Comparative Study between Curcumin and Nanocurcumin Loaded PLGA on Colon Carcinogenesis Induced Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Colon Cancer Induction Protocol
2.3. Curcumin Encapsulated PLGA Preparation
2.4. Animals and Housing
2.4.1. Animal Groups and Mode of Treatment
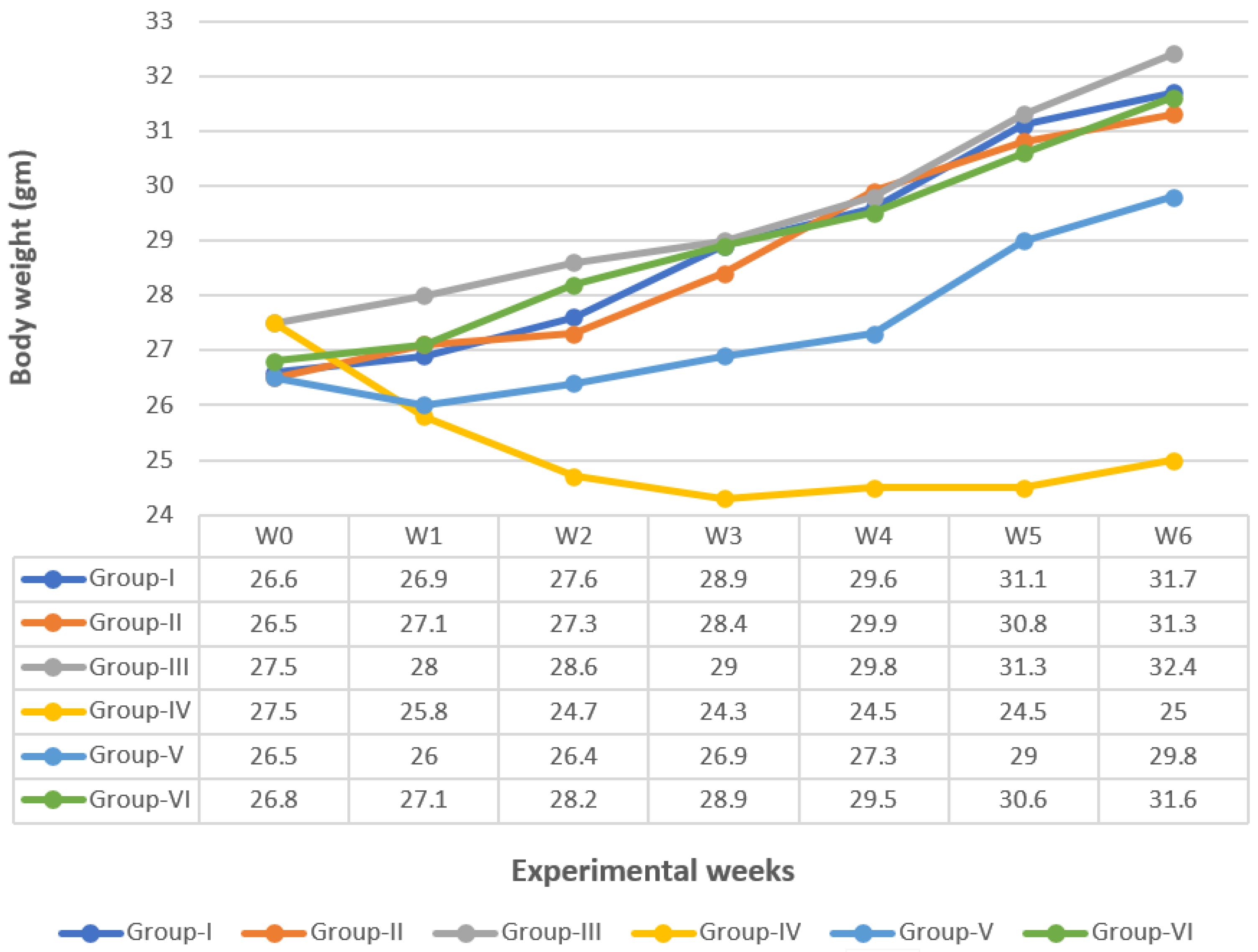
2.4.2. Body Weight Changes
2.5. Samples Collection and Tissue Preparation
2.5.1. Blood Sampling
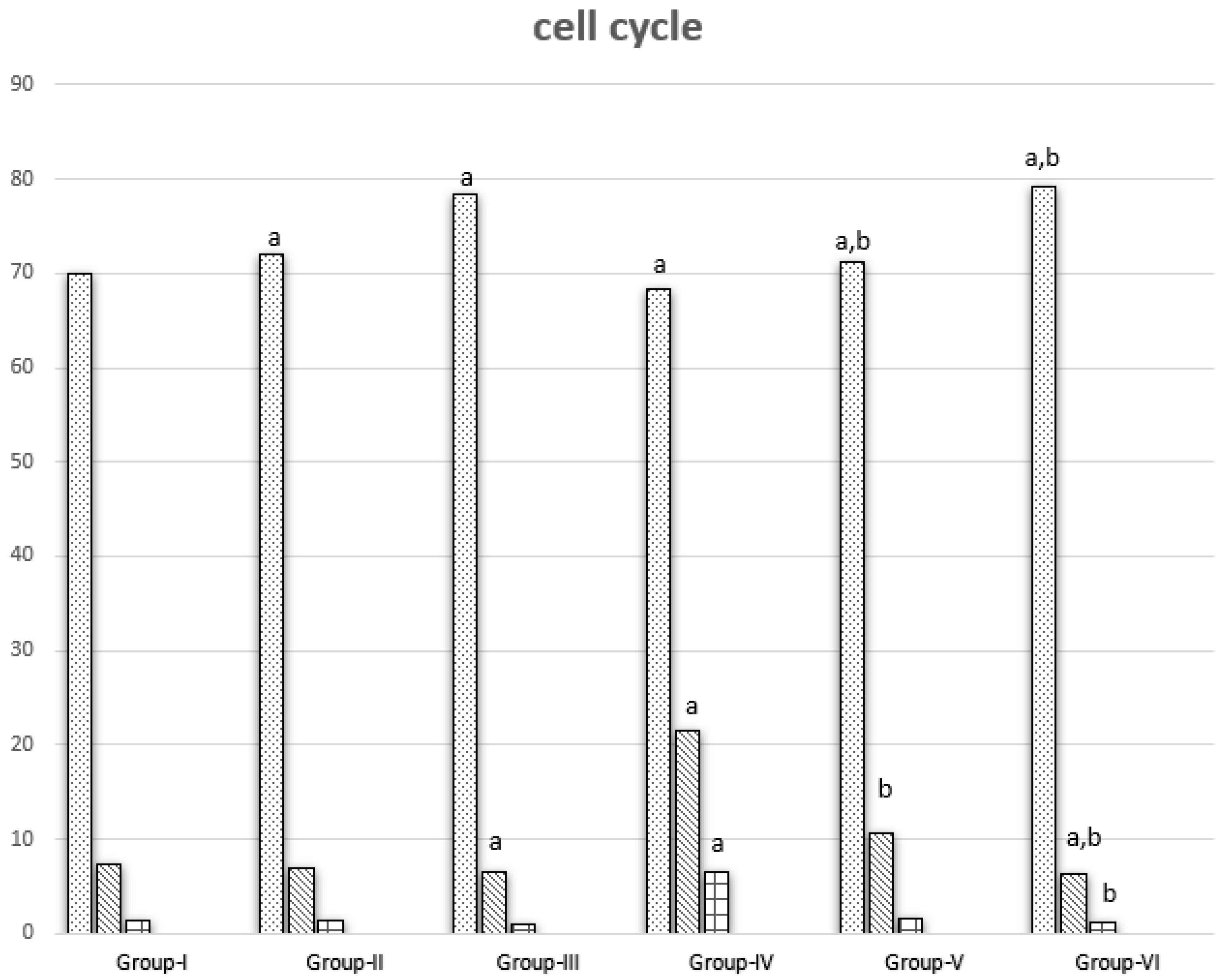
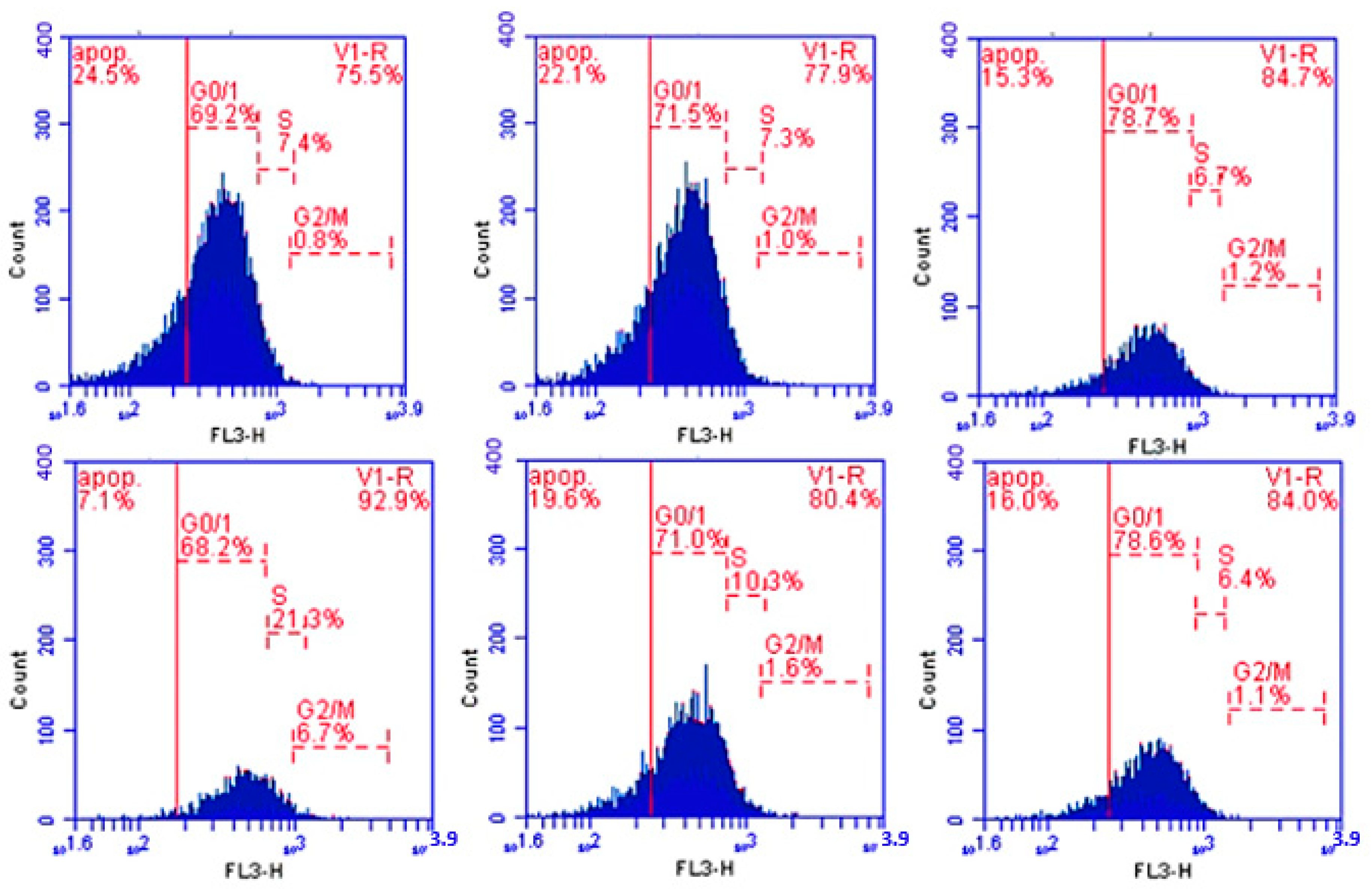
2.5.2. Determination of Cell Cycle
2.6. Hematoxylin and Eosin Stains
2.7. Immunohistochemical Detection of PCNA Polyclonal Antibody
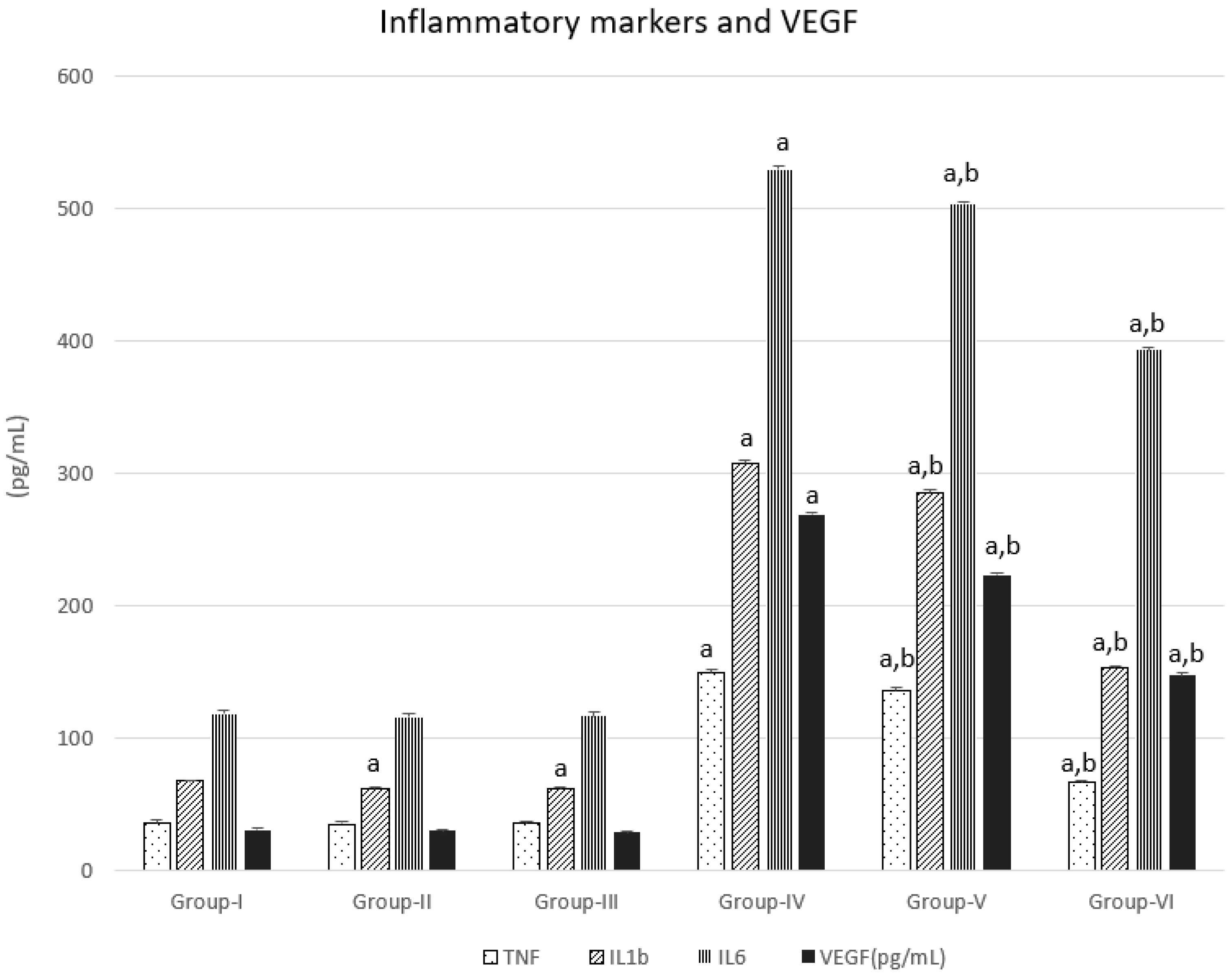
2.8. Determination of Inflammation Markers and VEGF
2.9. Statistical Analysis
3. Results
3.1. Body Weight Change
3.2. Inflammatory Markers and VEGF
3.3. Cell Cycle Results
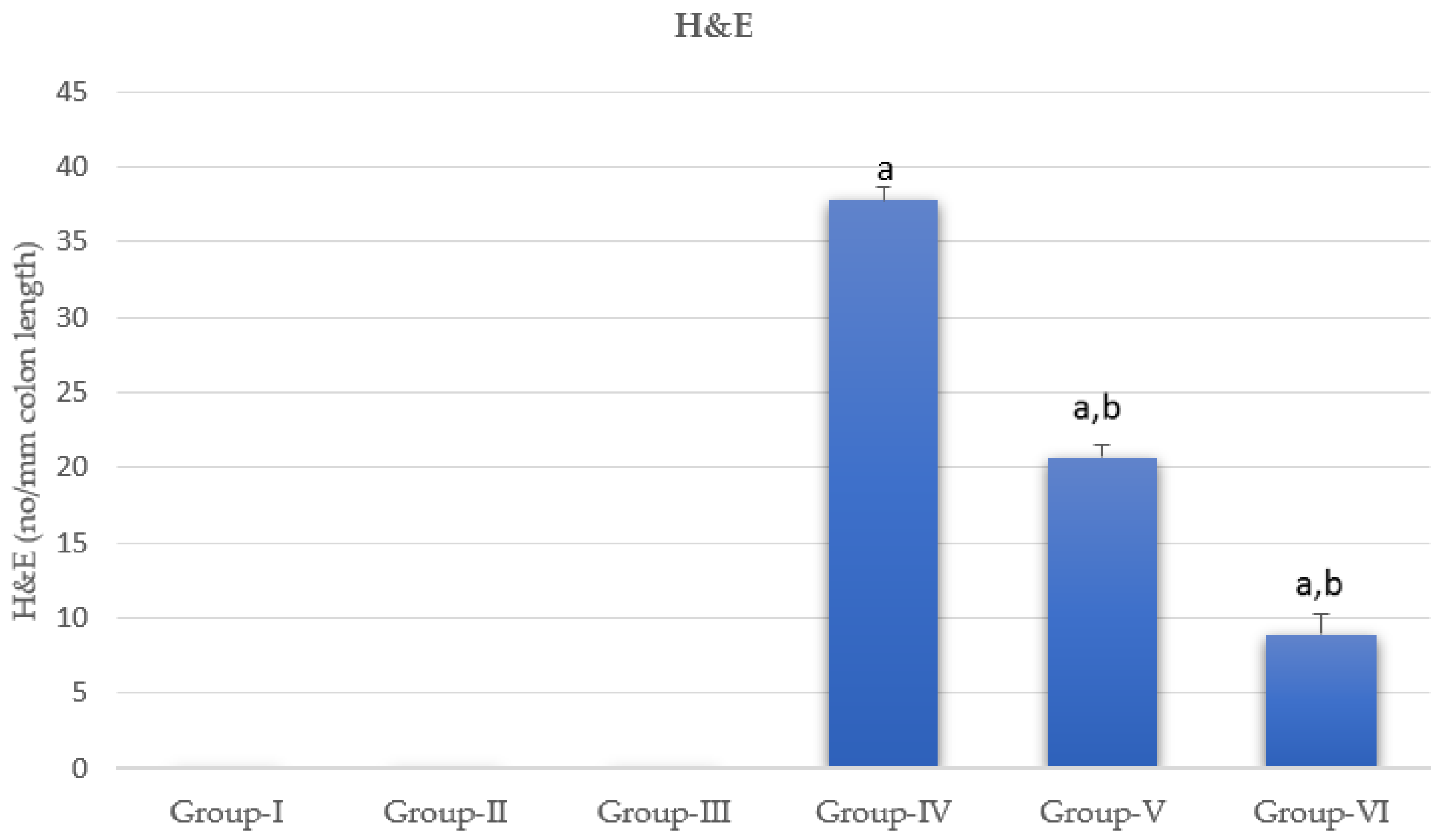
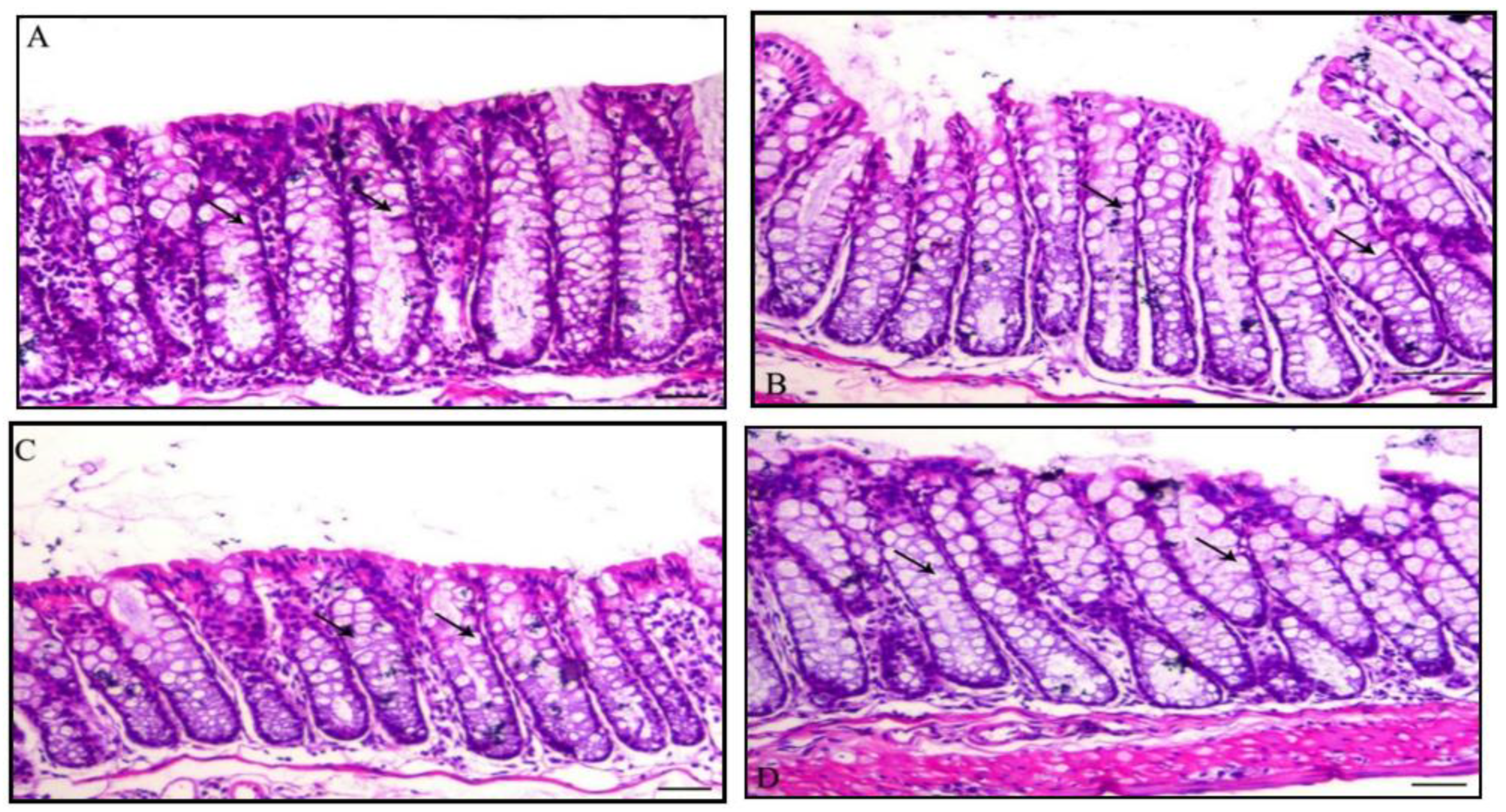
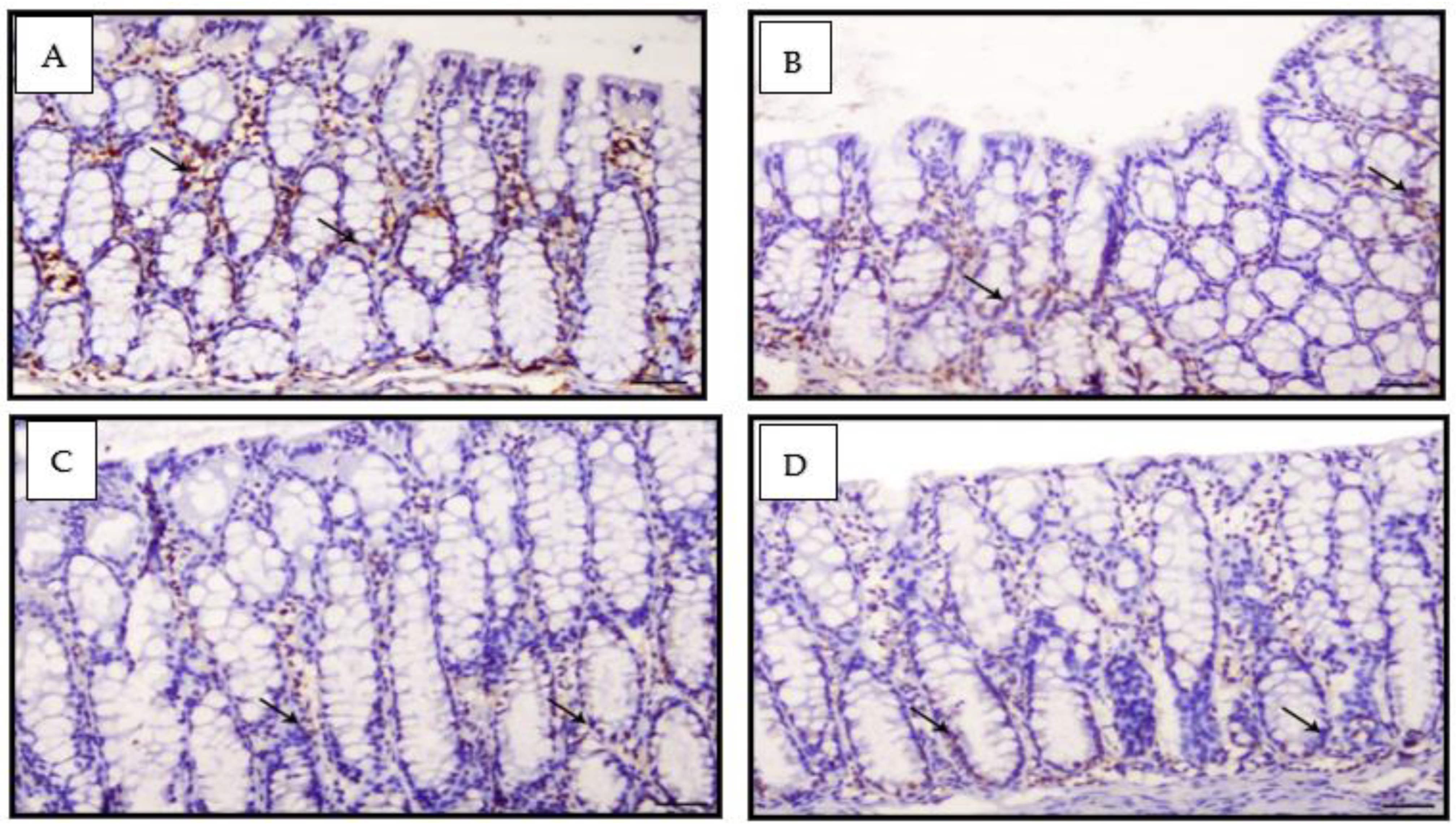
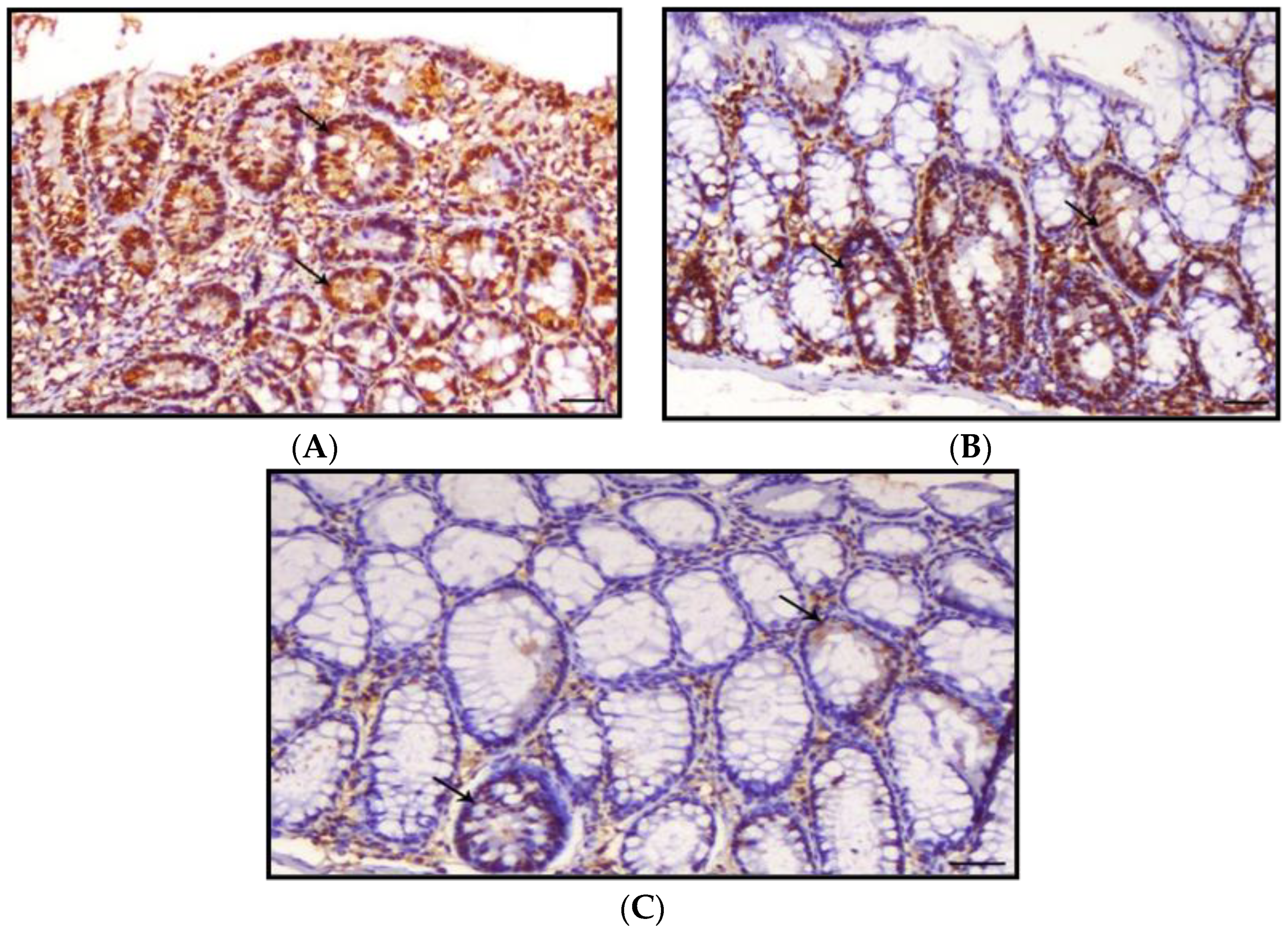
3.4. Histology of the Colon
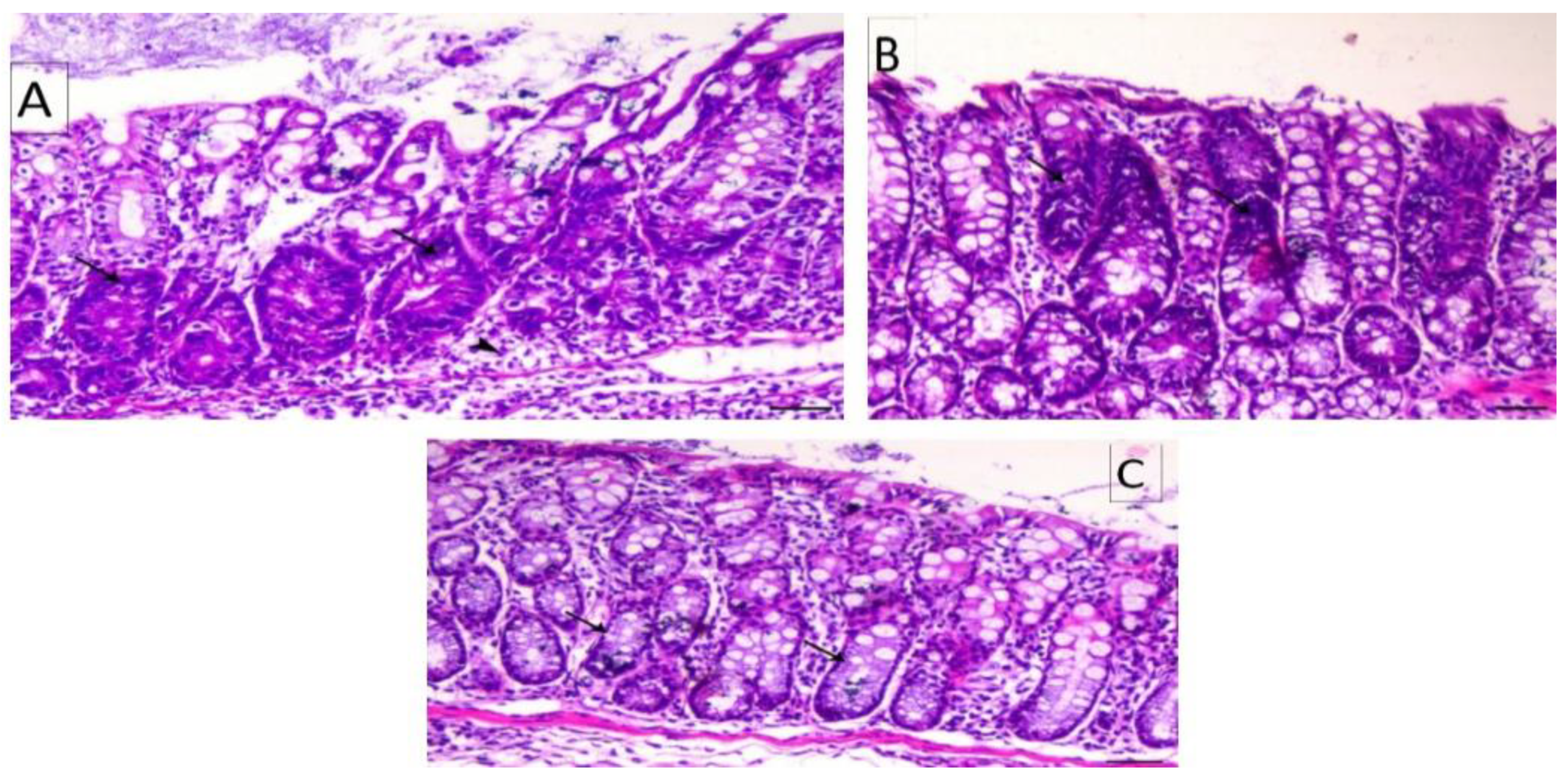
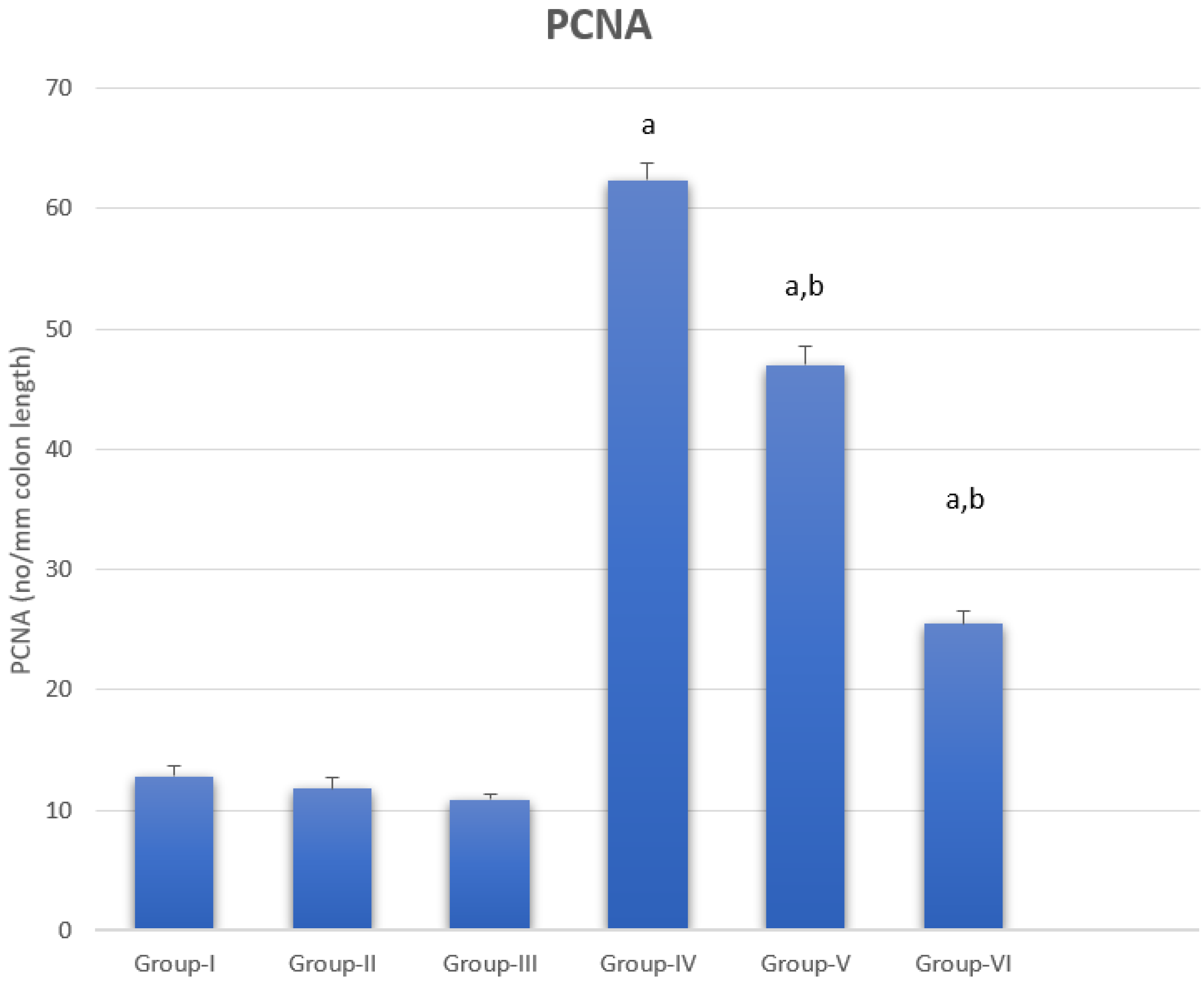
3.5. Proliferating Cell Nuclear Antigen
4. Discussion
5. Conclusions
- (1)
- Decreased G0/I phase in cell cycle;
- (2)
- Lowered inflammatory markers and VEGF levels;
- (3)
- Reduced intestinal crypt dysplasia and interstitial colitis linked with inflammatory cell infiltration, especially lymphocytes and macrophages;
- (4)
- PCNA expression was improved by CUR-loaded PLGA;
- (5)
- Curcumin’s bioavailability is severely reduced due to low serum levels, limited tissue distribution, apparent quick metabolism, and a short half-life. Longer circulation lengths, better permeability, and resistance to metabolic presystemic degradation were found in CUR @PLGA results;
- (6)
- Curcumin’s activity becomes more potent after being placed onto nanoparticles.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cooper, G. The Development and Causes of Cancer. In The Cell: A Molecular Approach; Sinauer Associates: Sunderland, MA, USA, 2000; ISBN 0878931066. Available online: https://www.ncbi.nlm.nih.gov/books/NBK9963/ (accessed on 8 April 2017).
- Fernández-Villa, T.; Álvarez-Álvarez, L.; Rubín-García, M.; Obón-Santacana, M.; Moreno, V. The role of dietary patterns in colorectal cancer: A 2019 update. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.L.; Wike, J.M.; Kato, I.; Lewis, D.R.; Michaud, F. A population-based study of colorectal cancer histology in the United States, 1998–2001. Cancer 2006, 107, 1128–1141. [Google Scholar] [CrossRef]
- Ahmad, R.; Srivastava, S.; Ghosh, S.; Khare, S.K. Phytochemical delivery through nanocarriers: A review. Colloids Surf. B Biointerfaces 2021, 197, 111389. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, S.; Cheng, X.; Wu, J.; Wu, L.; Wang, Y.; Wang, X.; Bao, J.; Yu, H. Curcumin induces autophagic cell death in human thyroid cancer cells. Toxicol. Vitr. 2022, 78, 105254. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Li, Z.; Gao, C.-Y.; Cho, C.H. Mechanisms of drug resistance in colon cancer and its therapeutic strategies. World J. Gastroenterol. 2016, 22, 6876. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Zarrabi, A.; Hashemi, F.; Zabolian, A.; Saleki, H.; Bagherian, M.; Azami, N.; Bejandi, A.K.; Hushmandi, K.; Ang, H.L. Polychemotherapy with curcumin and doxorubicin via biological nanoplatforms: Enhancing antitumor activity. Pharmaceutics 2020, 12, 1084. [Google Scholar] [CrossRef] [PubMed]
- Abadi, A.J.; Mirzaei, S.; Mahabady, M.K.; Hashemi, F.; Zabolian, A.; Hashemi, F.; Raee, P.; Aghamiri, S.; Ashrafizadeh, M.; Aref, A.R. Curcumin and its derivatives in cancer therapy: Potentiating antitumor activity of cisplatin and reducing side effects. Phytother. Res. 2021. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Surh, Y.-J.; Shishodia, S. The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Springer Science & Business Media: Berlin, Germany, 2007; Volume 595, ISBN 0387464018. [Google Scholar]
- Tunstall, R.; Sharma, R.; Perkins, S.; Sale, S.; Singh, R.; Farmer, P.; Steward, W.; Gescher, A. Cyclooxygenase-2 expression and oxidative DNA adducts in murine intestinal adenomas: Modification by dietary curcumin and implications for clinical trials. Eur. J. Cancer 2006, 42, 415–421. [Google Scholar] [CrossRef]
- Weir, N.M.; Selvendiran, K.; Kutala, V.K.; Tong, L.; Vishwanath, S.; Rajaram, M.; Tridandapani, S.; Anant, S.; Kuppusamy, P. Curcumin induces G2/M arrest and apoptosis in cisplatin-resistant human ovarian cancer cells by modulating Akt and p38 MAPK. Cancer Biol. Ther. 2007, 6, 178–184. [Google Scholar] [CrossRef] [Green Version]
- Maga, G.; Hübscher, U. Proliferating cell nuclear antigen (PCNA): A dancer with many partners. J. Cell Sci. 2003, 116, 3051–3060. [Google Scholar] [CrossRef] [Green Version]
- Kreuter, J. Colloidal Drug Delivery Systems; CRC Press: Boca Raton, FL, USA, 2014; Volume 66, ISBN 1498710565. [Google Scholar]
- Kabir, M.; Rahman, M.; Akter, R.; Behl, T.; Kaushik, D.; Mittal, V.; Pandey, P.; Akhtar, M.F.; Saleem, A.; Albadrani, G.M. Potential role of curcumin and its nanoformulations to treat various types of cancers. Biomolecules 2021, 11, 392. [Google Scholar] [CrossRef]
- Saleem, K.; Wani, W.A.; Haque, A.; Lone, M.N.; Hsieh, M.-F.; Jairajpuri, M.A.; Ali, I. Synthesis, DNA binding, hemolysis assays and anticancer studies of copper (II), nickel (II) and iron (III) complexes of a pyrazoline-based ligand. Future Med. Chem. 2013, 5, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Saleem, K.; Wesselinova, D.; Haque, A. Synthesis, DNA binding, hemolytic, and anti-cancer assays of curcumin I-based ligands and their ruthenium(III) complexes. Med. Chem. Res. 2013, 22, 1386–1398. [Google Scholar] [CrossRef]
- Jiang, T.; Charcosset, C. Premix membrane emulsification for the preparation of curcumin-loaded nanoemulsions. J. Food Eng. 2022, 316, 110836. [Google Scholar] [CrossRef]
- Ntoutoume, G.M.N.; Granet, R.; Mbakidi, J.P.; Brégier, F.; Léger, D.Y.; Fidanzi-Dugas, C.; Lequart, V.; Joly, N.; Liagre, B.; Chaleix, V. Development of curcumin–cyclodextrin/cellulose nanocrystals complexes: New anticancer drug delivery systems. Bioorg. Med. Chem. Lett. 2016, 26, 941–945. [Google Scholar] [CrossRef]
- Iurciuc-Tincu, C.-E.; Atanase, L.I.; Ochiuz, L.; Jérôme, C.; Sol, V.; Martin, P.; Popa, M. Curcumin-loaded polysaccharides-based complex particles obtained by polyelectrolyte complexation and ionic gelation. I-Particles obtaining and characterization. Int. J. Biol. Macromol. 2020, 147, 629–642. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, C.; Liu, X.; Mackie, A.; Zhang, M.; Dai, L.; Liu, J.; Mao, L.; Yuan, F.; Gao, Y. Co-encapsulation of curcumin and β-carotene in Pickering emulsions stabilized by complex nanoparticles: Effects of microfluidization and thermal treatment. Food Hydrocoll. 2022, 122, 107064. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, X.; Tian, T.; Zhang, Q.; Wen, Y.; Zhu, J.; Xiao, D.; Cui, W.; Lin, Y. Anti-inflammatory activity of curcumin-loaded tetrahedral framework nucleic acids on acute gouty arthritis. Bioact. Mater. 2022, 8, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.-Y.; Wang, S.P.-H.; Su, C.-H.; Tsai, T.-L.; Wu, P.-C.; Shieh, D.-B.; Chen, J.-H.; Hsieh, P.C.-H.; Yeh, C.-S. Stabilizer-free poly (lactide-co-glycolide) nanoparticles for multimodal biomedical probes. Biomaterials 2008, 29, 2104–2112. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Kyo, E.; Uesaka, T.; Katoh, O.; Watanabe, H. Prevention of development of N, N’-dimethylhydrazine-induced colon tumors by a water-soluble extract from cultured medium of Ganoderma lucidum (Rei-shi) mycelia in male ICR mice. Int. J. Mol. Med. 2002, 9, 113–117. [Google Scholar] [CrossRef]
- Sankar, P.; Telang, A.G.; Suresh, S.; Kesavan, M.; Kannan, K.; Kalaivanan, R.; Sarkar, S.N. Immunomodulatory effects of nanocurcumin in arsenic-exposed rats. Int. Immunopharmacol. 2013, 17, 65–70. [Google Scholar] [CrossRef]
- Satapathy, A.; Rao, M.V. Protective effect of Curcumin on 2, 4-Dichlorophenoxy acetic acid exerted Hepatotoxicity in Mice. Res. J. Pharm. Technol. 2018, 11, 637–642. [Google Scholar] [CrossRef]
- Oyeyemi, O.; Morenkeji, O.; Afolayan, F.; Dauda, K.; Busari, Z.; Meena, J.; Panda, A. Curcumin-artesunate based polymeric nanoparticle; antiplasmodial and toxicological evaluation in murine model. Front. Pharmacol. 2018, 9, 562. [Google Scholar] [CrossRef] [PubMed]
- Vindelov, L.L. Flow microfluorometric analysis of nuclear DNA in cells from solid tumors and cell suspensions. A new method for rapid isolation and straining of nuclei. Virchows Archiv. B Cell Pathol. 1977, 24, 227–242. [Google Scholar] [CrossRef]
- Goss, G. Theory and Practice of Histological Techniques. Am. J. Surg. Pathol. 2009, 33, 323. [Google Scholar] [CrossRef]
- Hall, P.A.; Levison, D.A.; Woods, A.L.; Yu, C.C.; Kellock, D.B.; Watkins, J.A.; Barnes, D.M.; Gillett, C.E.; Camplejohn, R.; Dover, R. Proliferating cell nuclear antigen (PCNA) immunolocalization in paraffin sections: An index of cell proliferation with evidence of deregulated expression in some neoplasms. J. Pathol. 1990, 162, 285–294. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Kohr, W.J.; Hass, P.E.; Moffat, B.; Spencer, S.A.; Henzel, W.J.; Bringman, T.; Nedwin, G.; Goeddel, D.; Harkins, R.N.; et al. Human tumor necrosis factor. Production, purification, and characterization. J. Biol. Chem. 1985, 260, 2345–2354. [Google Scholar] [CrossRef]
- Agay, D.; Andriollo-Sanchez, M.; Claeyssen, R.; Touvard, L.; Denis, J.; Roussel, A.-M.; Chancerelle, Y. Interleukin-6, TNF-alpha and interleukin-1 beta levels in blood and tissue in severely burned rats. Eur. Cytokine Netw. 2008, 19, 1–7. [Google Scholar] [CrossRef]
- Sato, T.; Wada, K.; Arahori, H.; Kuno, N.; Imoto, K.; Iwahashi-Shima, C.; Kusaka, S. Serum concentrations of bevacizumab (avastin) and vascular endothelial growth factor in infants with retinopathy of prematurity. Am. J. Ophthalmol. 2012, 153, 327–333. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Method, 7th ed.; Iowa State University Press: Ames, IA, USA, 1980; pp. 39–63. [Google Scholar]
- Yoon, H.; Liu, R.H. Effect of selected phytochemicals and apple extracts on NF-κB activation in human breast cancer MCF-7 cells. J. Agric. Food Chem. 2007, 55, 3167–3173. [Google Scholar] [CrossRef]
- Hamiza, O.O.; Rehman, M.U.; Tahir, M.; Khan, R.; Khan, A.Q.; Lateef, A.; Ali, F.; Sultana, S. Amelioration of 1, 2 Dimethylhydrazine (DMH) induced colon oxidative stress, inflammation and tumor promotion response by tannic acid in Wistar rats. Asian Pac. J. Cancer Prev. 2012, 13, 4393–4402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaikh, J.; Ankola, D.; Beniwal, V.; Singh, D.; Kumar, M.R. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur. J. Pharm. Sci. 2009, 37, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-M.; Jan, W.-C.; Chien, C.-F.; Lee, W.-C.; Lin, L.-C.; Tsai, T.-H. Optimised nano-formulation on the bioavailability of hydrophobic polyphenol, curcumin, in freely-moving rats. Food Chem. 2011, 127, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Al Obeed, O.A.; Alkhayal, K.A.; Al Sheikh, A.; Zubaidi, A.M.; Vaali-Mohammed, M.-A.; Boushey, R.; Mckerrow, J.H.; Abdulla, M.-H. Increased expression of tumor necrosis factor-α is associated with advanced colorectal cancer stages. World J. Gastroenterol. WJG 2014, 20, 18390. [Google Scholar] [CrossRef] [PubMed]
- Garza-Treviño, E.N.; Said-Fernández, S.L.; Martínez-Rodríguez, H.G. Understanding the colon cancer stem cells and perspectives on treatment. Cancer Cell Int. 2015, 15, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buhrmann, C.; Mobasheri, A.; Matis, U.; Shakibaei, M. Curcumin mediated suppression of nuclear factor-κB promotes chondrogenic differentiation of mesenchymal stem cells in a high-density co-culture microenvironment. Arthritis Res. Ther. 2010, 12, R127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer. Res. 2003, 23, 363–398. [Google Scholar] [PubMed]
- Cho, J.-W.; Lee, K.-S.; Kim, C.-W. Curcumin attenuates the expression of IL-1β, IL-6, and TNF-α as well as cyclin E in TNF-α-treated HaCaT cells; NF-κB and MAPKs as potential upstream targets. Int. J. Mol. Med. 2007, 19, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Asfour, W.; Almadi, S.; Haffar, L. Thymoquinone suppresses cellular proliferation, inhibits VEGF production and obstructs tumor progression and invasion in the rat model of DMH-induced colon carcinogenesis. Pharmacol. Pharm. 2013, 4, 26511. [Google Scholar] [CrossRef] [Green Version]
- Bates, D.; Hillman, N.; Pocock, T.; Neal, C. Regulation of microvascular permeability by vascular endothelial growth factors. J. Anat. 2002, 200, 523–534. [Google Scholar] [CrossRef]
- Hicklin, D.J.; Ellis, L.M. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J. Clin. Oncol. 2005, 23, 1011–1027. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.-K.; Kim, S.-H.; Jeong, J.-W.; Lee, Y.M.; Kim, H.-S.; Kim, S.-R.; Yun, I.; Bae, S.-K.; Kim, K.-W. Curcumin inhibits hypoxia-induced angiogenesis via down-regulation of HIF-1. Oncol. Rep. 2006, 15, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, J. Effects of curcumin on vessel formation insight into the pro-and antiangiogenesis of curcumin. Evid.-Based Complement. Altern. Med. 2019, 2019, 1390795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, P.; Nair, H.B.; Sung, B.; Kunnumakkara, A.B.; Yadav, V.R.; Tekmal, R.R.; Aggarwal, B.B. Design of Curcumin-Loaded PLGA Nanoparticles Formulation with Enhanced Cellular Uptake, and Increased Bioactivity In Vitro and Superior Bioavailability In Vivo; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar] [CrossRef] [Green Version]
- Bisht, S.; Feldmann, G.; Soni, S.; Ravi, R.; Karikar, C.; Maitra, A.; Maitra, A. Polymeric nanoparticle-encapsulated curcumin (“ nanocurcumin”): A novel strategy for human cancer therapy. J. Nanobiotechnol. 2007, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Sha, J.; Li, J.; Wang, W.; Pan, L.; Cheng, J.; Li, L.; Zhao, H.; Lin, W. Curcumin induces G0/G1 arrest and apoptosis in hormone independent prostate cancer DU-145 cells by down regulating Notch signaling. Biomed. Pharmacother. 2016, 84, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Tommonaro, G. Curcumin and cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Li, X.; Peng, Z.; Song, J.; Ren, J. Curcumin down-regulates PCNA, cyclin D1 and Bcl-xL expression in human keratinocyte cell lines. J. Med. Coll. PLA 2010, 25, 321–330. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elbassiouni, F.E.; El-Kholy, W.M.; Elhabibi, E.-S.M.; Albogami, S.; Fayad, E. Comparative Study between Curcumin and Nanocurcumin Loaded PLGA on Colon Carcinogenesis Induced Mice. Nanomaterials 2022, 12, 324. https://doi.org/10.3390/nano12030324
Elbassiouni FE, El-Kholy WM, Elhabibi E-SM, Albogami S, Fayad E. Comparative Study between Curcumin and Nanocurcumin Loaded PLGA on Colon Carcinogenesis Induced Mice. Nanomaterials. 2022; 12(3):324. https://doi.org/10.3390/nano12030324
Chicago/Turabian StyleElbassiouni, Farida E., Wafaa M. El-Kholy, El-Sayed M. Elhabibi, Sarah Albogami, and Eman Fayad. 2022. "Comparative Study between Curcumin and Nanocurcumin Loaded PLGA on Colon Carcinogenesis Induced Mice" Nanomaterials 12, no. 3: 324. https://doi.org/10.3390/nano12030324
APA StyleElbassiouni, F. E., El-Kholy, W. M., Elhabibi, E.-S. M., Albogami, S., & Fayad, E. (2022). Comparative Study between Curcumin and Nanocurcumin Loaded PLGA on Colon Carcinogenesis Induced Mice. Nanomaterials, 12(3), 324. https://doi.org/10.3390/nano12030324





