Cu-Doped Boron Nitride Nanosheets for Solid-Phase Extraction and Determination of Rhodamine B in Foods Matrix
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of the Adsorbents
2.2. Synthesis of Adsorbent
2.3. Characterization
2.4. Static Adsorption
2.5. SPE-HPLC Procedure
2.6. Sample Matrix Preparation
3. Results and Discussion
3.1. Sorbent Characterization
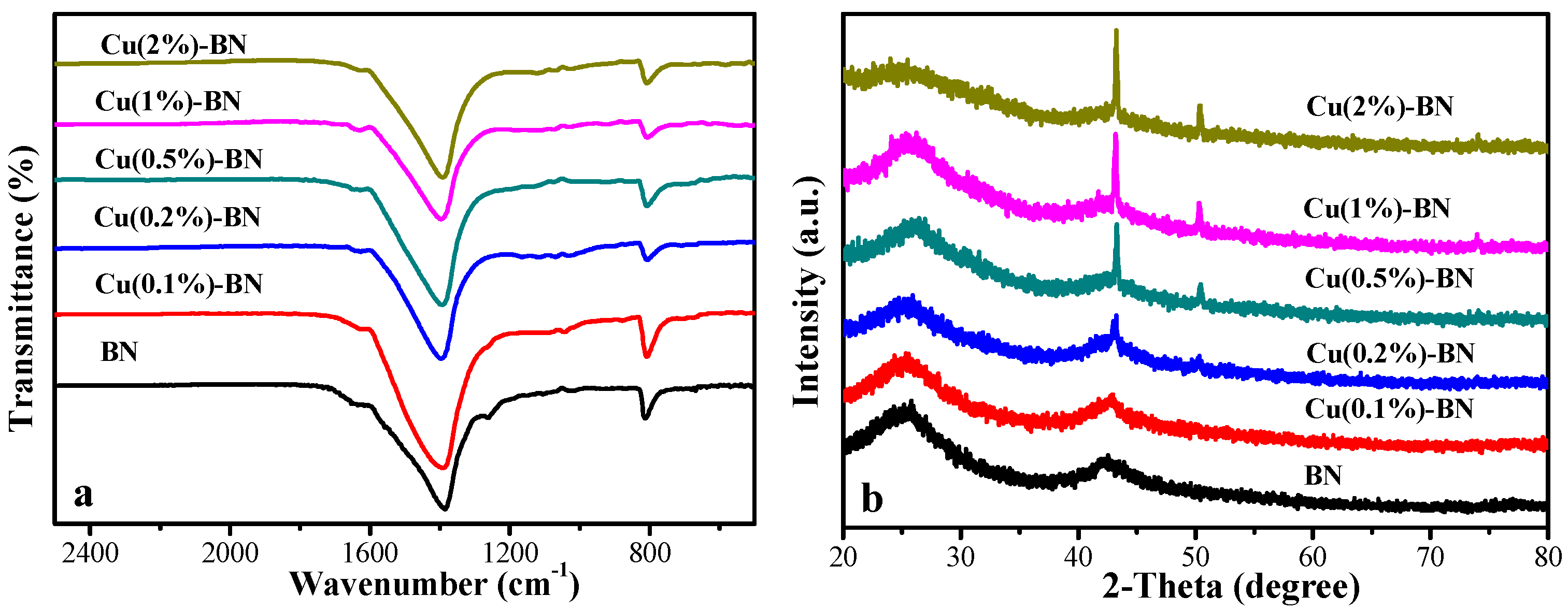
3.1.1. FTIR Spectrum and XRD Pattern
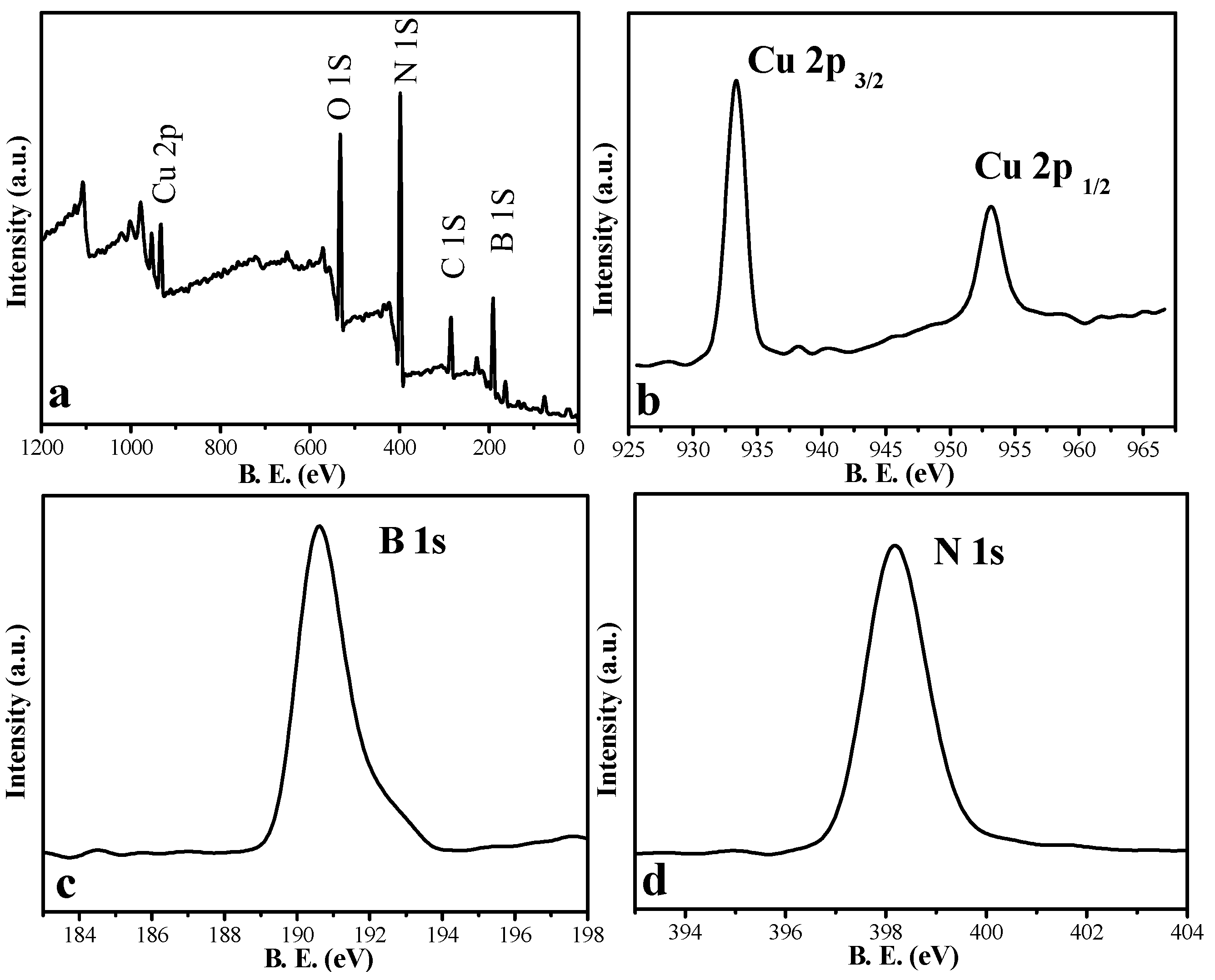
3.1.2. XPS Spectra
3.1.3. SEM and TEM Image
3.2. The Static Adsorption Behavior
3.2.1. Adsorption Kinetics
3.2.2. Adsorption Isotherms
3.2.3. Adsorption Thermodynamics
3.3. Optimization SPE Conditions
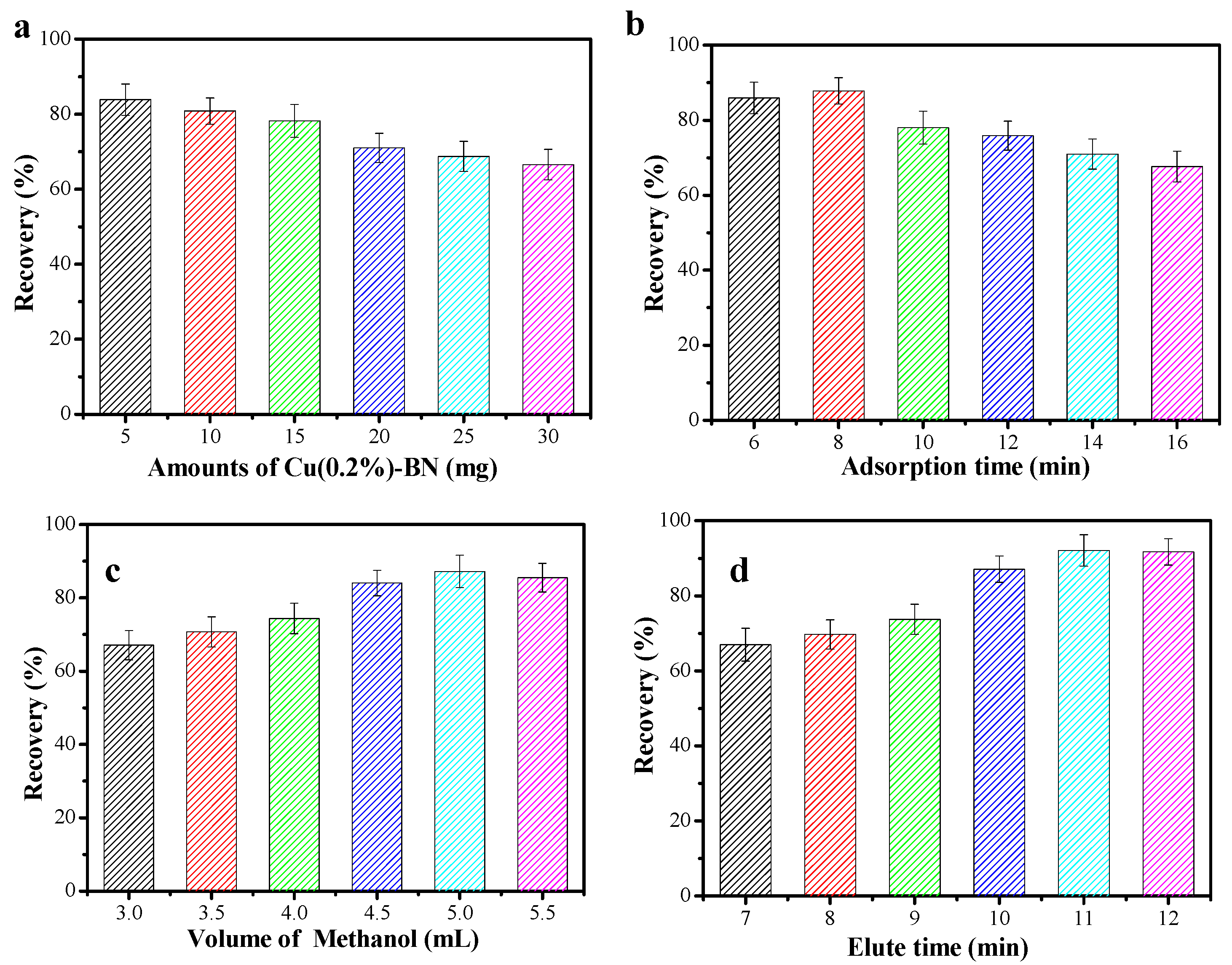
3.3.1. Extraction Conditions
3.3.2. Elution Conditions
3.4. Analysis of Spiked Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Wang, S.; Lu, Y.; Zhao, Y.; Zhang, Y.; Xu, G.; Zhang, J.; Fang, Z.; Xu, W.; Chen, X. Loading control of Metal-Organic Frameworks in Fe3O4@MOFs series composite adsorbents for optimizing dye adsorption. Ind. Eng. Chem. Res. 2019, 58, 22244–22249. [Google Scholar] [CrossRef]
- Ahmad, M.; Yousaf, M.; Nasir, A.; Bhatti, I.A.; Mahmood, A.; Fang, X.C.; Jian, X.; Kalantar-Zadeh, K.; Mahmood, N. Porous eleocharis@MnPE layered hybrid for synergistic adsorption and catalytic biodegradation of toxic azo dyes from industrial wastewater. Environ. Sci. Technol. 2019, 53, 2161–2170. [Google Scholar] [CrossRef] [PubMed]
- Elkady, M.F.; Hassan, H.S. Photocatalytic degradation of malachite green dye from aqueous solution using environmentally compatible Ag/ZnO polymeric nanofibers. Polymers 2021, 13, 2033. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, D.; Liu, W.; Zheng, L.; Wang, Y.; Liu, X.; Fan, M.; Gong, Z. Rapid screening of rhodamine B in food by hydrogel solid-phase extraction coupled with direct fluorescence detection. Food Chem. 2020, 316, 126378. [Google Scholar] [CrossRef]
- Xin, J.; Wang, X.; Li, N.; Liu, L.; Lian, Y.; Wang, M.; Zhao, R.-S. Recent applications of covalent organic frameworks and their multifunctional composites for food contaminant analysis. Food Chem. 2020, 330, 127255. [Google Scholar] [CrossRef]
- Jing, M.; Zhang, H.; Li, M.; Mao, Z.; Shi, X. Silver nanoparticle-decorated TiO2 nanotube array for solid-phase microextraction and SERS detection of antibiotic residue in milk. Spectrochim. Act. A 2021, 255, 119652. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Zhang, C.; Huang, D.; Wang, R.; Zeng, G.; Yan, M.; Xiong, W.; Zhou, C.; Cheng, M.; Xue, W.; et al. Recent progress in sustainable technologies for adsorptive and reactive removal of sulfonamides. Chem. Eng. J. 2020, 389, 123423. [Google Scholar] [CrossRef]
- Amini, S.; Ebrahimzadeh, H.; Seidi, S.; Jalilian, N. Preparation of Polyacrylonitrile/Ni-MOF electrospun nanofiber as an efficient fiber coating material for headspace solid-phase microextraction of diazinon and chlorpyrifos followed by CD-IMS analysis. Food Chem. 2021, 350, 129242. [Google Scholar] [CrossRef]
- Moinfar, S.; Khodayari, A.; Abdulrahman, S.S.; Aghaei, A.; Sohrabnezhad, S.; Jamil, L.A. Development of a SPE/GC–MS method for the determination of organophosphorus pesticides in food samples using syringe filters packed by GNP/MIL-101(Cr) nanocomposite. Food Chem. 2022, 371, 130997. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, Y.; Zhou, L.; Zhao, P.; Xiong, Z.; Yu, J. Magnetic solid-phase extraction based on zirconium-based metal–organic frameworks for simultaneous enantiomeric determination of eight chiral pesticides in water and fruit juices. Food Chem. 2022, 370, 131056. [Google Scholar] [CrossRef]
- Li, W.; Xue, Y.; Fu, X.; Ma, Z.; Feng, J. Covalent organic framework reinforced hollow fiber for solid-phase microextraction and determination of pesticides in foods. Food Control 2022, 133, 108587. [Google Scholar] [CrossRef]
- Ullah, L.; Zhao, G.; Hedin, N.; Ding, X.; Zhang, S.; Yao, X.; Nie, Y.; Zhang, Y. Highly efficient adsorption of benzothiophene from model fuel on a metal-organic framework modified with dodeca-tungstophosphoric acid. Chem. Eng. J. 2019, 362, 30–40. [Google Scholar] [CrossRef]
- Guo, H.L.; Wang, Y.K.; Qu, X.J.; Li, H.Y.; Yang, W.; Bai, Y.; Dang, D.B. Three-dimensional interpenetrating frameworks based on {P4Mo6} tetrameric clusters and filled with in situ generated alkyl viologens. Inorg. Chem. 2020, 59, 16430–16440. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, X.; Bi, F.; Zhang, X.; Yang, Y.; Wang, Y. A facile synthesis for uniform tablet-like TiO2/C derived from Materials of Institut Lavoisier-125(Ti) (MIL-125(Ti)) and their enhanced visible light-driven photodegradation of tetracycline. J. Colloid Interf. Sci. 2020, 571, 275–284. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, X.; Liu, X.; Li, C.; Zeng, S.; Wang, H.; Zhang, S. Fabrication of multilayered molecularly imprinted membrane for selective recognition and separation of artemisinin. ACS Sustain. Chem. Eng. 2019, 7, 3127–3137. [Google Scholar] [CrossRef]
- Liu, D.; Li, C.; Wu, J.; Liu, Y. Novel carbon -based sorbents for elemental mercury removal from gas streams: A review. Chem. Eng. J. 2020, 391, 123514. [Google Scholar] [CrossRef]
- Elkady, M.; Diab, K.E.; Shokry, H. Trimethoprim antibiotic adsorption from aqueous solution onto eco-friendly zr-metal organic framework material. Materials 2021, 14, 7545. [Google Scholar] [CrossRef]
- Diab, K.E.; Salama, E.; Hassan, H.S.; El-moneim, A.A.; Elkady, M.F. Bio-zirconium metal–organic framework regenerable bio-beads for the effective removal of organophosphates from polluted water. Polymers 2021, 13, 3869. [Google Scholar] [CrossRef]
- Weng, Q.; Zeng, L.; Chen, Z.; Han, Y.; Jiang, K.; Bando, Y.; Golberg, D. Hydrogen storage in carbon and oxygen co-doped porous boron nitrides. Adv. Funct. Mater. 2021, 31, 2007381. [Google Scholar] [CrossRef]
- Chao, Y.; Liu, M.; Pang, J.; Wu, P.; Jin, Y.; Li, X.; Luo, J.; Xiong, J.; Li, H.; Zhu, W. Gas-assisted exfoliation of boron nitride nanosheets enhancing adsorption performance. Ceram. Int. 2019, 45, 18838–18843. [Google Scholar] [CrossRef]
- Jia, J.; Wang, Y.; Feng, Y.; Hu, G.; Lin, J.; Huang, Y.; Zhang, Y.; Liu, Z.; Tang, C.; Yu, C. Hierarchically porous boron nitride/HKUST-1 hybrid materials: Synthesis, CO2 adsorption capacity, and CO2/N2 and CO2/CH4 selectivity. Ind. Eng. Chem. Res. 2021, 60, 2463–2471. [Google Scholar] [CrossRef]
- Zhu, W.; Wu, Z.; Foo, G.S.; Gao, X.; Zhou, M.; Liu, B.; Veith, G.M.; Wu, P.; Browning, K.L.; Lee, H.N.; et al. Taming interfacial electronic properties of platinum nanoparticles on vacancy-abundant boron nitride nanosheets for enhanced catalysis. Nature Commun. 2017, 8, 15291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Q.; Liang, J.; Fang, Y.; Guo, Z.; Du, Z.; Zhang, L.; Liu, Z.; Huang, Y.; Lin, J.; Tang, C. Nickel (II) modified porous boron nitride: An effective adsorbent for tetracycline removal from aqueous solution. Chem. Eng. J. 2020, 394, 124985. [Google Scholar] [CrossRef]
- Chao, Y.; Zhang, J.; Li, H.; Wu, P.; Li, X.; Chang, H.; He, J.; Wu, H.; Li, H.; Zhu, W. Synthesis of boron nitride nanosheets with N-defects for efficient tetracycline antibiotics adsorptive removal. Chem. Eng. J. 2020, 387, 124138. [Google Scholar] [CrossRef]
- Chao, Y.; Tang, B.; Luo, J.; Wu, P.; Tao, D.; Chang, H.; Chu, X.; Huang, Y.; Li, H.; Zhu, W. Hierarchical porous boron nitride with boron vacancies for improved adsorption performance to antibiotics. J. Colloid Interf. Sci. 2021, 584, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Li, M.J.; Li, N.; Xu, G.; Zhao, L.X.; Chen, X.; Zhao, Y.; Zhao, R.S. Magnetic boron nitride nanosheets as a novel magnetic solid-phase extraction adsorbent for the determination of plant growth regulators in tomatoes. Food Chem. 2021, 348, 129103. [Google Scholar] [CrossRef]
- Chao, Y.; Pang, J.; Bai, Y.; Wu, P.; Luo, J.; He, J.; Jin, Y.; Li, X.; Xiong, J.; Li, H.; et al. Graphene-like BN@SiO2 nanocomposites as efficient sorbents for solid-phase extraction of Rhodamine B and Rhodamine 6G from food samples. Food Chem. 2020, 320, 126666. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Chao, Y.; Chang, H.; Li, H.; Xiong, J.; He, M.; Zhang, Q.; Li, H.; Zhu, W. Tuning electronic properties of boron nitride nanoplate via doping carbon for enhanced adsorptive performance. J. Colloid Interf. Sci. 2017, 508, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Chao, Y.; Chang, H.; Li, H.; Xiong, J.; Zhang, Q.; Chen, G.; Qian, J.; Zhu, W.; Li, H. Silver Nanoparticle-Decorated Boron Nitride with Tunable Electronic Properties for Enhancement of Adsorption Performance. ACS Sustainable Chem. Eng. 2018, 6, 4948–4957. [Google Scholar] [CrossRef]
- Xiong, J.; Yang, L.; Chao, Y.; Pang, J.; Zhang, M.; Zhu, W.; Li, H. Boron nitride mesoporous nanowires with doped oxygen atoms for the remarkable adsorption desulfurization performance from fuels. ACS Sustain. Chem. Eng. 2016, 4, 4457–4464. [Google Scholar] [CrossRef]
- Li, H.; Ran, H.; Li, Y.; Lv, N.; Yin, J.; Zhang, J.; Wang, C.; Jiang, W.; Zhu, W.; Li, H.; et al. Comparative study of halogen-doped (X = Cl, Br, I) hexagonal boron nitride: A promising strategy to enhance the capacity of adsorptive desulfurization. J. Environ. Chem. Eng. 2021, 9, 105886. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Lv, N.; Yin, J.; Zhang, J.; Ran, H.; Zhang, M.; Jiang, W.; Zhu, W.; Li, H. Unraveling the effects of O-doping into h-BN on the adsorptive desulfurization performance by DFT calculations. J. Environ. Chem. Eng. 2021, 9, 105886. [Google Scholar] [CrossRef]
- Gong, W.Q.; Wu, X.L.; Li, Z.M.; Zhou, Y.; Zhu, W.; Tao, D.J. Sulfate ionic liquids impregnated 2D boron nitride nanosheets for trace SO2 capture with high capacity and selectivity. Sep. Purif. Technol. 2021, 270, 118824. [Google Scholar] [CrossRef]
- Wei, Y.; Luo, J.; Huang, Y.; Chao, Y.; Wu, P.; Wang, L.; Dai, L.; Zhang, M.; Li, H.; Zhu, W. Binary molten salts mediated defect engineering on hexagonal boron nitride catalyst with long-term stability for aerobic oxidative desulfurization. Appl. Surf. Sci. 2021, 558, 149724. [Google Scholar] [CrossRef]
- Wu, P.; Zhu, W.; Dai, B.; Chao, Y.; Li, C.; Li, H.; Zhang, M.; Jiang, W.; Li, H. Copper nanoparticles advance electron mobility of graphene-like boron nitride for enhanced aerobic oxidative desulfurization. Chem. Eng. J. 2016, 301, 123–131. [Google Scholar] [CrossRef]
- Ernawati, L.; Wahyuono, R.A.; Halim, A.; Noorain, R.; Widiyastuti, W.; Dewi, R.T.; Enomae, T. Hierarchically 3-D porous structure of silk fibroin-based biocomposite adsorbent for water pollutant removal. Environments 2021, 8, 127. [Google Scholar] [CrossRef]
- Ouakouak, A.; Abdelhamid, M.; Thouraya, B.; Chahinez, H.O.; Hocine, G.; Hamdi, N.; Syafiuddin, A.; Boopathy, R. Development of a novel adsorbent prepared from dredging sediment for effective removal of dye in aqueous solutions. Appl. Sci. 2021, 11, 10722. [Google Scholar] [CrossRef]
- Ali, A.; Alharthi, S.; Ahmad, B.; Naz, A.; Khan, I.; Mabood, F. Efficient removal of Pb(II) from aqueous medium using chemically modified silica monolith. Molecules 2021, 26, 6885. [Google Scholar] [CrossRef]
- Ejaz, U.; Wasim, A.A.; Khan, M.N.; Alzahrani, O.M.; Mahmoud, S.F.; El-Bahy, Z.M.; Sohail, M. Use of ionic liquid pretreated and fermented sugarcane bagasse as an adsorbent for congo red removal. Polymers 2021, 13, 3943. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Gotoh, T.; Nakai, S. Synthesis of oxidant functionalised cationic polymer hydrogel for enhanced removal of arsenic (III). Gels 2021, 7, 197. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Xiong, J.; Chao, Y.; Li, X.; Li, H.; Pang, J.; Zhu, F.; Zhu, W.; Li, H. Activated boron nitride ultrathin nanosheets for enhanced adsorption desulfurization performance. J. Taiwan Inst. Chem. Eng. 2018, 93, 245–252. [Google Scholar] [CrossRef]
- Luo, J.; Chao, Y.; Tang, Z.; Hua, M.; Li, X.; Wei, Y.; Ji, H.; Xiong, J.; Zhu, W.; Li, H. Design of lewis acid centers in bundlelike boron nitride for boosting adsorptive desulfurization performance. Ind. Eng. Chem. Res. 2019, 58, 13303–13312. [Google Scholar] [CrossRef]


| Sample | Added (ng/mL) | Recovery (%) | RSD (%, n = 3) |
|---|---|---|---|
| Chili powder | 100 | 91.0 | 4.3 |
| 500 | 89.8 | 3.1 | |
| Drinks | 100 | 92.1 | 3.0 |
| 500 | 95.4 | 3.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Zhou, Q.; Li, Y.; Pang, J. Cu-Doped Boron Nitride Nanosheets for Solid-Phase Extraction and Determination of Rhodamine B in Foods Matrix. Nanomaterials 2022, 12, 318. https://doi.org/10.3390/nano12030318
Liu F, Zhou Q, Li Y, Pang J. Cu-Doped Boron Nitride Nanosheets for Solid-Phase Extraction and Determination of Rhodamine B in Foods Matrix. Nanomaterials. 2022; 12(3):318. https://doi.org/10.3390/nano12030318
Chicago/Turabian StyleLiu, Fujie, Qihang Zhou, Yurui Li, and Jingyu Pang. 2022. "Cu-Doped Boron Nitride Nanosheets for Solid-Phase Extraction and Determination of Rhodamine B in Foods Matrix" Nanomaterials 12, no. 3: 318. https://doi.org/10.3390/nano12030318
APA StyleLiu, F., Zhou, Q., Li, Y., & Pang, J. (2022). Cu-Doped Boron Nitride Nanosheets for Solid-Phase Extraction and Determination of Rhodamine B in Foods Matrix. Nanomaterials, 12(3), 318. https://doi.org/10.3390/nano12030318





