Cl- and Al-Doped Argyrodite Solid Electrolyte Li6PS5Cl for All-Solid-State Lithium Batteries with Improved Ionic Conductivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Solid Electrolytes
2.2. Material Characterization
2.3. Measurement of Electrochemical Performance
3. Results and Discussion
3.1. Cl-Doped Solid Electrolyte
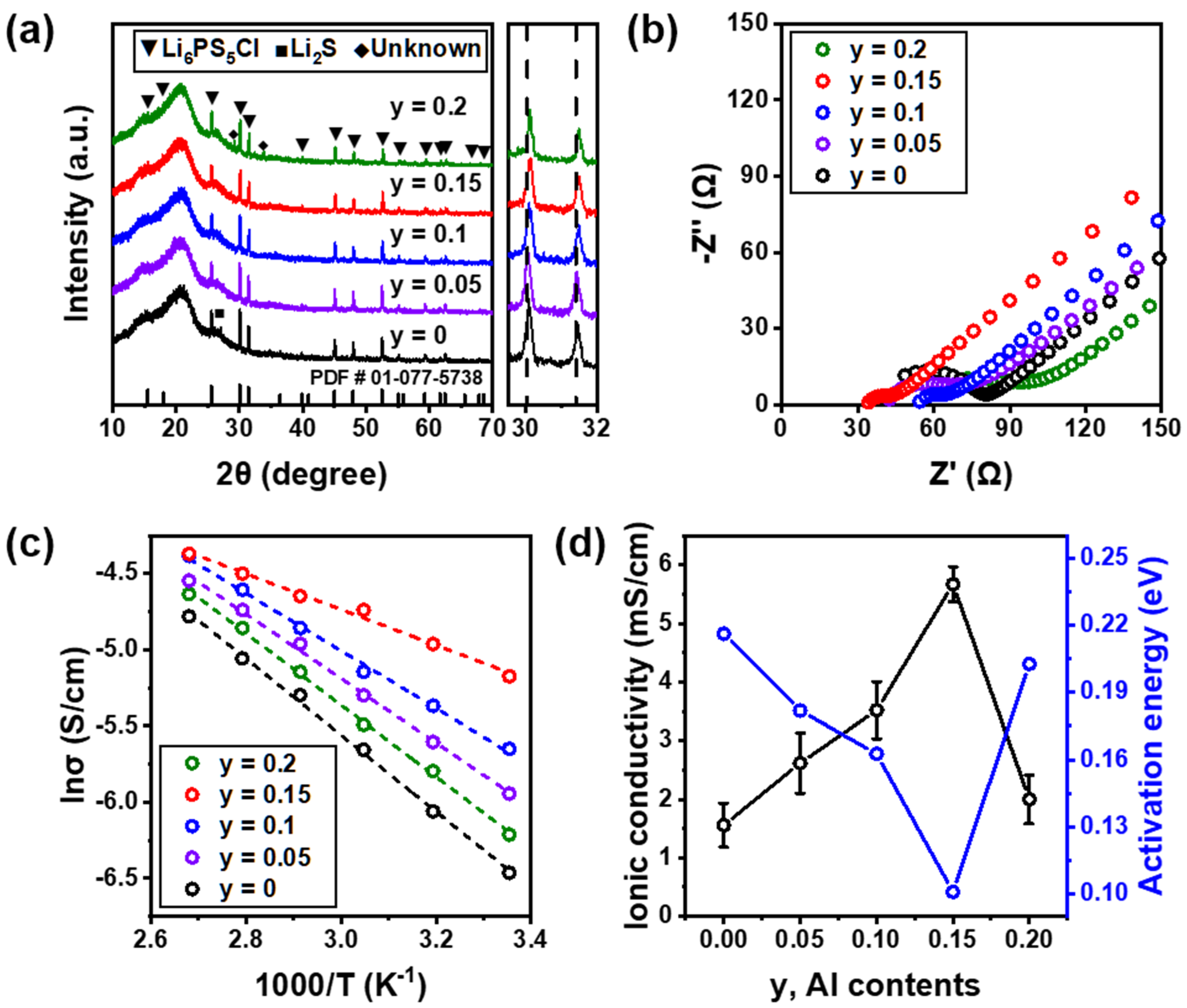
3.2. Al-Doped Solid Electrolyte
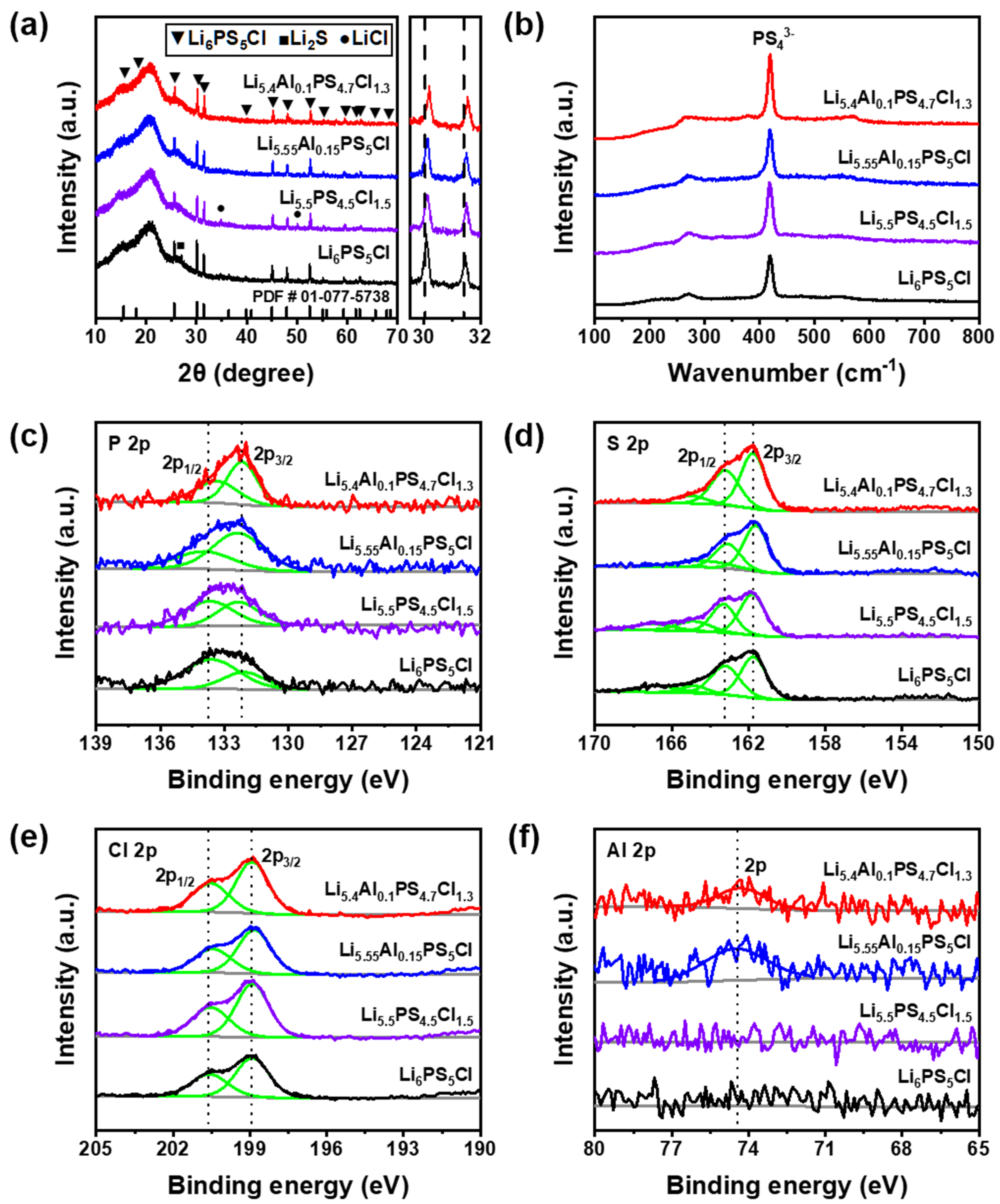
3.3. Structural Analysis of Co-Doped Solid Electrolyte
3.4. Electrochemical Properties of Co-Doped Solid Electrolyte
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ge, X.; Liu, S.; Qiao, M.; Du, Y.; Li, Y.; Bao, J.; Zhou, X. Enabling Superior Electrochemical Properties for Highly Efficient Potassium Storage by Impregnating Ultrafine Sb Nanocrystals Within Nanochannel-Containing Carbon Nanofibers. Angew. Chem. Int. Ed. Engl. 2019, 58, 14578–14583. [Google Scholar] [CrossRef] [PubMed]
- Tu, F.; Han, Y.; Du, Y.; Ge, X.; Weng, W.; Zhou, X.; Bao, J. Hierarchical Nanospheres Constructed by Ultrathin MoS2 Nanosheets Braced on Nitrogen-Doped Carbon Polyhedra for Efficient Lithium and Sodium Storage. ACS Appl. Mater. Interfaces 2019, 11, 2112–2119. [Google Scholar] [CrossRef] [PubMed]
- Larcher, D.; Tarascon, J.M. Towards Greener and More Sustainable Batteries for Electrical Energy Storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the Development of Advanced Li-Ion Batteries: A Review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Kato, Y.; Hori, S.; Saito, T.; Suzuki, K.; Hirayama, M.; Mitsui, A.; Yonemura, M.; Iba, H.; Kanno, R. High-Power All-Solid-State Batteries Using Sulfide Superionic Conductors. Nat. Energy 2016, 1, 16030. [Google Scholar] [CrossRef]
- Seino, Y.; Ota, T.; Takada, K.; Hayashi, A.; Tatsumisago, M. A Sulphide Lithium Super Ion Conductor Is Superior to Liquid Ion Conductors for Use in Rechargeable Batteries. Energy Environ. Sci. 2014, 7, 627–631. [Google Scholar] [CrossRef]
- Fergus, J.W. Ceramic and Polymeric Solid Electrolytes for Lithium-Ion Batteries. J. Power Sources 2010, 195, 4554–4569. [Google Scholar] [CrossRef]
- Ohsaki, T.; Kishi, T.; Kuboki, T.; Takami, N.; Shimura, N.; Sato, Y.; Sekino, M.; Satoh, A. Overcharge Reaction of Lithium-Ion Batteries. J. Power Sources 2005, 146, 97–100. [Google Scholar] [CrossRef]
- Zheng, F.; Kotobuki, M.; Song, S.; Lai, M.O.; Lu, L. Review on Solid Electrolytes for All-Solid-State Lithium-Ion Batteries. J. Power Sources 2018, 389, 198–213. [Google Scholar] [CrossRef]
- Tatsumisago, M.; Nagao, M.; Hayashi, A. Recent Development of Sulfide Solid Electrolytes and Interfacial Modification for All-Solid-State Rechargeable Lithium Batteries. J. Asian Ceram. Soc. 2013, 1, 17–25. [Google Scholar] [CrossRef]
- Takada, K. Progress and Prospective of Solid-State Lithium Batteries. Acta Mater. 2013, 61, 759–770. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Hoffert, M.I.; Caldeira, K.; Benford, G.; Criswell, D.R.; Green, C.; Herzog, H.; Jain, A.K.; Kheshgi, H.S.; Lackner, K.S.; Lewis, J.S.; et al. Advanced Technology Paths to Global Climate Stability: Energy for a Greenhouse Planet. Science 2002, 298, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Zhu, Y.; He, X.; Mo, Y.; Wang, C. Electrochemical Stability of Li10GeP2S12 and Li7La3Zr2O12 Solid Electrolytes. Adv. Energy Mater. 2016, 6, 1501590. [Google Scholar] [CrossRef]
- Yao, P.; Yu, H.; Ding, Z.; Liu, Y.; Lu, J.; Lavorgna, M.; Wu, J.; Liu, X. Review on Polymer-Based Composite Electrolytes for Lithium Batteries. Front. Chem. 2019, 7, 522. [Google Scholar] [CrossRef]
- Wolfenstine, J.; Allen, J.L.; Sakamoto, J.; Siegel, D.J.; Choe, H. Mechanical Behavior of Li-Ion-Conducting Crystalline Oxide-Based Solid Electrolytes: A Brief Review. Ionics 2018, 24, 1271–1276. [Google Scholar] [CrossRef]
- Adeli, P.; Bazak, J.D.; Huq, A.; Goward, G.R.; Nazar, L.F. Influence of Aliovalent Cation Substitution and Mechanical Compression on Li-Ion Conductivity and Diffusivity in Argyrodite Solid Electrolytes. Chem. Mater. 2021, 33, 146–157. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Zhang, X.; Liu, T.; Lin, Y.H.; Shen, Y.; Li, L.; Nan, C.W. High-Conductivity Argyrodite Li6PS5Cl Solid Electrolytes Prepared via Optimized Sintering Processes for All-Solid-State Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2018, 10, 42279–42285. [Google Scholar] [CrossRef]
- Kraft, M.A.; Culver, S.P.; Calderon, M.; Böcher, F.; Krauskopf, T.; Senyshyn, A.; Dietrich, C.; Zevalkink, A.; Janek, J.r.; Zeier, W.G. Influence of Lattice Polarizability on the Ionic Conductivity in the Lithium Superionic Argyrodites Li6PS5X (X = Cl, Br, I). J. Am. Chem. Soc. 2017, 139, 10909–10918. [Google Scholar] [CrossRef]
- Deiseroth, H.J.; Kong, S.T.; Eckert, H.; Vannahme, J.; Reiner, C.; Zaiss, T.; Schlosser, M. Li6PS5X: A Class of Crystalline Li-Rich Solids with an Unusually High Li+ Mobility. Angew. Chem. Int. Ed. Engl. 2008, 47, 755–758. [Google Scholar] [CrossRef]
- Yu, C.; Ganapathy, S.; Hageman, J.; Van Eijck, L.; Van Eck, E.R.H.; Zhang, L.; Schwietert, T.; Basak, S.; Kelder, E.M.; Wagemaker, M. Facile Synthesis toward the Optimal Structure-Conductivity Characteristics of the Argyrodite Li6PS5Cl Solid-State Electrolyte. ACS Appl. Mater. Interfaces 2018, 10, 33296–33306. [Google Scholar] [CrossRef]
- Boulineau, S.; Courty, M.; Tarascon, J.-M.; Viallet, V. Mechanochemical Synthesis of Li-Argyrodite Li6PS5X (X = Cl, Br, I) as Sulfur-Based Solid Electrolytes for All Solid State Batteries Application. Solid State Ionics 2012, 221, 1–5. [Google Scholar] [CrossRef]
- Yu, C.; van Eijck, L.; Ganapathy, S.; Wagemaker, M. Synthesis, Structure and Electrochemical Performance of the Argyrodite Li6PS5Cl Solid Electrolyte for Li-Ion Solid State Batteries. Electrochim. Acta 2016, 215, 93–99. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Jia, H.; Peng, L.; An, T.; Xie, J. Enhancing Ionic Conductivity of Solid Electrolyte by Lithium Substitution in Halogenated Li-Argyrodite. J. Power Sources 2020, 450, 227601. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Zheng, C.; Xia, Y.; Gan, Y.; Huang, H.; Liang, C.; He, X.; Tao, X.; Zhang, W. Silicon-Doped Argyrodite Solid Electrolyte Li6PS5I with Improved Ionic Conductivity and Interfacial Compatibility for High-Performance All-Solid-State Lithium Batteries. A.C.S. Appl. Mater. Interfaces 2020, 12, 41538–41545. [Google Scholar] [CrossRef]
- Yu, C.; Li, Y.; Willans, M.; Zhao, Y.; Adair, K.R.; Zhao, F.; Li, W.; Deng, S.; Liang, J.; Banis, M.N.; et al. Superionic Conductivity in Lithium Argyrodite Solid-State Electrolyte by Controlled Cl-Doping. Nano Energy 2020, 69, 104396. [Google Scholar] [CrossRef]
- Hikima, K.; Huy Phuc, N.H.H.; Tsukasaki, H.; Mori, S.; Muto, H.; Matsuda, A. High Ionic Conductivity of Multivalent Cation Doped Li6PS5Cl Solid Electrolytes Synthesized by Mechanical Milling. RSC Adv. 2020, 10, 22304–22310. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Yan, X.; Wang, H.; Liu, Y.; Yu, C.; Cao, X.; van Eijck, L.; Wen, B. All-in-One Improvement toward Li6PS5Br-Based Solid Electrolytes Triggered by Compositional Tune. J. Power Sources 2019, 410–411, 162–170. [Google Scholar] [CrossRef]
- Xuan, M.; Xiao, W.; Xu, H.; Shen, Y.; Li, Z.; Zhang, S.; Wang, Z.; Shao, G. Ultrafast Solid-State Lithium Ion Conductor Through Alloying Induced Lattice Softening of Li6PS5Cl. J. Mater. Chem. A 2018, 6, 19231–19240. [Google Scholar] [CrossRef]
- Adeli, P.; Bazak, J.D.; Park, K.H.; Kochetkov, I.; Huq, A.; Goward, G.R.; Nazar, L.F. Boosting solid-state diffusivity and conductivity in lithium superionic argyrodites by halide substitution. Angew. Chem. 2019, 131, 8773–8778. [Google Scholar] [CrossRef]
- Zeng, D.; Yao, J.; Zhang, L.; Xu, R.; Wang, S.; Yan, X.; Yu, C.; Wang, L. Promoting Favorable Interfacial Properties in Lithium-Based Batteries Using Chlorine-Rich Sulfide Inorganic Solid-State Electrolytes. Nat. Commun. 2022, 13, 1909. [Google Scholar] [CrossRef] [PubMed]
- de Klerk, N.J.; Rosłoń, I.; Wagemaker, M. Diffusion mechanism of Li argyrodite solid electrolytes for Li-ion batteries and prediction of optimized halogen doping: The effect of Li vacancies, halogens, and halogen disorder. Chem. Mater. 2016, 28, 7955–7963. [Google Scholar] [CrossRef]
- Liang, J.; Li, X.; Zhao, Y.; Goncharova, L.V.; Wang, G.; Adair, K.R.; Wang, C.; Li, R.; Zhu, Y.; Qian, Y.; et al. In Situ Li3PS4 Solid-State Electrolyte Protection Layers for Superior Long-Life and High-Rate Lithium-Metal Anodes. Adv. Mater. 2018, 30, e1804684. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.; Koerver, R.; Gaultois, M.W.; Kieslich, G.; Cibin, G.; Janek, J.; Zeier, W.G. Spectroscopic Characterization of Lithium Thiophosphates by XPS and XAS—A Model to Help Monitor Interfacial Reactions in All-Solid-State Batteries. Phys. Chem. Chem. Phys. 2018, 20, 20088–20095. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-H.; Li, W.-Z.; Fan, B.; Fan, P.; Luo, Z.-K.; Wang, F.; Zhang, X.-H.; Ma, H.-L.; Xue, B. Stabilizing Electrode/Electrolyte Interface in Li-S Batteries Using Liquid/Solid Li2S-P2S5 Hybrid Electrolyte. Appl. Surf. Sci. 2021, 546, 149034. [Google Scholar] [CrossRef]
- Tanibata, N.; Tsukasaki, H.; Deguchi, M.; Mori, S.; Hayashi, A.; Tatsumisago, M. A Novel Discharge–Charge Mechanism of a S–P2S5 Composite Electrode without Electrolytes in All-Solid-State Li/S Batteries. J. Mater. Chem. A 2017, 5, 11224–11228. [Google Scholar] [CrossRef]
- Strauss, F.; Stepien, D.; Maibach, J.; Pfaffmann, L.; Indris, S.; Hartmann, P.; Brezesinski, T. Influence of Electronically Conductive Additives on the Cycling Performance of Argyrodite-Based All-Solid-State Batteries. RSC. Adv. 2020, 10, 1114–1119. [Google Scholar] [CrossRef]
- Auvergniot, J.; Cassel, A.; Foix, D.; Viallet, V.; Seznec, V.; Dedryvère, R. Redox Activity of Argyrodite Li6PS5Cl Electrolyte in All-Solid-State Li-Ion Battery: An XPS Study. Solid State Ionics 2017, 300, 78–85. [Google Scholar] [CrossRef]


| Ionic Conductivity (mS/cm) | x, Cl Contents | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 0.2 | 0.3 | 0.4 | 0.5 | 0.7 | ||
| y, Al contents | 0 | 1.56 | - | 3.6 | - | 5.05 | 2.57 |
| 0.05 | 2.62 | 4.21 | 2.16 | 1.34 | - | - | |
| 0.1 | 3.52 | 4.6 | 7.29 | 3.83 | 0.64 | 1.24 | |
| 0.15 | 5.67 | - | 3.36 | 1.71 | 0.80 | - | |
| 0.2 | 2.00 | - | 2.49 | - | 1.32 | 0.88 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.J.; Kim, S.-I.; Son, M.; Lee, J.W.; Lee, D.H. Cl- and Al-Doped Argyrodite Solid Electrolyte Li6PS5Cl for All-Solid-State Lithium Batteries with Improved Ionic Conductivity. Nanomaterials 2022, 12, 4355. https://doi.org/10.3390/nano12244355
Choi YJ, Kim S-I, Son M, Lee JW, Lee DH. Cl- and Al-Doped Argyrodite Solid Electrolyte Li6PS5Cl for All-Solid-State Lithium Batteries with Improved Ionic Conductivity. Nanomaterials. 2022; 12(24):4355. https://doi.org/10.3390/nano12244355
Chicago/Turabian StyleChoi, Yeong Jun, Sun-I Kim, Mingyu Son, Jung Woo Lee, and Duck Hyun Lee. 2022. "Cl- and Al-Doped Argyrodite Solid Electrolyte Li6PS5Cl for All-Solid-State Lithium Batteries with Improved Ionic Conductivity" Nanomaterials 12, no. 24: 4355. https://doi.org/10.3390/nano12244355
APA StyleChoi, Y. J., Kim, S.-I., Son, M., Lee, J. W., & Lee, D. H. (2022). Cl- and Al-Doped Argyrodite Solid Electrolyte Li6PS5Cl for All-Solid-State Lithium Batteries with Improved Ionic Conductivity. Nanomaterials, 12(24), 4355. https://doi.org/10.3390/nano12244355








