Application of Piezoelectric Material and Devices in Bone Regeneration
Abstract
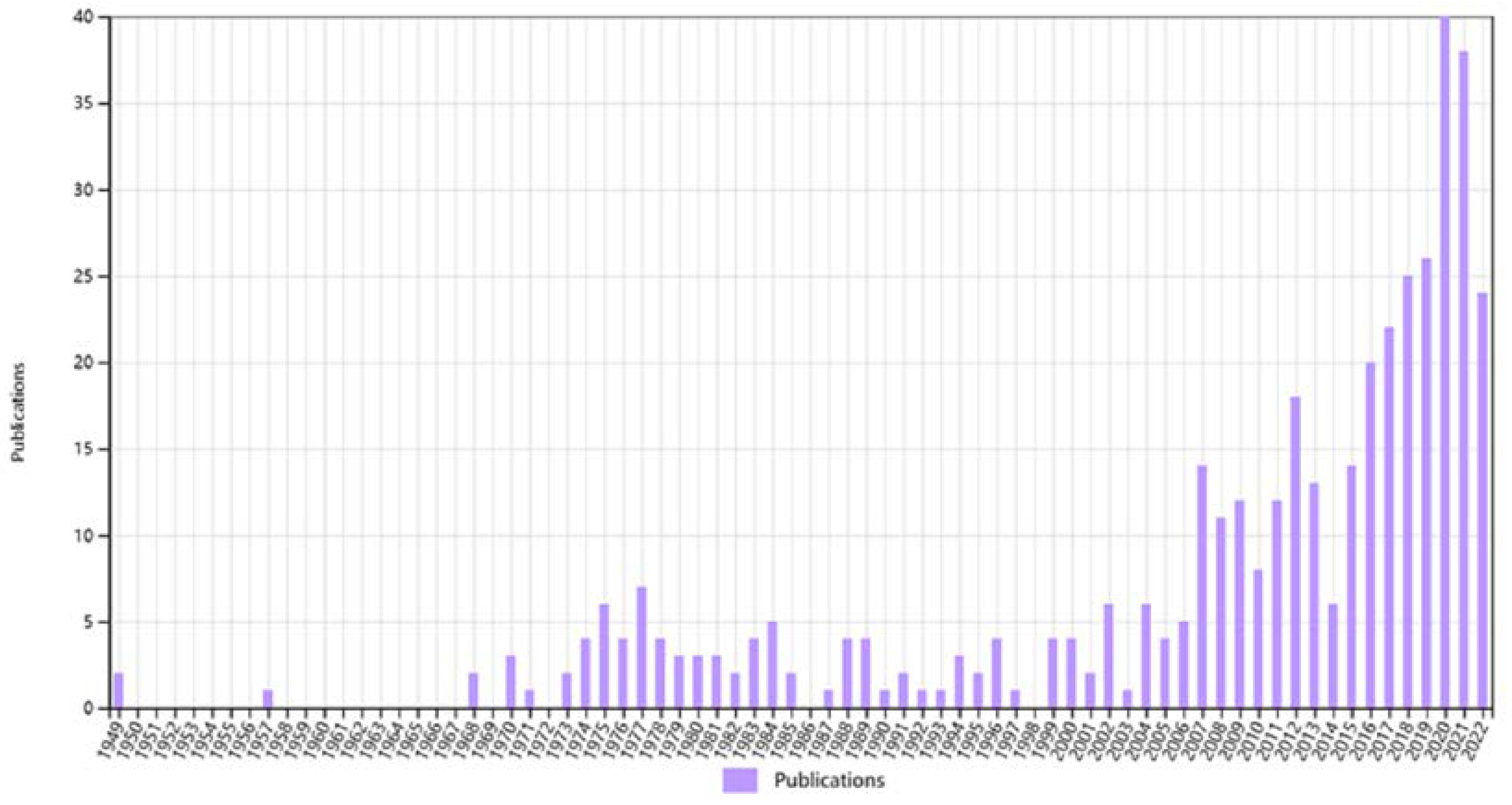
1. Introduction
2. Classification and Mechanism of Piezoelectric Material and Devices
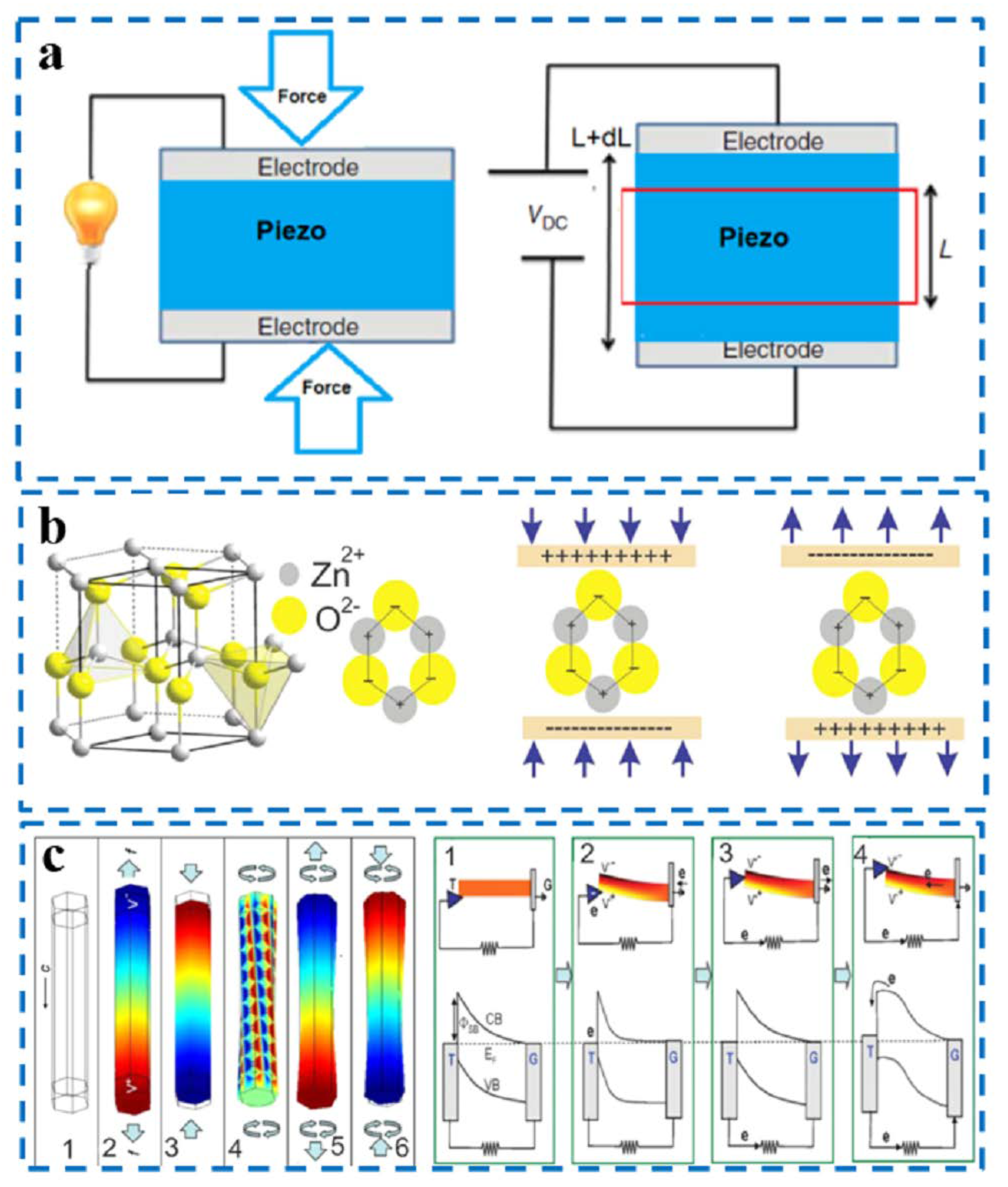
2.1. Mechanism of the Piezoelectric Effect
2.2. Classification of Piezoelectric Material
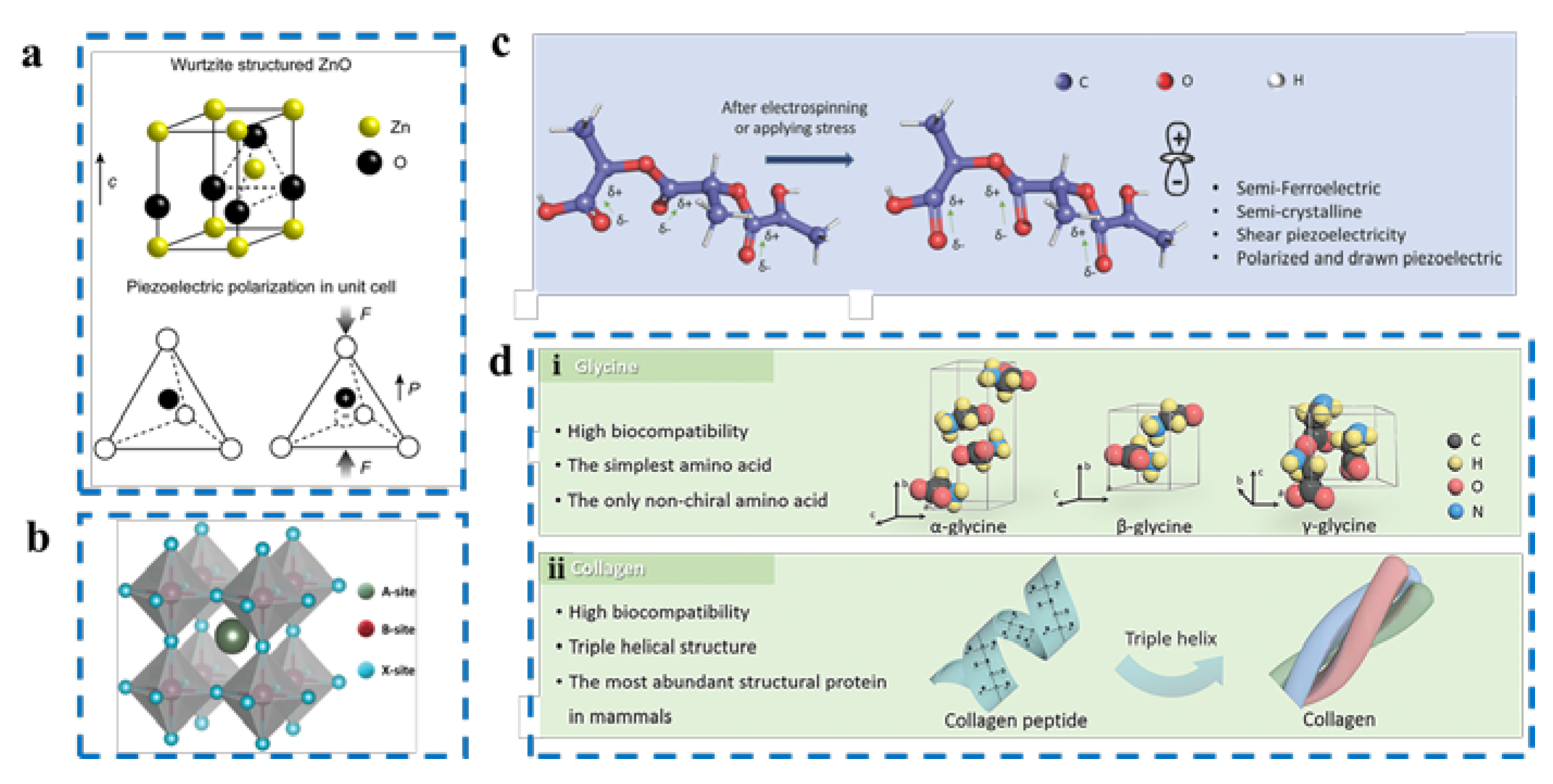
2.2.1. Piezoelectric Single Crystals
2.2.2. Piezoelectric Ceramics
2.2.3. Piezoelectric Polymers
2.2.4. Bio-Piezoelectric Materials
2.2.5. Composite Piezoelectric Materials
2.3. Classification of Piezoelectric Devices
3. Bone Regeneration Based on Piezoelectric Material and Devices
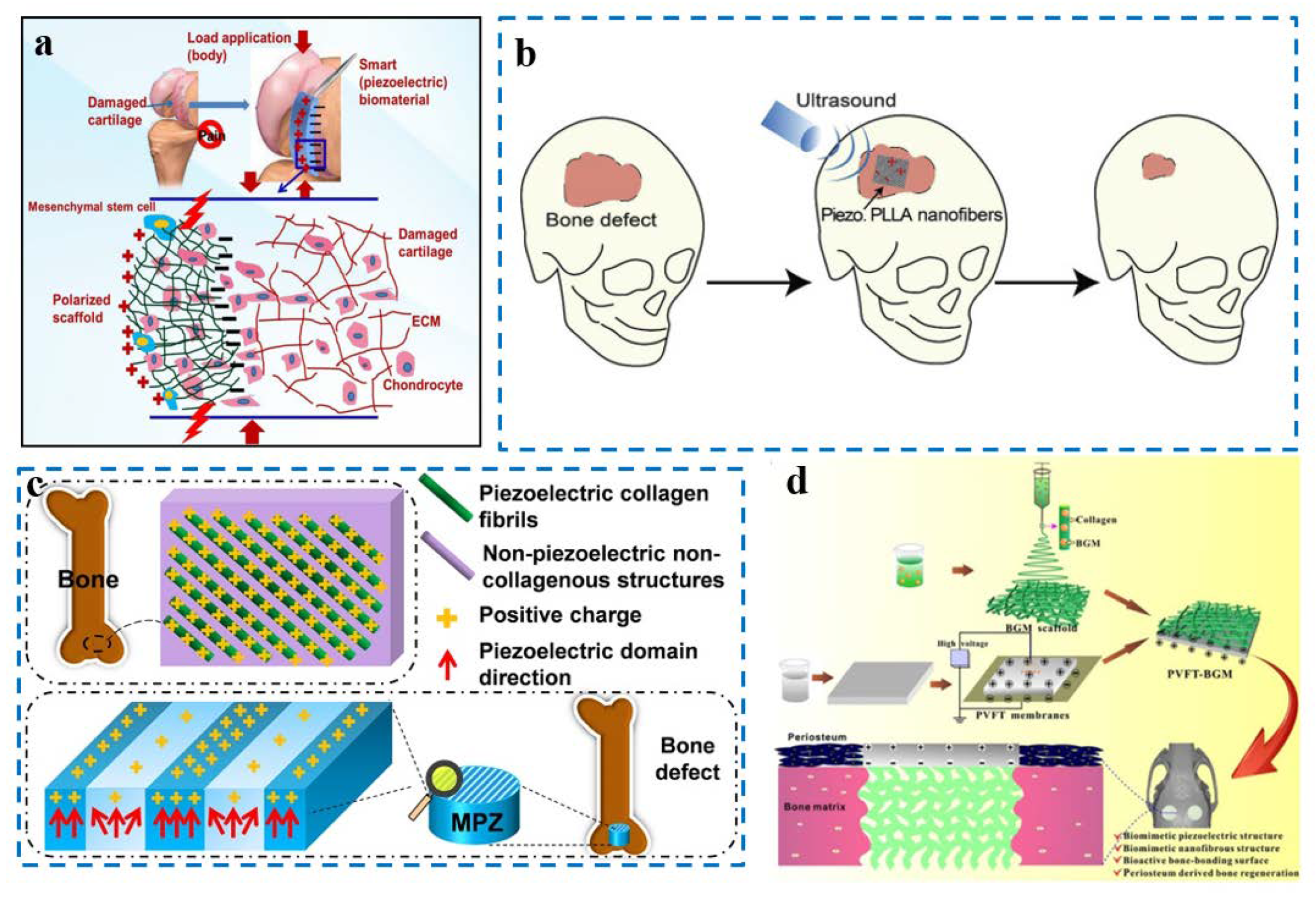
3.1. Piezoelectric Materials and Devices Applied in Cells
3.2. Piezoelectric Material and Devices Applied in Tissue
3.3. Piezoelectric Material and Devices Applied in Sensing and Repair Indicator Monitoring
4. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mohammadkhah, M.; Marinkovic, D.; Zehn, M.; Checa, S. A review on computer modeling of bone piezoelectricity and its application to bone adaptation and regeneration. Bone 2019, 127, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.; Correia, D.M.; Rodrigues, I.; Guardão, L.; Guimarães, S.; Soares, R.; Lanceros-Méndez, S. In vivo demonstration of the suitability of piezoelectric stimuli for bone reparation. Mater. Lett. 2017, 209, 118–121. [Google Scholar] [CrossRef]
- Silva, C.A.; Fernandes, M.M.; Ribeiro, C.; Lanceros-Mendez, S. Two- and three-dimensional piezoelectric scaffolds for bone tissue engineering. Colloids Surf. B Biointerfaces 2022, 218, 112708. [Google Scholar] [CrossRef] [PubMed]
- Vasquez-Sancho, F.; Abdollahi, A.; Damjanovic, D.; Catalan, G. Flexoelectricity in Bones. Adv. Mater. 2018, 30, 1705316. [Google Scholar] [CrossRef] [PubMed]
- Samadi, A.; Salati, M.A.; Safari, A.; Jouyandeh, M.; Barani, M.; Singh Chauhan, N.P.; Golab, E.G.; Zarrintaj, P.; Kar, S.; Seidi, F.; et al. Comparative review of piezoelectric biomaterials approach for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2022, 33, 1555–1594. [Google Scholar] [CrossRef] [PubMed]
- Tandon, B.; Blaker, J.J.; Cartmell, S.H. Piezoelectric materials as stimulatory biomedical materials and scaffolds for bone repair. Acta Biomater. 2018, 73, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; More, N.; Kalia, K.; Kapusetti, G. Piezoelectric smart biomaterials for bone and cartilage tissue engineering. Inflamm. Regen. 2018, 38, 2. [Google Scholar] [CrossRef]
- D’Alessandro, D.; Ricci, C.; Milazzo, M.; Strangis, G.; Forli, F.; Buda, G.; Petrini, M.; Berrettini, S.; Uddin, M.J.; Danti, S.; et al. Piezoelectric signals in vascularized bone regeneration. Biomolecules 2021, 11, 1731. [Google Scholar] [CrossRef]
- Fortuna, L.; Buscarino, A. Smart Materials. Materials 2022, 15, 6307. [Google Scholar] [CrossRef]
- Curie, P.C.J. Development by pressure of polar electricity in hemihedral crystals with inclined faces. Bull. Soc. 1880, 3, 90. [Google Scholar]
- Shamos, M.H.; Lavine, L.S. Piezoelectricity as a fundamental property of biological tissues. Nature 1967, 213, 267–269. [Google Scholar] [CrossRef]
- Park, J.B.; Kelly, B.J.; Kenner, G.H.; von Recum, A.F.; Grether, M.F.; Coffeen, W.W. Piezoelectric ceramic implants: In vivo results. J. Biomed. Mater. Res. 1981, 15, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Raza, W.; Li, X.; Gul, H.; Kim, K.-H. Piezoelectric energy harvesters for biomedical applications. Nano Energy 2019, 57, 879–902. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.L. Advances in piezotronic transistors and piezotronics. Nano Today 2021, 37, 101108. [Google Scholar] [CrossRef]
- Peng, B.; Lu, Q.; Tang, H.; Zhang, Y.; Cheng, Y.; Qiu, R.; Guo, Y.; Zhou, Z.; Liu, M. Large in-plane piezo-strain enhanced voltage control of magnetic anisotropy in Si-compatible multiferroic thin films. Mater. Horiz. 2022, 9, 3013–3021. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Kar-Narayan, S. Piezoelectric polymers: Theory, challenges and opportunities. Int. Mater. Rev. 2022, 67, 65–88. [Google Scholar] [CrossRef]
- Barros de Freitas, R.L.; Sakamoto, W.K.; Scarin Freitas, L.P.; Castro, F.; Lima Filho, A.P.; Kitano, C.; de Carvalho, A.A. Characterization of PZT/PVDF composite film as functional material. IEEE Sens. J. 2018, 18, 5067–5072. [Google Scholar] [CrossRef]
- Guo, S.-L.; Lai, S.-N.; Wu, J.M. Strain-induced ferroelectric heterostructure catalysts of hydrogen production through piezophototronic and piezoelectrocatalytic system. ACS Nano 2021, 15, 16106–16117. [Google Scholar] [CrossRef]
- Xu, Q.; Gao, X.; Zhao, S.; Liu, Y.-N.; Zhang, D.; Zhou, K.; Khanbareh, H.; Chen, W.; Zhang, Y.; Bowen, C. Construction of io-piezoelectric platforms: From structures and synthesis to applications. Adv. Mater. 2021, 33, 2008452. [Google Scholar] [CrossRef]
- Zheng, L.; Huo, X.; Wang, R.; Wang, J.; Jiang, W.; Cao, W. Large size lead-free (Na,K)(Nb,Ta)O-3 piezoelectric single crystal: Growth and full tensor properties. Crystengcomm 2013, 15, 7718–7722. [Google Scholar] [CrossRef]
- Maruska, H.P.; Tietjen, J.J. Preparation and properties of vapor-deposited single-crystalline gan. Appl. Phys. Lett. 1969, 15, 327–329. [Google Scholar] [CrossRef]
- Dingle, R.; Shaklee, K.L.; Leheny, R.F.; Zetterstrom, R.B. Stimulated emission and laser action in gallium nitride. Appl. Phys. Lett. 1971, 19, 5–7. [Google Scholar] [CrossRef]
- Qamar, A.; Dao, D.V.; Dinh, T.; Iacopi, A.; Walker, G.; Phan, H.-P.; Hold, L.; Dimitrijev, S. Piezo-Hall effect and fundamental piezo-Hall coefficients of single crystal n-type 3C-SiC(100) with low carrier concentration. Appl. Phys. Lett. 2017, 110, 162903. [Google Scholar] [CrossRef]
- Bagnall, D.M.; Chen, Y.F.; Zhu, Z.; Yao, T.; Koyama, S.; Shen, M.Y.; Goto, T. Optically pumped lasing of ZnO at room temperature. Appl. Phys. Lett. 1997, 70, 2230–2232. [Google Scholar] [CrossRef]
- Chu, S.; Wang, G.; Zhou, W.; Lin, Y.; Chernyak, L.; Zhao, J.; Kong, J.; Li, L.; Ren, J.; Liu, J. Electrically pumped waveguide lasing from ZnO nanowires. Nat. Nanotechnol. 2011, 6, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.Y.; Chen, T.Y. Fabrication of modified lead titanate piezoceramics with zero temperature coefficient and its application on SAW devices. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2004, 51, 663–667. [Google Scholar] [CrossRef]
- Cao, W.P.; Sheng, J.; Qiao, Y.L.; Jing, L.; Liu, Z.; Wang, J.; Li, W.L. Optimized strain with small hysteresis and high energy-storage density in Mn-doped NBT-ST system. J. Eur. Ceram. Soc. 2019, 39, 4046–4052. [Google Scholar] [CrossRef]
- Wu, H.-S.; Murti, B.T.; Singh, J.; Yang, P.-K.; Tsai, M.-L. Prospects of metal-free perovskites for piezoelectric applications. Adv. Sci. 2022, 9, 2104703. [Google Scholar] [CrossRef]
- Chorsi, M.T.; Curry, E.J.; Chorsi, H.T.; Das, R.; Baroody, J.; Purohit, P.K.; Ilies, H.; Nguyen, T.D. Piezoelectric biomaterials for sensors and actuators. Adv. Mater. 2019, 31, 1802084. [Google Scholar] [CrossRef]
- Chang, C.; Tran, V.H.; Wang, J.; Fuh, Y.K.; Lin, L. Direct-write piezoelectric polymeric nanogenerator with high energy conversion efficiency. Nano Lett 2010, 10, 726–731. [Google Scholar] [CrossRef]
- Datta, A.; Choi, Y.S.; Chalmers, E.; Ou, C.; Kar-Narayan, S. Piezoelectric nylon-11 nanowire arrays grown by template wetting for vibrational energy harvesting applications. Adv. Funct. Mater. 2017, 27, 1604262. [Google Scholar] [CrossRef]
- Pi, Z.; Zhang, J.; Wen, C.; Zhang, Z.-B.; Wu, D. Flexible piezoelectric nanogenerator made of poly(vinylidenefluoride-co-trifluoroethylene) (PVDF-TrFE) thin film. Nano Energy 2014, 7, 33–41. [Google Scholar] [CrossRef]
- Qian, J.; Peng, R.; Shen, Z.; Jiang, J.; Xue, F.; Yang, T.; Chen, L.; Shen, Y. Interfacial coupling boosts giant electrocaloric effects in relaxor polymer nanocomposites: In situ characterization and phase-field simulation. Adv. Mater. 2019, 31, 1801949. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.; Yang, S.; Yu, S.; Banerjee, A.; Myung, N.V.; Nam, J. Modulation of piezoelectric properties in electrospun PLLA nanofibers for application-specific self-powered stem cell culture platforms. Nano Energy 2021, 89, 106444. [Google Scholar] [CrossRef]
- Qu, X.; Ma, X.; Shi, B.; Li, H.; Zheng, L.; Wang, C.; Liu, Z.; Fan, Y.; Chen, X.; Li, Z.; et al. Refreshable braille display system based on triboelectric nanogenerator and dielectric elastomer. Adv. Funct. Mater. 2021, 31, 2006612. [Google Scholar] [CrossRef]
- Liu, K.; Cao, Y.; Wang, G.; Zhang, W.; Chen, W.; Gao, X. A novel photoacoustic spectroscopy gas sensor using a low cost polyvinylidene fluoride film. Sens. Actuators B-Chem. 2018, 277, 571–575. [Google Scholar] [CrossRef]
- Wang, A.; Hu, M.; Zhou, L.; Qiang, X. Self-powered wearable pressure sensors with enhanced piezoelectric properties of aligned p(VDF-TrFE)/MWCNT composites for monitoring human physiological and muscle motion signs. Nanomaterials 2018, 8, 1021. [Google Scholar] [CrossRef]
- Martin, A.J.P. Tribo-electricity in wool and hair. Proc. Phys. Soc. 1941, 53, 186–189. [Google Scholar] [CrossRef]
- Fukada, E.; Yasuda, I. On the piezoelectric effect of bone. J. Phys. Soc. Jpn. 1957, 12, 1158–1162. [Google Scholar] [CrossRef]
- Fukada, E.; Ueda, H.; Rinaldi, R. Piezoelectric and related properties of hydrated collag. Biophys. J. 1976, 16, 911–918. [Google Scholar] [CrossRef]
- Fukada, E.; Hara, K. Piezoelectric effect in blood vessel walls. J. Phys. Soc. Jpn. 1969, 26, 777–780. [Google Scholar] [CrossRef]
- Derossi, D.; Pastacaldi, P.; Domenici, C. Piezoelectric properties of dry human-skin. IEEE Trans. Electr. Insul. 1986, 21, 511–517. [Google Scholar] [CrossRef]
- Fukada, E.; Ueda, H. Piezoelectric effect in muscle. Jpn. J. Appl. Phys. 1970, 9, 844. [Google Scholar] [CrossRef]
- Athenstaedt, H. Permanent longitudinal electric polarization and pyroelectric behaviour of collagenous structures and nervous tissue in man and other vertebrates. Nature 1970, 228, 830–834. [Google Scholar] [CrossRef]
- Fukada, E. Piezoelectricity of biopolymers. Biorheology 1996, 33, 95–96. [Google Scholar] [CrossRef]
- Chow, W.S.; Ishak, Z.A.M. Smart polymer nanocomposites: A review. Express Polym. Lett. 2020, 14, 416–435. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Zhu, D.; Wang, Q.; Zhang, Y.; Zhu, Y.; Li, M. Magnetoelectric effect modulation in a PVDF/Metglas/PZT composite by applying DC electric fields on the PZT phase. J. Alloys Compd. 2016, 661, 38–42. [Google Scholar] [CrossRef]
- Wang, J.; Houwman, E.; Salm, C.; Minh, N.; Vergeer, K.; Schmitz, J. Process induced poling and plasma induced damage of thin film PZT. Microelectron. Eng. 2017, 177, 13–18. [Google Scholar] [CrossRef]
- Rasoolzadeh, M.; Sherafat, Z.; Vahedi, M.; Bagherzadeh, E. Structure dependent piezoelectricity in electrospun PVDF-SiC nanoenergy harvesters. J. Alloys Compd. 2022, 917, 165505. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Wang, Z.L. Fundamental theory of piezotronics. Adv. Mater. 2011, 23, 3004–3013. [Google Scholar] [CrossRef]
- Wu, W.; Wen, X.; Wang, Z.L. Taxel-addressable matrix of vertical-nanowire piezotronic transistors for active and adaptive tactile imaging. Science 2013, 340, 952–957. [Google Scholar] [CrossRef]
- Wen, X.; Wu, W.; Ding, Y.; Wang, Z.L. Piezotronic effect in flexible thin-film based devices. Adv. Mater. 2013, 25, 3371–3379. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hua, Q.; Yu, R.; Yang, Y.; Zhang, T.; Zhang, Y.; Pan, C. A bamboo-like gan microwire-based piezotronic memristor. Adv. Funct. Mater. 2016, 26, 5307–5314. [Google Scholar] [CrossRef]
- Wang, C.-H.; Liao, W.-S.; Ku, N.-J.; Li, Y.-C.; Chen, Y.-C.; Tu, L.-W.; Liu, C.-P. Effects of free carriers on piezoelectric nanogenerators and piezotronic devices made of gan nanowire arrays. Small 2014, 10, 4718–4725. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Dong, L.; Pan, C.; Niu, S.; Liu, H.; Liu, W.; Chua, S.; Chi, D.; Wang, Z.L. Piezotronic effect on the transport properties of GaN nanobelts for active flexible electronics. Adv. Mater. 2012, 24, 3532–3537. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Meguid, S.A. On the piezoelectric potential of gallium nitride nanotubes. Nano Energy 2015, 12, 322–330. [Google Scholar] [CrossRef]
- Yu, R.; Wang, X.; Wu, W.; Pan, C.; Bando, Y.; Fukata, N.; Hu, Y.; Peng, W.; Ding, Y.; Wang, Z.L. Temperature dependence of the piezophototronic effect in CdS nanowires. Adv. Funct. Mater. 2015, 25, 5277–5284. [Google Scholar] [CrossRef]
- Zhou, Y.S.; Wang, K.; Han, W.; Rai, S.C.; Zhang, Y.; Ding, Y.; Pan, C.; Zhang, F.; Zhou, W.; Wang, Z.L. Vertically aligned CdSe nanowire arrays for energy harvesting and piezotronic devices. ACS Nano 2012, 6, 6478–6482. [Google Scholar] [CrossRef]
- Peng, Y.; Que, M.; Lee, H.E.; Bao, R.; Wang, X.; Lu, J.; Yuan, Z.; Li, X.; Tao, J.; Sun, J.; et al. Achieving high-resolution pressure mapping via flexible GaN/ZnO nanowire LEDs array by piezo-phototronic effect. Nano Energy 2019, 58, 633–640. [Google Scholar] [CrossRef]
- Li, X.; Wei, X.; Xu, T.; Pan, D.; Zhao, J.; Chen, Q. Remarkable and crystal-structure-dependent piezoelectric and piezoresistive effects of InAs nanowires. Adv. Mater. 2015, 27, 2852–2858. [Google Scholar] [CrossRef]
- Ku, N.-J.; Huang, J.-H.; Wang, C.-H.; Fang, H.-C.; Liu, C.-P. Crystal face-dependent nanopiezotronics of an obliquely aligned inn nanorod array. Nano Lett. 2012, 12, 562–568. [Google Scholar] [CrossRef]
- Wu, J.M.; Chen, K.-H.; Zhang, Y.; Wang, Z.L. A self-powered piezotronic strain sensor based on single ZnSnO3 microbelts. RSC Adv. 2013, 3, 25184–25189. [Google Scholar] [CrossRef]
- Wu, J.M.; Chen, C.-Y.; Zhang, Y.; Chen, K.-H.; Yang, Y.; Hu, Y.; He, J.-H.; Wang, Z.L. Ultrahigh sensitive piezotronic strain sensors based on a ZnSnO3 nanowire/microwire. ACS Nano 2012, 6, 4369–4374. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.-C.; Yang, Y.; Lin, Z.-H.; Ding, Y.; Park, C.; Pradel, K.C.; Chen, L.-J.; Wang, Z.L. Nanogenerator based on zinc blende CdTe micro/nanowires. Nano Energy 2013, 2, 387–393. [Google Scholar] [CrossRef]
- Wang, Z.L.; Song, J.H. Piezoelectric nanogenerators based on zinc oxide nanowire arrays. Science 2006, 312, 242–246. [Google Scholar] [CrossRef]
- Zhang, Y.; Lingling, X.; Liu, Z.; Cui, X.; Xiang, Z.; Bai, J.; Jiang, D.; Xue, J.; Wang, C.; Lin, Y.; et al. Self-powered pulsed direct current stimulation system for enhancing osteogenesis in MC3T3-E1. Nano Energy 2021, 85, 106009. [Google Scholar] [CrossRef]
- Yu, B.; Qiao, Z.; Cui, J.; Lian, M.; Han, Y.; Zhang, X.; Wang, W.; Yu, X.; Yu, H.; Wang, X.; et al. A host-coupling bio-nanogenerator for electrically stimulated osteogenesis. Biomaterials 2021, 276, 120997. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, W.; Hao, X.; Peng, Y.; Zheng, Y.; Liu, J.; Kang, Y.; Zhao, F.; Luo, Z.; Guo, J.; et al. A novel approach to enhance bone regeneration by controlling the polarity of GaN/AlGaN heterostructures. Adv. Funct. Mater. 2021, 31, 2007487. [Google Scholar] [CrossRef]
- Qiao, Z.; Lian, M.; Liu, X.; Zhang, X.; Han, Y.; Ni, B.; Xu, R.; Yu, B.; Xu, Q.; Dai, K. Electreted sandwich membranes with persistent electrical stimulation for enhanced bone regeneration. ACS Appl. Mater. Interfaces 2022, 14, 31655–31666. [Google Scholar] [CrossRef]
- Jacob, J.; More, N.; Mounika, C.; Gondaliya, P.; Kalia, K.; Kapusetti, G. Smart piezoelectric nanohybrid of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and barium titanate for stimulated cartilage regeneration. ACS Appl. Bio Mater. 2019, 2, 4922–4931. [Google Scholar] [CrossRef]
- Das, R.; Curry, E.J.; Le, T.T.; Awale, G.; Liu, Y.; Li, S.; Contreras, J.; Bednarz, C.; Millender, J.; Xin, X.; et al. Biodegradable nanofiber bone-tissue scaffold as remotely-controlled and self-powering electrical stimulator. Nano Energy 2020, 76, 105028. [Google Scholar] [CrossRef]
- Yu, P.; Ning, C.; Zhang, Y.; Tan, G.; Lin, Z.; Liu, S.; Wang, X.; Yang, H.; Li, K.; Yi, X.; et al. Bone-inspired spatially specific piezoelectricity induces bone regeneration. Theranostics 2017, 7, 3387–3397. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zhang, C.; Liu, J.; Liu, L.; Cao, X.; Chen, X.; Lei, B.; Shao, L. Periosteum structure/function-mimicking bioactive scaffolds with piezoelectric/chem/nano signals for critical-sized bone regeneration. Chem. Eng. J. 2020, 402, 126203. [Google Scholar] [CrossRef]
- Ahmadi, N.; Kharaziha, M.; Labbaf, S. Core–shell fibrous membranes of PVDF–Ba0.9Ca0.1TiO3/PVA with osteogenic and piezoelectric properties for bone regeneration. Biomed. Mater. 2020, 15, 015007. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.; Kwon, J.; Liang, B.; Cho, H.; Soghrati, S. Finite element analysis of the impact of bone nanostructure on its piezoelectric response. Biomech. Model. Mechanobiol. 2021, 20, 1689–1708. [Google Scholar] [CrossRef] [PubMed]
- Marino, A.A.; Gross, B.D. Piezoelectricity in cementum, dentine and bone. Arch. Oral Biol. 1989, 34, 507–509. [Google Scholar] [CrossRef] [PubMed]
- Akbari, N.; Khorshidi, S.; Karkhaneh, A. Effect of piezoelectricity of nanocomposite electrospun scaffold on cell behavior in bone tissue engineering. Iran. Polym. J. 2022, 31, 919–930. [Google Scholar] [CrossRef]
- More, N.; Kapusetti, G. Piezoelectric material—A promising approach for bone and cartilage regeneration. Med. Hypotheses 2017, 108, 10–16. [Google Scholar] [CrossRef]
- Polley, C.; Distler, T.; Detsch, R.; Lund, H.; Springer, A.; Boccaccini, A.R.; Seitz, H. 3D Printing of piezoelectric barium titanate-hydroxyapatite scaffolds with interconnected porosity for bone tissue engineering. Materials 2020, 13, 1773. [Google Scholar] [CrossRef]
- Liu, W.; Yang, D.; Wei, X.; Guo, S.; Wang, N.; Tang, Z.; Lu, Y.; Shen, S.; Shi, L.; Li, X.; et al. Fabrication of piezoelectric porous BaTiO3 scaffold to repair large segmental bone defect in sheep. J. Biomater. Appl. 2020, 35, 544–552. [Google Scholar] [CrossRef]
- Nelson, B.D.; Karipott, S.S.; Guldberg, R.E.; Ong, K.G. A piezoelectric bone fixation plate for in vivo application and monitoring of mechanical loading during fracture healing. Meas. Sci. Technol. 2020, 31, 095703. [Google Scholar] [CrossRef]
- Makino, T.; Nakamura, T.; Bustamante, L.; Takayanagi, S.; Koyama, D.; Matsukawa, M. Piezoelectric and inversely piezoelectric responses of bone tissue plates in the megahertz range. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Tinoco, H.A.; Cardona, C.I.; Peña, F.M.; Gomez, J.P.; Roldan-Restrepo, S.I.; Velasco-Mejia, M.A.; Barco, D.R. Evaluation of a piezo-actuated sensor for monitoring elastic variations of its support with impedance-based measurements. Sensors 2019, 19, 184. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Bhalla, S.; Madan, A. Shape memory alloy actuation of non-bonded piezo sensor configuration for bone diagnosis and impedance based analysis. Biomed. Eng. Lett. 2019, 9, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Krech, E.D.; Cadel, E.S.; Barrett, R.M.; Friis, E.A. Effect of compliant layers within piezoelectric composites on power generation providing electrical stimulation in low frequency applications. J. Mech. Behav. Biomed. Mater. 2018, 88, 340–345. [Google Scholar] [CrossRef]
- Hosokawa, A. Change of piezoelectric signal in cancellous bone with ultrasound irradiation angle. Jpn. J. Appl. Phys. 2020, 59, SKKE03. [Google Scholar] [CrossRef]
- Li, Y.; Liao, C.; Tjong, S.C. Electrospun polyvinylidene fluoride-based fibrous scaffolds with piezoelectric characteristics for bone and neural tissue engineering. Nanomaterials 2019, 9, 952. [Google Scholar] [CrossRef]
- Lakes, R. The role of gradient effects in the piezoelectricity of bone. IEEE Trans. Biomed. Eng. 1980, BME-27, 282–283. [Google Scholar] [CrossRef]
- Hosokawa, A.; Matsukawa, M. Piezoelectric and opto-acoustic material properties of bone. In Bone Quantitative Ultrasound: New Horizons; Laugier, P., Grimal, Q., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 319–346. [Google Scholar]
- KÖNig, C.W.; TrÜBenbach, J.; BÖHm, P.; Fritz, J.A.N.; Duda, S.H.; Pereira, P.L. Magnetic resonance-guided transcortical biopsy of bone marrow lesions using a magnetic resonance imaging-compatible piezoelectric power drill. Investig. Radiol. 2003, 38, 159–163. [Google Scholar] [CrossRef]
- Shuai, C.; Liu, G.; Yang, Y.; Yang, W.; He, C.; Wang, G.; Liu, Z.; Qi, F.; Peng, S. Functionalized BaTiO3 enhances piezoelectric effect towards cell response of bone scaffold. Colloids Surf. B Biointerfaces 2020, 185, 110587. [Google Scholar] [CrossRef]
- Shokrollahi, H.; Salimi, F.; Doostmohammadi, A. The fabrication and characterization of barium titanate/akermanite nano-bio-ceramic with a suitable piezoelectric coefficient for bone defect recovery. J. Mech. Behav. Biomed. Mater. 2017, 74, 365–370. [Google Scholar] [CrossRef]
- Gorodzha, S.N.; Muslimov, A.R.; Syromotina, D.S.; Timin, A.S.; Tcvetkov, N.Y.; Lepik, K.V.; Petrova, A.V.; Surmeneva, M.A.; Gorin, D.A.; Sukhorukov, G.B.; et al. A comparison study between electrospun polycaprolactone and piezoelectric poly(3-hydroxybutyrate-co-3-hydroxyvalerate) scaffolds for bone tissue engineering. Colloids Surf. B Biointerfaces 2017, 160, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Manohar, C.S.; Kumar, B.S.; Sadhu, S.P.P.; Srimadh, S.K.; Muthukumar, V.S.; Venketesh, S.; Varma, K.B.R. Novel lead-free biocompatible piezoelectric hydroxyapatite (HA)—BCZT (Ba0.85Ca0.15Zr0.1Ti0.9O3) nanocrystal composites for bone regeneration. Nanotechnol. Rev. 2019, 8, 61–78. [Google Scholar] [CrossRef]
- Pekovits, K.; Wildburger, A.; Payer, M.; Hutter, H.; Jakse, N.; Dohr, G. Evaluation of Graft Cell Viability—Efficacy of Piezoelectric Versus Manual Bone Scraper Technique. J. Oral Maxillofac. Surg. 2012, 70, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Stroe, M.C.; Crolet, J.M.; Racila, M. Mechanotransduction in cortical bone and the role of piezoelectricity: A numerical approach. Comput. Methods Biomech. Biomed. Eng. 2013, 16, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Balasandaram, I.; Bridle, C.; Holmes, S. Piezoelectric surgery—Primary bone grafting in craniofacial trauma revisited. Int. J. Oral Maxillofac. Surg. 2015, 44, 1016–1017. [Google Scholar] [CrossRef]
- Gellner, V.; Koele, W.; Wolf, A.; Gerstenberger, C.; Mokry, M.; Stammberger, H.; Tomazic, P.V. A piezoelectric device for bone work in endoscopic anterior skull base surgery—A feasibility study in 15 patients. Clin. Otolaryngol. 2017, 42, 927–931. [Google Scholar] [CrossRef]
- Samadi, A.; Hasanzadeh, R.; Azdast, T.; Abdollahi, H.; Zarrintaj, P.; Saeb, M.R. Piezoelectric performance of microcellular polypropylene foams fabricated using foam injection molding as a potential scaffold for bone tissue engineering. J. Macromol. Sci. Part B 2020, 59, 376–389. [Google Scholar] [CrossRef]
- Park, I.-Y.; Shimizu, Y.; O’Connor, K.N.; Puria, S.; Cho, J.-H. Comparisons of electromagnetic and piezoelectric floating-mass transducers in human cadaveric temporal bones. Hear. Res. 2011, 272, 187–192. [Google Scholar] [CrossRef]
- Zheng, T.; Zhao, H.; Huang, Y.; Gao, C.; Zhang, X.; Cai, Q.; Yang, X. Piezoelectric calcium/manganese-doped barium titanate nanofibers with improved osteogenic activity. Ceram. Int. 2021, 47, 28778–28789. [Google Scholar] [CrossRef]
- Reis, J.; Frias, C.; Capela-Silva, F.; Potes, J.; Botelho, L.; Castro, C.; Marques, A.; Simões, J. Piezoelectric actuators for bone mechanical stimulation: Exploring the concept. J. Biomech. 2012, 45, S344. [Google Scholar] [CrossRef][Green Version]
- Aschero, G.; Gizdulich, P.; Mango, F.; Romano, S.M. Converse piezoelectric effect detected in fresh cow femur bone. J. Biomech. 1996, 29, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Mouraret, S.; Houschyar, K.S.; Hunter, D.J.; Smith, A.A.; Jew, O.S.; Girod, S.; Helms, J.A. Cell viability after osteotomy and bone harvesting: Comparison of piezoelectric surgery and conventional bur. Int. J. Oral Maxillofac. Surg. 2014, 43, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Augello, M.; Deibel, W.; Nuss, K.; Cattin, P.; Jürgens, P. Comparative microstructural analysis of bone osteotomies after cutting by computer-assisted robot-guided laser osteotome and piezoelectric osteotome: An in vivo animal study. Lasers Med. Sci. 2018, 33, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.M. A new mechanical stimulator for cultured bone cells using piezoelectric actuator. J. Biomech. 1999, 32, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Younes, R.; Nasseh, I.; Lahoud, P.; Wassef, E.; Dagher, M. Bone lid technique using a piezoelectric device for the treatment of a mandibular bony lesion. Case Rep. Dent. 2017, 2017, 9315070. [Google Scholar] [CrossRef] [PubMed]


| Materials/Devices | Properties | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| sm-PENG | Promote osteogenic differentiation | Memory of shape | Lack of validation of animal experiments. | [66] |
| ISPG | Host-coupled bio-nanogenerator (HCBG) configured with a self-powered regional electrical environment for bone regeneration | Achieved biomechanical energy scavenging and electrical stimulation therapy | In vivo degradation properties are unclear, and if surgical removal is required, it will cause secondary damage to the organism. | [67] |
| GaN/AlGaN | Enhance bio regeneration | Rapid and superior bone repair in vivo | The specific mechanism of action can be studied in more depth. | [68] |
| PHBV | Simulate the structure and piezoelectric coefficient of natural cartilage | Promote the regeneration of cartilage | The specific mechanism of action can be studied in more depth. | [70] |
| PLLA | Remote-controlled electrical stimulation | Repair mouse skull defects | The output performance of the device can continue to be optimized. | [71] |
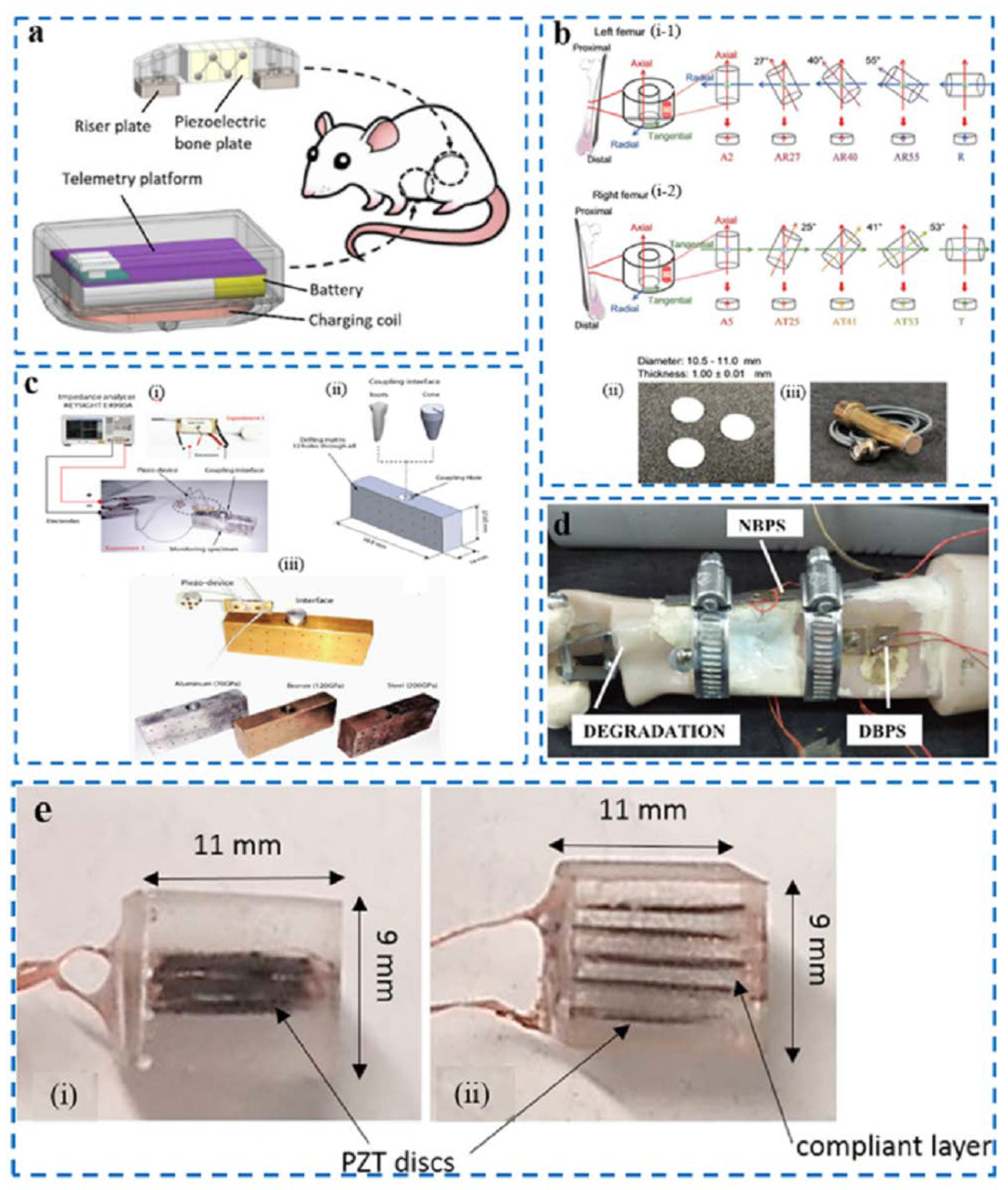
| Piezoelectric fixation plate | Using piezoelectric materials as both sensors and brakes | Realize the mechanical vibration of non-connected areas and evaluate the effectiveness of the treatment | Inconvenient to carry. | [81] |
| An ultrasonic transducer using bovine femoral material | Measure the ultrasonic radiation and reception of bone | Measure the piezoelectric properties of the bone in the megahertz segment | In vivo degradation properties are unclear, and if surgical removal is required, it will cause secondary damage to the organism. | [82] |
| A piezoelectric sensing device for biological applications on bones. | Uses mechanical electrical impedance technology | Detect the healing of the bone injury | Lack of validation of animal experiments. | [83] |
| NBPS | Avoid the mechanical tightening of clamps through screws that may cause discomfort to patients and damage the PZT patch | Provides quantification of parameters during bone degeneration | The explanation of the mechanism needs to be improved. | [84] |
| CLACS | Generate direct current under the frequency and load of bone healing | Solve slow bone healing and nonunion | The explanation of the mechanism needs to be improved. | [85] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Ji, J.; Lv, Y.; Li, Z.; Luo, D. Application of Piezoelectric Material and Devices in Bone Regeneration. Nanomaterials 2022, 12, 4386. https://doi.org/10.3390/nano12244386
Yang C, Ji J, Lv Y, Li Z, Luo D. Application of Piezoelectric Material and Devices in Bone Regeneration. Nanomaterials. 2022; 12(24):4386. https://doi.org/10.3390/nano12244386
Chicago/Turabian StyleYang, Chunyu, Jianying Ji, Yujia Lv, Zhou Li, and Dan Luo. 2022. "Application of Piezoelectric Material and Devices in Bone Regeneration" Nanomaterials 12, no. 24: 4386. https://doi.org/10.3390/nano12244386
APA StyleYang, C., Ji, J., Lv, Y., Li, Z., & Luo, D. (2022). Application of Piezoelectric Material and Devices in Bone Regeneration. Nanomaterials, 12(24), 4386. https://doi.org/10.3390/nano12244386










