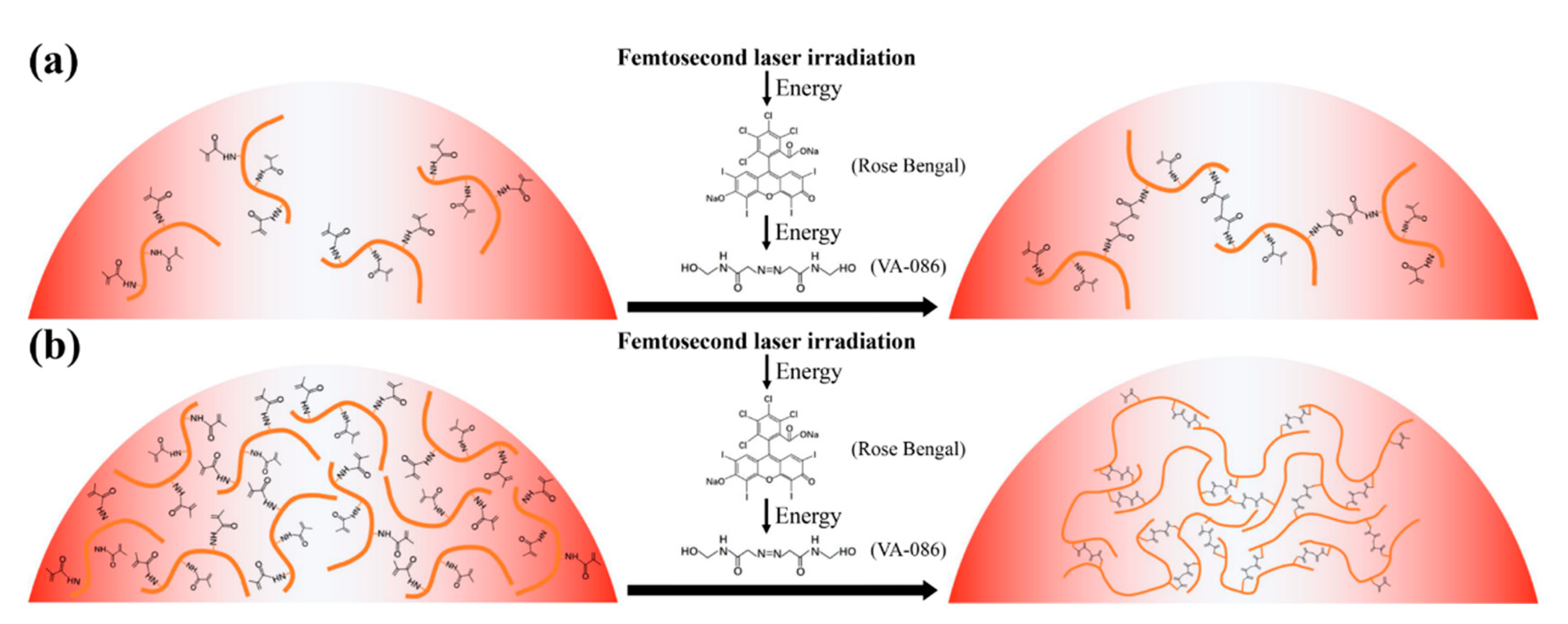
2.1. Configuration of Photosensitive GelMA Hydrogel Solutions
2.2. Biological Properties of GelMA Hydrogel Solutions
2.3. TPP Fabrication
2.4. Cell Attachment of 3D Scaffolds
3. Results and Discussion
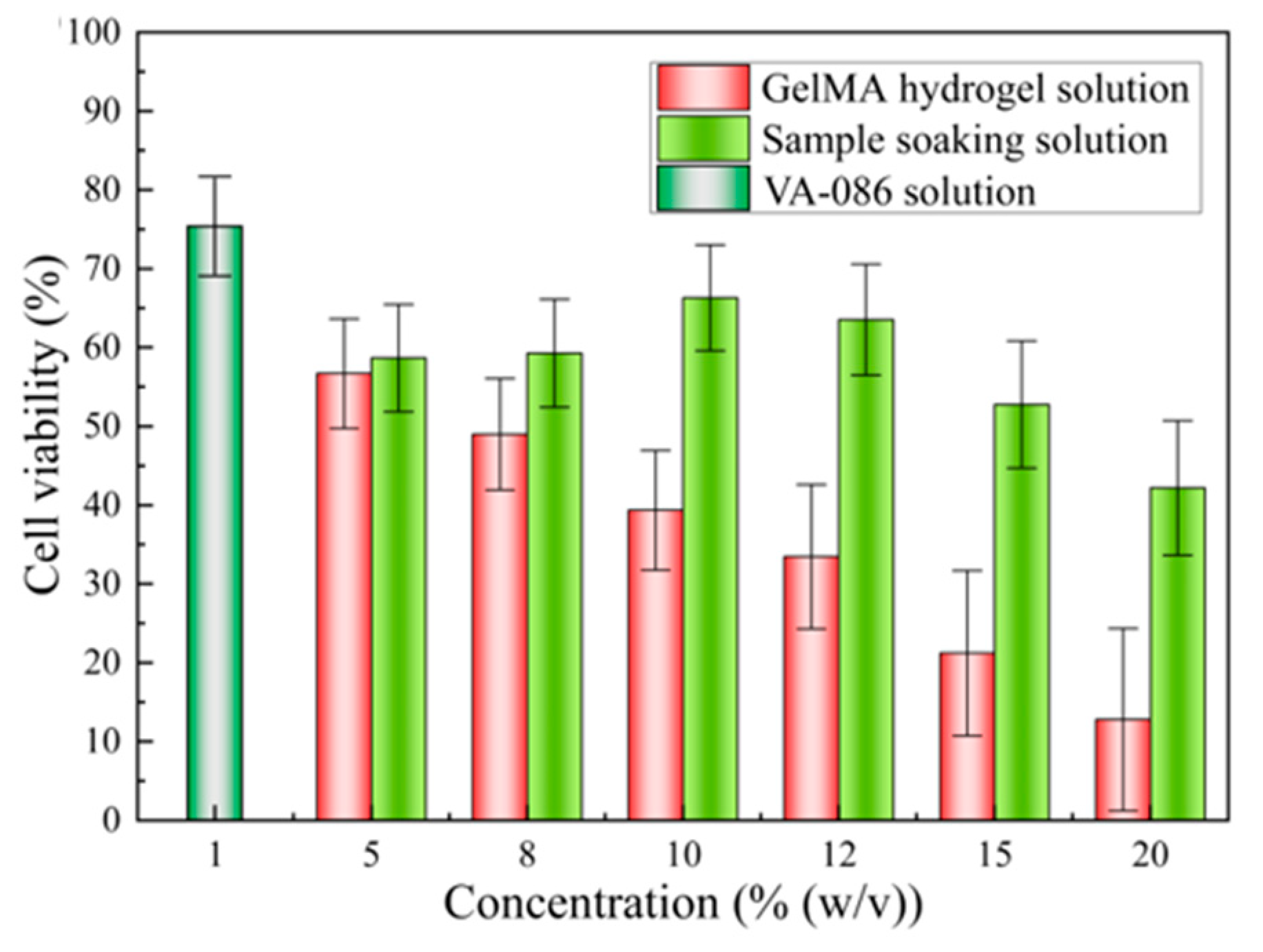
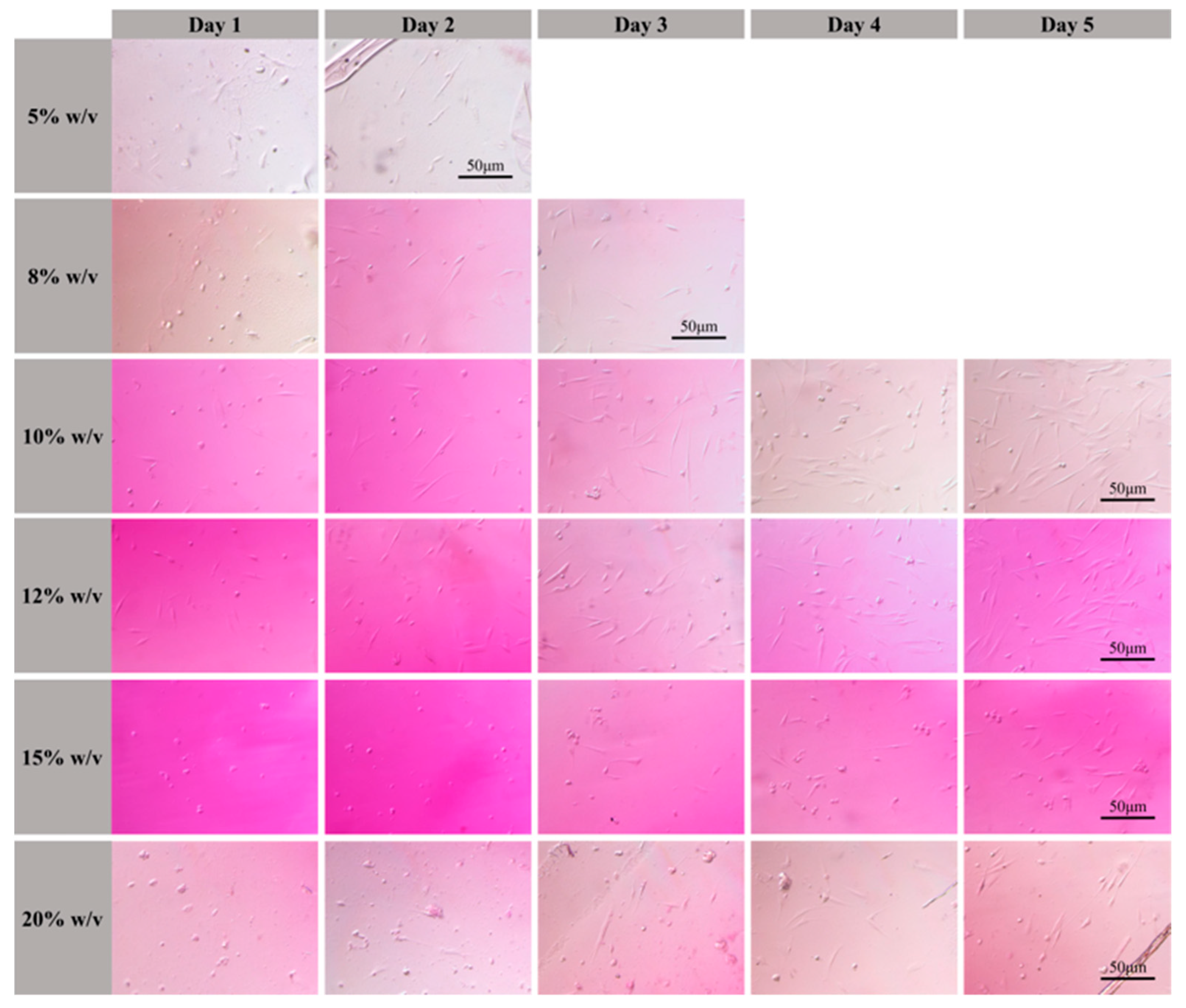
3.1. Biological Properties of GelMA Hydrogel Solutions
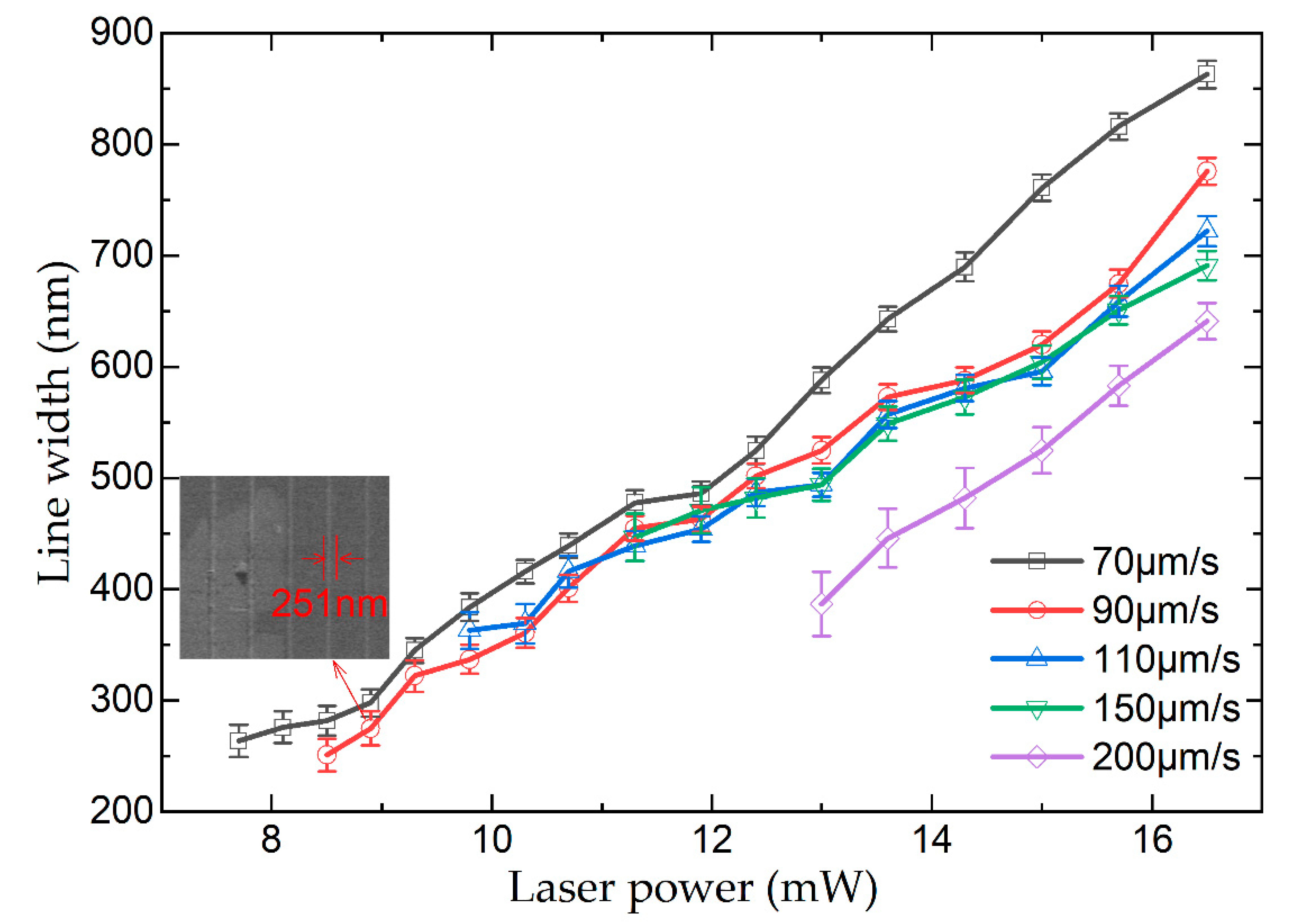
3.2. Resolution of Polymer Lines
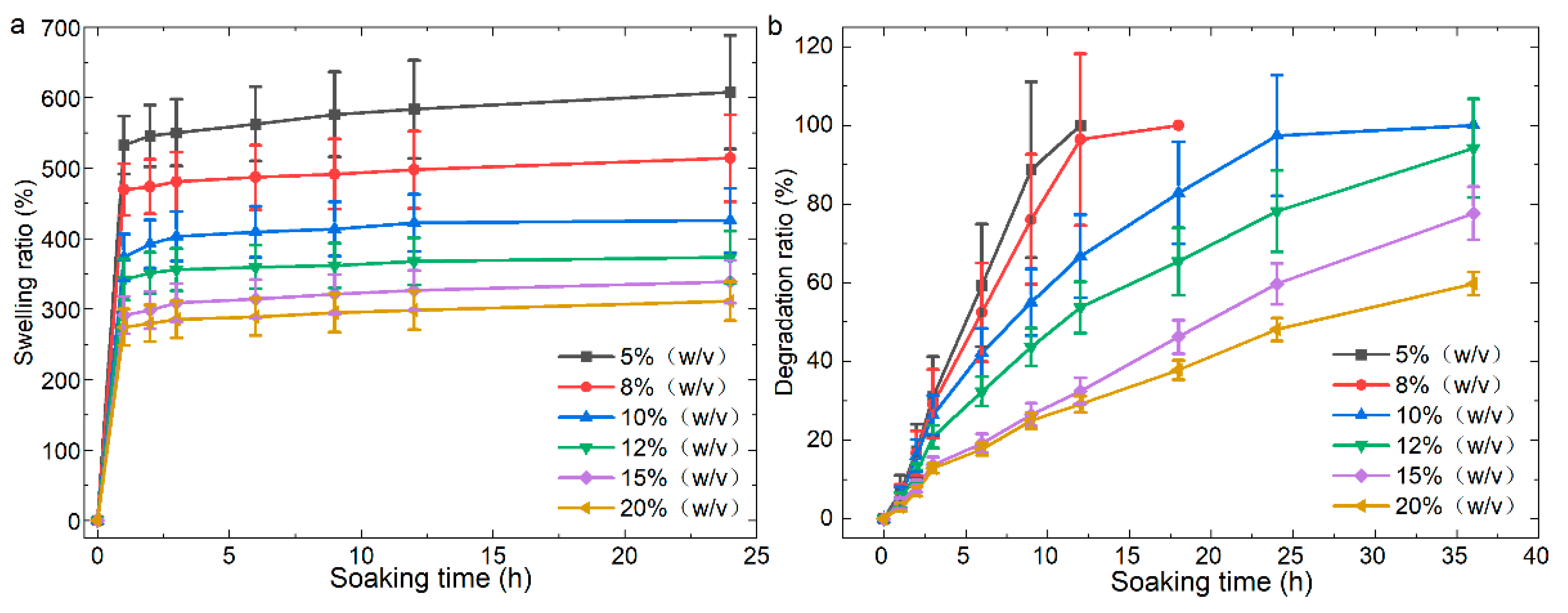
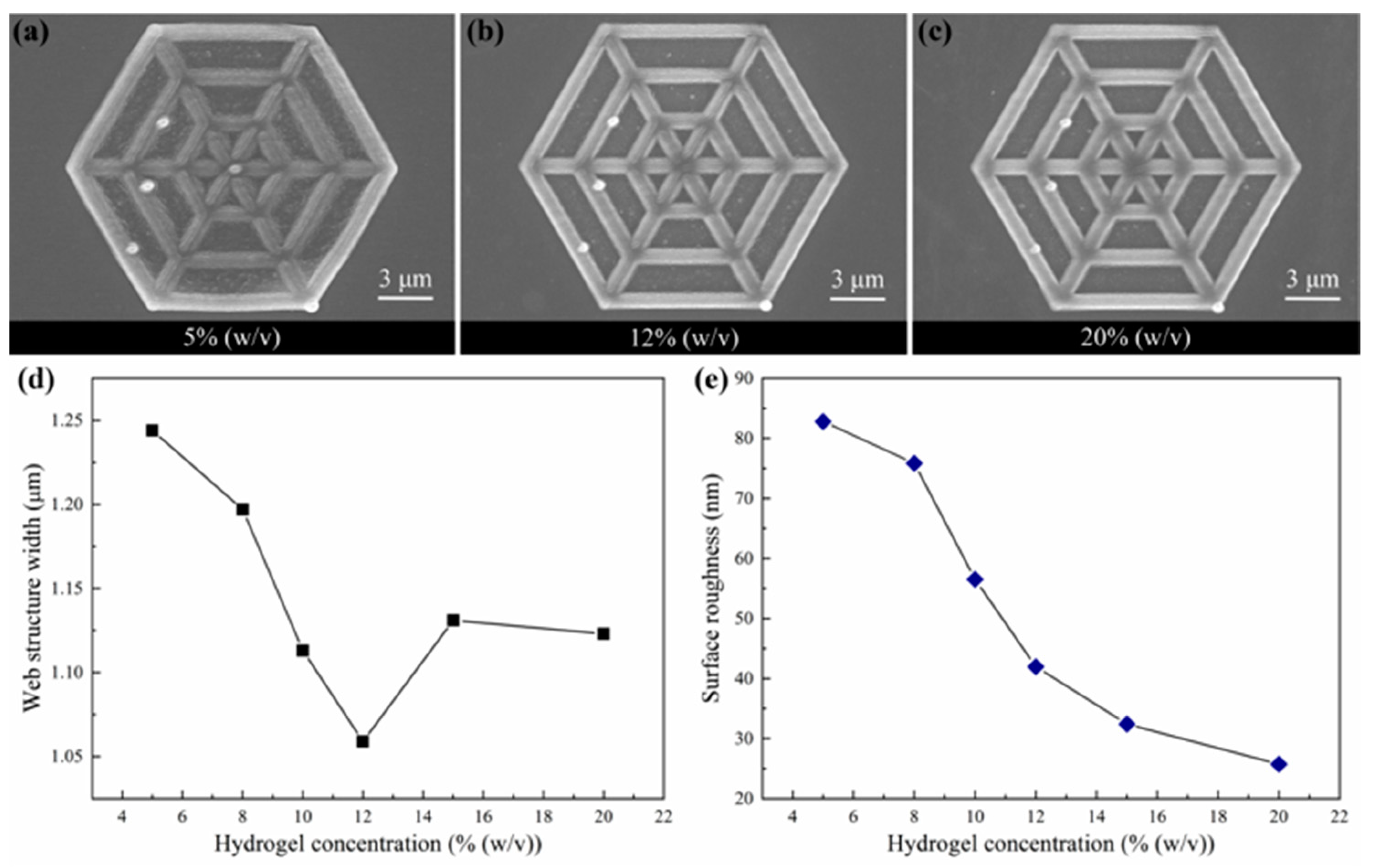
3.3. Effect of GelMA Hydrogel Concentration on the Properties of the 3D Scaffolds
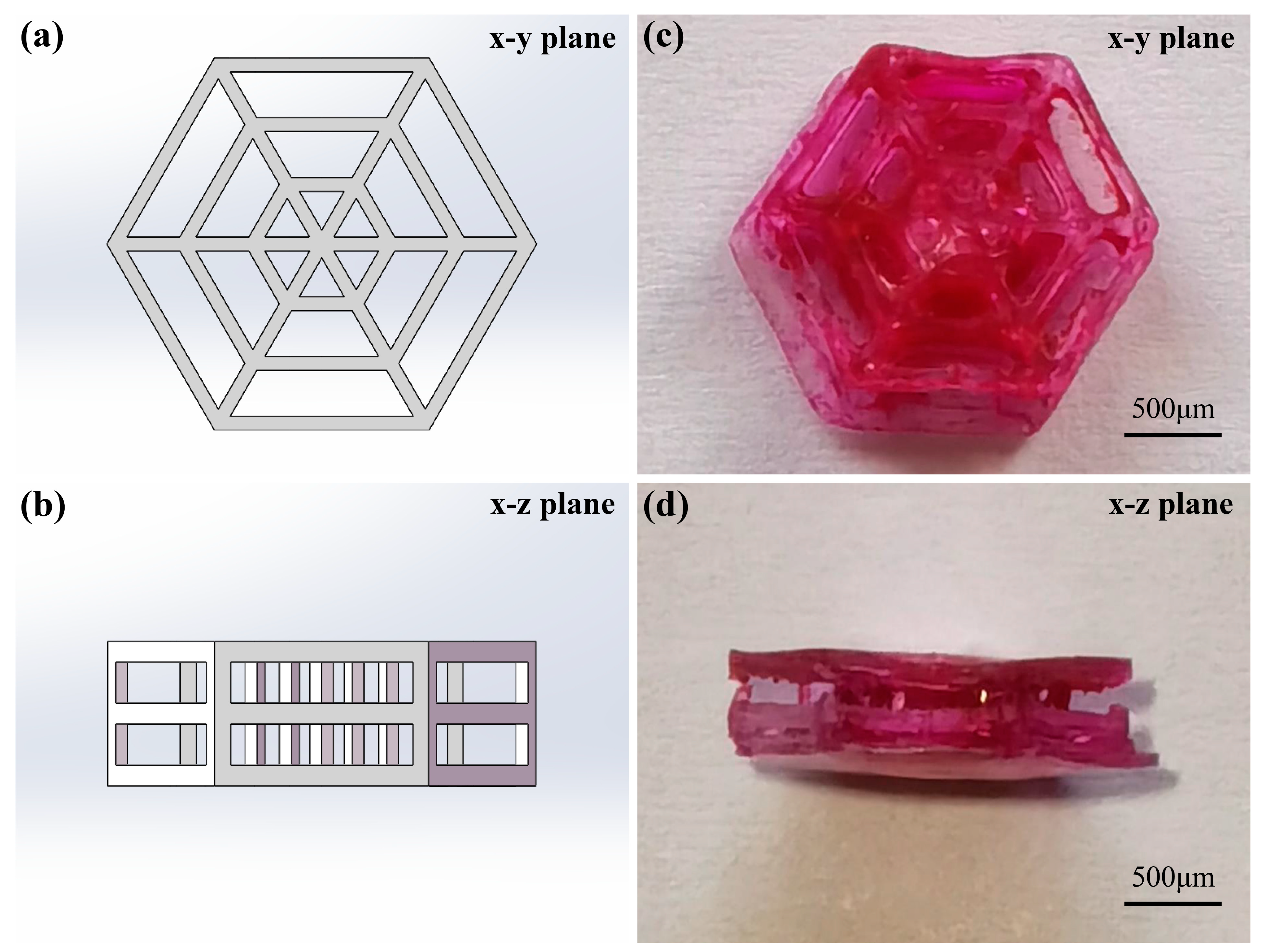
3.4. Fabrication of 3D Scaffolds
3.5. Cell Shape on 3D Scaffolds
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Fiqi, A.; Kim, J.H.; Kim, H.W. Novel bone-mimetic nanohydroxyapatite/collagen porous scaffolds biomimetically mineralized from surface silanized mesoporous nanobioglass/collagen hybrid scaffold: Physicochemical, mechanical and in vivo evaluations. Mater. Sci. Eng. C 2020, 110, 110660. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Ding, Y.; Liang, Z.; Sun, H.; Zheng, F.; Zhu, Z.; Fong, H. Three-dimensional monolithic porous structures assembled from fragmented electrospun nanofiber mats/membranes: Methods, properties, and applications. Prog. Mater. Sci. 2020, 112, 100656. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, J.; Fan, D. Fabrication of high-strength and porous hybrid scaffolds based on nano-hydroxyapatite and human-like collagen for bone tissue regeneration. Polymers 2020, 12, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswal, T. Biopolymers for tissue engineering applications: A review. Mater. Today Proc. 2021, 41, 397–402. [Google Scholar] [CrossRef]
- Bao, W.; Li, M.; Yang, Y.; Wan, Y.; Wang, X.; Bi, N.; Li, C. Advancements and frontiers in the high performance of natural hydrogels for cartilage tissue engineering. Front. Chem. 2020, 8, 53. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Xue, B.; Cao, Y. 100th anniversary of macromolecular science viewpoint: Synthetic protein hydrogels. ACS Macro Lett. 2020, 9, 512–524. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Ramesh, S. Fundamental concepts of hydrogels: Synthesis, properties, and their applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Batalov, I.; Stevens, K.R.; DeForest, C.A. Photopatterned biomolecule immobilization to guide three-dimensional cell fate in natural protein-based hydrogels. Proc. Natl. Acad. Sci. USA 2021, 118, e2014194118. [Google Scholar] [CrossRef]
- Mandal, A.; Clegg, J.R.; Anselmo, A.C.; Mitragotri, S. Hydrogels in the clinic. Bioeng. Transl. Med. 2020, 5, e10158. [Google Scholar] [CrossRef] [Green Version]
- Yoon, D.M.; Fisher, J.P. Natural and synthetic polymeric scaffolds. In Biomedical Materials; Springer: Cham, Switzerland, 2021; pp. 257–283. [Google Scholar]
- Dong, Q.; Li, Y.; Jiang, H.; Zhou, X.; Liu, H.; Lu, M.; Bai, J. 3D-cubic interconnected porous Mg-based scaffolds for bone repair. J. Magnes. Alloy. 2021, 9, 1329–1338. [Google Scholar] [CrossRef]
- Zhang, W.C.; Zheng, M.L.; Liu, J.; Jin, F.; Dong, X.Z.; Guo, M.; Li, T. Modulation of Cell Behavior by 3D Biocompatible Hydrogel Microscaffolds with Precise Configuration. Nanomaterials 2021, 11, 2325. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, P.; Shamloo, A. Fabrication of a novel 3D scaffold for cartilage tissue repair: In-vitro and in-vivo study. Mater. Sci. Eng. C 2021, 128, 112285. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Shafiee, A. Engineering bioactive scaffolds for skin regeneration. Small 2021, 17, 2101384. [Google Scholar] [CrossRef] [PubMed]
- Langridge, B.; Griffin, M.; Butler, P.E. Regenerative medicine for skeletal muscle loss: A review of current tissue engineering approaches. J. Mater. Sci. Mater. Med. 2021, 32, 15. [Google Scholar] [CrossRef]
- Wang, X.; Yu, Y.; Yang, C.; Shao, C.; Shi, K.; Shang, L.; Zhao, Y. Microfluidic 3D Printing Responsive Scaffolds with Biomimetic Enrichment Channels for Bone Regeneration. Adv. Funct. Mater. 2021, 31, 2105190. [Google Scholar] [CrossRef]
- Jang, J.; Park, H.J.; Kim, S.W.; Kim, H.; Park, J.Y.; Na, S.J.; Cho, D.W. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials 2017, 112, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Shahzadi, S.; Ishtiaq, I.; Aslam, K. 3D bioprinting–a step towards heart tissue regeneration. J. Appl. Biotechnol. Bioeng. 2021, 8, 1–4. [Google Scholar] [CrossRef]
- Tse, C.; Whiteley, R.; Yu, T.; Stringer, J.; MacNeil, S.; Haycock, J.W.; Smith, P.J. Inkjet printing Schwann cells and neuronal analogue NG108-15 cells. Biofabrication 2016, 8, 015017. [Google Scholar] [CrossRef]
- Blaeser, A.; Duarte Campos, D.F.; Puster, U.; Richtering, W.; Stevens, M.M.; Fischer, H. Controlling shear stress in 3D bioprinting is a key factor to balance printing resolution and stem cell integrity. Adv. Healthc. Mater. 2016, 5, 326–333. [Google Scholar] [CrossRef]
- Aytac, Z.; Dubey, N.; Daghrery, A.; Ferreira, J.A.; de Souza Araújo, I.J.; Castilho, M.; Bottino, M.C. Innovations in craniofacial bone and periodontal tissue engineering–from electrospinning to converged biofabrication. Int. Mater. Rev. 2021, 1–38. [Google Scholar] [CrossRef]
- Isaacson, A.; Swioklo, S.; Connon, C.J. 3D bioprinting of a corneal stroma equivalent. Exp. Eye Res. 2018, 173, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Bejoy, A.M.; Makkithaya, K.N.; Hunakunti, B.B.; Hegde, A.; Krishnamurthy, K.; Sarkar, A.; Mazumder, N. An insight on advances and applications of 3d bioprinting: A review. Bioprinting 2021, 24, e00176. [Google Scholar] [CrossRef]
- Ye, W.; Li, H.; Yu, K.; Xie, C.; Wang, P.; Zheng, Y.; Gao, Q. 3D printing of gelatin methacrylate-based nerve guidance conduits with multiple channels. Mater. Des. 2020, 192, 108757. [Google Scholar] [CrossRef]
- Shen, Y.; Tang, H.; Huang, X.; Hang, R.; Zhang, X.; Wang, Y.; Yao, X. DLP printing photocurable chitosan to build bio-constructs for tissue engineering. Carbohydr. Polym. 2020, 235, 115970. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, W.; Mille, L.S.; Ching, T.; Luo, Z.; Tang, G.; Zhang, Y.S. Digital Light Processing Based Bioprinting with Composable Gradients. Adv. Mater. 2021, 34, 2107038. [Google Scholar] [CrossRef] [PubMed]
- Morris, V.B.; Nimbalkar, S.; Younesi, M.; McClellan, P.; Akkus, O. Mechanical properties, cytocompatibility and manufacturability of chitosan: PEGDA hybrid-gel scaffolds by stereolithography. Ann. Biomed. Eng. 2017, 45, 286–296. [Google Scholar] [CrossRef]
- Kawata, S.; Sun, H.B.; Tanaka, T.; Takada, K. Finer features for functional microdevices. Nature 2001, 412, 697–698. [Google Scholar] [CrossRef]
- Tromayer, M.; Gruber, P.; Markovic, M.; Rosspeintner, A.; Vauthey, E.; Redl, H.; Liska, R. A biocompatible macromolecular two-photon initiator based on hyaluronan. Polym. Chem. 2017, 8, 451–460. [Google Scholar] [CrossRef] [Green Version]
- Brigo, L.; Urciuolo, A.; Giulitti, S.; Della Giustina, G.; Tromayer, M.; Liska, R.; Brusatin, G. 3D high-resolution two-photon crosslinked hydrogel structures for biological studies. Acta Biomater. 2017, 55, 373–384. [Google Scholar] [CrossRef]
- Ovsianikov, A.; Mühleder, S.; Torgersen, J.; Li, Z.; Qin, X.H.; Van Vlierberghe, S.; Stampfl, J. Laser photofabrication of cell-containing hydrogel constructs. Langmuir 2014, 30, 3787–3794. [Google Scholar] [CrossRef]
- Pennacchio, F.A.; Fedele, C.; De Martino, S.; Cavalli, S.; Vecchione, R.; Netti, P.A. Three-dimensional microstructured azobenzene-containing gelatin as a photoactuable cell confining system. ACS Appl. Mater. Interfaces 2018, 10, 91–97. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Chen, L.; Shi, Z.; Chen, J. Micro/Nanoarchitectonics of 3D Printed Scaffolds with Excellent Biocompatibility Prepared Using Femtosecond Laser Two-Photon Polymerization for Tissue Engineering Applications. Nanomaterials 2022, 12, 391. https://doi.org/10.3390/nano12030391
Yuan Y, Chen L, Shi Z, Chen J. Micro/Nanoarchitectonics of 3D Printed Scaffolds with Excellent Biocompatibility Prepared Using Femtosecond Laser Two-Photon Polymerization for Tissue Engineering Applications. Nanomaterials. 2022; 12(3):391. https://doi.org/10.3390/nano12030391
Chicago/Turabian StyleYuan, Yanping, Lei Chen, Ziyuan Shi, and Jimin Chen. 2022. "Micro/Nanoarchitectonics of 3D Printed Scaffolds with Excellent Biocompatibility Prepared Using Femtosecond Laser Two-Photon Polymerization for Tissue Engineering Applications" Nanomaterials 12, no. 3: 391. https://doi.org/10.3390/nano12030391
APA StyleYuan, Y., Chen, L., Shi, Z., & Chen, J. (2022). Micro/Nanoarchitectonics of 3D Printed Scaffolds with Excellent Biocompatibility Prepared Using Femtosecond Laser Two-Photon Polymerization for Tissue Engineering Applications. Nanomaterials, 12(3), 391. https://doi.org/10.3390/nano12030391




