Laboratory Investigation of Nanofluid-Assisted Polymer Flooding in Carbonate Reservoirs
Abstract
1. Introduction
2. Materials and Methods
2.1. Core Samples
2.2. Crude Oil
2.3. Brines
2.4. Polymer
2.5. Silica Nanoparticles
2.6. Fluid Preparation
2.7. Zeta Potential Measurements
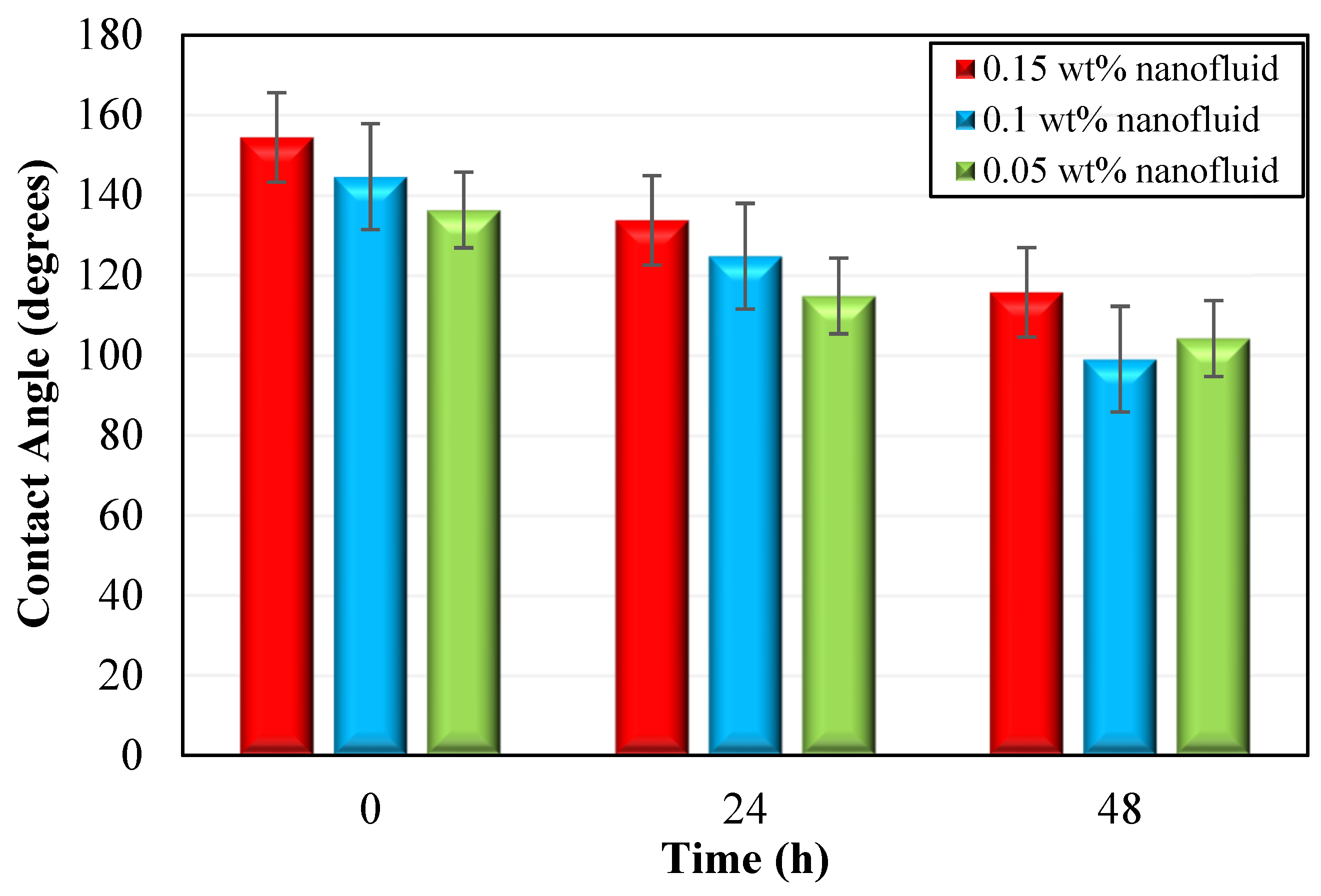
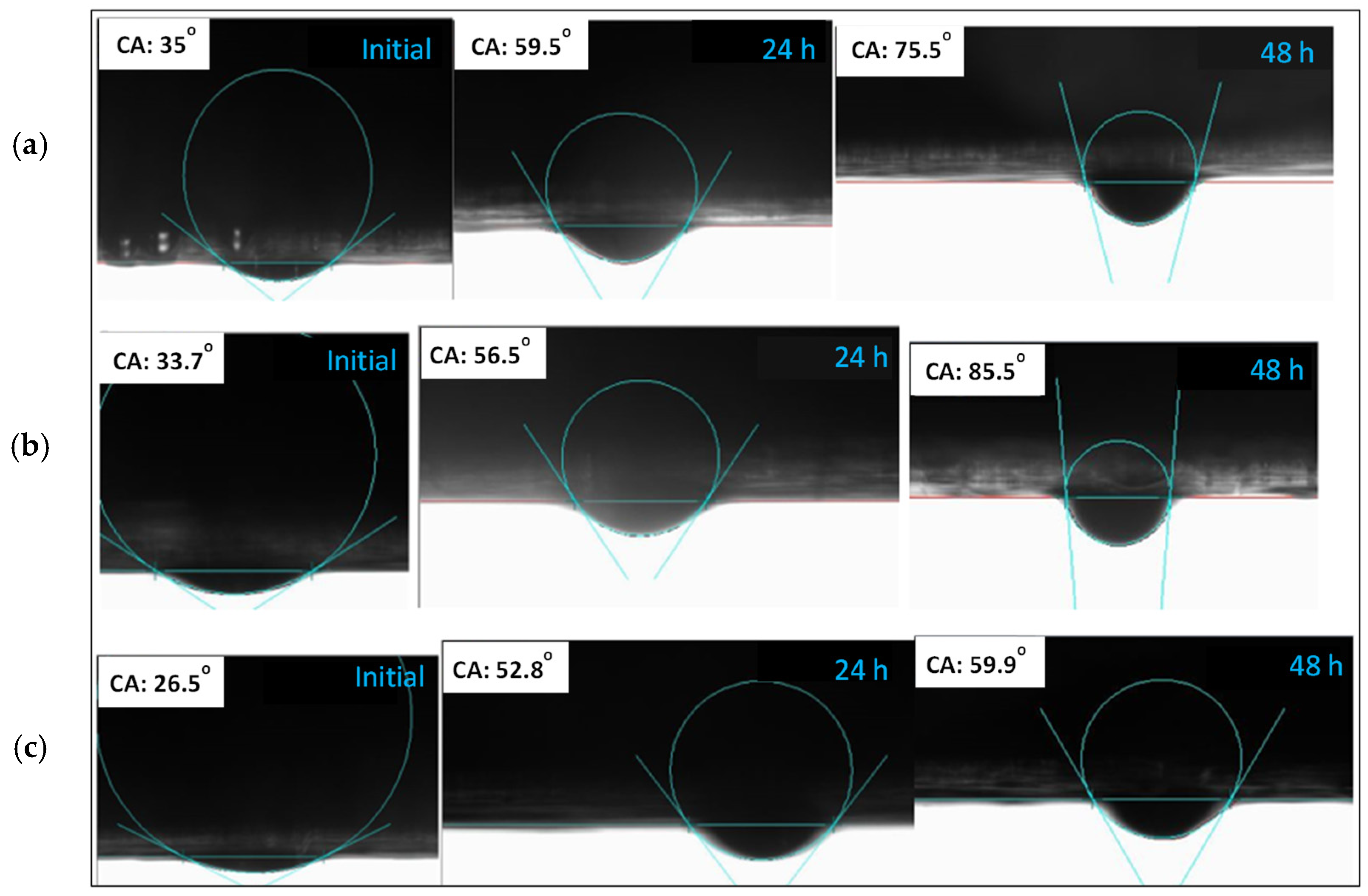
2.8. Contact Angle Measurements
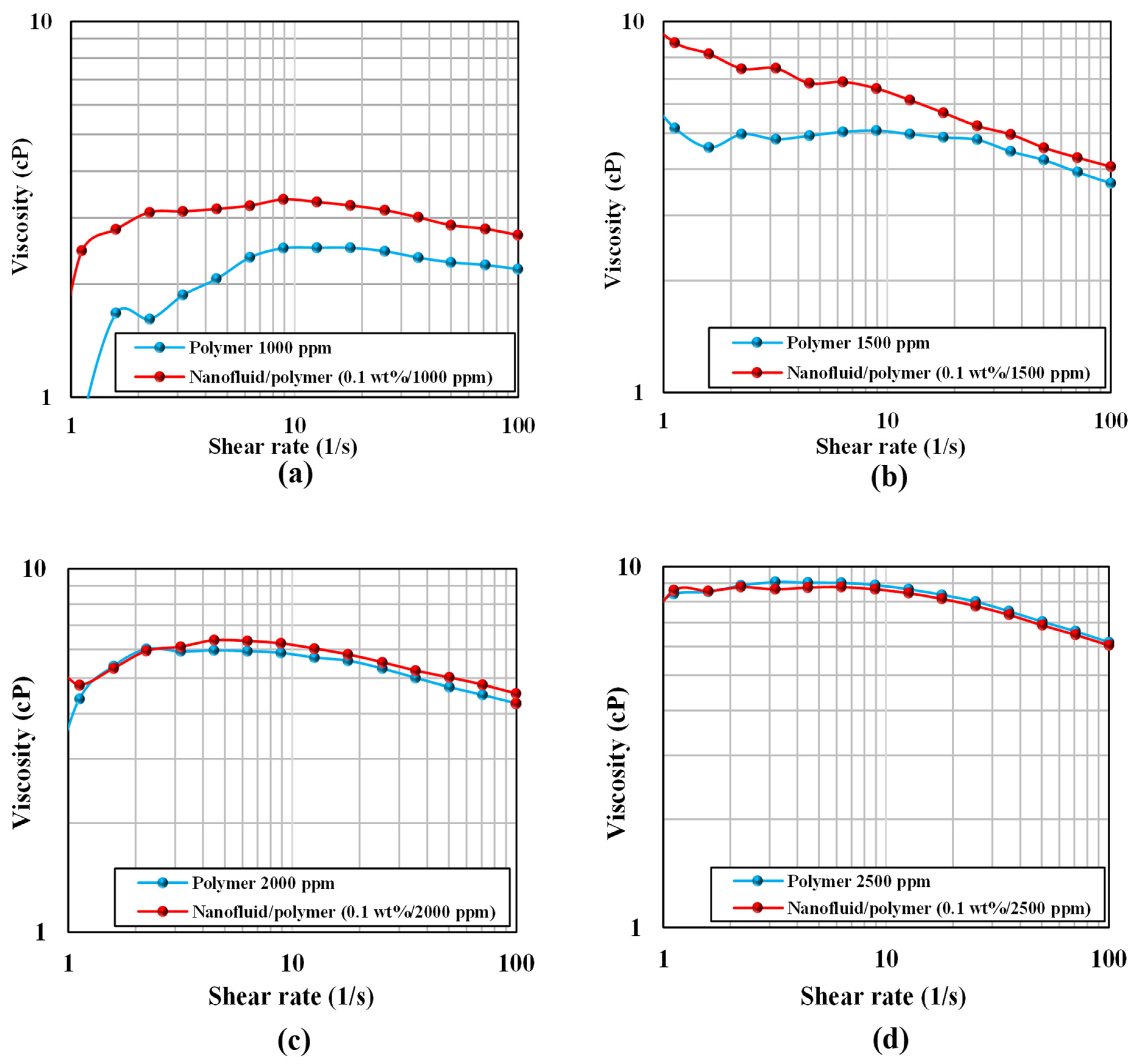
2.9. Rheology Measurements
2.10. Coreflooding Experiments
3. Results and Discussion
3.1. Zeta Potential Results
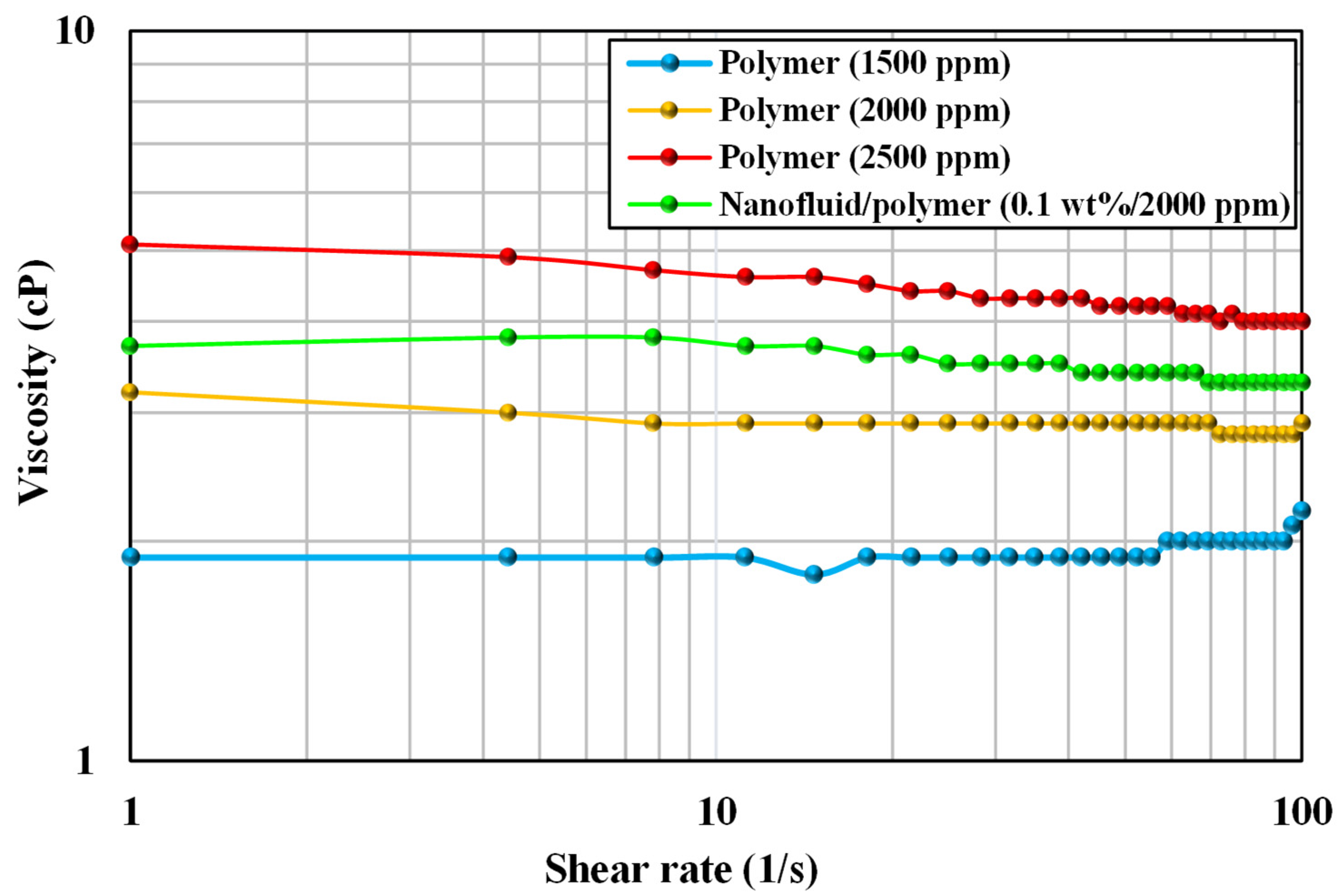
3.2. Optimization of Nanofluid and Polymer Concentration
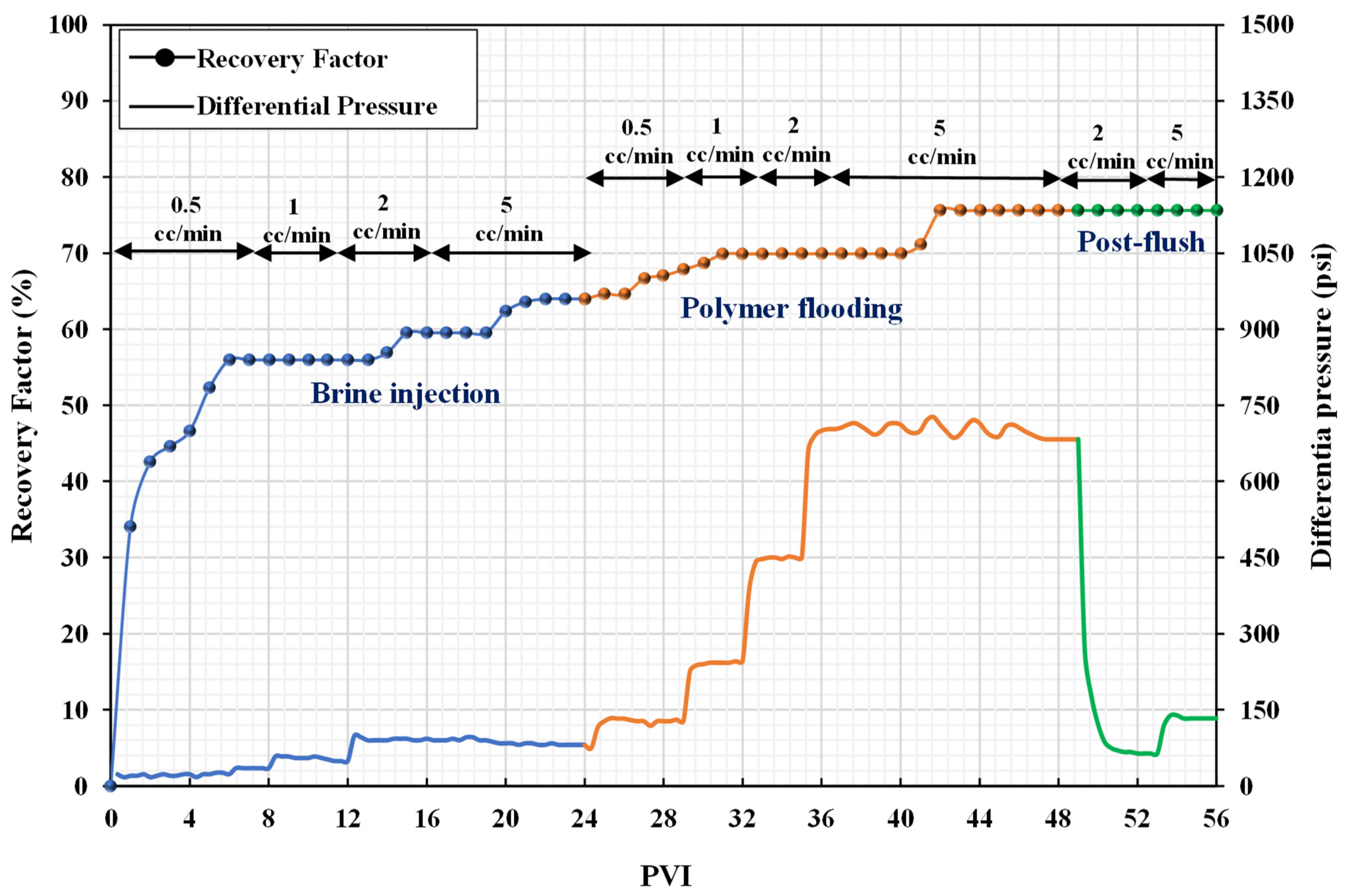
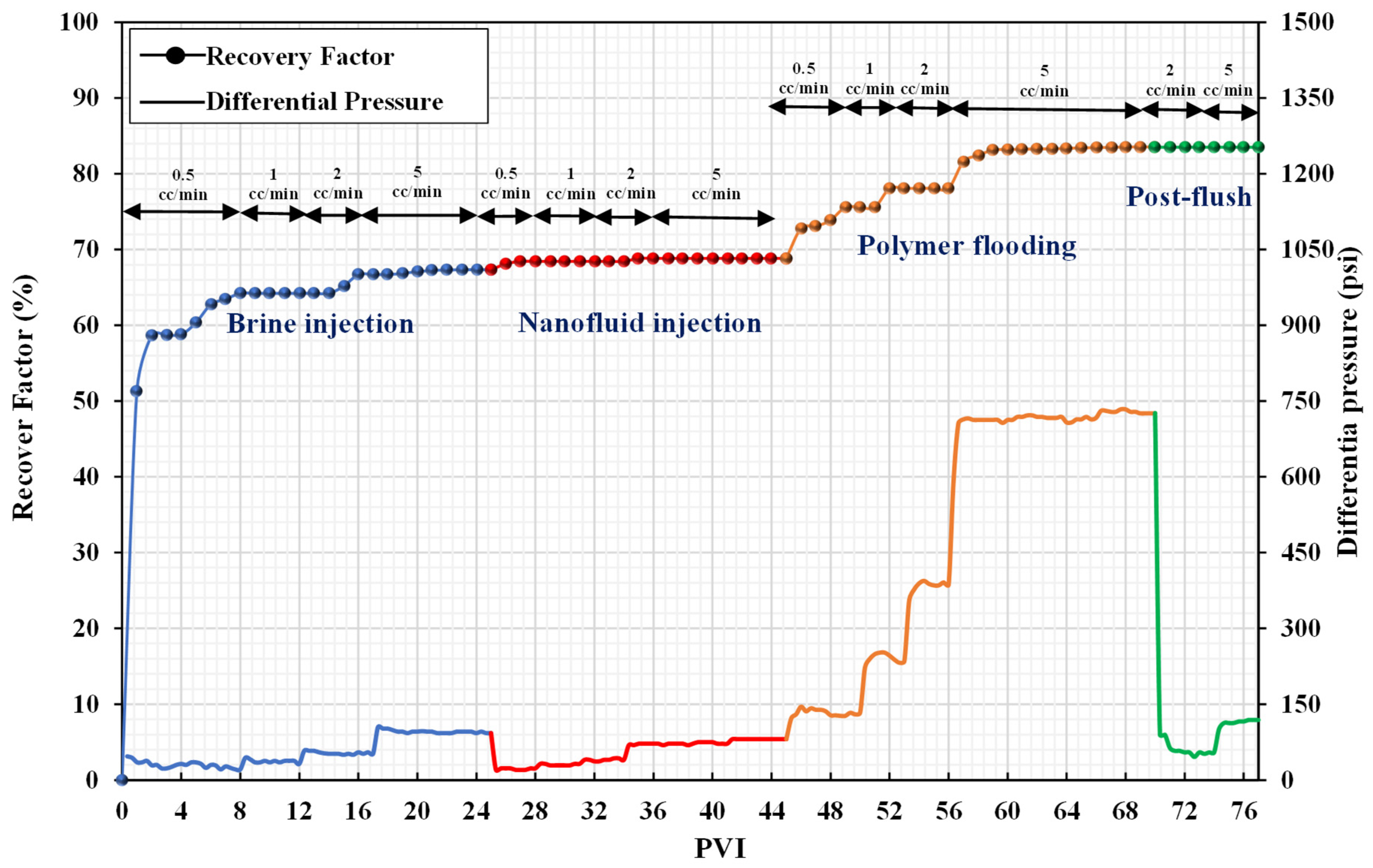
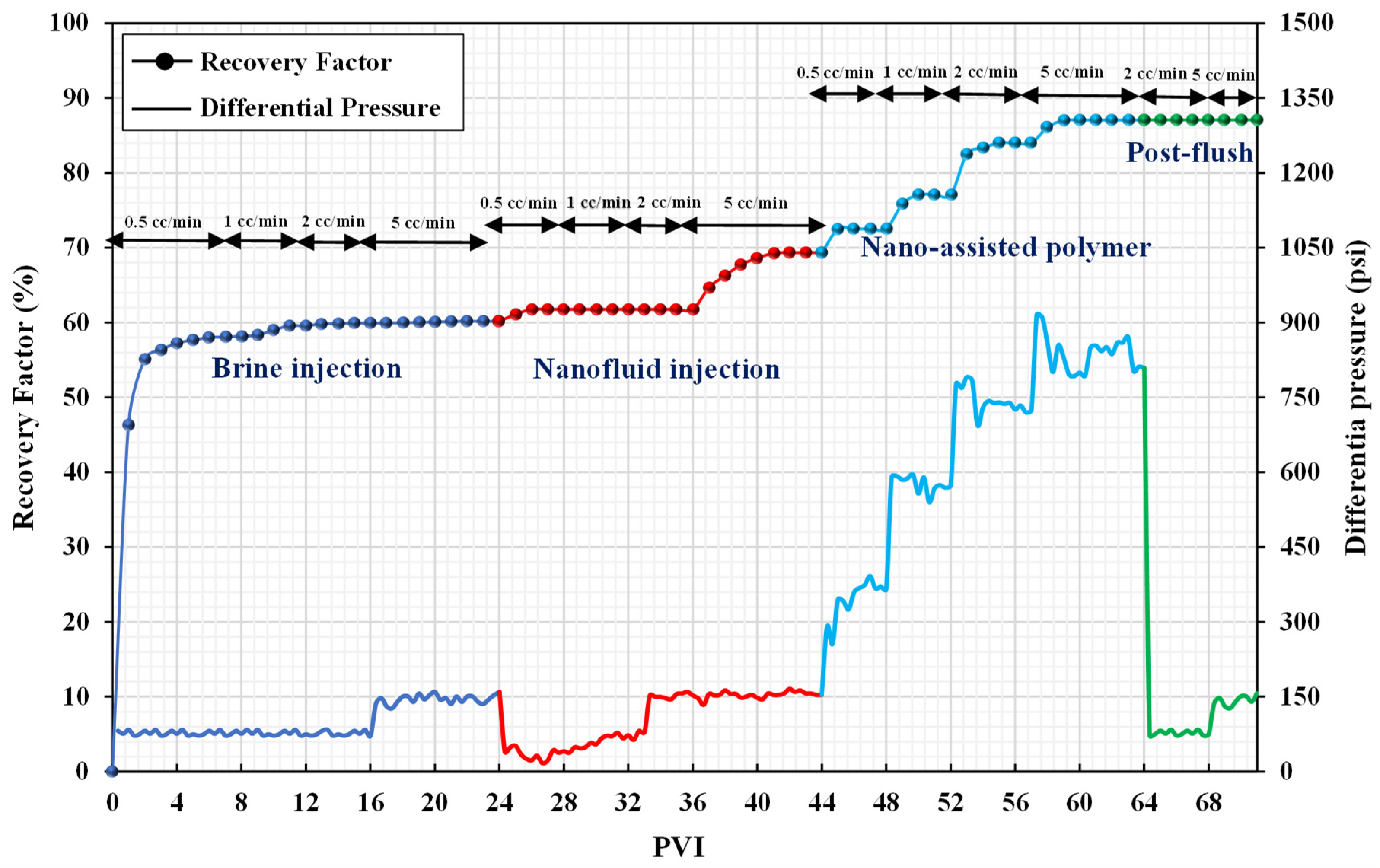
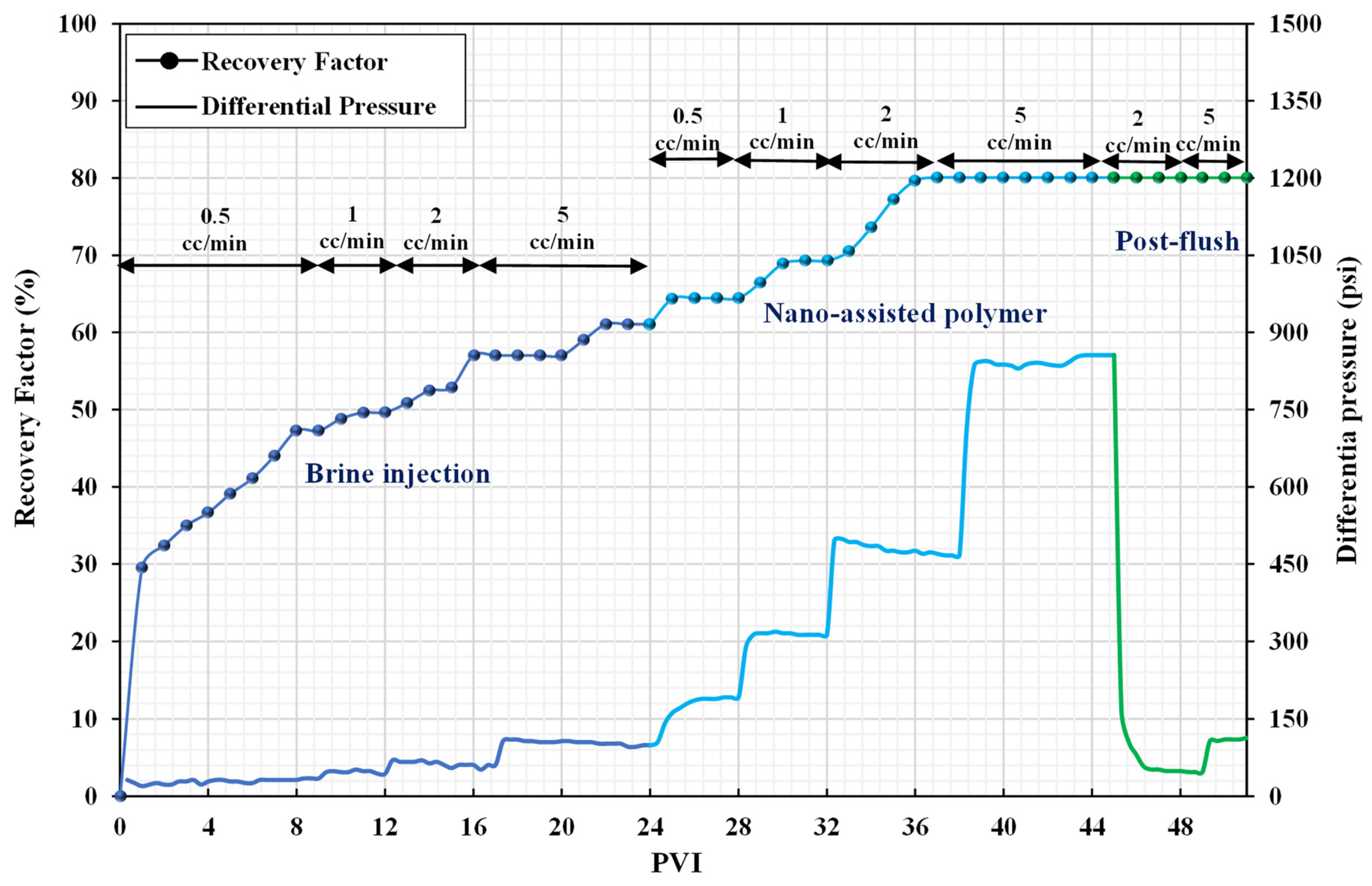
3.3. Core Flooding Experiments
3.4. Selection of the Optimum Oil Displacement Scenario
4. Conclusions
- Rock wettability can be changed using silica nanofluids. The wettability of carbonate rock was most effectively changed from an oil-wet to a water-wet with a deviation of 45.6° state at 0.1 wt% nanoparticles, making it the optimum concentration for oil displacement.
- Rheology experiments proved that adding silica nanoparticles to a polymer solution increased the fluid viscosity by 27.6% at 80 °C.
- Contact angle measurements and rheological experiment results showed that a nanofluid-assisted polymer solution containing 0.1 wt% silica nanoparticles and 2000 ppm polymer was the best selection.
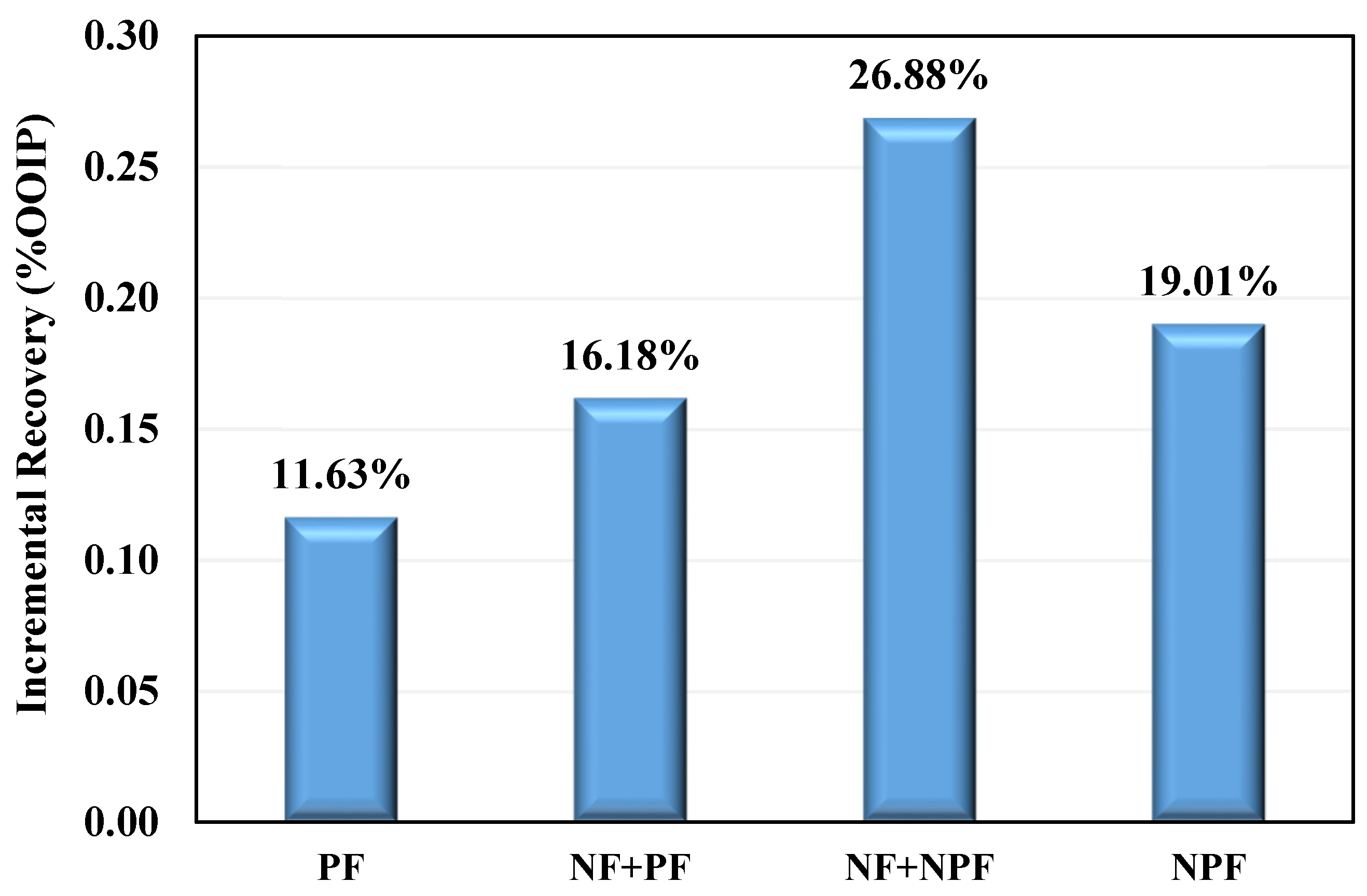
- As an effective EOR technique, injecting silica nanofluid followed by a nano-assisted polymer resulted in a maximum incremental oil recovery of 26.88% in the third scenario.
- Corresponding RF and RRF values of 6.83 and 1.04 the addition of silica nanoparticles to the polymer prevents polymer retention, therefore, injection fluid viscosity increased in porous media with no permeability reduction; Thus, the combination of silica nanoparticles and the polymer is more effective than pure polymer solution due to synergy of different mechanisms.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alagorni, A.H.; Yaacob, Z.B.; Nour, A.H. An Overview of Oil Production Stages: Enhanced Oil Recovery Techniques and Nitrogen Injection. Int. J. Environ. Sci. Dev. 2015, 6, 693. [Google Scholar] [CrossRef]
- Al-Mutairi, S.M.; Kokal, S.L. EOR Potential in the Middle East: Current and Future Trends. In Proceedings of the SPE EUROPEC/EAGE Annual Conference and Exhibition, Vienna, Austria, 23–26 May 2011; OnePetro: Richardson, TX, USA, 2011. [Google Scholar]
- Sun, X.; Zhang, Y.; Chen, G.; Gai, Z. Application of Nanoparticles in Enhanced Oil Recovery: A Critical Review of Recent Progress. Energies 2017, 10, 345. [Google Scholar] [CrossRef]
- Willhite, G.P.; Dominguez, J.G. Mechanisms of Polymer Retention in Porous Media. In Improved Oil Recovery by Surfactant and Polymer Flooding; Elsevier: Amsterdam, The Netherlands, 1977; pp. 511–554. [Google Scholar]
- Samanta, A.; Ojha, K.; Sarkar, A.; Mandal, A. Mobility Control and Enhanced Oil Recovery Using Partially Hydrolysed Polyacrylamide (PHPA). Int. J. Oil Gas Coal Technol. 2013, 6, 245–258. [Google Scholar] [CrossRef]
- Salih, T.A.; Sahi, S.H.; Hameed, O.K. Rheological Evaluation of Polymer (Sav 10) for Polymer Flooding Applications. Iraqi J. Chem. Pet. Eng. 2016, 17, 37–46. [Google Scholar]
- Wang, J.; Dong, M. Optimum Effective Viscosity of Polymer Solution for Improving Heavy Oil Recovery. J. Pet. Sci. Eng. 2009, 67, 155–158. [Google Scholar] [CrossRef]
- Shakeel, M.; Pourafshary, P.; Rehan Hashmet, M. Hybrid Engineered Water–Polymer Flooding in Carbonates: A Review of Mechanisms and Case Studies. Appl. Sci. 2020, 10, 6087. [Google Scholar] [CrossRef]
- Shakeel, M.; Samanova, A.; Pourafshary, P.; Hashmet, M.R. Experimental Analysis of Oil Displacement by Hybrid Engineered Water/Chemical EOR Approach in Carbonates. J. Pet. Sci. Eng. 2021, 207, 109297. [Google Scholar] [CrossRef]
- Li, G.; Zhai, L.; Xu, G.; Shen, Q.; Mao, H.; Pei, M. Current Tertiary Oil Recovery in China. J. Dispers. Sci. Technol. 2000, 21, 367–408. [Google Scholar] [CrossRef]
- Kong, X.; Ohadi, M.M. Applications of Micro and Nano Technologies in the Oil and Gas Industry—An Overview of the Recent Progress. In Proceedings of the Abu Dhabi International Petroleum Exhibition and Conference, Abu Dhabi, United Arab Emirates, 1–4 November 2010; OnePetro: Richardson, TX, USA, 2010. [Google Scholar]
- Muneer, R.; Rehan Hashmet, M.; Pourafshary, P. Fine Migration Control in Sandstones: Surface Force Analysis and Application of DLVO Theory. ACS Omega 2020, 5, 31624–31639. [Google Scholar] [CrossRef]
- Muneer, R.; Hashmet, M.R.; Pourafshary, P. DLVO Modeling to Predict Critical Salt Concentration to Initiate Fines Migration Pre-and Post-Nanofluid Treatment in Sandstones. SPE J. 2022, 27, 1915–1929. [Google Scholar] [CrossRef]
- Muneer, R.; Hashmet, M.R.; Pourafshary, P. Application of DLVO Modeling to Study the Effect of Silica Nanofluid to Reduce Critical Salt Concentration in Sandstones. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1186, 12001. [Google Scholar] [CrossRef]
- Muneer, R.; Pourafshary, P.; Hashmet, M.R. Application of DLVO Modeling to Predict Critical PH for Fines Migration Pre-and Post-SiO2 and MgO Nanofluid Treatments in Sandstones. J. Fluid Flow Heat Mass Transf. 2022, 9, 106. [Google Scholar] [CrossRef]
- Rueda, E.; Akarri, S.; Torsæter, O.; Moreno, R.B.Z.L. Experimental Investigation of the Effect of Adding Nanoparticles to Polymer Flooding in Water-Wet Micromodels. Nanomaterials 2020, 10, 1489. [Google Scholar] [CrossRef] [PubMed]
- Ogolo, N.A.; Olafuyi, O.A.; Onyekonwu, M.O. Enhanced Oil Recovery Using Nanoparticles. In Proceedings of the SPE Saudi Arabia Section Technical Symposium and Exhibition, Al Khobar, Saudi Arabia, 8–11 April 2012; OnePetro: Richardson, TX, USA, 2012. [Google Scholar]
- Buitrago-Rincon, D.L.; Sadtler, V.; Mercado, R.A.; Roques-Carmes, T.; Benyahia, L.; Durand, A.; Ferji, K.; Marchal, P.; Pedraza-Avella, J.A.; Lemaitre, C. Effect of Silica Nanoparticles in Xanthan Gum Solutions: Evolution of Viscosity over Time. Nanomaterials 2022, 12, 1906. [Google Scholar] [CrossRef] [PubMed]
- Aminian, A.; ZareNezhad, B. Wettability Alteration in Carbonate and Sandstone Rocks Due to Low Salinity Surfactant Flooding. J. Mol. Liq. 2019, 275, 265–280. [Google Scholar] [CrossRef]
- Sheshdeh, M.J. A Review Study of Wettability Alteration Methods with Regard to Nano-Materials Application. In Proceedings of the SPE Bergen One Day Seminar, Bergen, Norway, 5 April 2015; OnePetro: Richardson, TX, USA, 2015. [Google Scholar]
- Eltoum, H.; Yang, Y.-L.; Hou, J.-R. The Effect of Nanoparticles on Reservoir Wettability Alteration: A Critical Review. Pet. Sci. 2021, 18, 136–153. [Google Scholar] [CrossRef]
- Carpenter, C. A Study of Wettability-Alteration Methods with Nanomaterials Application. J. Pet. Technol. 2015, 67, 74–75. [Google Scholar] [CrossRef]
- Maghzi, A.; Mohammadi, S.; Ghazanfari, M.H.; Kharrat, R.; Masihi, M. Monitoring Wettability Alteration by Silica Nanoparticles during Water Flooding to Heavy Oils in Five-Spot Systems: A Pore-Level Investigation. Exp. Therm. Fluid Sci. 2012, 40, 168–176. [Google Scholar] [CrossRef]
- Lim, S.; Horiuchi, H.; Nikolov, A.D.; Wasan, D. Nanofluids Alter the Surface Wettability of Solids. Langmuir 2015, 31, 5827–5835. [Google Scholar] [CrossRef]
- Wang, J.; Cao, X.; Fang, L.; You, X.; Wong, K.; Cao, S.; Xiao, C.; Cai, S.; Huang, Y.; Zhang, X. MoS2 Nanoflower Supported Pt Nanoparticle as an Efficient Electrocatalyst for Ethanol Oxidation Reaction. Int. J. Hydrogen Energy 2019, 44, 16411–16423. [Google Scholar] [CrossRef]
- Issakhov, M.; Shakeel, M.; Pourafshary, P.; Aidarova, S.; Sharipova, A. Hybrid Surfactant-Nanoparticles Assisted CO2 Foam Flooding for Improved Foam Stability: A Review of Principles and Applications. Pet. Res. 2021, 7, 186–203. [Google Scholar] [CrossRef]
- Du, C.; Chang, Z.; Yu, H.; Zhu, Y.; Ma, Y.; Ma, G.; Yan, Y.; Wang, C.; Wang, W.; Cheng, Y. Magnetic Quantum Dots-Stabilized Foam Fluid for Enhanced Oil Recovery. Chem. Eng. J. 2022, 450, 138334. [Google Scholar] [CrossRef]
- Bila, A.; Stensen, J.Å.; Torsæter, O. Experimental Investigation of Polymer-Coated Silica Nanoparticles for Enhanced Oil Recovery. Nanomaterials 2019, 9, 822. [Google Scholar] [CrossRef] [PubMed]
- Gbadamosi, A.O.; Junin, R.; Manan, M.A.; Yekeen, N.; Agi, A.; Oseh, J.O. Recent Advances and Prospects in Polymeric Nanofluids Application for Enhanced Oil Recovery. J. Ind. Eng. Chem. 2018, 66, 1–19. [Google Scholar] [CrossRef]
- ShamsiJazeyi, H.; Miller, C.A.; Wong, M.S.; Tour, J.M.; Verduzco, R. Polymer-coated Nanoparticles for Enhanced Oil Recovery. J. Appl. Polym. Sci. 2014, 131, 40576. [Google Scholar] [CrossRef]
- Rodriguez Pin, E.; Roberts, M.; Yu, H.; Huh, C.; Bryant, S.L. Enhanced Migration of Surface-Treated Nanoparticles in Sedimentary Rocks. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 4–7 October 2009; OnePetro: Richardson, TX, USA, 2009. [Google Scholar]
- Zhang, T.; Murphy, M.J.; Yu, H.; Bagaria, H.G.; Yoon, K.Y.; Neilson, B.M.; Bielawski, C.W.; Johnston, K.P.; Huh, C.; Bryant, S.L. Investigation of Nanoparticle Adsorption during Transport in Porous Media. SPE J. 2015, 20, 667–677. [Google Scholar] [CrossRef]
- Ponnapati, R.; Karazincir, O.; Dao, E.; Ng, R.; Mohanty, K.K.; Krishnamoorti, R. Polymer-Functionalized Nanoparticles for Improving Waterflood Sweep Efficiency: Characterization and Transport Properties. Ind. Eng. Chem. Res. 2011, 50, 13030–13036. [Google Scholar] [CrossRef]
- Behzadi, A.; Mohammadi, A. Environmentally Responsive Surface-Modified Silica Nanoparticles for Enhanced Oil Recovery. J. Nanopart. Res. 2016, 18, 266. [Google Scholar] [CrossRef]
- Saha, R.; Uppaluri, R.V.S.; Tiwari, P. Silica Nanoparticle Assisted Polymer Flooding of Heavy Crude Oil: Emulsification, Rheology, and Wettability Alteration Characteristics. Ind. Eng. Chem. Res. 2018, 57, 6364–6376. [Google Scholar] [CrossRef]
- Maghzi, A.; Mohebbi, A.; Kharrat, R.; Ghazanfari, M.H. An Experimental Investigation of Silica Nanoparticles Effect on the Rheological Behavior of Polyacrylamide Solution to Enhance Heavy Oil Recovery. Pet. Sci. Technol. 2013, 31, 500–508. [Google Scholar] [CrossRef]
- Luo, J.H.; Liu, Y.Z.; Zhu, P. Polymer Solution Properties and Displacement Mechanisms. In Enhanced Oil Recovery—Polymer Flooding; Shen, P.-P., Liu, Y.-Z., Liu, H.-R., Eds.; Gulf Professional Publishing: Oxford, UK, 2006; pp. 1–72. [Google Scholar]
- Otsubo, Y.; Umeya, K. Rheological Properties of Silica Suspensions in Polyacrylamide Solutions. J. Rheol. 1984, 28, 95–108. [Google Scholar] [CrossRef]
- Kamibayashi, M.; Ogura, H.; Otsubo, Y. Viscosity Behavior of Silica Suspensions Flocculated by Associating Polymers. J. Colloid Interface Sci. 2005, 290, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Zaman, A.A.; Delorme, N. Effect of Polymer Bridging on Rheological Properties of Dispersions of Charged Silica Particles in the Presence of Low-Molecular-Weight Physically Adsorbed Poly (Ethylene Oxide). Rheol. Acta 2002, 41, 408–417. [Google Scholar] [CrossRef]
- Aalaie, J. Rheological Behavior of Polyacrylamide/Laponite Nanoparticle Suspensions in Electrolyte Media. J. Macromol. Sci. Part B 2012, 51, 1139–1147. [Google Scholar] [CrossRef]
- Maurya, N.K.; Mandal, A. Studies on Behavior of Suspension of Silica Nanoparticle in Aqueous Polyacrylamide Solution for Application in Enhanced Oil Recovery. Pet. Sci. Technol. 2016, 34, 429–436. [Google Scholar] [CrossRef]
- Zhangaliyev, M.M.; Hashmet, M.R.; Pourafshary, P. Laboratory Investigation of Hybrid Nano-Assisted-Polymer Method for EOR Applications in Carbonate Reservoirs. In Proceedings of the Offshore Technology Conference Asia, Kuala Lumpur, Malaysia, 22–25 March 2022; OnePetro: Richardson, TX, USA, 2022. [Google Scholar]
- Hashmet, M.R.; Qaiser, Y.; Mathew, E.S.; AlAmeri, W.; AlSumaiti, A.M. Injection of Polymer for Improved Sweep Efficiency in High Temperature High Salinity Carbonate Reservoirs: Linear X-Ray Aided Flood Front Monitoring. In Proceedings of the SPE Kingdom of Saudi Arabia Annual Technical Symposium and Exhibition, Dammam, Saudi Arabia, 24–27 April 2017; OnePetro: Richardson, TX, USA, 2017. [Google Scholar]
- Zeyghami, M.; Kharrat, R.; Ghazanfari, M.H. Investigation of the Applicability of Nano Silica Particles as a Thickening Additive for Polymer Solutions Applied in EOR Processes. Energy Sources Part A Recover. Util. Environ. Eff. 2014, 36, 1315–1324. [Google Scholar] [CrossRef]
- Teklu, T.W.; Alameri, W.; Kazemi, H.; Graves, R.M. Contact Angle Measurements on Conventional and Unconventional Reservoir Cores. In Proceedings of the Unconventional Resources Technology Conference, San Antonio, TX, USA, 20–22 July 2015; OnePetro: Richardson, TX, USA, 2015; pp. 2297–2311. [Google Scholar]
- Salaudeen, I.; Hashmet, M.R.; Pourafshary, P. Catalytic Effects of Temperature and Silicon Dioxide Nanoparticles on the Acceleration of Production from Carbonate Rocks. Nanomaterials 2021, 11, 1642. [Google Scholar] [CrossRef]
- Roustaei, A.; Saffarzadeh, S.; Mohammadi, M. An Evaluation of Modified Silica Nanoparticles’ Efficiency in Enhancing Oil Recovery of Light and Intermediate Oil Reservoirs. Egypt. J. Pet. 2013, 22, 427–433. [Google Scholar] [CrossRef]

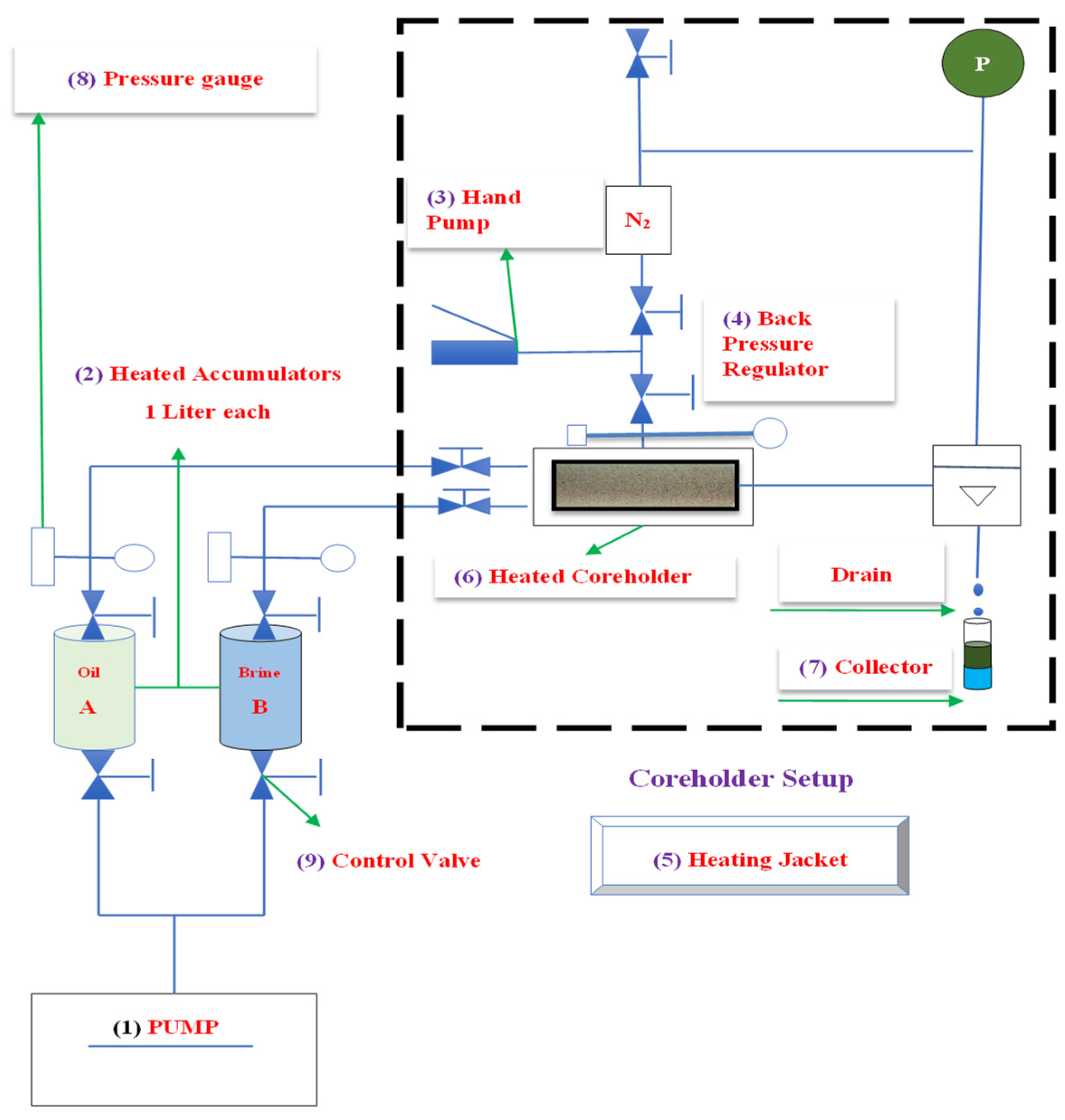
| Core No. | Length (cm) | Dry Weight (g) | Vp (mL) | Vb (mL) | ϕ (%) |
|---|---|---|---|---|---|
| 1 | 7.95 | 200.28 | 17.09 | 90.68 | 18.85 |
| 2 | 7.27 | 182.04 | 16.13 | 82.93 | 19.45 |
| 3 | 7.13 | 180.09 | 15.09 | 81.33 | 18.56 |
| 4 | 7.15 | 178.44 | 15.36 | 81.56 | 18.84 |
| Core No. | Soi (%) | Swi (%) | kabs (mD) | keff-oil (mD) |
|---|---|---|---|---|
| 1 | 72.3 | 27.7 | 30.27 | 14.92 |
| 2 | 79.9 | 20.1 | 39.23 | 23.92 |
| 3 | 79.5 | 20.5 | 43.43 | 24.06 |
| 4 | 80.25 | 19.75 | 25.03 | 15.25 |
| Temperature (°C) | Dynamic Viscosity (cp) | Density (g/cm3) |
|---|---|---|
| 25 | 5.66 | 0.84 |
| 80 | 2.89 | 0.81 |
| Ions | Formation Water (ppm) | Injection Brine (ppm) |
|---|---|---|
| Na+ and K+ | 81,600 | 13,600 |
| Ca2+ | 1470 | 1590 |
| Mg2+ | 9540 | 245 |
| Cl− | 90,370 | 15,062 |
| TDS | 182,980 | 40,000 |
| Nanoparticle | Size (nm) | Specific Surface Area (m2/g) | Morphology | Density (g/cm3) | Purity |
|---|---|---|---|---|---|
| Silicon dioxide (SiO2) | 10–20 | 640 | Spherical | 2.4 | 99.50% |
| Coreflood No. | Injection Sequence |
|---|---|
| 1 | Brine → Polymer → Post-flush |
| 2 | Brine → Nanofluid → Polymer → Post-flush |
| 3 | Brine → Nanofluid → Nanofluid-polymer → Post-flush |
| 4 | Brine → Nanofluid-polymer → Post-flush |
| Silica Nanofluid (wt%) | Zeta Potential (no Salt), mV | Zeta Potential (with Salt), mV |
|---|---|---|
| 0.05 | −39.7 | −4.06 |
| 0.1 | −42.5 | −6.3 |
| 0.15 | −39.5 | −3.12 |
| Solution | Temperature | Shear Rate, 1/s | Polymer Concentration, ppm | Viscosity, cP |
|---|---|---|---|---|
| Polymer | 25 | 10 | 1000 | 2.5 |
| 1500 | 5 | |||
| 2000 | 5.75 | |||
| 2500 | 8.7 |
| Solution | Temperature, °C | Shear Rate, 1/s | SiO2 Concentration, wt% | Polymer Concentration, ppm | Viscosity, cP |
|---|---|---|---|---|---|
| Nano-assisted polymer | 25 | 10 | 0.1 | 1000 | 3.32 |
| 1500 | 6.3 | ||||
| 2000 | 6.15 | ||||
| 2500 | 8.63 |
| Solution | Temperature, °C | Shear Rate, 1/s | Polymer Concentration, ppm | Viscosity, cP |
|---|---|---|---|---|
| Polymer | 80 | 10 | 1500 | 1.9 |
| 2000 | 3.1 | |||
| 2500 | 4.6 |
| Sr. No. | Injection Fluid | Incremental Recovery (%) | Recovery Mechanism | Total Incremental Recovery (%) |
|---|---|---|---|---|
| coreflood-1 | polymer | 11.63 | mobility control + viscous forces | 11.63 |
| coreflood-2 | nanofluid | 1.47 | wettability alteration | 16.18 |
| polymer | 14.71 | mobility control + viscous forces | ||
| coreflood-3 | nanofluid | 9.15 | wettability alteration | 26.88 |
| nanofluid-assisted polymer | 17.7 | better performance of the polymer in the presence of nanofluid | ||
| coreflood-4 | nanofluid-assisted polymer | 19.01 | wettability alteration + better performance of the polymer in the presence of nanofluid | 19.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulasbek, K.; Hashmet, M.R.; Pourafshary, P.; Muneer, R. Laboratory Investigation of Nanofluid-Assisted Polymer Flooding in Carbonate Reservoirs. Nanomaterials 2022, 12, 4258. https://doi.org/10.3390/nano12234258
Ulasbek K, Hashmet MR, Pourafshary P, Muneer R. Laboratory Investigation of Nanofluid-Assisted Polymer Flooding in Carbonate Reservoirs. Nanomaterials. 2022; 12(23):4258. https://doi.org/10.3390/nano12234258
Chicago/Turabian StyleUlasbek, Kassymzhomart, Muhammad Rehan Hashmet, Peyman Pourafshary, and Rizwan Muneer. 2022. "Laboratory Investigation of Nanofluid-Assisted Polymer Flooding in Carbonate Reservoirs" Nanomaterials 12, no. 23: 4258. https://doi.org/10.3390/nano12234258
APA StyleUlasbek, K., Hashmet, M. R., Pourafshary, P., & Muneer, R. (2022). Laboratory Investigation of Nanofluid-Assisted Polymer Flooding in Carbonate Reservoirs. Nanomaterials, 12(23), 4258. https://doi.org/10.3390/nano12234258






