Mechanisms of Formation, Structure, and Dynamics of Lipoprotein Discs Stabilized by Amphiphilic Copolymers: A Comprehensive Review
Abstract
1. Introduction
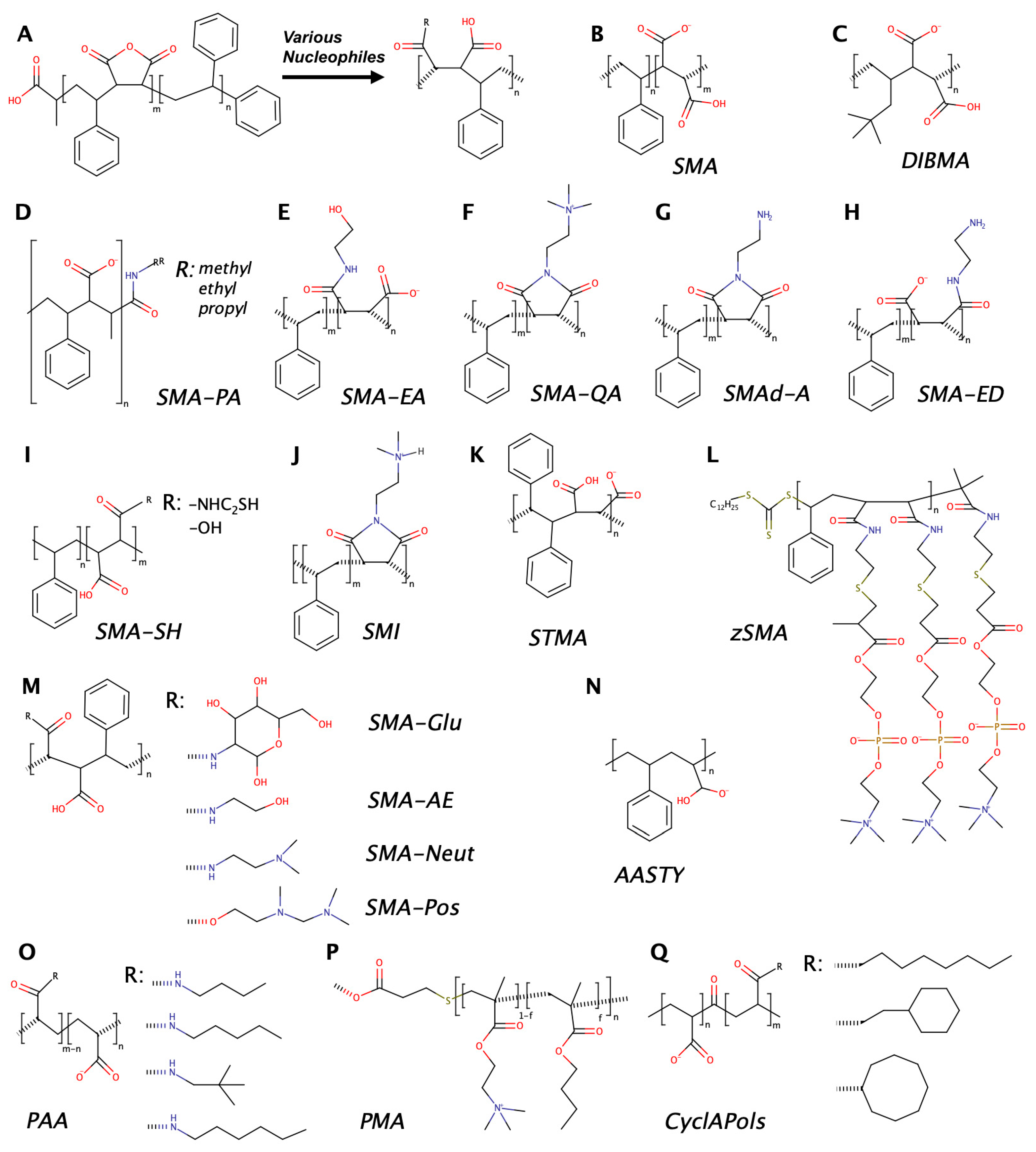
2. Amphiphilic Copolymers Used for Preparation of Lipodiscs
2.1. Types of Amphiphilic Copolymers
| Polymer Type, Ratio of Hydrophobic:Hydrophilic Units | Mn, kDa | Solubilization Conditions | Ð | Disc Size, nm | Reference |
|---|---|---|---|---|---|
| SMA variants | |||||
| SMA (Xiran SZ 20010), 4.3:1 | 2.5 | pH = 7–9, Ca2+ ≤ 2 mM | 2.9 | 7–10 | [20,21] |
| SMA (Xiran SZ 25010), 3:1 | 4 | pH = 6–9, Ca2+ ≤ 2 mM | 2.5 | 7–10 | [20,21] |
| SMA (Xiran SZ 30010), 2.3:1 | 2.5 | pH = 5.5–9, Ca2+ ≤ 2 mM | 2.6 | 7–10 | [20,21] |
| SMA (Xiran SZ 40005), 1.2:1 | 2 | pH > 6, Ca2+ ≤ 2 mM | 2.5 | 7–10 | [20,21] |
| SMA (RAFT), 2:1 | 5.4–18 | pH > 6, Ca2+ ≤ 2 mM | 1.28–1.31 | 27–28 | [22] |
| SMA (RAFT), 3:1 | 6.4–22 | pH > 6, Ca2+ ≤ 2 mM | 1.25–1.29 | 9–10 | [22] |
| SMA (RAFT), 4:1 | 7.4–28 | pH > 6, Ca2+ ≤ 2 mM | 1.25–1.28 | 31–33 | [22] |
| Styrene−maleic anhydride copolymer derivatives (SMADs) | |||||
| SMA-MA, 1:1 | 5.8 | pH > 5, Ca2+ ≤ 8 mM | 2.5 | 14 | [16] |
| SMA-EtA, 1:1 | 6.2 | pH = 5–10, Ca2+ ≤ 24 mM | 2.5 | 25 | [16] |
| SMA-PA, 1:1 | 6.5 | pH = 5–10, Ca2+ ≤ 12.5 mM | 2.5 | 32 | [16] |
| SMA-EA, 1.3:1 | 2 1 | pH > =3.3, up to 21 mM for Ca2+ and 30 mM for Mg2+ | 10–60 | [23] | |
| SMA-QA, 1.3:1 | 2.1 1 | pH = 2.5–10, Ca2+ up to 200 mM | 10–30 | [24] | |
| SMAd-A, 1.3:1 | 1.8 1 | pH < 6, Mg2+/Ca2+ up to 200 mM | ~3–~20 | [25] | |
| SMA-ED, 1.3:1 | 1.8 1 | pH > 7 or pH < 5, Mg2+/Ca2+ 10 (pH = 8.5)- 200 mM | ~4–~10 | [25] | |
| SMA-SH, 2:1 | 7.5 | stable at pH = 8 | polydisp. | 11–15 | [19] |
| SMI, 2:1 | 2.7 | pH < 7.8, Ca2+ 100+ mM | 2.8 | 6–11 | [26] |
| zSMA, 1:1 | 12–44 | pH > 4, Ca2+ up to 20 mM | 1.1–1.2 | 8–30 | [27] |
| SMA-Glu, 2:1 | 42.1 | pH > 3, Mg2+ > 100 mM | 6.93 | 10–28 | [18] |
| SMA-Neut, 2:1 | 6.9 | pH = 3–9, Mg2+ > 100 mM | 1.46 | 15–60 | [18] |
| SMA-AE, 2:1 | 18.3 | pH = 3–9, Mg2+ > 100 mM | 1.72 | 10–28 | [18] |
| SMA-Pos, 2:1 | 11.1 | pH < 3 or pH > 9, Mg2+ > 100 mM | 1.43 | 10–28 | [18] |
| SMA-Pos, 3:1 | 21.9 | pH < 3 or pH > 9, Mg2+ > 100 mM | 1.33 | 15–60 | [18] |
| Non-SMA-based polymers | |||||
| PAA (non-aromatic polyacrylic acid), pentyl-derivative | 2.5 2 | pH > 6, Ca2+/Mg2+ < 3.5 mM, 5.5 mM | 8–16 | [9] | |
| PAA (non-aromatic polyacrylic acid), hexyl-derivative | 2.5 2 | pH > 6, Ca2+/Mg2+ < 2 mM, 2 mM | 7–14 | [9] | |
| PAA (non-aromatic polyacrylic acid), neopentyl-derivative | 2.7 2 | pH > 6.5, Ca2+/Mg2+ < 2 mM, 5.5 mM | 10–17 | [9] | |
| PMA (polymethacrylate) | 1.7–14 | stable at pH = 5.3–7.3 | 10–20 | [28] | |
| DIBMA, 1:1 | 8.5–15 | pH ≥ 6.5, Ca2+/Mg2+ ≤ 20 mM | 1.4 | 15–20 | [29] |
| AASTY, 1:~1 | 6.6–8.9 | pH = 6.5+, Ca2+ ≤ 7 mM | 1.14–1.21 | <10 | [30] |
| CyclAPols, 1:1 | 4.8–5.0 | stable at pH = 7 | 2.0 | <40 | [31] |
| STMA, 1:1 | 4.4–5.8 | pH = 5–10, Ca2+ ≤ 2.5 mM | 1.2–1.5 | 20 | [32] |
2.2. Influence of Polymer Concentration, Type and External Factors on Lipodisc Size and Solubilization Efficiency
2.2.1. Effect of Polymer Concentration
2.2.2. Effect of Polymer Length and Molecular Weight
2.2.3. Effect of Mono- and Divalent Ions
2.2.4. Effect of pH and Polymer Charge
2.2.5. Effects of Monomer Size and Chemical Nature
2.2.6. Effects of Embedded Proteins
2.2.7. Effects of Lipid Types and Phase
3. Formation, Structure and Dynamics of Lipodiscs
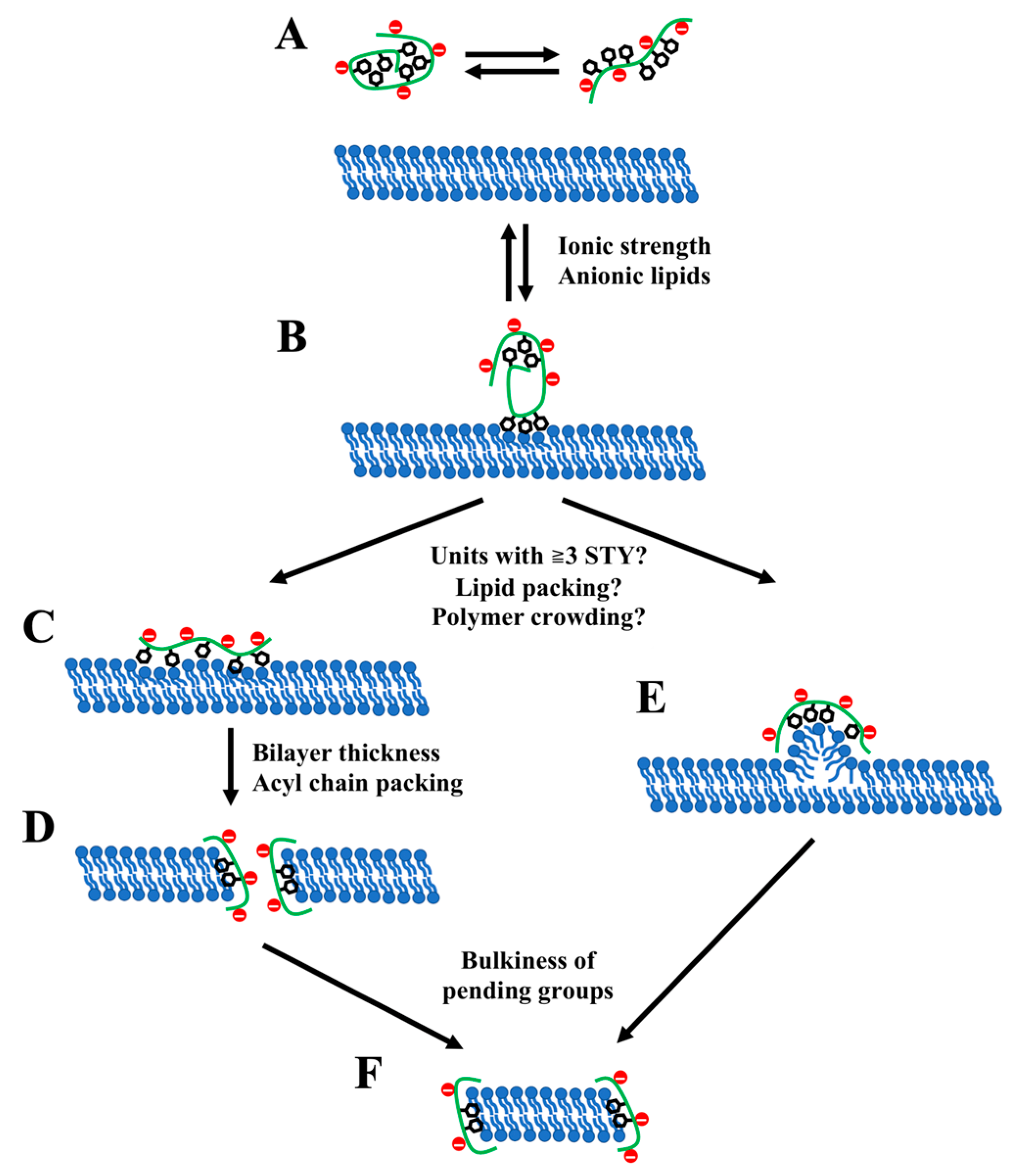
3.1. Membrane Binding
3.2. Insertion of Polymers into a Membrane and Membrane Solubilization
3.3. Formation of Lipodiscs and Lipodisc Morphology
3.4. Dynamics of Lipids in Lipodiscs
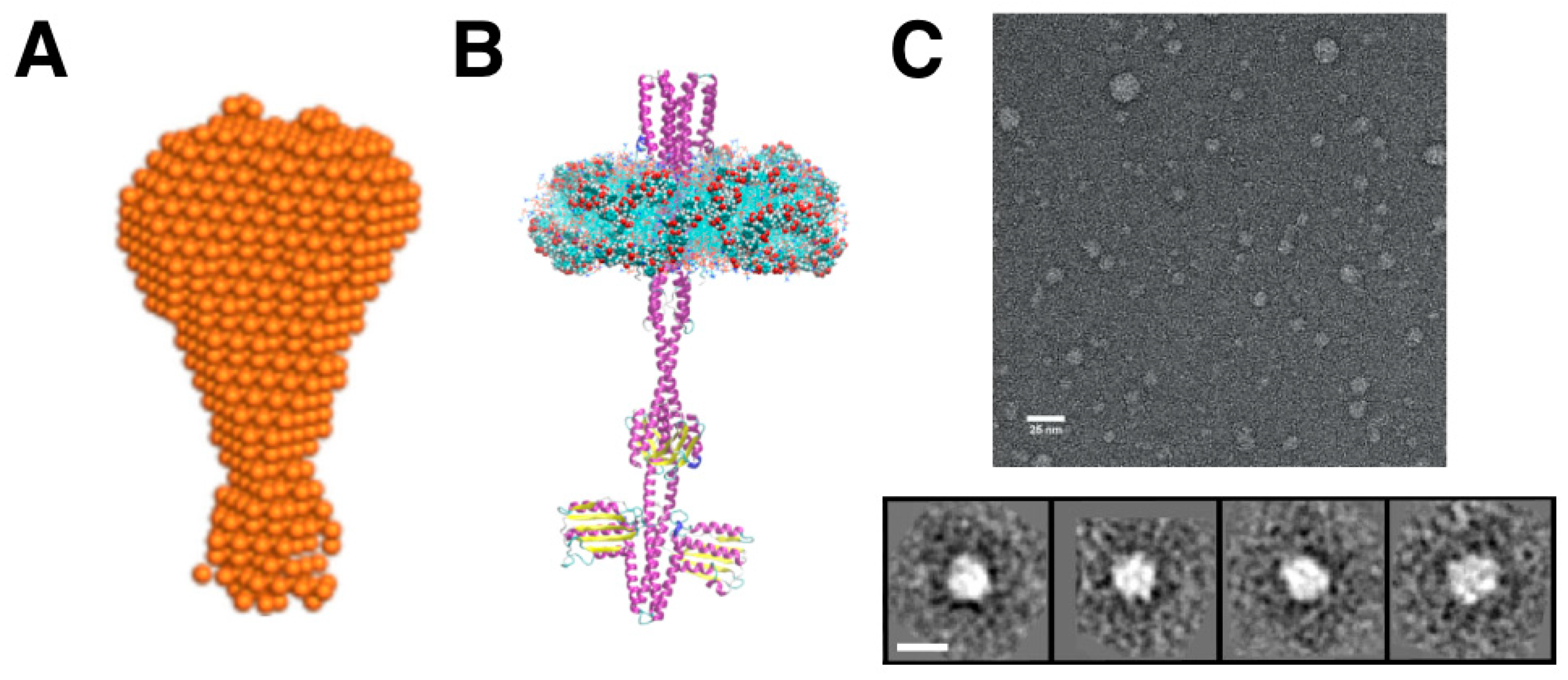
4. Applications of Lipodiscs in Structural Biology
4.1. Electron Paramagnetic Resonance
4.2. Nuclear Magnetic Resonance
4.3. Small-Angle Scattering
4.4. X-ray Crystallography
4.5. Electron Microscopy
5. Further Investigations, Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MPs | membrane proteins |
| SMA | copolymer of polystyrene and maleic anhydride |
| XFEL | X-ray free-electron laser |
| SMAD | styrene–maleic anhydride copolymer derivative |
| DIBMA | diisobutylene–maleic acid copolymer |
| DLS | dynamic light scattering |
| SEC | size-exclusion chromatography |
| EPR | electron paramagnetic resonance |
| SAXS/SANS | small angle x-ray/neutron scattering |
| SMALP | styrene–maleic anhydride copolymer lipoprotein particle |
| MSP | membrane scaffolding protein |
| MACPs | maleic acid-containing alternating copolymers |
| LC | liquid chromatography |
| MS | mass spectrometry |
References
- Palsdottir, H.; Hunte, C. Lipids in Membrane Protein Structures. Biochim. Biophys. Acta-Biomembr. 2004, 1666, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.W. Lipid-Protein Interactions in Biological Membranes: A Dynamic Perspective. Biochim. Biophys. Acta-Biomembr. 2012, 1818, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Dörr, J.M.; Scheidelaar, S.; Koorengevel, M.C.; Dominguez, J.J.; Schäfer, M.; van Walree, C.A.; Antoinette Killian, J. The Styrene–Maleic Acid Copolymer: A Versatile Tool in Membrane Research. Eur. Biophys. J. 2016, 45, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Tribet, C.; Audebert, R.; Popot, J.L. Amphipols: Polymers That Keep Membrane Proteins Soluble in Aqueous Solutions. Proc. Natl. Acad. Sci. USA 1996, 93, 15047–15050. [Google Scholar] [CrossRef]
- Zoonens, M.; Popot, J.-L. Amphipols for Each Season. J. Membr. Biol. 2014, 247, 759–796. [Google Scholar] [CrossRef]
- Dürr, U.H.N.; Soong, R.; Ramamoorthy, A. When Detergent Meets Bilayer: Birth and Coming of Age of Lipid Bicelles. Prog. Nucl. Magn. Reson. Spectrosc. 2013, 69, 1–22. [Google Scholar] [CrossRef][Green Version]
- Sligar, S.G.; Denisov, I.G. Nanodiscs: A Toolkit for Membrane Protein Science. Protein Sci. 2021, 30, 297–315. [Google Scholar] [CrossRef]
- Xue, M.; Cheng, L.; Faustino, I.; Guo, W.; Marrink, S.J. Molecular Mechanism of Lipid Nanodisk Formation by Styrene-Maleic Acid Copolymers. Biophys. J. 2018, 115, 494–502. [Google Scholar] [CrossRef]
- Hardin, N.Z.; Ravula, T.; Mauro, G.D.; Ramamoorthy, A. Hydrophobic Functionalization of Polyacrylic Acid as a Versatile Platform for the Development of Polymer Lipid Nanodisks. Small 2019, 15, e1804813. [Google Scholar] [CrossRef]
- Gulamhussein, A.A.; Uddin, R.; Tighe, B.J.; Poyner, D.R.; Rothnie, A.J. A Comparison of SMA (Styrene Maleic Acid) and DIBMA (Di-Isobutylene Maleic Acid) for Membrane Protein Purification. Biochim. Biophys. Acta-Biomembr. 2020, 1862, 183281. [Google Scholar] [CrossRef]
- Bada Juarez, J.F.; Harper, A.J.; Judge, P.J.; Tonge, S.R.; Watts, A. From Polymer Chemistry to Structural Biology: The Development of SMA and Related Amphipathic Polymers for Membrane Protein Extraction and Solubilisation. Chem. Phys. Lipids 2019, 221, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Overduin, M.; Esmaili, M. Memtein: The Fundamental Unit of Membrane-Protein Structure and Function. Chem. Phys. Lipids 2019, 218, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Overduin, M.; Esmaili, M. Native Nanodiscs and the Convergence of Lipidomics, Metabolomics, Interactomics and Proteomics. NATO Adv. Sci. Inst. Ser. E Appl. Sci. 2019, 9, 1230. [Google Scholar] [CrossRef]
- Overduin, M.; Trieber, C.; Prosser, R.S.; Picard, L.-P.; Sheff, J.G. Structures and Dynamics of Native-State Transmembrane Protein Targets and Bound Lipids. Membranes 2021, 11, 451. [Google Scholar] [CrossRef] [PubMed]
- Farrelly, M.D.; Martin, L.L.; Thang, S.H. Polymer Nanodiscs and Their Bioanalytical Potential. Chemistry 2021, 27, 12922–12939. [Google Scholar] [CrossRef] [PubMed]
- Esmaili, M.; Acevedo-Morantes, C.; Wille, H.; Overduin, M. The Effect of Hydrophobic Alkyl Sidechains on Size and Solution Behaviors of Nanodiscs Formed by Alternating Styrene Maleamic Copolymer. Biochim. Biophys. Acta-Biomembr. 2020, 1862, 183360. [Google Scholar] [CrossRef]
- Ravula, T.; Hardin, N.Z.; Di Mauro, G.M.; Ramamoorthy, A. Styrene Maleic Acid Derivates to Enhance the Applications of Bio-Inspired Polymer Based Lipid-Nanodiscs. Eur. Polym. J. 2018, 108, 597–602. [Google Scholar] [CrossRef]
- Burridge, K.M.; Harding, B.D.; Sahu, I.D.; Kearns, M.M.; Stowe, R.B.; Dolan, M.T.; Edelmann, R.E.; Dabney-Smith, C.; Page, R.C.; Konkolewicz, D.; et al. Simple Derivatization of RAFT-Synthesized Styrene-Maleic Anhydride Copolymers for Lipid Disk Formulations. Biomacromolecules 2020, 21, 1274–1284. [Google Scholar] [CrossRef]
- Lindhoud, S.; Carvalho, V.; Pronk, J.W.; Aubin-Tam, M.-E. SMA-SH: Modified Styrene-Maleic Acid Copolymer for Functionalization of Lipid Nanodiscs. Biomacromolecules 2016, 17, 1516–1522. [Google Scholar] [CrossRef]
- Scheidelaar, S.; Koorengevel, M.C.; van Walree, C.A.; Dominguez, J.J.; Dörr, J.M.; Killian, J.A. Effect of Polymer Composition and PH on Membrane Solubilization by Styrene-Maleic Acid Copolymers. Biophys. J. 2016, 111, 1974–1986. [Google Scholar] [CrossRef]
- Craig, A.F.; Sahu, I.D.; Dabney-Smith, C.; Konkolewicz, D.; Lorigan, G.A. 16. Styrene-Maleic Acid Copolymers: A New Tool for Membrane Biophysics. In Characterization of Biological Membranes; De Gruyter: Berlin, Germany, 2019; pp. 477–496. ISBN 9783110544657. [Google Scholar]
- Craig, A.F.; Clark, E.E.; Sahu, I.D.; Zhang, R.; Frantz, N.D.; Al-Abdul-Wahid, M.S.; Dabney-Smith, C.; Konkolewicz, D.; Lorigan, G.A. Tuning the Size of Styrene-Maleic Acid Copolymer-Lipid Nanoparticles (SMALPs) Using RAFT Polymerization for Biophysical Studies. Biochim. Biophys. Acta-Biomembr. 2016, 1858, 2931–2939. [Google Scholar] [CrossRef] [PubMed]
- Ravula, T.; Ramadugu, S.K.; Di Mauro, G.; Ramamoorthy, A. Bioinspired, Size-Tunable Self-Assembly of Polymer-Lipid Bilayer Nanodiscs. Angew. Chem. Int. Ed. Engl. 2017, 56, 11466–11470. [Google Scholar] [CrossRef] [PubMed]
- Ravula, T.; Hardin, N.Z.; Ramadugu, S.K.; Cox, S.J.; Ramamoorthy, A. Formation of PH-Resistant Monodispersed Polymer-Lipid Nanodiscs. Angew. Chem. Int. Ed. Engl. 2018, 57, 1342–1345. [Google Scholar] [CrossRef] [PubMed]
- Ravula, T.; Hardin, N.Z.; Ramadugu, S.K.; Ramamoorthy, A. PH Tunable and Divalent Metal Ion Tolerant Polymer Lipid Nanodiscs. Langmuir 2017, 33, 10655–10662. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.C.L.; Tognoloni, C.; Charlton, J.; Bragginton, É.C.; Rothnie, A.J.; Sridhar, P.; Wheatley, M.; Knowles, T.J.; Arnold, T.; Edler, K.J.; et al. An Acid-Compatible Co-Polymer for the Solubilization of Membranes and Proteins into Lipid Bilayer-Containing Nanoparticles. Nanoscale 2018, 10, 10609–10619. [Google Scholar] [CrossRef]
- Fiori, M.C.; Jiang, Y.; Altenberg, G.A.; Liang, H. Polymer-Encased Nanodiscs with Improved Buffer Compatibility. Sci. Rep. 2017, 7, 7432. [Google Scholar] [CrossRef]
- Yasuhara, K.; Arakida, J.; Ravula, T.; Ramadugu, S.K.; Sahoo, B.; Kikuchi, J.-I.; Ramamoorthy, A. Spontaneous Lipid Nanodisc Fomation by Amphiphilic Polymethacrylate Copolymers. J. Am. Chem. Soc. 2017, 139, 18657–18663. [Google Scholar] [CrossRef]
- Oluwole, A.O.; Danielczak, B.; Meister, A.; Babalola, J.O.; Vargas, C.; Keller, S. Solubilization of Membrane Proteins into Functional Lipid-Bilayer Nanodiscs Using a Diisobutylene/Maleic Acid Copolymer. Angew. Chem. Int. Ed. Engl. 2017, 56, 1919–1924. [Google Scholar] [CrossRef]
- Smith, A.A.A.; Autzen, H.E.; Faust, B.; Mann, J.L.; Muir, B.W.; Howard, S.; Postma, A.; Spakowitz, A.J.; Cheng, Y.; Appel, E.A. Lipid Nanodiscs via Ordered Copolymers. Chem 2020, 6, 2782–2795. [Google Scholar] [CrossRef]
- Marconnet, A.; Michon, B.; Le Bon, C.; Giusti, F.; Tribet, C.; Zoonens, M. Solubilization and Stabilization of Membrane Proteins by Cycloalkane-Modified Amphiphilic Polymers. Biomacromolecules 2020, 21, 3459–3467. [Google Scholar] [CrossRef]
- Esmaili, M.; Brown, C.J.; Shaykhutdinov, R.; Acevedo-Morantes, C.; Wang, Y.L.; Wille, H.; Gandour, R.D.; Turner, S.R.; Overduin, M. Homogeneous Nanodiscs of Native Membranes Formed by Stilbene-Maleic-Acid Copolymers. Nanoscale 2020, 12, 16705–16709. [Google Scholar] [CrossRef] [PubMed]
- Voskoboynikova, N.; Margheritis, E.G.; Kodde, F.; Rademacher, M.; Schowe, M.; Budke-Gieseking, A.; Psathaki, O.-E.; Steinhoff, H.-J.; Cosentino, K. Evaluation of DIBMA Nanoparticles of Variable Size and Anionic Lipid Content as Tools for the Structural and Functional Study of Membrane Proteins. Biochim. Biophys. Acta-Biomembr. 2021, 1863, 183588. [Google Scholar] [CrossRef] [PubMed]
- Scheidelaar, S.; Koorengevel, M.C.; Pardo, J.D.; Meeldijk, J.D.; Breukink, E.; Killian, J.A. Molecular Model for the Solubilization of Membranes into Nanodisks by Styrene Maleic Acid Copolymers. Biophys. J. 2015, 108, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Sahu, I.D.; Liu, L.; Osatuke, A.; Comer, R.G.; Dabney-Smith, C.; Lorigan, G.A. Characterizing the Structure of Lipodisq Nanoparticles for Membrane Protein Spectroscopic Studies. Biochim. Biophys. Acta-Biomembr. 2015, 1848, 329–333. [Google Scholar] [CrossRef]
- Denisov, I.G.; Sligar, S.G. Nanodiscs in Membrane Biochemistry and Biophysics. Chem. Rev. 2017, 117, 4669–4713. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, R.; Ackermann, R.; Im, S.-C.; Waskell, L.; Schwendeman, A.; Ramamoorthy, A. Reconstitution of the Cytb5-CytP450 Complex in Nanodiscs for Structural Studies Using NMR Spectroscopy. Angew. Chem. Int. Ed. 2016, 55, 4497–4499. [Google Scholar] [CrossRef]
- Domínguez Pardo, J.J.; Koorengevel, M.C.; Uwugiaren, N.; Weijers, J.; Kopf, A.H.; Jahn, H.; van Walree, C.A.; van Steenbergen, M.J.; Killian, J.A. Membrane Solubilization by Styrene-Maleic Acid Copolymers: Delineating the Role of Polymer Length. Biophys. J. 2018, 115, 129–138. [Google Scholar] [CrossRef]
- Ball, L.E.; Riley, L.J.; Hadasha, W.; Pfukwa, R.; Smith, C.J.I.; Dafforn, T.R.; Klumperman, B. Influence of DIBMA Polymer Length on Lipid Nanodisc Formation and Membrane Protein Extraction. Biomacromolecules 2021, 22, 763–772. [Google Scholar] [CrossRef]
- Orekhov, P.S.; Bozdaganyan, M.E.; Voskoboynikova, N.; Mulkidjanian, A.Y.; Steinhoff, H.-J.; Shaitan, K.V. Styrene/Maleic Acid Copolymers Form SMALPs by Pulling Lipid Patches out of the Lipid Bilayer. Langmuir 2019, 35, 3748–3758. [Google Scholar] [CrossRef]
- Kopf, A.H.; Lijding, O.; Elenbaas, B.O.W.; Koorengevel, M.C.; van Walree, C.A.; Antoinette Killian, J. Synthesis and Evaluation of a Novel Library of Alternating Amphipathic Copolymers to Solubilize and Study Membrane Proteins. ChemRxiv 2021. [Google Scholar] [CrossRef]
- Morrison, K.A.; Akram, A.; Mathews, A.; Khan, Z.A.; Patel, J.H.; Zhou, C.; Hardy, D.J.; Moore-Kelly, C.; Patel, R.; Odiba, V.; et al. Membrane Protein Extraction and Purification Using Styrene-Maleic Acid (SMA) Copolymer: Effect of Variations in Polymer Structure. Biochem. J 2016, 473, 4349–4360. [Google Scholar] [CrossRef] [PubMed]
- Swainsbury, D.J.K.; Scheidelaar, S.; Foster, N.; van Grondelle, R.; Killian, J.A.; Jones, M.R. The Effectiveness of Styrene-Maleic Acid (SMA) Copolymers for Solubilisation of Integral Membrane Proteins from SMA-Accessible and SMA-Resistant Membranes. Biochim. Biophys. Acta-Biomembr. 2017, 1859, 2133–2143. [Google Scholar] [CrossRef] [PubMed]
- Dörr, J. Membrane Solubilization by Styrene–Maleic Acid Copolymers: Towards New Applications in Membrane Protein Research. Ph.D. thesis, Utrecht University, Utrecht, the Netherlands, 2017. ISBN 9789039367353. [Google Scholar]
- Grethen, A.; Glueck, D.; Keller, S. Role of Coulombic Repulsion in Collisional Lipid Transfer Among SMA(2:1)-Bounded Nanodiscs. J. Membr. Biol. 2018, 251, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Oluwole, A.O.; Klingler, J.; Danielczak, B.; Babalola, J.O.; Vargas, C.; Pabst, G.; Keller, S. Formation of Lipid-Bilayer Nanodiscs by Diisobutylene/Maleic Acid (DIBMA) Copolymer. Langmuir 2017, 33, 14378–14388. [Google Scholar] [CrossRef]
- Danielczak, B.; Keller, S. Collisional Lipid Exchange among DIBMA-Encapsulated Nanodiscs (DIBMALPs). Eur. Polym. J. 2018, 109, 206–213. [Google Scholar] [CrossRef]
- Kourghi, M.; Nourmohammadi, S.; Pei, J.V.; Qiu, J.; McGaughey, S.; Tyerman, S.D.; Byrt, C.S.; Yool, A.J. Divalent Cations Regulate the Ion Conductance Properties of Diverse Classes of Aquaporins. Int. J. Mol. Sci. 2017, 18, 2323. [Google Scholar] [CrossRef]
- Zimmermann, I.; Marabelli, A.; Bertozzi, C.; Sivilotti, L.G.; Dutzler, R. Inhibition of the Prokaryotic Pentameric Ligand-Gated Ion Channel ELIC by Divalent Cations. PLoS Biol. 2012, 10, e1001429. [Google Scholar] [CrossRef]
- Lee, S.K.; Shanmughapriya, S.; Mok, M.C.Y.; Dong, Z.; Tomar, D.; Carvalho, E.; Rajan, S.; Junop, M.S.; Madesh, M.; Stathopulos, P.B. Structural Insights into Mitochondrial Calcium Uniporter Regulation by Divalent Cations. Cell Chem. Biol. 2016, 23, 1157–1169. [Google Scholar] [CrossRef]
- Ye, L.; Neale, C.; Sljoka, A.; Lyda, B.; Pichugin, D.; Tsuchimura, N.; Larda, S.T.; Pomès, R.; García, A.E.; Ernst, O.P.; et al. Mechanistic Insights into Allosteric Regulation of the A2A Adenosine G Protein-Coupled Receptor by Physiological Cations. Nat. Commun. 2018, 9, 1372. [Google Scholar] [CrossRef]
- Pollock, N.L.; Lee, S.C.; Patel, J.H.; Gulamhussein, A.A.; Rothnie, A.J. Structure and Function of Membrane Proteins Encapsulated in a Polymer-Bound Lipid Bilayer. Biochim. Biophys. Acta-Biomembr. 2018, 1860, 809–817. [Google Scholar] [CrossRef]
- Hawkins, O.P.; Jahromi, C.P.T.; Gulamhussein, A.A.; Nestorow, S.; Bahra, T.; Shelton, C.; Owusu-Mensah, Q.K.; Mohiddin, N.; O’Rourke, H.; Ajmal, M.; et al. Membrane Protein Extraction and Purification Using Partially-Esterified SMA Polymers. Biochim. Biophys. Acta-Biomembr. 2021, 1863, 183758. [Google Scholar] [CrossRef] [PubMed]
- Brady, N.G.; Workman, C.E.; Cawthon, B.; Bruce, B.D.; Long, B.K. Protein Extraction Efficiency and Selectivity of Esterified Styrene–Maleic Acid Copolymers in Thylakoid Membranes. Biomacromolecules 2021, 22, 2544–2553. [Google Scholar] [CrossRef] [PubMed]
- Danielczak, B.; Meister, A.; Keller, S. Influence of Mg2+ and Ca2+ on Nanodisc Formation by Diisobutylene/Maleic Acid (DIBMA) Copolymer. Chem. Phys. Lipids 2019, 221, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Pal, T.K.; Guha, S.K. Probing Molecular Interactions of Poly(Styrene-Co-Maleic Acid) with Lipid Matrix Models to Interpret the Therapeutic Potential of the Co-Polymer. Biochim. Biophys. Acta-Biomembr. 2012, 1818, 537–550. [Google Scholar] [CrossRef]
- Ravula, T.; Hardin, N.Z.; Bai, J.; Im, S.-C.; Waskell, L.; Ramamoorthy, A. Effect of Polymer Charge on Functional Reconstitution of Membrane Proteins in Polymer Nanodiscs. Chem. Commun. 2018, 54, 9615–9618. [Google Scholar] [CrossRef]
- Thomas, J.L.; Devlin, B.P.; Tirrell, D.A. Kinetics of Membrane Micellization by the Hydrophobic Polyelectrolyte Poly( 2-Ethylacrylic Acid). Biochim. Biophys. Acta-Biomembr. 1996, 1278, 73–78. [Google Scholar] [CrossRef][Green Version]
- Chung, J.C.; Gross, D.J.; Thomas, J.L.; Tirrell, D.A.; Opsahl-Ong, L.R. PH-Sensitive, Cation-Selective Channels Formed by a Simple Synthetic Polyelectrolyte in Artificial Bilayer Membranes. Macromolecules 1996, 29, 4636–4641. [Google Scholar] [CrossRef]
- Scheidelaar, S. Solulization of Lipids and Membrane Proteins into Nanodiscs. Mode of Action and Applications of Styrene--Maleic Acid Copolymers. Ph.D. Thesis, Utrecht University, Utrecht, The Netherlands, 2016. ISBN 978-90-393-6635-6. [Google Scholar]
- Swainsbury, D.J.K.; Scheidelaar, S.; van Grondelle, R.; Killian, J.A.; Jones, M.R. Bacterial Reaction Centers Purified with Styrene Maleic Acid Copolymer Retain Native Membrane Functional Properties and Display Enhanced Stability. Angew. Chem. Int. Ed. Engl. 2014, 53, 11803–11807. [Google Scholar] [CrossRef]
- Long, A.R.; O’Brien, C.C.; Malhotra, K.; Schwall, C.T.; Albert, A.D.; Watts, A.; Alder, N.N. A Detergent-Free Strategy for the Reconstitution of Active Enzyme Complexes from Native Biological Membranes into Nanoscale Discs. BMC Biotechnol. 2013, 13, 41. [Google Scholar] [CrossRef]
- Orwick-Rydmark, M.; Lovett, J.E.; Graziadei, A.; Lindholm, L.; Hicks, M.R.; Watts, A. Detergent-Free Incorporation of a Seven-Transmembrane Receptor Protein into Nanosized Bilayer Lipodisq Particles for Functional and Biophysical Studies. Nano Lett. 2012, 12, 4687–4692. [Google Scholar] [CrossRef]
- Paulin, S.; Jamshad, M.; Dafforn, T.R.; Garcia-Lara, J.; Foster, S.J.; Galley, N.F.; Roper, D.I.; Rosado, H.; Taylor, P.W. Surfactant-Free Purification of Membrane Protein Complexes from Bacteria: Application to the Staphylococcal Penicillin-Binding Protein Complex PBP2/PBP2a. Nanotechnology 2014, 25, 285101. [Google Scholar] [CrossRef] [PubMed]
- Bjørnestad, V.A.; Orwick-Rydmark, M.; Lund, R. Understanding the Structural Pathways for Lipid Nanodisc Formation: How Styrene Maleic Acid Copolymers Induce Membrane Fracture and Disc Formation. Langmuir 2021, 37, 6178–6188. [Google Scholar] [CrossRef]
- Marie, E.; Sagan, S.; Cribier, S.; Tribet, C. Amphiphilic Macromolecules on Cell Membranes: From Protective Layers to Controlled Permeabilization. J. Membr. Biol. 2014, 247, 861–881. [Google Scholar] [CrossRef]
- Jamshad, M.; Grimard, V.; Idini, I.; Knowles, T.J.; Dowle, M.R.; Schofield, N.; Sridhar, P.; Lin, Y.-P.; Finka, R.; Wheatley, M.; et al. Structural Analysis of a Nanoparticle Containing a Lipid Bilayer Used for Detergent-Free Extraction of Membrane Proteins. Nano Res. 2015, 8, 774–789. [Google Scholar] [CrossRef] [PubMed]
- Tonge, S.R.; Tighe, B.J. Responsive Hydrophobically Associating Polymers: A Review of Structure and Properties. Adv. Drug Deliv. Rev. 2001, 53, 109–122. [Google Scholar] [CrossRef]
- Colbasevici, A.; Voskoboynikova, N.; Orekhov, P.S.; Bozdaganyan, M.E.; Karlova, M.G.; Sokolova, O.S.; Klare, J.P.; Mulkidjanian, A.Y.; Shaitan, K.V.; Steinhoff, H.-J. Lipid Dynamics in Nanoparticles Formed by Maleic Acid-Containing Copolymers: EPR Spectroscopy and Molecular Dynamics Simulations. Biochim. Biophys. Acta-Biomembr. 2020, 1862, 183207. [Google Scholar] [CrossRef] [PubMed]
- Teramura, Y.; Kaneda, Y.; Totani, T.; Iwata, H. Behavior of Synthetic Polymers Immobilized on a Cell Membrane. Biomaterials 2008, 29, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Yessine, M.-A.; Leroux, J.-C. Membrane-Destabilizing Polyanions: Interaction with Lipid Bilayers and Endosomal Escape of Biomacromolecules. Adv. Drug Deliv. Rev. 2004, 56, 999–1021. [Google Scholar] [CrossRef]
- Chen, R.; Khormaee, S.; Eccleston, M.E.; Slater, N.K.H. The Role of Hydrophobic Amino Acid Grafts in the Enhancement of Membrane-Disruptive Activity of PH-Responsive Pseudo-Peptides. Biomaterials 2009, 30, 1954–1961. [Google Scholar] [CrossRef]
- Henry, S.M.; El-Sayed, M.E.H.; Pirie, C.M.; Hoffman, A.S.; Stayton, P.S. PH-Responsive Poly(Styrene-Alt-Maleic Anhydride) Alkylamide Copolymers for Intracellular Drug Delivery. Biomacromolecules 2006, 7, 2407–2414. [Google Scholar] [CrossRef]
- Khormaee, S.; Choi, Y.; Shen, M.J.; Xu, B.; Wu, H.; Griffiths, G.L.; Chen, R.; Slater, N.K.H.; Park, J.K. Endosomolytic Anionic Polymer for the Cytoplasmic Delivery of SiRNAs in Localized Applications. Adv. Funct. Mater. 2013, 23, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Nelson, A.; Coldrick, Z.; Chen, R. The Effects of Substituent Grafting on the Interaction of PH-Responsive Polymers with Phospholipid Monolayers. Langmuir 2011, 27, 8530–8539. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.J. The Dipole Potential of Phospholipid Membranes and Methods for Its Detection. Adv. Colloid Interface Sci. 2001, 89–90, 263–281. [Google Scholar] [CrossRef]
- Battaglia, M.R.; Buckingham, A.D.; Williams, J.H. The Electric Quadrupole Moments of Benzene and Hexafluorobenzene. Chem. Phys. Lett. 1981, 78, 421–423. [Google Scholar] [CrossRef]
- Caparotta, M.; Puiatti, M.; Masone, D. Artificial Stabilization of the Fusion Pore by Intra-Organelle Styrene-Maleic Acid Copolymers. Soft Matter 2021, 17, 8314–8321. [Google Scholar] [CrossRef]
- Orwick Rydmark, M.; Christensen, M.K.; Köksal, E.S.; Kantarci, I.; Kustanovich, K.; Yantchev, V.; Jesorka, A.; Gözen, I. Styrene Maleic Acid Copolymer Induces Pores in Biomembranes. Soft Matter 2019, 15, 7934–7944. [Google Scholar] [CrossRef]
- Pizzirusso, A.; De Nicola, A.; Sevink, G.J.A.; Correa, A.; Cascella, M.; Kawakatsu, T.; Rocco, M.; Zhao, Y.; Celino, M.; Milano, G. Biomembrane Solubilization Mechanism by Triton X-100: A Computational Study of the Three Stage Model. Phys. Chem. Chem. Phys. 2017, 19, 29780–29794. [Google Scholar] [CrossRef]
- Lichtenberg, D.; Ahyayauch, H.; Alonso, A.; Goñi, F.M. Detergent Solubilization of Lipid Bilayers: A Balance of Driving Forces. Trends Biochem. Sci. 2013, 38, 85–93. [Google Scholar] [CrossRef]
- Kragh-Hansen, U.; le Maire, M.; Møller, J.V. The Mechanism of Detergent Solubilization of Liposomes and Protein-Containing Membranes. Biophys. J. 1998, 75, 2932–2946. [Google Scholar] [CrossRef]
- Stuart, M.C.A.; Boekema, E.J. Two Distinct Mechanisms of Vesicle-to-Micelle and Micelle-to-Vesicle Transition Are Mediated by the Packing Parameter of Phospholipid-Detergent Systems. Biochim. Biophys. Acta-Biomembr. 2007, 1768, 2681–2689. [Google Scholar] [CrossRef]
- Helenius, A.; Simons, K. Solubilization of Membranes by Detergents. Biochim. Biophys. Acta-Biomembr. 1975, 415, 29–79. [Google Scholar] [CrossRef]
- Lichtenberg, D.; Ahyayauch, H.; Goñi, F.M. The Mechanism of Detergent Solubilization of Lipid Bilayers. Biophys. J. 2013, 105, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, A.; Pirri, G.; Bozzi, A.; Di Giulio, A.; Aschi, M.; Rinaldi, A.C. Antimicrobial Peptides: Natural Templates for Synthetic Membrane-Active Compounds. Cell. Mol. Life Sci. 2008, 65, 2450–2460. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Olubummo, A.; Binder, W.H. Beyond the Lipid -Bilayer: Interaction of Polymers and Nanoparticles with Membranes. Soft Matter 2012, 8, 4849–4864. [Google Scholar] [CrossRef]
- Binder, W.H. Polymer-Induced Transient Pores in Lipid Membranes. Angew. Chem. Int. Ed. Engl. 2008, 47, 3092–3095. [Google Scholar] [CrossRef] [PubMed]
- Wimley, W.C. Describing the Mechanism of Antimicrobial Peptide Action with the Interfacial Activity Model. ACS Chem. Biol. 2010, 5, 905–917. [Google Scholar] [CrossRef]
- Voskoboynikova, N.; Mosslehy, W.; Colbasevici, A.; Ismagulova, T.T.; Bagrov, D.V.; Akovantseva, A.A.; Timashev, P.S.; Mulkidjanian, A.Y.; Bagratashvili, V.N.; Shaitan, K.V.; et al. Characterization of an Archaeal Photoreceptor/Transducer Complex from Natronomonas Pharaonis Assembled within Styrene–Maleic Acid Lipid Particles. RSC Adv. 2017, 7, 51324–51334. [Google Scholar] [CrossRef]
- Bagrov, D.V.; Voskoboynikova, N.; Armeev, G.A. Characterization of Lipodisc Nanoparticles Containing Sensory Rhodopsin Ii and Its Cognate Transducer from Natronomonas Pharaonis. Biophysics 2016, 942–949. [Google Scholar] [CrossRef]
- Guo, R.; Sumner, J.; Qian, S. Structure of Diisobutylene Maleic Acid Copolymer (DIBMA) and Its Lipid Particle as a “Stealth” Membrane-Mimetic for Membrane Protein Research. ACS Appl. Bio Mater. 2021, 4, 4760–4768. [Google Scholar] [CrossRef]
- Voskoboynikova, N.; Orekhov, P.; Bozdaganyan, M.; Kodde, F.; Rademacher, M.; Schowe, M.; Budke-Gieseking, A.; Brickwedde, B.; Psathaki, O.-E.; Mulkidjanian, A.Y.; et al. Lipid Dynamics in Diisobutylene-Maleic Acid (Dibma) Lipid Particles in Presence of Sensory Rhodopsin Ii. Int. J. Mol. Sci. 2021, 22, 2548. [Google Scholar] [CrossRef]
- Sahoo, B.R.; Genjo, T.; Moharana, K.C.; Ramamoorthy, A. Self-Assembly of Polymer-Encased Lipid Nanodiscs and Membrane Protein Reconstitution. J. Phys. Chem. B 2019, 123, 4562–4570. [Google Scholar] [CrossRef] [PubMed]
- Orwick, M.C.; Judge, P.J.; Procek, J.; Lindholm, L.; Graziadei, A.; Engel, A.; Gröbner, G.; Watts, A. Detergent-Free Formation and Physicochemical Characterization of Nanosized Lipid-Polymer Complexes: Lipodisq. Angew. Chem. Int. Ed. Engl. 2012, 51, 4653–4657. [Google Scholar] [CrossRef] [PubMed]
- Domínguez Pardo, J.J.; van Walree, C.A.; Egmond, M.R.; Koorengevel, M.C.; Killian, J.A. Nanodiscs Bounded by Styrene-Maleic Acid Allow Trans-Cis Isomerization of Enclosed Photoswitches of Azobenzene Labeled Lipids. Chem. Phys. Lipids 2019, 220, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Cuevas Arenas, R.; Danielczak, B.; Martel, A.; Porcar, L.; Breyton, C.; Ebel, C.; Keller, S. Fast Collisional Lipid Transfer among Polymer-Bounded Nanodiscs. Sci. Rep. 2017, 7, 45875. [Google Scholar] [CrossRef]
- Schmidt, V.; Sturgis, J.N. Modifying Styrene-Maleic Acid Co-Polymer for Studying Lipid Nanodiscs. Biochim. Biophys. Acta-Biomembr. 2018, 1860, 777–783. [Google Scholar] [CrossRef]
- Hubbell, W.L.; McConnell, H.M. Molecular Motion in Spin-Labeled Phospholipids and Membranes. J. Am. Chem. Soc. 1971, 93, 314–326. [Google Scholar] [CrossRef]
- Hemminga, M.A.; Berliner, L. ESR Spectroscopy in Membrane Biophysics; Springer Science & Business Media: New York, NY, USA, 2007; ISBN 9780387493671. [Google Scholar]
- Sahu, I.D.; McCarrick, R.M.; Troxel, K.R.; Zhang, R.; Smith, H.J.; Dunagan, M.M.; Swartz, M.S.; Rajan, P.V.; Kroncke, B.M.; Sanders, C.R.; et al. DEER EPR Measurements for Membrane Protein Structures via Bifunctional Spin Labels and Lipodisq Nanoparticles. Biochemistry 2013, 52, 6627–6632. [Google Scholar] [CrossRef][Green Version]
- Sahu, I.D.; Kroncke, B.M.; Zhang, R.; Dunagan, M.M.; Smith, H.J.; Craig, A.; McCarrick, R.M.; Sanders, C.R.; Lorigan, G.A. Structural Investigation of the Transmembrane Domain of KCNE1 in Proteoliposomes. Biochemistry 2014, 53, 6392–6401. [Google Scholar] [CrossRef]
- Stepien, P.; Polit, A.; Wisniewska-Becker, A. Comparative EPR Studies on Lipid Bilayer Properties in Nanodiscs and Liposomes. Biochim. Biophys. Acta-Biomembr. 2015, 1848, 60–66. [Google Scholar] [CrossRef]
- Bali, A.P.; Sahu, I.D.; Craig, A.F.; Clark, E.E.; Burridge, K.M.; Dolan, M.T.; Dabney-Smith, C.; Konkolewicz, D.; Lorigan, G.A. Structural Characterization of Styrene-Maleic Acid Copolymer-Lipid Nanoparticles (SMALPs) Using EPR Spectroscopy. Chem. Phys. Lipids 2019, 220, 6–13. [Google Scholar] [CrossRef]
- Hoffmann, M.; Eisermann, J.; Schöffmann, F.A.; Das, M.; Vargas, C.; Keller, S.; Hinderberger, D. Influence of Different Polymer Belts on Lipid Properties in Nanodiscs Characterized by CW EPR Spectroscopy. Biochim. Biophys. Acta-Biomembr. 2021, 1863, 183681. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Haselberger, D.; Hofmann, T.; Müller, L.; Janson, K.; Meister, A.; Das, M.; Vargas, C.; Keller, S.; Kastritis, P.L.; et al. Nanoscale Model System for the Human Myelin Sheath. Biomacromolecules 2021, 22, 3901–3912. [Google Scholar] [CrossRef] [PubMed]
- Szundi, I.; Pitch, S.G.; Chen, E.; Farrens, D.L.; Kliger, D.S. Styrene-Maleic Acid Copolymer Effects on the Function of the GPCR Rhodopsin in Lipid Nanoparticles. Biophys. J. 2021, 120, 4337–4348. [Google Scholar] [CrossRef]
- Tedesco, D.; Maj, M.; Malarczyk, P.; Cingolani, A.; Zaffagnini, M.; Wnorowski, A.; Czapiński, J.; Benelli, T.; Mazzoni, R.; Bartolini, M.; et al. Application of the SMALP Technology to the Isolation of GPCRs from Low-Yielding Cell Lines. Biochim. Biophys. Acta-Biomembr. 2021, 1863, 183641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gui, M.; Wang, Z.-F.; Gorgulla, C.; Yu, J.J.; Wu, H.; Sun, Z.-Y.J.; Klenk, C.; Merklinger, L.; Morstein, L.; et al. Cryo-EM Structure of an Activated GPCR-G Protein Complex in Lipid Nanodiscs. Nat. Struct. Mol. Biol. 2021, 28, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Harwood, C.; Sykes, D.A.; Hoare, B.; Heydenreich, F.M.; Uddin, R.; Poyner, D.R.; Briddon, S.J.; Veprintsev, D.B. Functional Solubilisation of the Β2-Adrenoceptor (Β 2AR) Using Diisobutylene Maleic Acid (DIBMA). iScience 2021, 24, 103362. [Google Scholar] [CrossRef]
- Wheatley, M.; Charlton, J.; Jamshad, M.; Routledge, S.J.; Bailey, S.; La-Borde, P.J.; Azam, M.T.; Logan, R.T.; Bill, R.M.; Dafforn, T.R.; et al. GPCR-Styrene Maleic Acid Lipid Particles (GPCR-SMALPs): Their Nature and Potential. Biochem. Soc. Trans. 2016, 44, 619–623. [Google Scholar] [CrossRef]
- Hothersall, J.D.; Jones, A.Y.; Dafforn, T.R.; Perrior, T.; Chapman, K.L. Releasing the Technical “shackles” on GPCR Drug Discovery: Opportunities Enabled by Detergent-Free Polymer Lipid Particle (PoLiPa) Purification. Drug Discov. Today 2020, 25, 1944–1956. [Google Scholar] [CrossRef]
- Hardy, D.; Bill, R.M.; Rothnie, A.J.; Jawhari, A. Stabilization of Human Multidrug Resistance Protein 4 (MRP4/ABCC4) Using Novel Solubilization Agents. SLAS Discov. 2019, 24, 1009–1017. [Google Scholar] [CrossRef]
- Baeta, T.; Giandoreggio-Barranco, K.; Ayala, I.; Moura, E.C.C.M.; Sperandeo, P.; Polissi, A.; Simorre, J.-P.; Laguri, C. LptB2FG Is an ABC Transporter with Adenylate Kinase Activity Regulated by LptC/A Recruitment. bioRxiv 2021. [Google Scholar] [CrossRef]
- Horsey, A.J.; Briggs, D.A.; Holliday, N.D.; Briddon, S.J.; Kerr, I.D. Application of Fluorescence Correlation Spectroscopy to Study Substrate Binding in Styrene Maleic Acid Lipid Copolymer Encapsulated ABCG2. Biochim. Biophys. Acta-Biomembr. 2020, 1862, 183218. [Google Scholar] [CrossRef] [PubMed]
- Gulati, S.; Jamshad, M.; Knowles, T.J.; Morrison, K.A.; Downing, R.; Cant, N.; Collins, R.; Koenderink, J.B.; Ford, R.C.; Overduin, M.; et al. Detergent-Free Purification of ABC (ATP-Binding-Cassette) Transporters. Biochem. J 2014, 461, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Karlova, M.G.; Voskoboynikova, N.; Gluhov, G.S.; Abramochkin, D.; Malak, O.A.; Mulkidzhanyan, A.; Loussouarn, G.; Steinhoff, H.-J.; Shaitan, K.V.; Sokolova, O.S. Detergent-Free Solubilization of Human Kv Channels Expressed in Mammalian Cells. Chem. Phys. Lipids 2019, 219, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Cymes, G.D.; Grosman, C. Structure and Function at the Lipid-Protein Interface of a Pentameric Ligand-Gated Ion Channel. Proc. Natl. Acad. Sci. USA 2021, 118, e2100164118. [Google Scholar] [CrossRef] [PubMed]
- Krajewska, M.; Koprowski, P. Solubilization, Purification, and Functional Reconstitution of Human ROMK Potassium Channel in Copolymer Styrene-Maleic Acid (SMA) Nanodiscs. Biochim. Biophys. Acta-Biomembr. 2021, 1863, 183555. [Google Scholar] [CrossRef] [PubMed]
- Brady, N.G.; Li, M.; Ma, Y.; Gumbart, J.C.; Bruce, B.D. Non-Detergent Isolation of a Cyanobacterial Photosystem I Using Styrene Maleic Acid Alternating Copolymers. RSC Adv. 2019, 9, 31781–31796. [Google Scholar] [CrossRef]
- Cherepanov, D.A.; Brady, N.G.; Shelaev, I.V.; Nguyen, J.; Gostev, F.E.; Mamedov, M.D.; Nadtochenko, V.A.; Bruce, B.D. PSI-SMALP, a Detergent-Free Cyanobacterial Photosystem I, Reveals Faster Femtosecond Photochemistry. Biophys. J. 2020, 118, 337–351. [Google Scholar] [CrossRef]
- Dörr, J.M.; Koorengevel, M.C.; Schäfer, M.; Prokofyev, A.V.; Scheidelaar, S.; van der Cruijsen, E.A.W.; Dafforn, T.R.; Baldus, M.; Killian, J.A. Detergent-Free Isolation, Characterization, and Functional Reconstitution of a Tetrameric K+ Channel: The Power of Native Nanodiscs. Proc. Natl. Acad. Sci. USA 2014, 111, 18607–18612. [Google Scholar] [CrossRef]
- Qiu, W.; Fu, Z.; Xu, G.G.; Grassucci, R.A.; Zhang, Y.; Frank, J.; Hendrickson, W.A.; Guo, Y. Structure and Activity of Lipid Bilayer within a Membrane-Protein Transporter. Proc. Natl. Acad. Sci. USA 2018, 115, 12985–12990. [Google Scholar] [CrossRef]
- Lee, S.C.; Collins, R.; Lin, Y.-P.; Jamshad, M.; Broughton, C.; Harris, S.A.; Hanson, B.S.; Tognoloni, C.; Parslow, R.A.; Terry, A.E.; et al. Nano-Encapsulated Escherichia Coli Divisome Anchor ZipA, and in Complex with FtsZ. Sci. Rep. 2019, 9, 18712. [Google Scholar] [CrossRef]
- Tascón, I.; Sousa, J.S.; Corey, R.A.; Mills, D.J.; Griwatz, D.; Aumüller, N.; Mikusevic, V.; Stansfeld, P.J.; Vonck, J.; Hänelt, I. Structural Basis of Proton-Coupled Potassium Transport in the KUP Family. Nat. Commun. 2020, 11, 626. [Google Scholar] [CrossRef] [PubMed]
- Jamshad, M.; Charlton, J.; Lin, Y.-P.; Routledge, S.J.; Bawa, Z.; Knowles, T.J.; Overduin, M.; Dekker, N.; Dafforn, T.R.; Bill, R.M.; et al. G-Protein Coupled Receptor Solubilization and Purification for Biophysical Analysis and Functional Studies, in the Total Absence of Detergent. Biosci. Rep. 2015, 35, e00188. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, I.A.; Sjöstrand, D.; Li, F.; Björck, M.; Schäfer, J.; Östbye, H.; Högbom, M.; von Ballmoos, C.; Lander, G.C.; Ädelroth, P.; et al. Isolation of Yeast Complex IV in Native Lipid Nanodiscs. Biochim. Biophys. Acta-Biomembr. 2016, 1858, 2984–2992. [Google Scholar] [CrossRef] [PubMed]
- Logez, C.; Damian, M.; Legros, C.; Dupré, C.; Guéry, M.; Mary, S.; Wagner, R.; M’Kadmi, C.; Nosjean, O.; Fould, B.; et al. Detergent-Free Isolation of Functional G Protein-Coupled Receptors into Nanometric Lipid Particles. Biochemistry 2016, 55, 38–48. [Google Scholar] [CrossRef]
- Voskoboynikova, N.; Karlova, M.; Kurre, R.; Mulkidjanian, A.Y.; Shaitan, K.V.; Sokolova, O.S.; Steinhoff, H.-J.; Heinisch, J.J. A Three-Dimensional Model of the Yeast Transmembrane Sensor Wsc1 Obtained by SMA-Based Detergent-Free Purification and Transmission Electron Microscopy. J. Fungi 2021, 7, 118. [Google Scholar] [CrossRef]
- Rehan, S.; Jaakola, V.-P. Expression, Purification and Functional Characterization of Human Equilibrative Nucleoside Transporter Subtype-1 (HENT1) Protein from Sf9 Insect Cells. Protein Expr. Purif. 2015, 114, 99–107. [Google Scholar] [CrossRef]
- Yu, J.; Zhu, H.; Lape, R.; Greiner, T.; Du, J.; Lü, W.; Sivilotti, L.; Gouaux, E. Mechanism of Gating and Partial Agonist Action in the Glycine Receptor. Cell 2021, 184, 957–968.e21. [Google Scholar] [CrossRef]
- Dörr, J.M.; van Coevorden-Hameete, M.H.; Hoogenraad, C.C.; Killian, J.A. Solubilization of Human Cells by the Styrene-Maleic Acid Copolymer: Insights from Fluorescence Microscopy. Biochim. Biophys. Acta-Biomembr. 2017, 1859, 2155–2160. [Google Scholar] [CrossRef]
- Bada Juarez, J.F.; Muñoz-García, J.C.; Inácio Dos Reis, R.; Henry, A.; McMillan, D.; Kriek, M.; Wood, M.; Vandenplas, C.; Sands, Z.; Castro, L.; et al. Detergent-Free Extraction of a Functional Low-Expressing GPCR from a Human Cell Line. Biochim. Biophys. Acta-Biomembr. 2020, 1862, 183152. [Google Scholar] [CrossRef]
- Korotych, O.; Mondal, J.; Gattás-Asfura, K.M.; Hendricks, J.; Bruce, B.D. Evaluation of Commercially Available Styrene-Co-Maleic Acid Polymers for the Extraction of Membrane Proteins from Spinach Chloroplast Thylakoids. Eur. Polym. J. 2019, 114, 485–500. [Google Scholar] [CrossRef]
- Reading, E.; Hall, Z.; Martens, C.; Haghighi, T.; Findlay, H.; Ahdash, Z.; Politis, A.; Booth, P.J. Interrogating Membrane Protein Conformational Dynamics within Native Lipid Compositions. Angew. Chem. Int. Ed. Engl. 2017, 56, 15654–15657. [Google Scholar] [CrossRef]
- Jakubec, M.; Bariås, E.; Furse, S.; Govasli, M.L.; George, V.; Turcu, D.; Iashchishyn, I.A.; Morozova-Roche, L.A.; Halskau, Ø. Cholesterol-Containing Lipid Nanodiscs Promote an α-Synuclein Binding Mode That Accelerates Oligomerization. FEBS J. 2021, 288, 1887–1905. [Google Scholar] [CrossRef] [PubMed]
- Teo, A.C.K.; Lee, S.C.; Pollock, N.L.; Stroud, Z.; Hall, S.; Thakker, A.; Pitt, A.R.; Dafforn, T.R.; Spickett, C.M.; Roper, D.I. Analysis of SMALP Co-Extracted Phospholipids Shows Distinct Membrane Environments for Three Classes of Bacterial Membrane Protein. Sci. Rep. 2019, 9, 1813. [Google Scholar] [CrossRef]
- Hoi, K.K.; Bada Juarez, J.F.; Judge, P.J.; Yen, H.-Y.; Wu, D.; Vinals, J.; Taylor, G.F.; Watts, A.; Robinson, C.V. Detergent-Free Lipodisq Nanoparticles Facilitate High-Resolution Mass Spectrometry of Folded Integral Membrane Proteins. Nano Lett. 2021, 21, 2824–2831. [Google Scholar] [CrossRef] [PubMed]
- Grime, R.L.; Goulding, J.; Uddin, R.; Stoddart, L.A.; Hill, S.J.; Poyner, D.R.; Briddon, S.J.; Wheatley, M. Single Molecule Binding of a Ligand to a G-Protein-Coupled Receptor in Real Time Using Fluorescence Correlation Spectroscopy, Rendered Possible by Nano-Encapsulation in Styrene Maleic Acid Lipid Particles. Nanoscale 2020, 12, 11518–11525. [Google Scholar] [CrossRef] [PubMed]
- de Jonge, P.A.; Smit Sibinga, D.J.C.; Boright, O.A.; Costa, A.R.; Nobrega, F.L.; Brouns, S.J.J.; Dutilh, B.E. Development of Styrene Maleic Acid Lipid Particles as a Tool for Studies of Phage-Host Interactions. J. Virol. 2020, 94. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, N.; Peetz, O.; Ahdash, Z.; Tascón, I.; Booth, P.J.; Mikusevic, V.; Diskowski, M.; Politis, A.; Hellmich, Y.; Hänelt, I.; et al. Native Mass Spectrometry Goes More Native: Investigation of Membrane Protein Complexes Directly from SMALPs. Chem. Commun. 2018, 54, 13702–13705. [Google Scholar] [CrossRef]
- Pitch, S.G.; Yao, W.; Szundi, I.; Fay, J.; Chen, E.; Shumate, A.; Kliger, D.S.; Farrens, D.L. Functional Integrity of Membrane Protein Rhodopsin Solubilized by Styrene-Maleic Acid Copolymer. Biophys. J. 2021, 120, 3508–3515. [Google Scholar] [CrossRef]
- Brown, C.J.; Trieber, C.; Overduin, M. Structural Biology of Endogenous Membrane Protein Assemblies in Native Nanodiscs. Curr. Opin. Struct. Biol. 2021, 69, 70–77. [Google Scholar] [CrossRef]
- Sahu, I.D.; Zhang, R.; Dunagan, M.M.; Craig, A.F.; Lorigan, G.A. Characterization of KCNE1 inside Lipodisq Nanoparticles for EPR Spectroscopic Studies of Membrane Proteins. J. Phys. Chem. B 2017, 121, 5312–5321. [Google Scholar] [CrossRef]
- Sahu, I.D.; Dixit, G.; Reynolds, W.D.; Kaplevatsky, R.; Harding, B.D.; Jaycox, C.K.; McCarrick, R.M.; Lorigan, G.A. Characterization of the Human KCNQ1 Voltage Sensing Domain (VSD) in Lipodisq Nanoparticles for Electron Paramagnetic Resonance (EPR) Spectroscopic Studies of Membrane Proteins. J. Phys. Chem. B 2020, 124, 2331–2342. [Google Scholar] [CrossRef] [PubMed]
- Mosslehy, W.; Voskoboynikova, N.; Colbasevici, A.; Ricke, A.; Klose, D.; Klare, J.P.; Mulkidjanian, A.Y.; Steinhoff, H.-J. Conformational Dynamics of Sensory Rhodopsin II in Nanolipoprotein and Styrene-Maleic Acid Lipid Particles. Photochem. Photobiol. 2019, 95, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Radoicic, J.; Park, S.H.; Opella, S.J. Macrodiscs Comprising SMALPs for Oriented Sample Solid-State NMR Spectroscopy of Membrane Proteins. Biophys. J. 2018, 115, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Beriashvili, D.; Spencer, N.R.; Dieckmann, T.; Overduin, M.; Palmer, M. Characterization of Multimeric Daptomycin Bound to Lipid Nanodiscs Formed by Calcium-Tolerant Styrene-Maleic Acid Copolymer. Biochim. Biophys. Acta-Biomembr. 2020, 1862, 183234. [Google Scholar] [CrossRef] [PubMed]
- Bersch, B.; Dörr, J.M.; Hessel, A.; Killian, J.A.; Schanda, P. Proton-Detected Solid-State NMR Spectroscopy of a Zinc Diffusion Facilitator Protein in Native Nanodiscs. Angew. Chem. Int. Ed. Engl. 2017, 56, 2508–2512. [Google Scholar] [CrossRef] [PubMed]
- Danmaliki, G.I.; Hwang, P.M. Solution NMR Spectroscopy of Membrane Proteins. Biochim. Biophys. Acta-Biomembr. 2020, 1862, 183356. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.F.; McCrea, P.D.; Zaccaï, G.; Engelman, D.M. Assessment of the Aggregation State of Integral Membrane Proteins in Reconstituted Phospholipid Vesicles Using Small Angle Neutron Scattering. J. Mol. Biol. 1997, 273, 1004–1019. [Google Scholar] [CrossRef]
- Skar-Gislinge, N.; Kynde, S.A.R.; Denisov, I.G.; Ye, X.; Lenov, I.; Sligar, S.G.; Arleth, L. Small-Angle Scattering Determination of the Shape and Localization of Human Cytochrome P450 Embedded in a Phospholipid Nanodisc Environment. Acta Crystallogr. D Biol. Crystallogr. 2015, 71, 2412–2421. [Google Scholar] [CrossRef]
- Pérez, J.; Koutsioubas, A. Memprot: A Program to Model the Detergent Corona around a Membrane Protein Based on SEC–SAXS Data. Acta Crystallogr. D Biol. Crystallogr. 2015, 71, 86–93. [Google Scholar] [CrossRef]
- Kynde, S.A.R.; Skar-Gislinge, N.; Pedersen, M.C.; Midtgaard, S.R.; Simonsen, J.B.; Schweins, R.; Mortensen, K.; Arleth, L. Small-Angle Scattering Gives Direct Structural Information about a Membrane Protein inside a Lipid Environment. Acta Crystallogr. D Biol. Crystallogr. 2014, 70, 371–383. [Google Scholar] [CrossRef]
- Molodenskiy, D.S.; Svergun, D.I.; Mertens, H.D.T. MPBuilder: A PyMOL Plugin for Building and Refinement of Solubilized Membrane Proteins Against Small Angle X-ray Scattering Data. J. Mol. Biol. 2021, 433, 166888. [Google Scholar] [CrossRef] [PubMed]
- Orioli, S.; Henning Hansen, C.G.; Arleth, L. Ab Initio Determination of the Shape of Membrane Proteins in a Nanodisc. Acta Crystallogr. D Struct. Biol. 2021, 77, 176–193. [Google Scholar] [CrossRef] [PubMed]
- Franke, D.; Svergun, D.I. DAMMIF, a Program for Rapid Ab-Initio Shape Determination in Small-Angle Scattering. J. Appl. Crystallogr. 2009, 42, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Ryzhykau, Y.L.; Orekhov, P.S.; Rulev, M.I.; Vlasov, A.V.; Melnikov, I.A.; Volkov, D.A.; Nikolaev, M.Y.; Zabelskii, D.V.; Murugova, T.N.; Chupin, V.V.; et al. Molecular Model of a Sensor of Two-Component Signaling System. Sci. Rep. 2021, 11, 10774. [Google Scholar] [CrossRef]
- Ryzhykau, Y.L.; Vlasov, A.V.; Orekhov, P.S.; Rulev, M.I.; Rogachev, A.V.; Vlasova, A.D.; Kazantsev, A.S.; Verteletskiy, D.P.; Skoi, V.V.; Brennich, M.E.; et al. Ambiguities in and Completeness of SAS Data Analysis of Membrane Proteins: The Case of the Sensory Rhodopsin II-Transducer Complex. Acta Crystallogr. D Struct. Biol. 2021, 77, 1386–1400. [Google Scholar] [CrossRef]
- Nazarenko, V.; Remeeva, A.; Ryzhykau, Y.; Orekhov, P.; Semenov, O.; Goncharov, I.; Yudenko, A.; Gushchin, I. Small Angle X-ray Scattering Study of a Histidine Kinase Embedded in Styrene-maleic Acid Copolymer Lipid Particles. FASEB J. 2021, 35. [Google Scholar] [CrossRef]
- Gushchin, I.; Aleksenko, V.A.; Orekhov, P.; Goncharov, I.M.; Nazarenko, V.V.; Semenov, O.; Remeeva, A.; Gordeliy, V. Nitrate- and Nitrite-Sensing Histidine Kinases: Function, Structure, and Natural Diversity. Int. J. Mol. Sci. 2021, 22, 5933. [Google Scholar] [CrossRef]
- Moraes, I.; Evans, G.; Sanchez-Weatherby, J.; Newstead, S.; Stewart, P.D.S. Membrane Protein Structure Determination - the next Generation. Biochim. Biophys. Acta-Biomembr. 2014, 1838, 78–87. [Google Scholar] [CrossRef]
- Bill, R.M.; Henderson, P.J.F.; Iwata, S.; Kunji, E.R.S.; Michel, H.; Neutze, R.; Newstead, S.; Poolman, B.; Tate, C.G.; Vogel, H. Overcoming Barriers to Membrane Protein Structure Determination. Nat. Biotechnol. 2011, 29, 335–340. [Google Scholar] [CrossRef]
- Caffrey, M.; Cherezov, V. Crystallizing Membrane Proteins Using Lipidic Mesophases. Nat. Protoc. 2009, 4, 706–731. [Google Scholar] [CrossRef]
- Broecker, J.; Eger, B.T.; Ernst, O.P. Crystallogenesis of Membrane Proteins Mediated by Polymer-Bounded Lipid Nanodiscs. Structure 2017, 25, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Polovinkin, V.; Gushchin, I.; Sintsov, M.; Round, E.; Balandin, T.; Chervakov, P.; Shevchenko, V.; Utrobin, P.; Popov, A.; Borshchevskiy, V.; et al. High-Resolution Structure of a Membrane Protein Transferred from Amphipol to a Lipidic Mesophase. J. Membr. Biol. 2014, 247, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, M.; Round, E.; Gushchin, I.; Polovinkin, V.; Balandin, T.; Kuzmichev, P.; Shevchenko, V.; Borshchevskiy, V.; Kuklin, A.; Round, A.; et al. Integral Membrane Proteins Can Be Crystallized Directly from Nanodiscs. Cryst. Growth Des. 2017, 17, 945–948. [Google Scholar] [CrossRef]
- Nakane, T.; Kotecha, A.; Sente, A.; McMullan, G.; Masiulis, S.; Brown, P.M.G.E.; Grigoras, I.T.; Malinauskaite, L.; Malinauskas, T.; Miehling, J.; et al. Single-Particle Cryo-EM at Atomic Resolution. Nature 2020, 587, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.; Shao, S.; Murray, J.; Hegde, R.S.; Ramakrishnan, V. Structural Basis for Stop Codon Recognition in Eukaryotes. Nature 2015, 524, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Hite, R.K.; MacKinnon, R. Structural Titration of Slo2.2, a Na+-Dependent K+ Channel. Cell 2017, 168, 390–399.e11. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Gennis, R.B. Single-Particle Cryo-EM Studies of Transmembrane Proteins in SMA Copolymer Nanodiscs. Chem. Phys. Lipids 2019, 221, 114–119. [Google Scholar] [CrossRef]
- Postis, V.; Rawson, S.; Mitchell, J.K.; Lee, S.C.; Parslow, R.A.; Dafforn, T.R.; Baldwin, S.A.; Muench, S.P. The Use of SMALPs as a Novel Membrane Protein Scaffold for Structure Study by Negative Stain Electron Microscopy. Biochim. Biophys. Acta-Biomembr. 2015, 1848, 496–501. [Google Scholar] [CrossRef]
- Parmar, M.; Rawson, S.; Scarff, C.A.; Goldman, A.; Dafforn, T.R.; Muench, S.P.; Postis, V.L.G. Using a SMALP Platform to Determine a Sub-Nm Single Particle Cryo-EM Membrane Protein Structure. Biochim. Biophys. Acta-Biomembr. 2018, 1860, 378–383. [Google Scholar] [CrossRef]
- Sun, C.; Benlekbir, S.; Venkatakrishnan, P.; Wang, Y.; Hong, S.; Hosler, J.; Tajkhorshid, E.; Rubinstein, J.L.; Gennis, R.B. Structure of the Alternative Complex III in a Supercomplex with Cytochrome Oxidase. Nature 2018, 557, 123–126. [Google Scholar] [CrossRef]
- Yoder, N.; Gouaux, E. The His-Gly Motif of Acid-Sensing Ion Channels Resides in a Reentrant ‘Loop’ Implicated in Gating and Ion Selectivity. eLife 2020, 9, e56527. [Google Scholar]
- Flegler, V.J.; Rasmussen, A.; Rao, S.; Wu, N.; Zenobi, R.; Sansom, M.S.P.; Hedrich, R.; Rasmussen, T.; Böttcher, B. The MscS-like Channel YnaI Has a Gating Mechanism Based on Flexible Pore Helices. Proc. Natl. Acad. Sci. USA 2020, 117, 28754–28762. [Google Scholar] [CrossRef] [PubMed]
- Oates, J.; Watts, A. Uncovering the Intimate Relationship between Lipids, Cholesterol and GPCR Activation. Curr. Opin. Struct. Biol. 2011, 21, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Chorev, D.S.; Robinson, C.V. The Importance of the Membrane for Biophysical Measurements. Nat. Chem. Biol. 2020, 16, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Seibert, M.M.; Ekeberg, T.; Maia, F.R.N.C.; Svenda, M.; Andreasson, J.; Jönsson, O.; Odić, D.; Iwan, B.; Rocker, A.; Westphal, D.; et al. Single Mimivirus Particles Intercepted and Imaged with an X-ray Laser. Nature 2011, 470, 78–81. [Google Scholar] [CrossRef]
- Shelby, M.L.; Gilbile, D.; Grant, T.D.; Bauer, W.J.; Segelke, B.; He, W.; Evans, A.C.; Crespo, N.; Fischer, P.; Pakendorf, T.; et al. Crystallization of ApoA1 and ApoE4 Nanolipoprotein Particles and Initial XFEL-Based Structural Studies. Crystals 2020, 10, 886. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orekhov, P.S.; Bozdaganyan, M.E.; Voskoboynikova, N.; Mulkidjanian, A.Y.; Karlova, M.G.; Yudenko, A.; Remeeva, A.; Ryzhykau, Y.L.; Gushchin, I.; Gordeliy, V.I.; et al. Mechanisms of Formation, Structure, and Dynamics of Lipoprotein Discs Stabilized by Amphiphilic Copolymers: A Comprehensive Review. Nanomaterials 2022, 12, 361. https://doi.org/10.3390/nano12030361
Orekhov PS, Bozdaganyan ME, Voskoboynikova N, Mulkidjanian AY, Karlova MG, Yudenko A, Remeeva A, Ryzhykau YL, Gushchin I, Gordeliy VI, et al. Mechanisms of Formation, Structure, and Dynamics of Lipoprotein Discs Stabilized by Amphiphilic Copolymers: A Comprehensive Review. Nanomaterials. 2022; 12(3):361. https://doi.org/10.3390/nano12030361
Chicago/Turabian StyleOrekhov, Philipp S., Marine E. Bozdaganyan, Natalia Voskoboynikova, Armen Y. Mulkidjanian, Maria G. Karlova, Anna Yudenko, Alina Remeeva, Yury L. Ryzhykau, Ivan Gushchin, Valentin I. Gordeliy, and et al. 2022. "Mechanisms of Formation, Structure, and Dynamics of Lipoprotein Discs Stabilized by Amphiphilic Copolymers: A Comprehensive Review" Nanomaterials 12, no. 3: 361. https://doi.org/10.3390/nano12030361
APA StyleOrekhov, P. S., Bozdaganyan, M. E., Voskoboynikova, N., Mulkidjanian, A. Y., Karlova, M. G., Yudenko, A., Remeeva, A., Ryzhykau, Y. L., Gushchin, I., Gordeliy, V. I., Sokolova, O. S., Steinhoff, H.-J., Kirpichnikov, M. P., & Shaitan, K. V. (2022). Mechanisms of Formation, Structure, and Dynamics of Lipoprotein Discs Stabilized by Amphiphilic Copolymers: A Comprehensive Review. Nanomaterials, 12(3), 361. https://doi.org/10.3390/nano12030361









