The Effect of Anodizing Bath Composition on the Electronic Properties of Anodic Ta-Nb Mixed Oxides
Abstract
1. Introduction
2. Materials and Methods
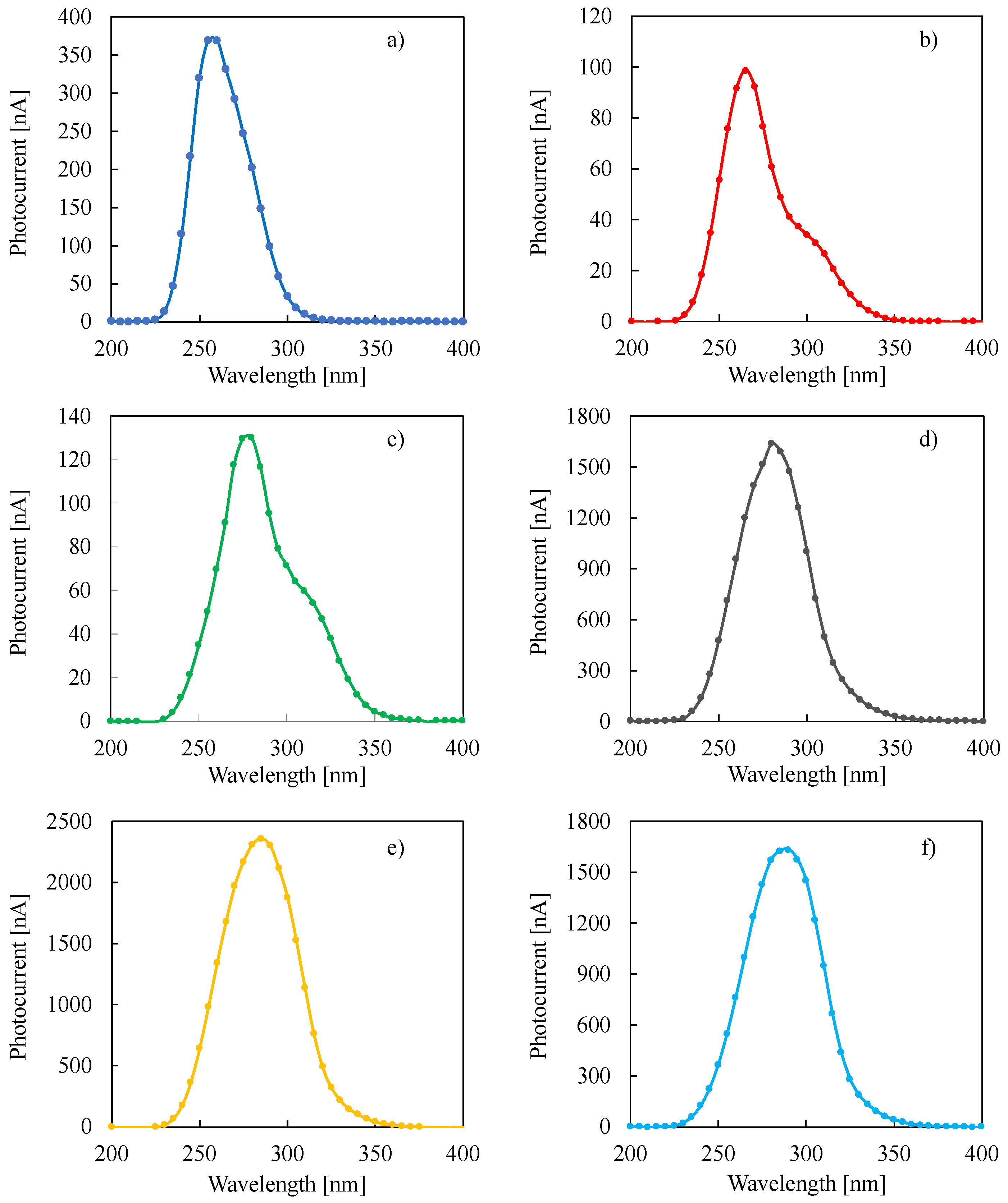
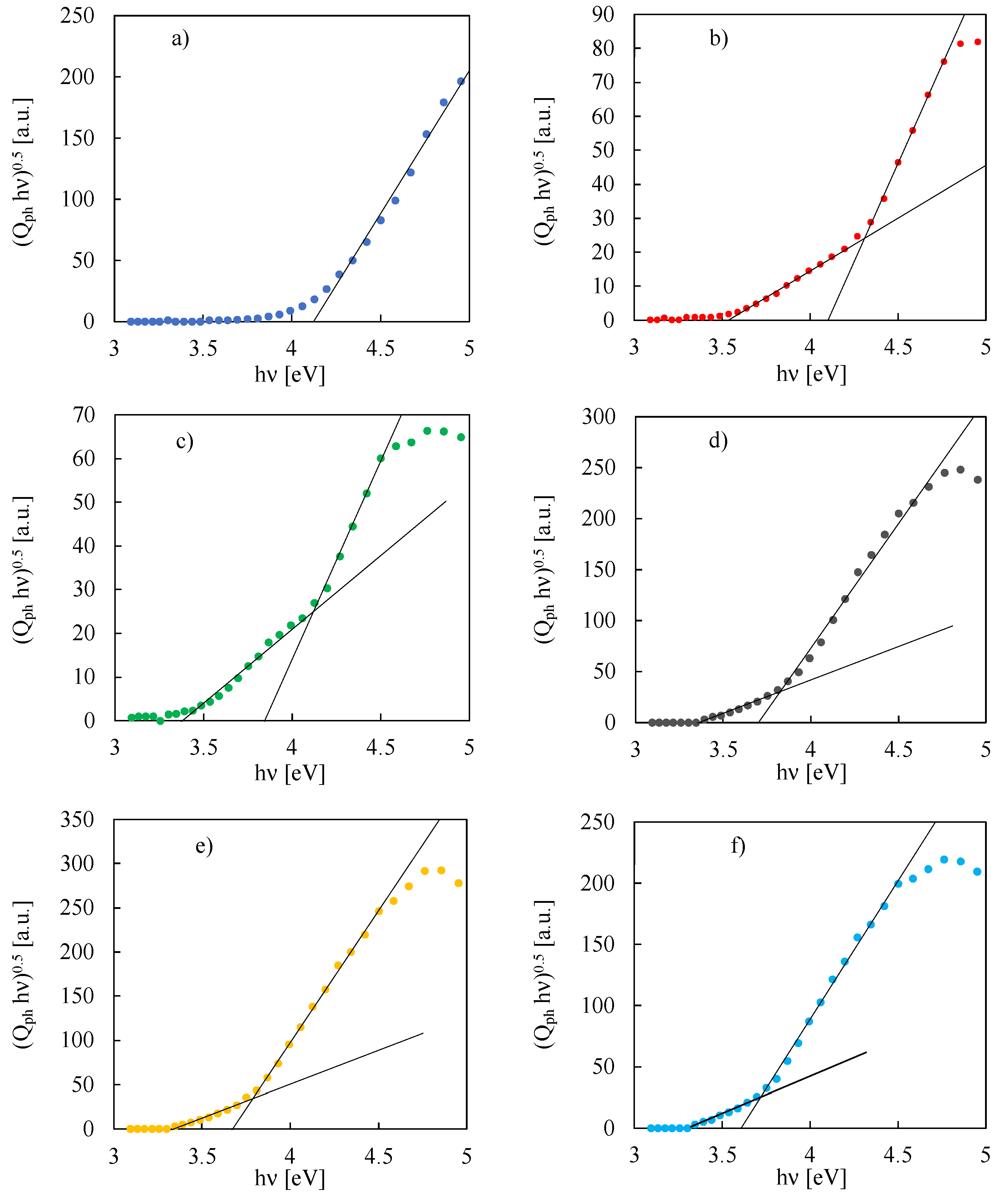
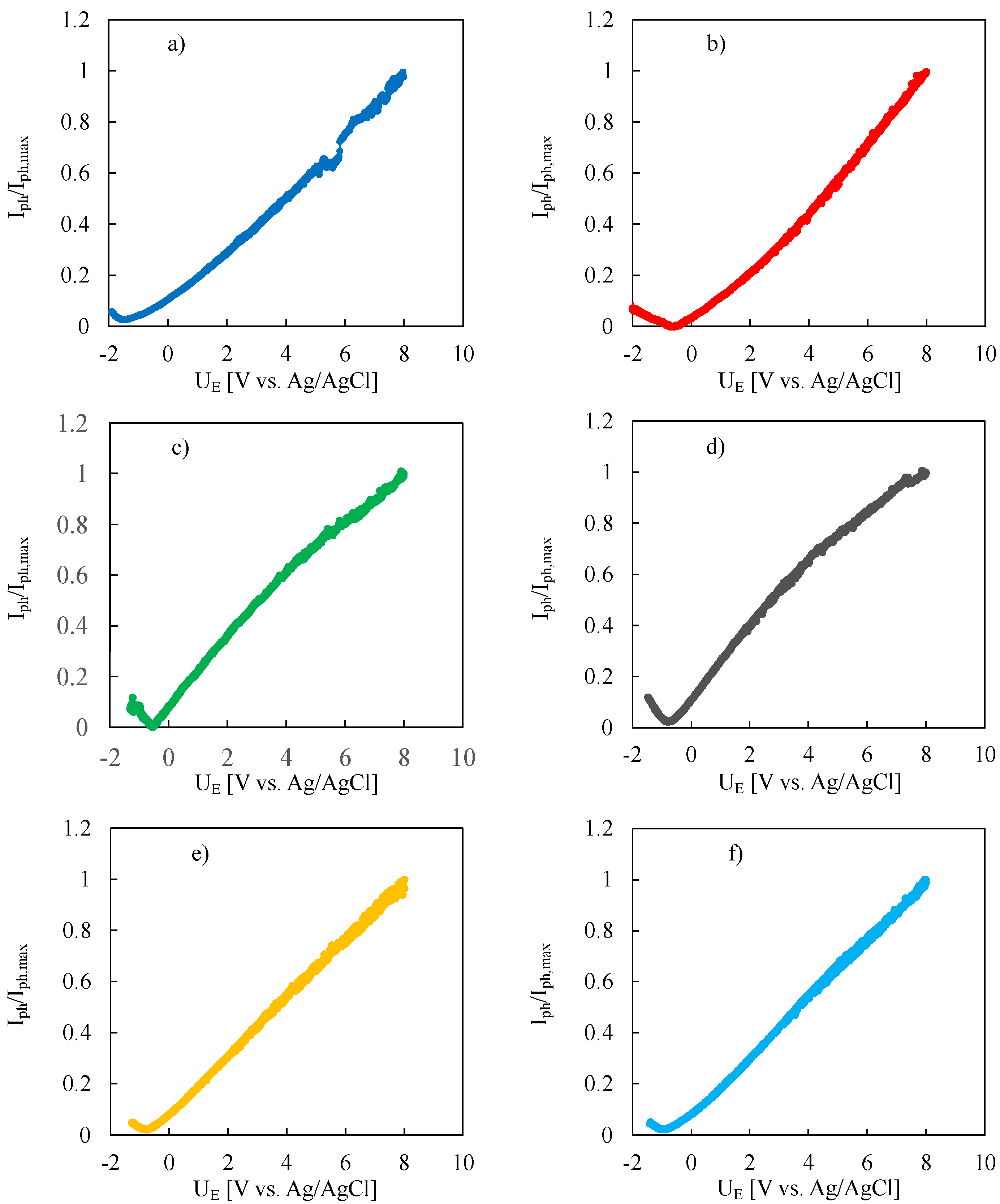
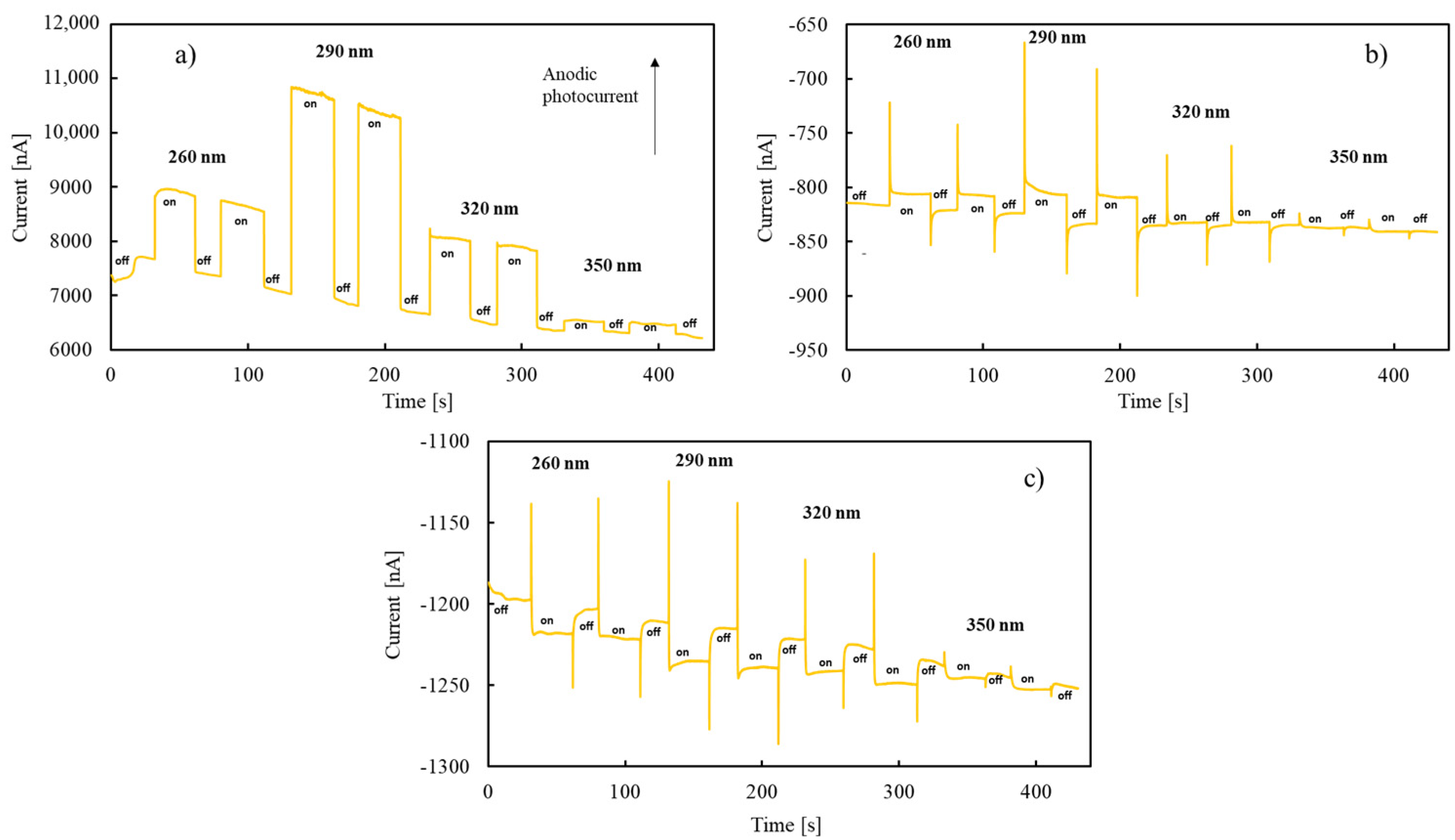
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Young, L. Anodic Oxide Films; Academic Press: London, UK, 1961. [Google Scholar]
- Lohrengel, M.M. Thin Anodic Oxide Layers on Aluminium and Other Valve Metals: High Field Regime. Mater. Sci. Eng. R Rep. 1993, 11, 243–294. [Google Scholar] [CrossRef]
- di Franco, F.; Zaffora, A.; Santamaria, M.; di Quarto, F. Anodization and Anodic Oxides. In Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry; Wandelt, K., Ed.; Elsevier: Waltham, MA, USA, 2018; pp. 26–40. ISBN 9780124095472. [Google Scholar]
- Robertson, J.; Wallace, R.M. High-K Materials and Metal Gates for CMOS Applications. Mater. Sci. Eng. R Rep. 2015, 88, 1–41. [Google Scholar] [CrossRef]
- Zaffora, A.; di Franco, F.; Santamaria, M.; Habazaki, H.; di Quarto, F. The Influence of Composition on Band Gap and Dielectric Constant of Anodic Al-Ta Mixed Oxides. Electrochim. Acta 2015, 180, 666–678. [Google Scholar] [CrossRef]
- Franke, E.; Trimble, C.L.; DeVries, M.J.; Woollam, J.A.; Schubert, M.; Frost, F. Dielectric Function of Amorphous Tantalum Oxide from the Far Infrared to the Deep Ultraviolet Spectral Region Measured by Spectroscopic Ellipsometry. J. Appl. Phys. 2000, 88, 5166–5174. [Google Scholar] [CrossRef]
- Guo, Y.; Robertson, J. Oxygen Vacancy Defects in Ta2O5 Showing Long-Range Atomic Re-Arrangements. Appl. Phys. Lett. 2014, 104, 3–8. [Google Scholar] [CrossRef]
- French, R.H. Electronic Band Structure of Al2O3, with Comparison to Alon and AIN. J. Am. Ceram. Soc. 1990, 73, 477–489. [Google Scholar] [CrossRef]
- Gaskins, J.T.; Hopkins, P.E.; Merrill, D.R.; Bauers, S.R.; Hadland, E.; Johnson, D.C.; Koh, D.; Yum, J.H.; Banerjee, S.; Nordell, B.J.; et al. Review—Investigation and Review of the Thermal, Mechanical, Electrical, Optical, and Structural Properties of Atomic Layer Deposited High- k Dielectrics: Beryllium Oxide, Aluminum Oxide, Hafnium Oxide, and Aluminum Nitride. ECS J. Solid State Sci. Technol. 2017, 6, N189–N208. [Google Scholar] [CrossRef]
- Scaduto, G.; Santamaria, M.; Bocchetta, P.; di Quarto, F. The Effect of Hydration Layers on the Anodic Growth and on the Dielectric Properties of Al2O3 for Electrolytic Capacitors. Thin Solid Films 2014, 550, 128–134. [Google Scholar] [CrossRef]
- di Franco, F.; Santamaria, M.; di Quarto, F.; la Mantia, F.; de Sá, A.I.; Rangel, C.M. Dielectric Properties of Al-Nb Amorphous Mixed Oxides. ECS J. Solid State Sci. Technol. 2013, 2, N205–N210. [Google Scholar] [CrossRef][Green Version]
- di Franco, F.; Zampardi, G.; Santamaria, M.; di Quarto, F.; Habazaki, H. Characterization of the Solid State Properties of Anodic Oxides on Magnetron Sputtered Ta, Nb and Ta-Nb Alloys. J. Electrochem. Soc. 2012, 159, C33–C39. [Google Scholar] [CrossRef]
- Mardare, A.I.; Ludwig, A.; Savan, A.; Hassel, A.W. Electrochemistry on Binary Valve Metal Combinatorial Libraries: Niobium-Tantalum Thin Films. Electrochim. Acta 2014, 140, 366–375. [Google Scholar] [CrossRef]
- Komiyama, S.; Tsuji, E.; Aoki, Y.; Habazaki, H.; Santamaria, M.; di Quarto, F.; Skeldon, P.; Thompson, G.E. Growth and Field Crystallization of Anodic Films on Ta–Nb Alloys. J. Solid State Electrochem. 2012, 16, 1595–1604. [Google Scholar] [CrossRef]
- Zaffora, A.; di Quarto, F.; Habazaki, H.; Valov, I.; Santamaria, M. Electrochemically Prepared Oxides for Resistive Switching Memories. Faraday Discuss. 2019, 213, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Vorobjova, A.I.; Tishkevich, D.I.; Outkina, E.A.; Shimanovich, D.L.; Razanau, I.U.; Zubar, T.I.; Bondaruk, A.A.; Zheleznova, E.K.; Dong, M.; Aloraini, D.A.; et al. A Study of Ta2O5 Nanopillars with Ni Tips Prepared by Porous Anodic Alumina Through-Mask Anodization. Nanomaterials 2022, 12, 1344. [Google Scholar] [CrossRef] [PubMed]
- Tishkevich, D.I.; Vorobjova, A.I.; Vinnik, D.A. Formation and Corrosion Behavior of Nickel/Alumina Nanocomposites. Solid State Phenom. 2020, 299, 100–106. [Google Scholar] [CrossRef]
- Banerjee, P.; Perez, I.; Henn-Lecordier, L.; Lee, S.B.; Rubloff, G.W. Nanotubular Metal–Insulator–Metal Capacitor Arrays for Energy Storage. Nat. Nanotechnol. 2009, 4, 292–296. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Uosaki, K. Preparation of Tantalum Anodic Oxide Film in Citric Acid Solution—Evidence and Effects of Citrate Anion Incorporation. J. Electrochem. Sci. Technol. 2013, 4, 163–170. [Google Scholar] [CrossRef]
- Ono, S.; Kuramochi, K.; Asoh, H. Effects of Electrolyte PH and Temperature on Dielectric Properties of Anodic Oxide Films Formed on Niobium. Corros Sci. 2009, 51, 1513–1518. [Google Scholar] [CrossRef]
- di Franco, F.; Zaffora, A.; Santamaria, M. Band Gap Narrowing and Dielectric Constant Enhancement of (NbxTa(1-x))2O5 by Electrochemical Nitrogen Doping. Electrochim. Acta 2018, 265, 326–335. [Google Scholar] [CrossRef]
- Zaffora, A.; di Franco, F.; di Quarto, F.; Santamaria, M. Optimization of Anodizing Process of Tantalum for Ta2O5-Based Capacitors. J. Solid State Electrochem. 2020, 24, 2953–2962. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; Pergamon Press: Oxford, UK, 1966. [Google Scholar]
- Zaffora, A.; di Franco, F.; di Quarto, F.; Macaluso, R.; Mosca, M.; Habazaki, H.; Santamaria, M. The Effect of Nb Incorporation on the Electronic Properties of Anodic HfO2. ECS J. Solid State Sci. Technol. 2017, 6, N25–N31. [Google Scholar] [CrossRef][Green Version]
- di Quarto, F.; Gentile, C.; Piazza, S.; Sunseri, C. A Photoelectrochemical Study on Anodic Tantalum Oxide Films. Corros Sci. 1993, 35, 801–808. [Google Scholar] [CrossRef]
- Mott, N.F.; Davis, E.A. Electronic Processes in Non-Crystalline Materials, 2nd ed.; Clarendon Press: Oxford, UK, 1979. [Google Scholar]
- di Franco, F.; Santamaria, M.; di Quarto, F.; Tsuji, E.; Habazaki, H. The Influence of Nitrogen Incorporation on the Optical Properties of Anodic Ta2O5. Electrochim. Acta 2012, 59, 382–386. [Google Scholar] [CrossRef]
- Zaffora, A.; Santamaria, M.; di Franco, F.; Habazaki, H.; di Quarto, F. Photoelectrochemical Evidence of Nitrogen Incorporation during Anodizing Sputtering-Deposited Al-Ta Alloys. Phys. Chem. Chem. Phys. 2016, 18, 351–360. [Google Scholar] [CrossRef]
- Zaffora, A.; Santamaria, M.; di Franco, F.; Habazaki, H.; di Quarto, F. Photoelectrochemical Evidence of Inhomogeneous Composition at Nm Length Scale of Anodic Films on Valve Metals Alloys. Electrochim. Acta 2016, 201, 333–339. [Google Scholar] [CrossRef]
- Shimizu, K.; Habazaki, H.; Skeldon, P.; Thompson, G.E.; Wood, G.C. Migration of Oxalate Ions in Anodic Alumina. Electrochim. Acta 2001, 46, 4379–4382. [Google Scholar] [CrossRef]
- Nishio, K.; Sasaki, R. Anodization of Al in Neutral Oxalate Solution to Form Unusually Fine Nanoporous Film. Chem. Lett. 2019, 48, 1126–1129. [Google Scholar] [CrossRef]
- Brudzisz, A.M.; Giziński, D.; Stępniowski, W.J. Incorporation of Ions into Nanostructured Anodic Oxides—Mechanism and Functionalities. Molecules 2021, 26, 6378. [Google Scholar] [CrossRef]
- Shimizu, K.; Habazaki, H.; Skeldon, P.; Thompson, G.E. Radiofrequency GDOES: A Powerful Technique for Depth Profiling Analysis of Thin Films. Surf. Interface Anal. 2003, 35, 564–574. [Google Scholar] [CrossRef]
- Habazaki, H.; Teraoka, M.; Aoki, Y.; Skeldon, P.; Thompson, G.E. Formation of Porous Anodic Titanium Oxide Films in Hot Phosphate/Glycerol Electrolyte. Electrochim. Acta 2010, 55, 3939–3943. [Google Scholar] [CrossRef]
- Habazaki, H.; Fushimi, K.; Shimizu, K.; Skeldon, P.; Thompson, G.E. Fast Migration of Fluoride Ions in Growing Anodic Titanium Oxide. Electrochem. Commun. 2007, 9, 1222–1227. [Google Scholar] [CrossRef]
- Habazaki, H.; Uozumi, M.; Konno, H.; Shimizu, K.; Skeldon, P.; Thompson, G.E. Crystallization of Anodic Titania on Titanium and Its Alloys. Corros Sci. 2003, 45, 2063–2073. [Google Scholar] [CrossRef]
- Lu, Q.; Skeldon, P.; Thompson, G.E.; Masheder, D.; Habazaki, H.; Shimizu, K. Transport Numbers of Metal and Oxygen Species in Anodic Tantala. Corros Sci. 2004, 46, 2817–2824. [Google Scholar] [CrossRef]
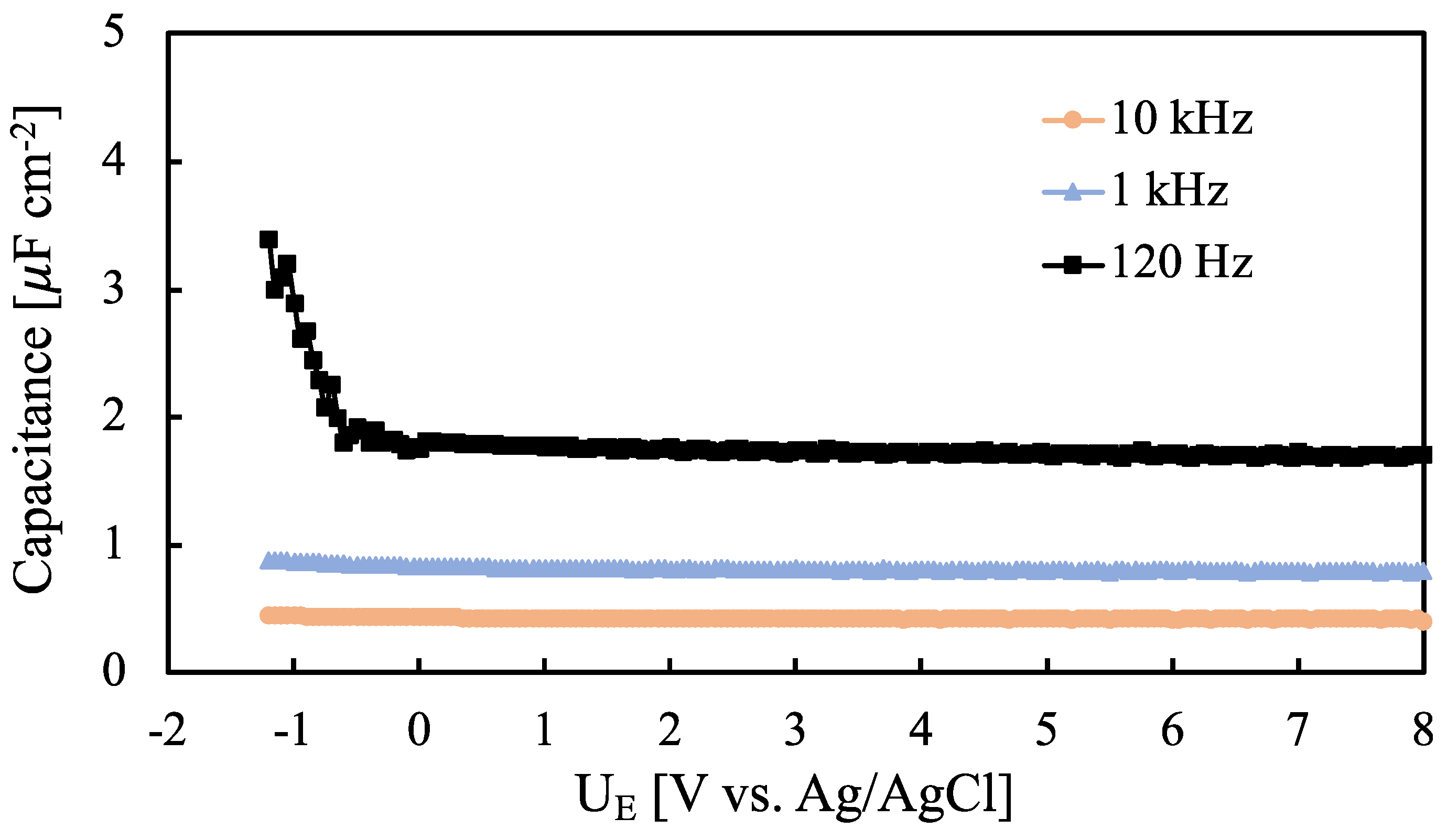
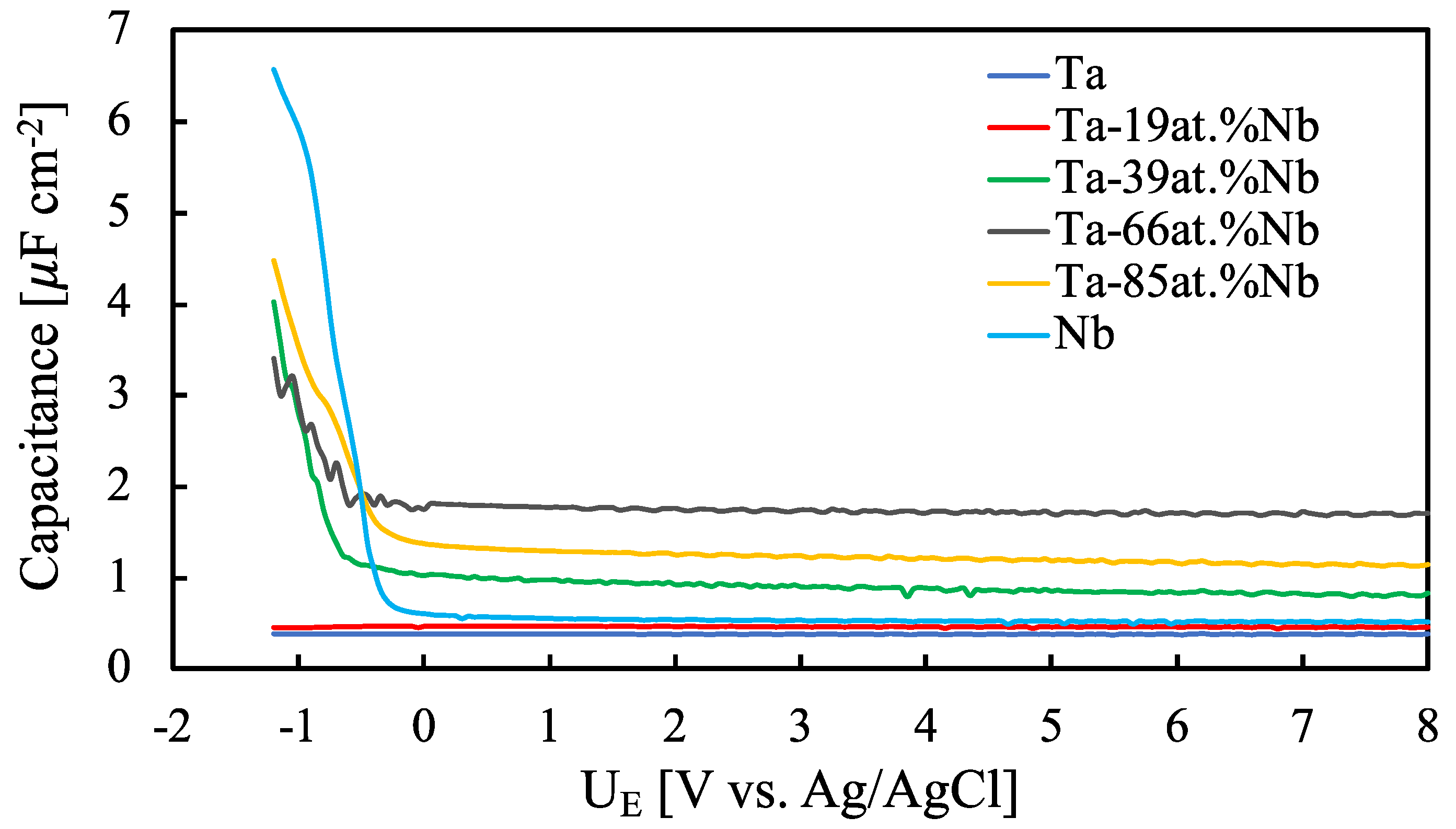
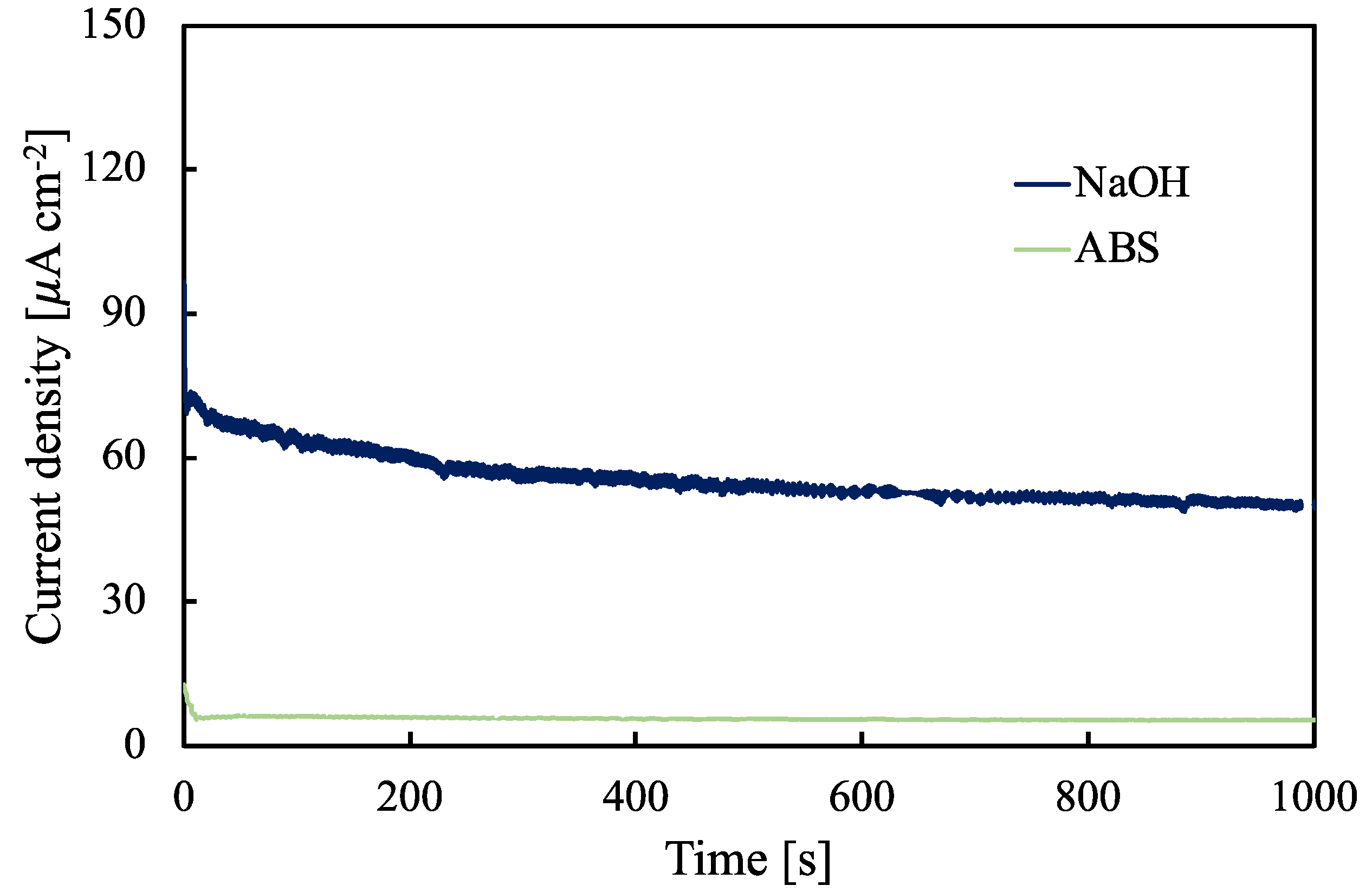
| Sample | i [µA cm−2] | E [MV cm−1] | k [nm V−1] | d [nm] |
|---|---|---|---|---|
| Nb | 410 | 3.7 | 2.7 | 135 |
| Ta-85at.%Nb | 395 | 4.0 | 2.5 | 124 |
| Ta-66at.%Nb | 370 | 4.5 | 1.9 | 112 |
| Ta-39at.%Nb | 363 | 4.7 | 2.1 | 106 |
| Ta-19at.%Nb | 304 | 5.8 | 1.7 | 86 |
| Ta | 289 | 6.2 | 1.6 | 81 |
| Base Alloy | Eg,opt [eV] | ELS [eV] |
|---|---|---|
| Ta | 4.12 | n.d. |
| Ta-19at.%Nb | 4.10 | 3.54 |
| Ta-39at.%Nb | 3.85 | 3.38 |
| Ta-66at.%Nb | 3.70 | 3.37 |
| Ta-85at.%Nb | 3.67 | 3.34 |
| Nb | 3.61 | 3.31 |
| Base Alloy | UFB vs. (Ag/AgCl) [V] |
|---|---|
| Ta | −0.90 |
| Ta-19at.%Nb | −0.45 |
| Ta-39at.%Nb | −0.40 |
| Ta-66at.%Nb | −0.45 |
| Ta-85at.%Nb | −0.45 |
| Nb | −0.50 |
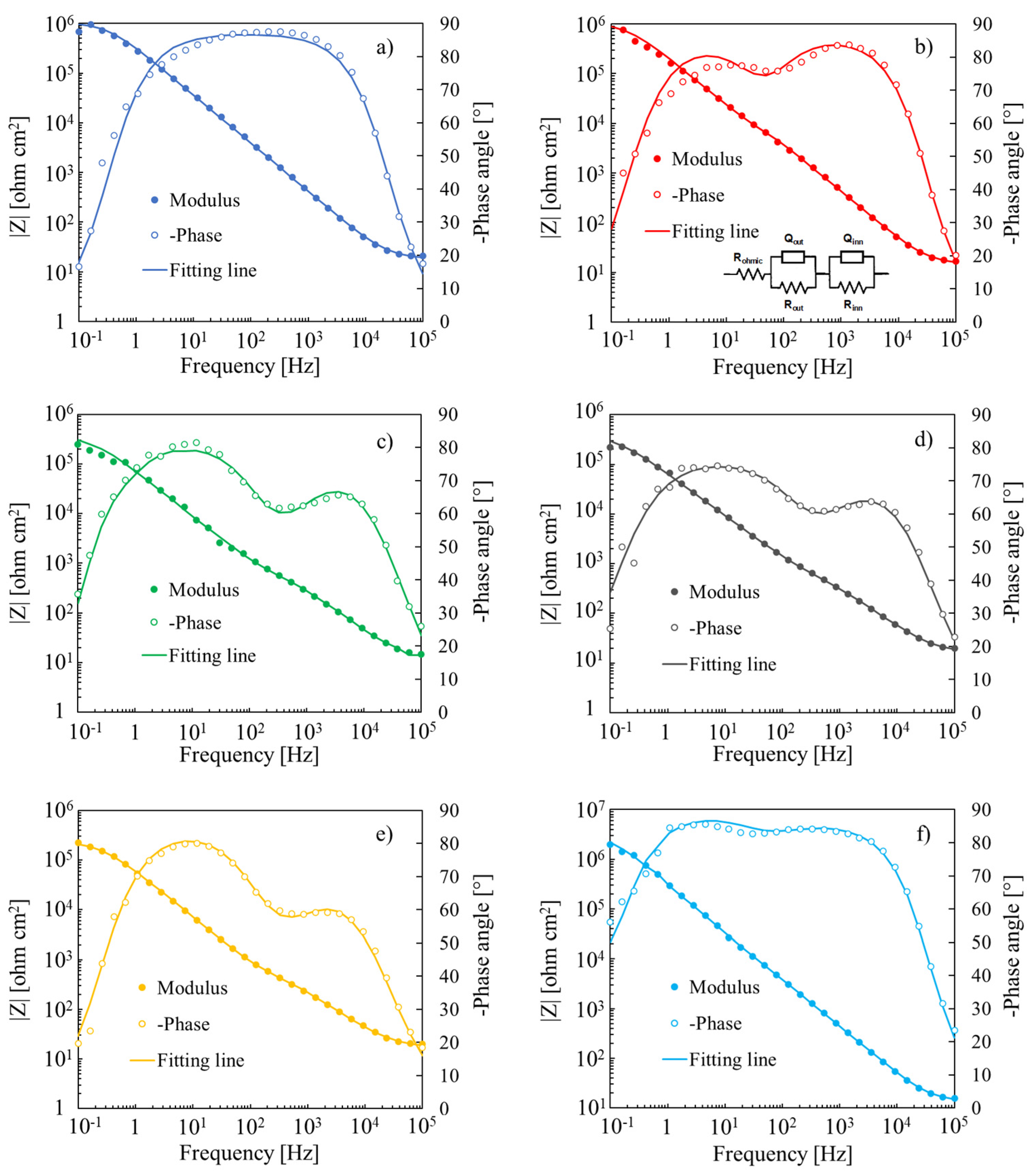
| Base Alloy | Rohmic [Ω cm2] | Rout [Ω cm2] | Qout [S sα cm−2] | α | Rinn [Ω cm2] | Qinn [S sα cm−2] | α |
|---|---|---|---|---|---|---|---|
| Ta | 19 | - | - | - | 1 × 106 | 5.0 × 10−7 | 0.97 |
| Ta-19at.%Nb | 15 | 1 × 106 | 8.3 × 10−7 | 0.95 | 2 × 103 | 1.3 × 10−6 | 0.99 |
| Ta-39at.%Nb | 12 | 4 × 105 | 2.5 × 10−6 | 0.92 | 3 × 102 | 3.1 × 10−6 | 0.85 |
| Ta-66at.%Nb | 16 | 4 × 105 | 3.1 × 10−6 | 0.86 | 3 × 102 | 3.7 × 10−6 | 0.84 |
| Ta-85at.%Nb | 17 | 2 × 105 | 2.8 × 10−6 | 0.94 | 3 × 102 | 5.4 × 10−6 | 0.80 |
| Nb | 14 | 3 × 106 | 5.1 × 10−7 | 0.99 | 1 × 103 | 5.4 × 10−6 | 0.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tranchida, G.; Zaffora, A.; Di Franco, F.; Santamaria, M. The Effect of Anodizing Bath Composition on the Electronic Properties of Anodic Ta-Nb Mixed Oxides. Nanomaterials 2022, 12, 4439. https://doi.org/10.3390/nano12244439
Tranchida G, Zaffora A, Di Franco F, Santamaria M. The Effect of Anodizing Bath Composition on the Electronic Properties of Anodic Ta-Nb Mixed Oxides. Nanomaterials. 2022; 12(24):4439. https://doi.org/10.3390/nano12244439
Chicago/Turabian StyleTranchida, Giada, Andrea Zaffora, Francesco Di Franco, and Monica Santamaria. 2022. "The Effect of Anodizing Bath Composition on the Electronic Properties of Anodic Ta-Nb Mixed Oxides" Nanomaterials 12, no. 24: 4439. https://doi.org/10.3390/nano12244439
APA StyleTranchida, G., Zaffora, A., Di Franco, F., & Santamaria, M. (2022). The Effect of Anodizing Bath Composition on the Electronic Properties of Anodic Ta-Nb Mixed Oxides. Nanomaterials, 12(24), 4439. https://doi.org/10.3390/nano12244439









