Electron-Beam-Induced Fluorination Cycle for Long-Term Preservation of Graphene under Ambient Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Device Fabrication
2.2. Graphene Aging and Fluorination/Defluorination
2.3. Characterizations
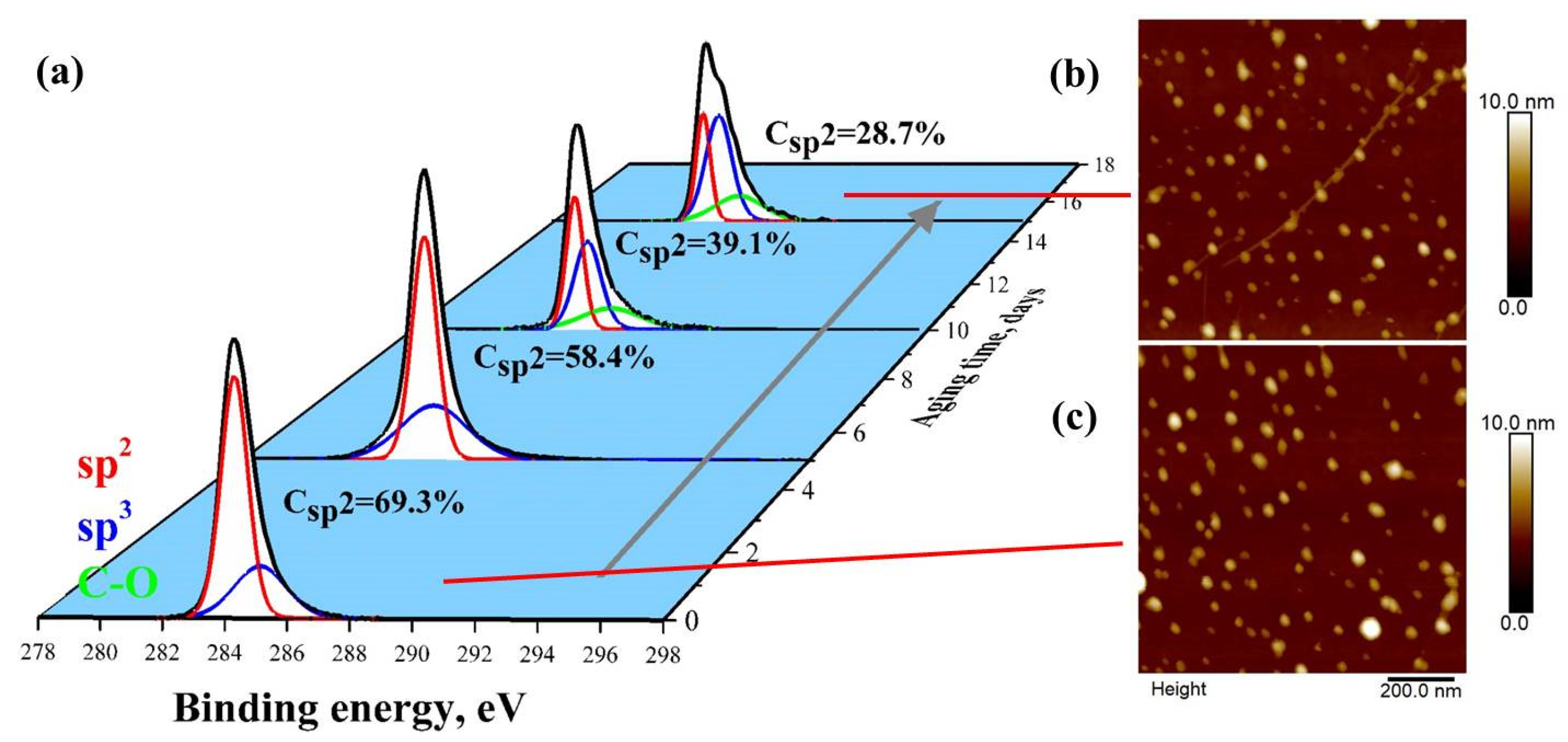
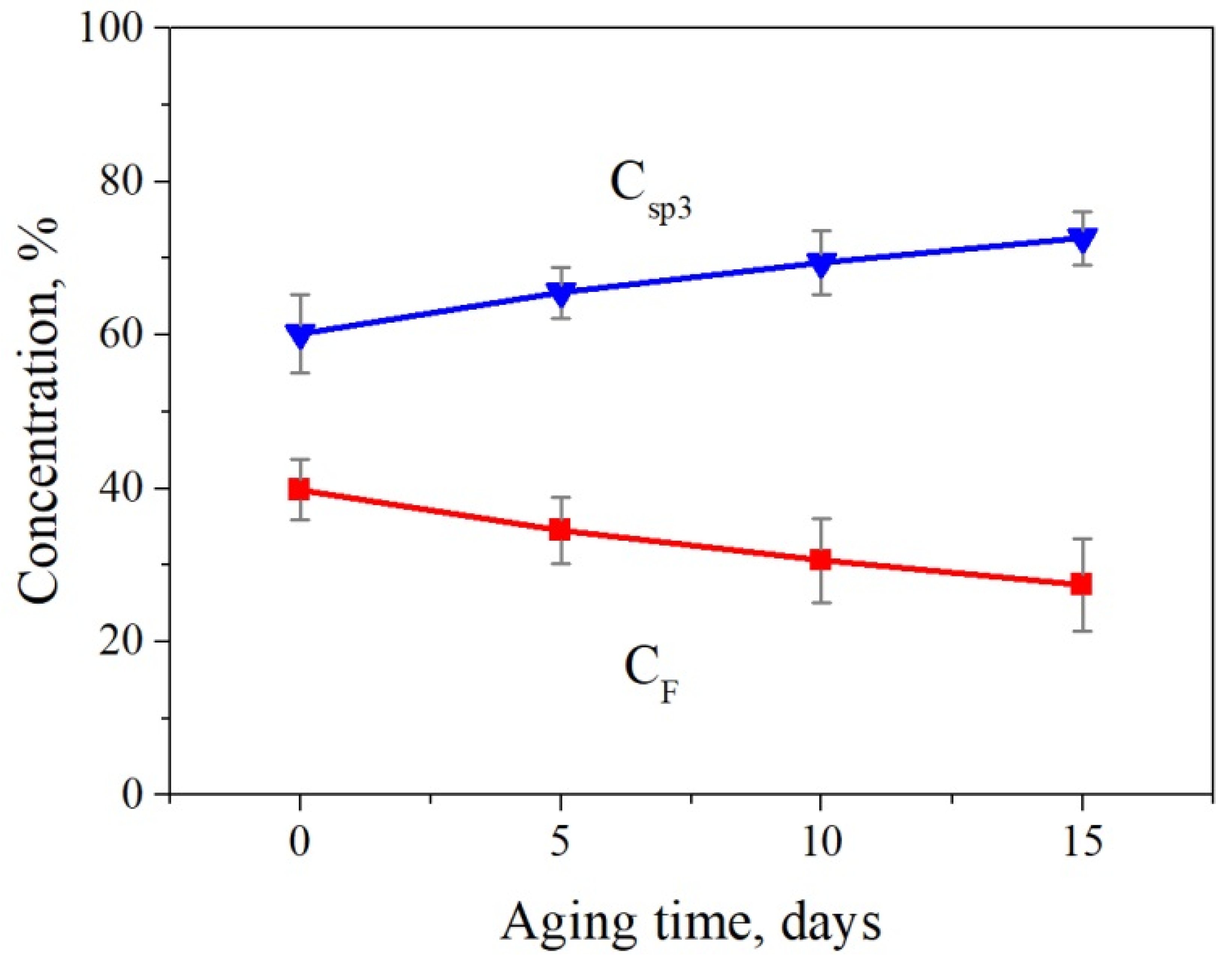
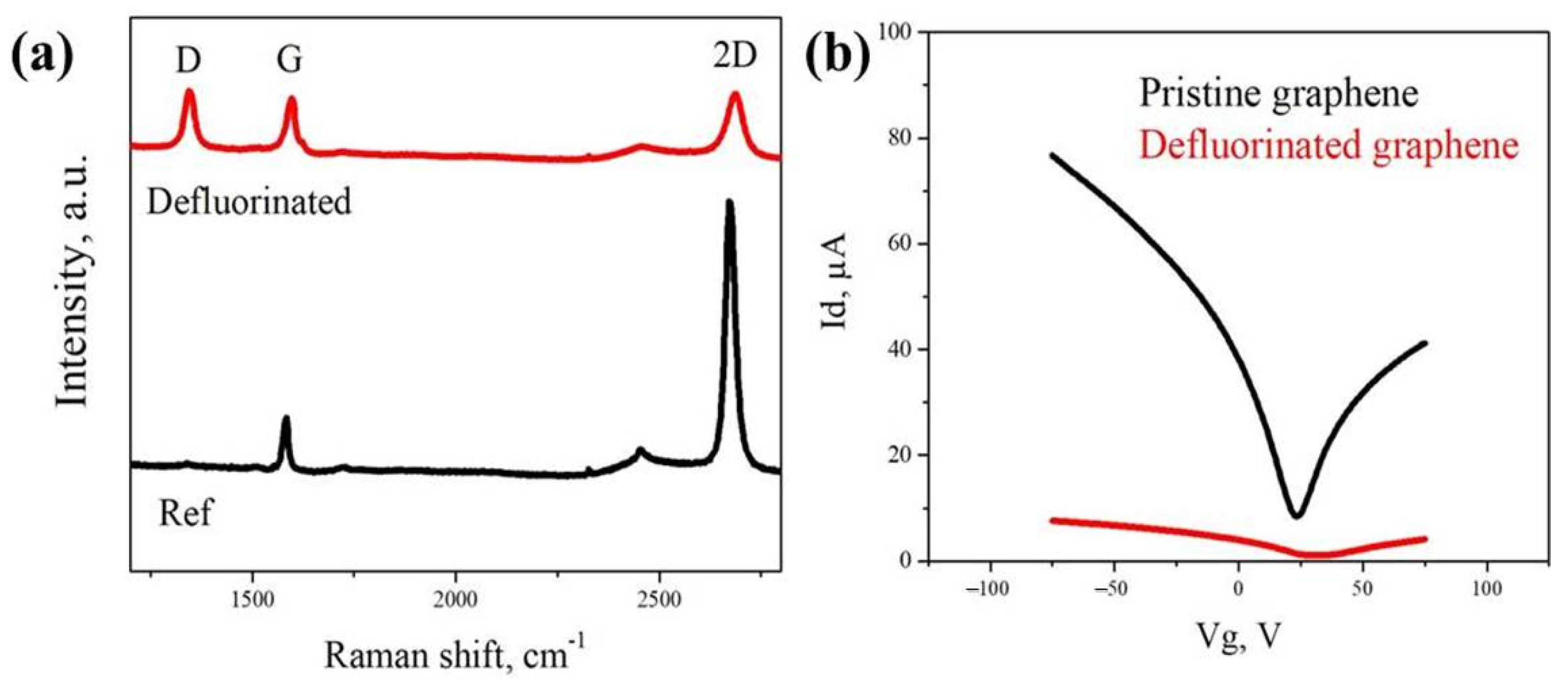
3. Results
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bonaccorso, F.; Sun, Z.; Hasan, T.; Ferrari, A.C. Graphene photonics and optoelectronics. Nat. Photonics 2010, 4, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.-Y.; Tang, T.-C.; Amadei, C.A.; Marsden, A.J.; Verdaguer, A.; Wilson, N.; Chiesa, M. A nanoscopic approach to studying evolution in graphene wettability. Carbon 2014, 80, 784–792. [Google Scholar] [CrossRef]
- Decker, R.; Wang, Y.; Brar, V.W.; Regan, W.; Tsai, H.Z.; Wu, Q.; Gannett, W.; Zettl, A.; Crommie, M.F. Local Electronic Properties of Graphene on a BN Substrate via Scanning Tunneling Microscopy. Nano Lett. 2011, 11, 2291–2295. [Google Scholar] [CrossRef] [PubMed]
- Zan, R.; Bangert, U.; Ramasse, Q.; Novoselov, K.S. Metal-Graphene Interaction Studied via Atomic Resolution Scanning Transmission Electron Microscopy. Nano Lett. 2011, 11, 1087–1092. [Google Scholar] [CrossRef]
- Papadakis, R.; Li, H.; Bergman, J.; Lundstedt, A.; Jorner, K.; Ayub, R.; Haldar, S.; Jahn, B.O.; Denisova, A.; Zietz, B.; et al. Metal-free photochemical silylations and transfer hydrogenations of benzenoid hydrocarbons and graphene. Nat. Commun. 2016, 7, 12962. [Google Scholar] [CrossRef]
- Meyer, J.C.; Girit, C.O.; Crommie, M.F.; Zettl, A. Imaging and dynamics of light atoms and molecules on graphene. Nature 2008, 454, 319–322. [Google Scholar] [CrossRef] [Green Version]
- Bachmatiuk, A.; Zhao, J.; Gorantla, S.M.; Martinez, I.G.G.; Wiedermann, J.; Lee, C.; Eckert, J.; Rummeli, M.H. Low Voltage Transmission Electron Microscopy of Graphene. Small 2015, 11, 515–542. [Google Scholar] [CrossRef]
- Dyck, O.; Kim, S.; Kalinin, S.V.; Jesse, S. Mitigating e-beam-induced hydrocarbon deposition on graphene for atomic-scale scanning transmission electron microscopy studies. J. Vac. Sci. Technol. B 2018, 36, 011801. [Google Scholar] [CrossRef]
- Smith, P.J.; Lindley, P.M. Analysis of organic contamination in semiconductor processing. Aip Conf. Proc. 1998, 449, 133–139. [Google Scholar]
- Kurokawa, A.; Odaka, K.; Azuma, Y.; Fujimoto, T.; Kojima, I. Diagnosis and cleaning of carbon contamination on SiO2 thin film. J. Surf. Anal. 2009, 15, 337–340. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.C.; Jeon, D.Y.; Chung, H.J.; Woo, Y.; Shin, J.K.; Seo, S. The structural and electrical evolution of graphene by oxygen plasma-induced disorder. Nanotechnology 2009, 20, 375703. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.T.; Wang, Y.J.; Kozbial, A.; Shenoy, G.; Zhou, F.; McGinley, R.; Ireland, P.; Morganstein, B.; Kunkel, A.; Surwade, S.P.; et al. Effect of airborne contaminants on the wettability of supported graphene and graphite. Nat. Mater. 2013, 12, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Lu, C.C.; Yeh, C.H.; Jin, C.H.; Suenaga, K.; Chiu, P.W. Graphene Annealing: How Clean Can It Be? Nano Lett. 2012, 12, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Duan, T.B.; Haldar, S.; Sanyal, B.; Eriksson, O.; Jafri, S.H.M.; Hajjar-Garreau, S.; Simon, L.; Leifer, K. Direct writing of lateral fluorographene nanopatterns with tunable bandgaps and its application in new generation of moiré superlattice. Appl. Phys. Rev. 2020, 7, 011403. [Google Scholar] [CrossRef]
- Burkert, S.; Kuntzsch, M.; Bellmann, C.; Uhlmann, P.; Stamm, M. Tuning of surface properties of thin polymer films by electron beam treatment. Appl. Surf. Sci. 2009, 255, 6256–6261. [Google Scholar] [CrossRef]
- Son, B.H.; Kim, H.S.; Jeong, H.; Park, J.Y.; Lee, S.; Ahn, Y.H. Electron beam induced removal of PMMA layer used for graphene transfer. Sci. Rep. 2017, 7, 18058. [Google Scholar] [CrossRef] [Green Version]
- Bart, M.; Harland, P.W.; Hudson, J.E.; Vallance, C. Absolute total electron impact ionization cross-sections for perfluorinated hydrocarbons and small halocarbons. Phys. Chem. Chem. Phys. 2001, 3, 800–806. [Google Scholar] [CrossRef]
- Withers, F.; Bointon, T.H.; Dubois, M.; Russo, S.; Craciun, M.F. Nanopatterning of Fluorinated Graphene by Electron Beam Irradiation. Nano Lett. 2011, 11, 3912–3916. [Google Scholar] [CrossRef]
- Boukhvalov, D.W.; Katsnelson, M.I. Enhancement of Chemical Activity in Corrugated Graphene. J. Phys. Chem. C 2009, 113, 14176–14178. [Google Scholar] [CrossRef] [Green Version]
- Mishchenko, A.; Eckmann, A.; Grigorieva, I.V.; Novoselov, K.S. Fluorination Clusters on Graphene Resolved by Conductive AFM. In Nanomaterials for Security; NATO Science for Peace and Security Series A: Chemistry and Biology; Springer: Dordrecht, The Netherlands, 2016; pp. 19–24. [Google Scholar] [CrossRef]
- Teweldebrhan, D.; Balandin, A.A. Modification of graphene properties due to electron-beam irradiation. Appl. Phys. Lett. 2009, 94, 013101. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.S.; Fujita, D.; Hanagata, N. Monitoring electron-beam irradiation effects on graphenes by temporal Auger electron spectroscopy. Nanotechnology 2010, 21, 265705. [Google Scholar] [CrossRef] [PubMed]
- Song, H.S.; Li, S.L.; Miyazaki, H.; Sato, S.; Hayashi, K.; Yamada, A.; Yokoyama, N.; Tsukagoshi, K. Origin of the relatively low transport mobility of graphene grown through chemical vapor deposition. Sci Rep. 2012, 2, 337. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, T.; Li, H.; Leifer, K. Electron-Beam-Induced Fluorination Cycle for Long-Term Preservation of Graphene under Ambient Conditions. Nanomaterials 2022, 12, 383. https://doi.org/10.3390/nano12030383
Duan T, Li H, Leifer K. Electron-Beam-Induced Fluorination Cycle for Long-Term Preservation of Graphene under Ambient Conditions. Nanomaterials. 2022; 12(3):383. https://doi.org/10.3390/nano12030383
Chicago/Turabian StyleDuan, Tianbo, Hu Li, and Klaus Leifer. 2022. "Electron-Beam-Induced Fluorination Cycle for Long-Term Preservation of Graphene under Ambient Conditions" Nanomaterials 12, no. 3: 383. https://doi.org/10.3390/nano12030383
APA StyleDuan, T., Li, H., & Leifer, K. (2022). Electron-Beam-Induced Fluorination Cycle for Long-Term Preservation of Graphene under Ambient Conditions. Nanomaterials, 12(3), 383. https://doi.org/10.3390/nano12030383







