Postproduction Approach to Enhance the External Quantum Efficiency for Red Light-Emitting Diodes Based on Silicon Nanocrystals
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Preparation of SiQD Ink
2.3. Device Fabrication
2.4. Characterization
2.5. Observation and Analysis
2.6. Calculation of EQE and Optical Power Density
3. Results
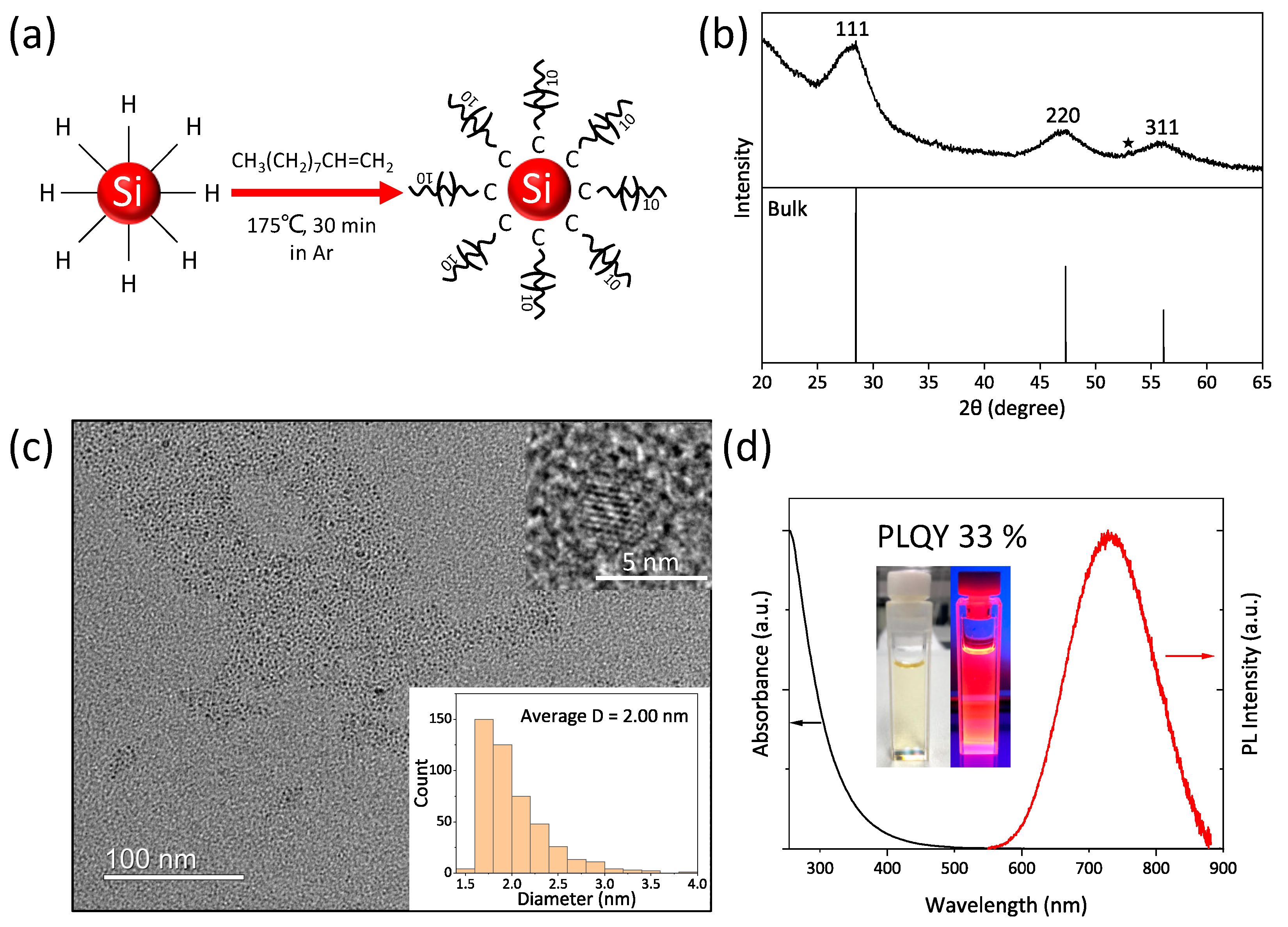
3.1. Hydrophobic SiQD Ink
3.2. Si-iQLED Fabrication
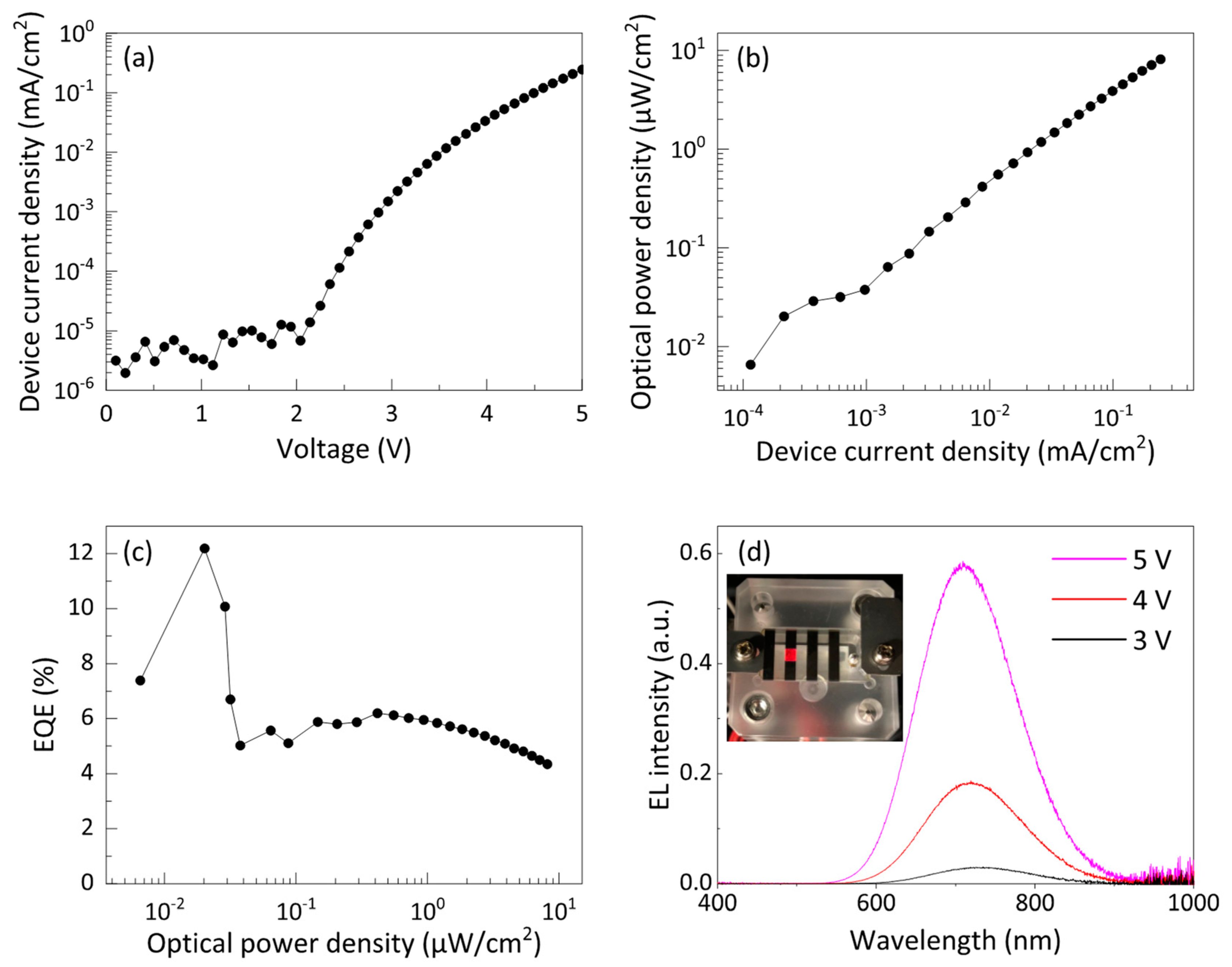
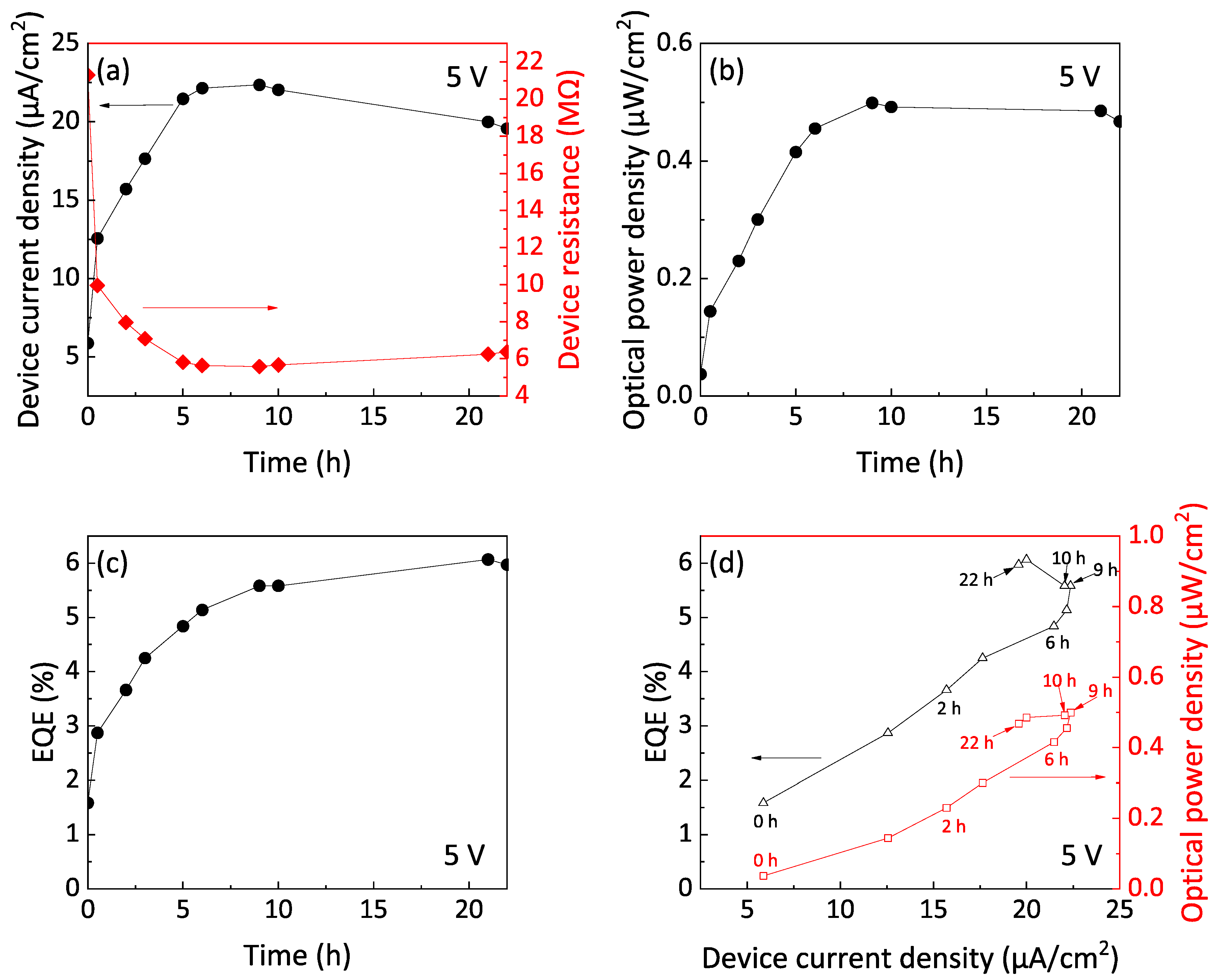
3.3. Device Performance of Si-iQLED
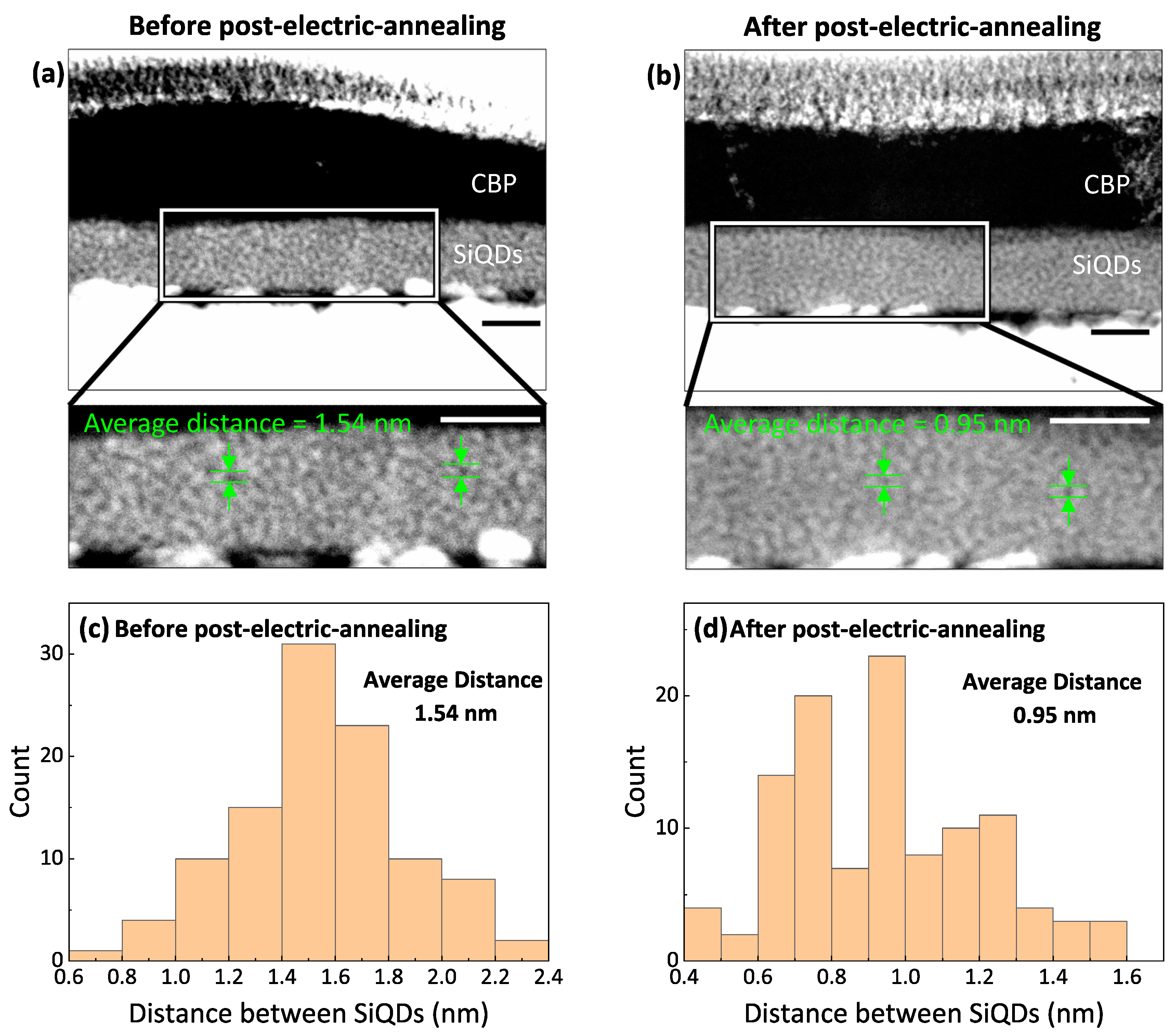
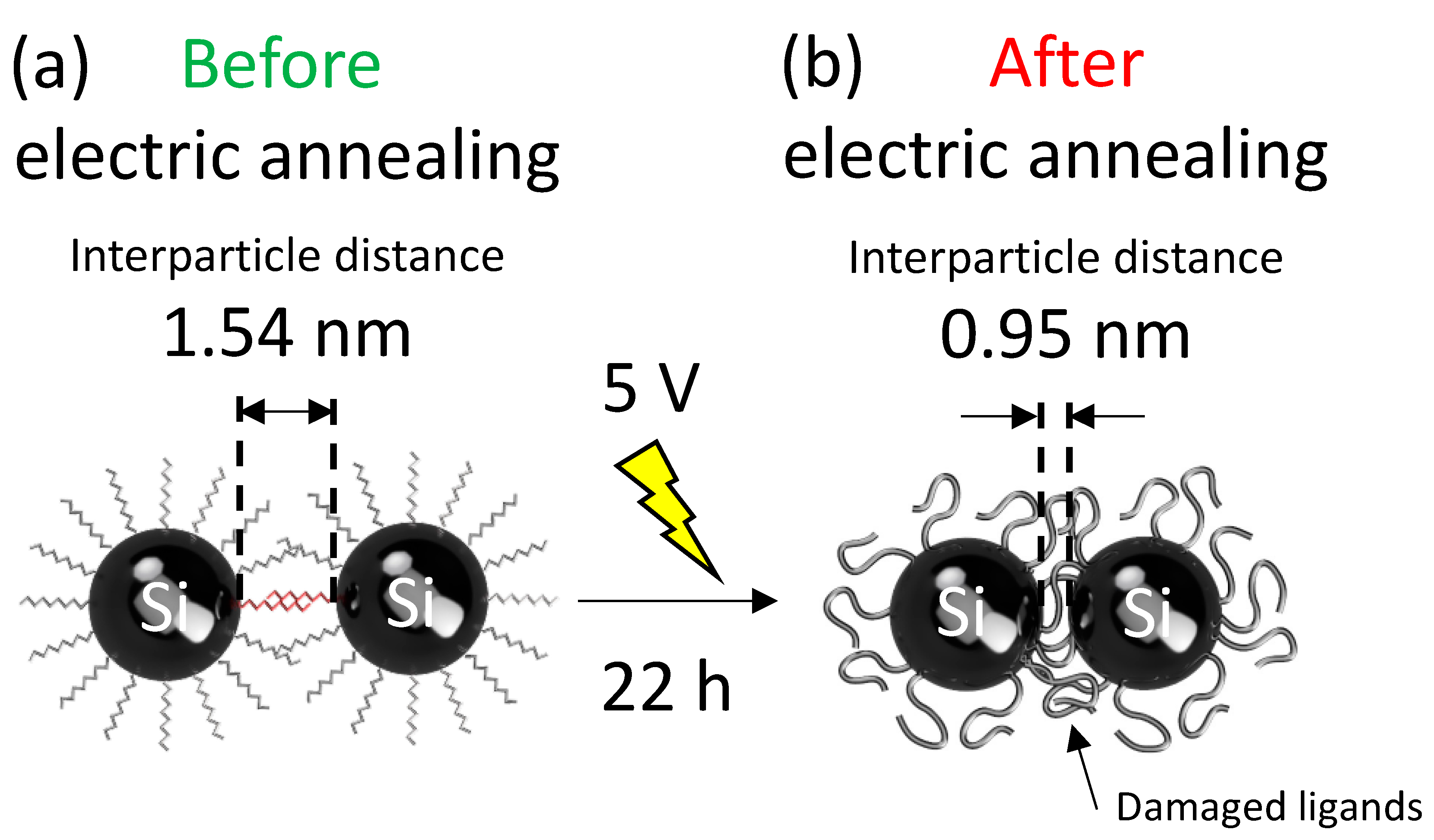
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Won, Y.-H.; Cho, O.; Kim, T.; Chung, D.-Y.; Kim, T.; Chung, H.; Jang, H.; Lee, J.; Kim, D.; Jang, E. Highly efficient and stable InP/ZnSe/ZnS quantum dot light-emitting diodes. Nature 2019, 575, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, X.; Xin, W.; Yao, D.; Liu, Y.; Zhang, L.; Liu, W.; Zhang, W.; Zheng, W.; Yang, B.; et al. Facile Synthesis of Cu-In-S/ZnS Core/Shell Quantum Dots in 1-Dodecanethiol for Efficient Light-Emitting Diodes with an External Quantum Efficiency of 7.8%. Chem. Mater. 2018, 30, 8939–8947. [Google Scholar] [CrossRef]
- Motomura, G.; Ogura, K.; Kameyama, T.; Torimoto, T.; Uematsu, T.; Kuwabata, S.; Tsuzuki, T. Efficient quantum-dot light-emitting diodes using ZnS-AgInS2 solid-solution quantum dots in combination with organic charge-transport materials. Appl. Phys. Lett. 2020, 116, 093302. [Google Scholar] [CrossRef]
- Liang, H.; Yuan, F.; Johnston, A.; Gao, C.; Choubisa, H.; Gao, Y.; Wang, Y.; Sagar, L.K.; Sun, B.; Li, P.; et al. High Color Purity Lead-Free Perovskite Light-Emitting Diodes via Sn Stabilization. Adv. Sci. 2020, 7, 1903213. [Google Scholar] [CrossRef]
- Yao, L.; Yu, T.; Ba, L.; Meng, H.; Fang, X.; Wang, Y.; Li, L.; Rong, X.; Wang, S.; Wang, X.; et al. Efficient silicon quantum dots light emitting diodes with an inverted device structure. J. Mater. Chem. C 2016, 4, 673–677. [Google Scholar] [CrossRef]
- Ghosh, B.; Yamada, H.; Chinnathambi, S.; Özbilgin, I.N.G.; Shirahata, N. Inverted Device Architecture for Enhanced Performance of Flexible Silicon Quantum Dot Light-Emitting Diode. J. Phys. Chem. Lett. 2018, 9, 5400–5407. [Google Scholar] [CrossRef]
- Tu, C.-C.; Tang, L.; Huang, J.; Voutsas, A.; Lin, L.Y. Visible electroluminescence from hybrid colloidal silicon quantum dot-organic light-emitting diodes. Appl. Phys. Lett. 2011, 98, 213102. [Google Scholar] [CrossRef]
- Cheng, K.-Y.; Anthony, R.; Kortshagen, U.R.; Holmes, R.J. High-Efficiency Silicon Nanocrystal Light-Emitting Devices. Nano Lett. 2011, 11, 1952–1956. [Google Scholar] [CrossRef]
- Puzzo, D.P.; Henderson, E.J.; Helander, M.G.; Wang, Z.; Ozin, G.A.; Lu, Z. Visible Colloidal Nanocrystal Silicon Light-Emitting Diode. Nano Lett. 2011, 11, 1585–1590. [Google Scholar] [CrossRef]
- Mastronardi, M.L.; Henderson, E.J.; Puzzo, D.P.; Chang, Y.; Wang, Z.B.; Helander, M.G.; Jeong, J.; Kherani, N.P.; Lu, Z.; Ozin, G.A. Silicon Nanocrystal OLEDs: Effect of Organic Capping Group on Performance. Small 2012, 8, 3647–3654. [Google Scholar] [CrossRef]
- Maier-Flaig, F.; Rinck, J.; Stephan, M.; Bocksrocker, T.; Bruns, M.; Kübel, C.; Powell, A.; Ozin, G.A.; Lemmer, U. Multicolor Silicon Light-Emitting Diodes (SiLEDs). Nano Lett. 2013, 13, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Xu, Y.; Sakata, T.; Saitow, K.-I. Designing Efficient Si Quantum Dots and LEDs by Quantifying Ligand Effects. ACS Appl. Mater. Interfaces 2022, 14, 1373–1388. [Google Scholar] [CrossRef]
- Xu, Y.; Terada, S.; Xin, Y.; Ueda, H.; Saitow, K.-I. Ligand Effects on Photoluminescence and Electroluminescence of Silicon Quantum Dots for Light-Emitting Diodes. ACS Appl. Nano Mater. 2022, 5, 7787–7797. [Google Scholar] [CrossRef]
- Terada, S.; Ueda, H.; Ono, T.; Saitow, K.-I. Orange–Red Si Quantum Dot LEDs from Recycled Rice Husks. ACS Sustain. Chem. Eng. 2022, 10, 1765–1776. [Google Scholar] [CrossRef]
- Maier-Flaig, F.; Kübel, C.; Rinck, J.; Bocksrocker, T.; Scherer, T.; Prang, R.; Powell, A.; Ozin, G.A.; Lemmer, U. Looking Inside a Working SiLED. Nano Lett. 2013, 13, 3539–3545. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, B.; Shirahata, N. All-Inorganic Red-Light Emitting Diodes Based on Silicon Quantum Dots. Crystals 2019, 9, 385. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, S.; Gu, W.; Zhang, Y.; Qiao, X.; Ni, Z.; Pi, X.; Yang, D. Light-Emitting Diodes Based on Colloidal Silicon Quantum Dots with Octyl and Phenylpropyl Ligands. ACS Appl. Mater. Interfaces 2018, 10, 5959–5966. [Google Scholar] [CrossRef]
- Mock, J.; Groß, E.; Kloberg, M.J.; Rieger, B.; Becherer, M. Surface Engineering of Silicon Quantum Dots: Does the Ligand Length Impact the Optoelectronic Properties of Light-Emitting Diodes? Adv. Photonics Res. 2021, 2, 2100083. [Google Scholar] [CrossRef]
- Yamada, H.; Saitoh, N.; Ghosh, B.; Masuda, Y.; Yoshizawa, N.; Shirahata, N. Improved Brightness and Color Tunability of Solution-Processed Silicon Quantum Dot Light-Emitting Diodes. J. Phys. Chem. C 2020, 124, 23333–23342. [Google Scholar] [CrossRef]
- Watanabe, J.; Yamada, H.; Sun, H.-T.; Moronaga, T.; Ishii, Y.; Shirahata, N. Silicon Quantum Dots for Light-Emitting Diodes Extending to the NIR-II Window. ACS Appl. Nano Mater. 2021, 4, 11651–11660. [Google Scholar] [CrossRef]
- Cheng, K.-Y.; Anthony, R.; Kortshagen, U.R.; Holmes, R.J. Hybrid Silicon Nanocrystal−Organic Light-Emitting Devices for Infrared Electroluminescence. Nano Lett. 2010, 10, 1154–1157. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, B.; Shirahata, N. Colloidal silicon quantum dots: Synthesis and luminescence tuning from the near-UV to the near-IR range. Sci. Technol. Adv. Mater. 2014, 15, 014207. [Google Scholar] [CrossRef] [PubMed]
- Canham, L. Introductory lecture: Origins and applications of efficient visible photoluminescence from silicon-based nanostructures. Faraday Discuss. 2020, 222, 10–81. [Google Scholar] [CrossRef] [PubMed]
- Hessel, C.M.; Reid, D.; Panthani, M.G.; Rasch, M.R.; Goodfellow, B.W.; Wei, J.; Fujii, H.; Akhavan, V.; Korgel, B.A. Synthesis of Ligand-Stabilized Silicon Nanocrystals with Size-Dependent Photoluminescence Spanning Visible to Near-Infrared Wavelengths. Chem. Mater. 2012, 24, 393–401. [Google Scholar] [CrossRef]
- Shirahata, N.; Nakamura, J.; Inoue, J.-I.; Ghosh, B.; Nemoto, K.; Nemoto, Y.; Takeguchi, M.; Masuda, Y.; Tanaka, M.; Ozin, G.A. Emerging Atomic Energy Levels in Zero-Dimensional Silicon Quantum Dots. Nano Lett. 2020, 20, 1491–1498. [Google Scholar] [CrossRef]
- Sugimoto, H.; Fujii, M.; Imakita, K.; Hayashi, S.; Akamatsu, K. Codoping n- and p-Type Impurities in Colloidal Silicon Nanocrystals: Controlling Luminescence Energy from below Bulk Band Gap to Visible Range. J. Phys. Chem. C 2013, 117, 11850–11857. [Google Scholar] [CrossRef]
- Kortshagen, U.R.; Sankaran, R.M.; Pereira, R.N.; Girshick, S.L.; Wu, J.J.; Aydil, E.S. Nonthermal Plasma Synthesis of Nanocrystals: Fundamental Principles, Materials, and Applications. Chem. Rev. 2016, 116, 11061–11127. [Google Scholar] [CrossRef]
- Sangghaleh, F.; Sychugov, I.; Yang, Z.; Veinot, J.G.C.; Linnros, J. Near-Unity Internal Quantum Efficiency of Luminescent Silicon Nanocrystals with Ligand Passivation. ACS Nano 2015, 9, 7097–7104. [Google Scholar] [CrossRef]
- Mastronardi, M.L.; Maier-Flaig, F.; Faulkner, D.; Henderson, E.J.; Kübel, C.; Lemmer, U.; Ozin, G.A. Size-Dependent Absolute Quantum Yields for Size-Separated Colloidally-Stable Silicon Nanocrystals. Nano Lett. 2012, 12, 337–342. [Google Scholar] [CrossRef]
- Botas, A.M.P.; Anthony, R.J.; Wu, J.; Rowe, D.J.; Silva, N.J.O.; Kortshagen, U.; Pereira, R.N.; Ferreira, R.A.S. Influence of the surface termination on the light emission of crystalline silicon nanoparticles. Nanotechnology 2016, 27, 325703. [Google Scholar] [CrossRef]
- Ghosh, B.; Takeguchi, M.; Nakamura, J.; Nemoto, Y.; Hamaoka, T.; Chandra, S.; Shirahata, N. Origin of the Photoluminescence Quantum Yields Enhanced by Alkane-Termination of Freestanding Silicon Nanocrystals: Temperature-Dependence of Optical Properties. Sci. Rep. 2016, 6, 36951. [Google Scholar] [CrossRef]
- Kůsová, K.; Hapala, P.; Valenta, J.; Jelínek, P.; Cibulka, O.; Ondič, L.; Pelant, I. Direct Bandgap Silicon: Tensile-Strained Silicon Nanocrystals. Adv. Mater. Interfaces 2014, 1, 1300042. [Google Scholar] [CrossRef]
- Dohnalová, K.; Poddubny, A.N.; Prokofiev, A.A.; De Boer, W.D.; Umesh, C.P.; Paulusse, J.M.; Zuilhof, H.; Gregorkiewicz, T. Surface brightens up Si quantum dots: Direct bandgap-like size-tunable emission. Light Sci. Appl. 2013, 2, e47. [Google Scholar] [CrossRef]
- Ghosh, B.; Masuda, Y.; Wakayama, Y.; Imanaka, Y.; Inoue, J.-I.; Hashi, K.; Deguchi, K.; Yamada, H.; Sakka, Y.; Ohki, S.; et al. Hybrid White Light Emitting Diode Based on Silicon Nanocrystals. Adv. Funct. Mater. 2014, 24, 7151–7160. [Google Scholar] [CrossRef]
- You, H.R.; Park, J.Y.; Lee, D.H.; Kim, Y.; Choi, J. Recent Research Progress in Surface Ligand Exchange of PbS Quantum Dots for Solar Cell Application. Appl. Sci. 2020, 10, 975. [Google Scholar] [CrossRef]
- Chandra, S.; Masuda, Y.; Shirahata, N.; Winnik, F.M. Transition-Metal-Doped NIR-Emitting Silicon Nanocrystals. Angew. Chem. Int. Ed. 2017, 56, 6157–6160. [Google Scholar] [CrossRef] [PubMed]
- Supran, G.J.S. Enhancing Quantum-Dot Luminescence in Visible and Infrared Light Emitting Devices. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2016. [Google Scholar]
- Dasog, M.; Kehrle, J.; Rieger, B.; Veinot, J.G.C. Silicon Nanocrystals and Silicon-Polymer Hybrids: Synthesis, Surface Engineering, and Applications. Angew. Chem. Int. Ed. 2016, 55, 2322–2339. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, J.; Chen, H.; Samanta, A.; Linnros, J.; Yang, Z.; Sychugov, I. Low-Cost Synthesis of Silicon Quantum Dots with Near-Unity Internal Quantum Efficiency. J. Phys. Chem. Lett. 2021, 12, 8909–8916. [Google Scholar] [CrossRef] [PubMed]
- Shirahata, N. Effect of the surface coverage of an alkyl carboxylic acid monolayer on waterborne and cellular uptake behaviors for silicon quantum dots. Sci. Rep. 2022, 12, 17211. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Qian, C.; He, L.; Ghuman, K.K.; Wong, A.P.Y.; Jia, J.; Jelle, A.A.; O’Brien, P.G.; Reyes, L.M.; Wood, T.E.; et al. Heterogeneous reduction of carbon dioxide by hydride-terminated silicon nanocrystals. Nat. Commun. 2016, 7, 12553. [Google Scholar] [CrossRef]
- Gu, W.; Liu, X.; Pi, X.; Dai, X.; Zhao, S.; Yao, L.; Li, D.; Jin, Y.; Xu, M.; Yang, D.; et al. Silicon-Quantum-Dot Light-Emitting Diodes With Interlayer-Enhanced Hole Transport. IEEE Photonics J. 2017, 9, 4500610. [Google Scholar] [CrossRef]
- Wang, D.-C.; Chen, J.-R.; Zhu, J.; Lu, C.-T.; Lu, M. On the spectral difference between electroluminescence and photoluminescence of Si nanocrystals: A mechanism study of electroluminescence. J. NanoPart. Res. 2013, 15, 2063. [Google Scholar] [CrossRef]
- Priolo, F.; Gregorkiewicz, T.; Galli, M.; Krauss, T.F. Silicon nanostructures for photonics and photovoltaics. Nat. Nanotechnol. 2014, 9, 19–32. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, H.; Watanabe, J.; Nemoto, K.; Sun, H.-T.; Shirahata, N. Postproduction Approach to Enhance the External Quantum Efficiency for Red Light-Emitting Diodes Based on Silicon Nanocrystals. Nanomaterials 2022, 12, 4314. https://doi.org/10.3390/nano12234314
Yamada H, Watanabe J, Nemoto K, Sun H-T, Shirahata N. Postproduction Approach to Enhance the External Quantum Efficiency for Red Light-Emitting Diodes Based on Silicon Nanocrystals. Nanomaterials. 2022; 12(23):4314. https://doi.org/10.3390/nano12234314
Chicago/Turabian StyleYamada, Hiroyuki, Junpei Watanabe, Kazuhiro Nemoto, Hong-Tao Sun, and Naoto Shirahata. 2022. "Postproduction Approach to Enhance the External Quantum Efficiency for Red Light-Emitting Diodes Based on Silicon Nanocrystals" Nanomaterials 12, no. 23: 4314. https://doi.org/10.3390/nano12234314
APA StyleYamada, H., Watanabe, J., Nemoto, K., Sun, H.-T., & Shirahata, N. (2022). Postproduction Approach to Enhance the External Quantum Efficiency for Red Light-Emitting Diodes Based on Silicon Nanocrystals. Nanomaterials, 12(23), 4314. https://doi.org/10.3390/nano12234314






