Cu-Ag Nanocomposite Pastes for Low Temperature Bonding and Flexible Interlayer-Interconnections
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Fabrication of Nanocomposite Pastes
2.2. Bonding Process
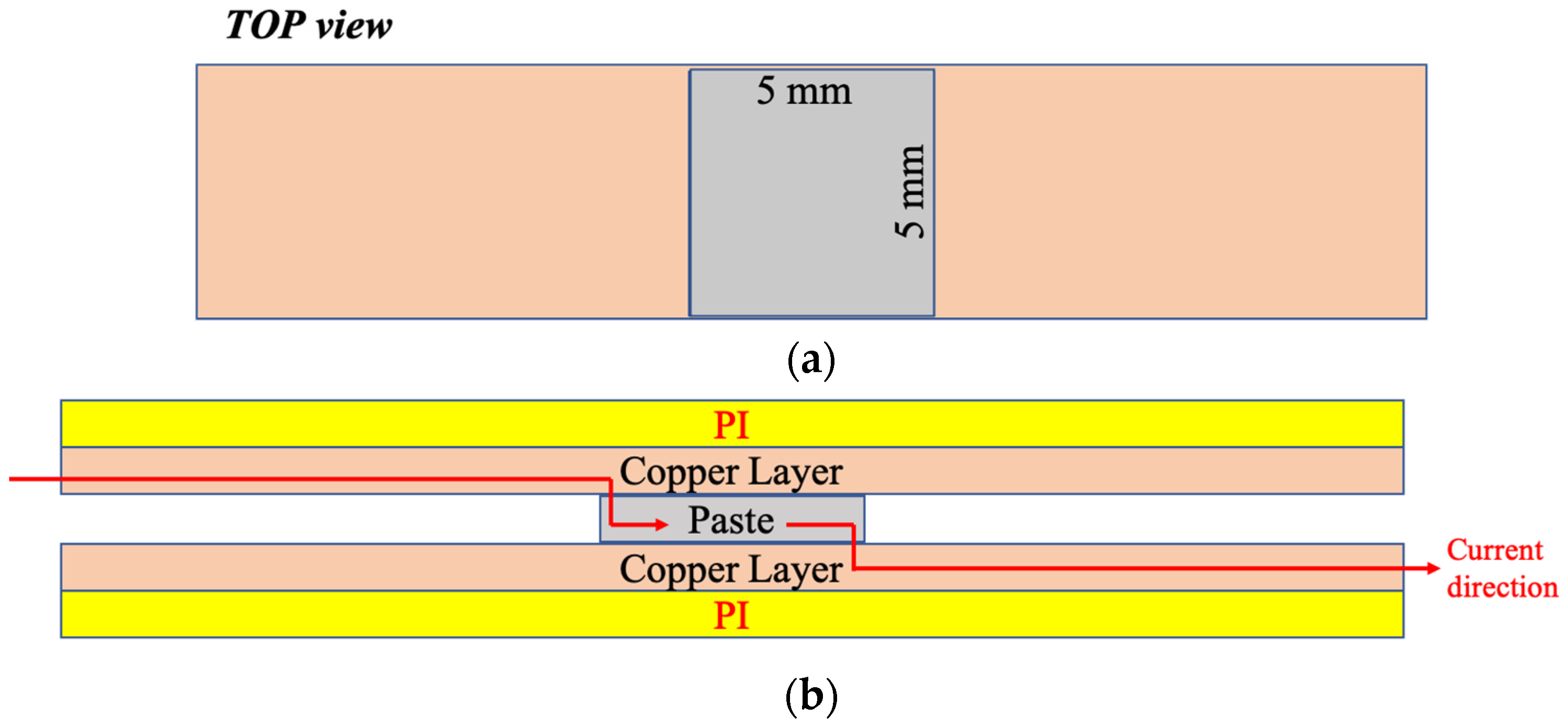
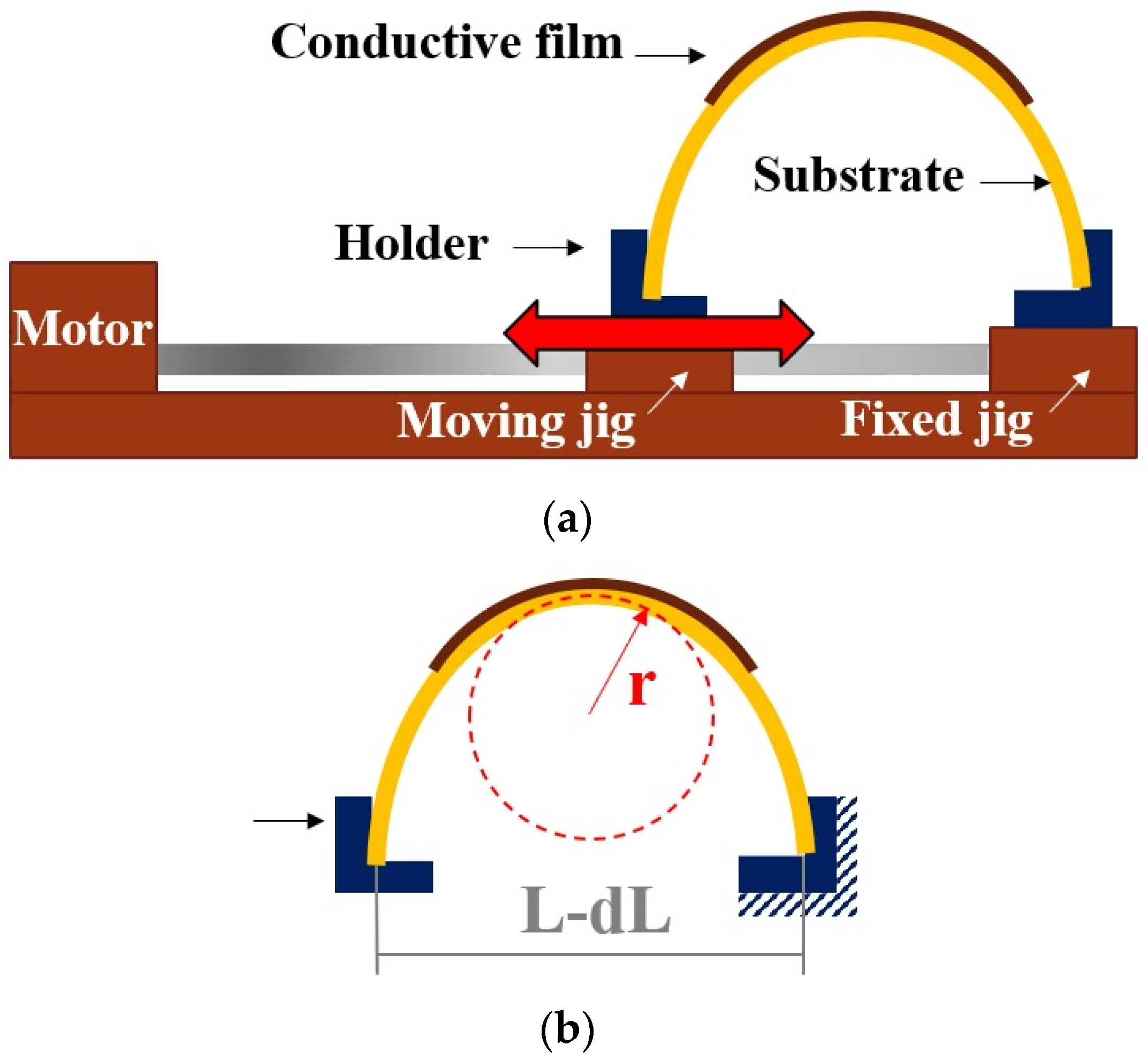
2.3. Mechanical Testing for Rigid and Flexible Joints
3. Results and Discussion
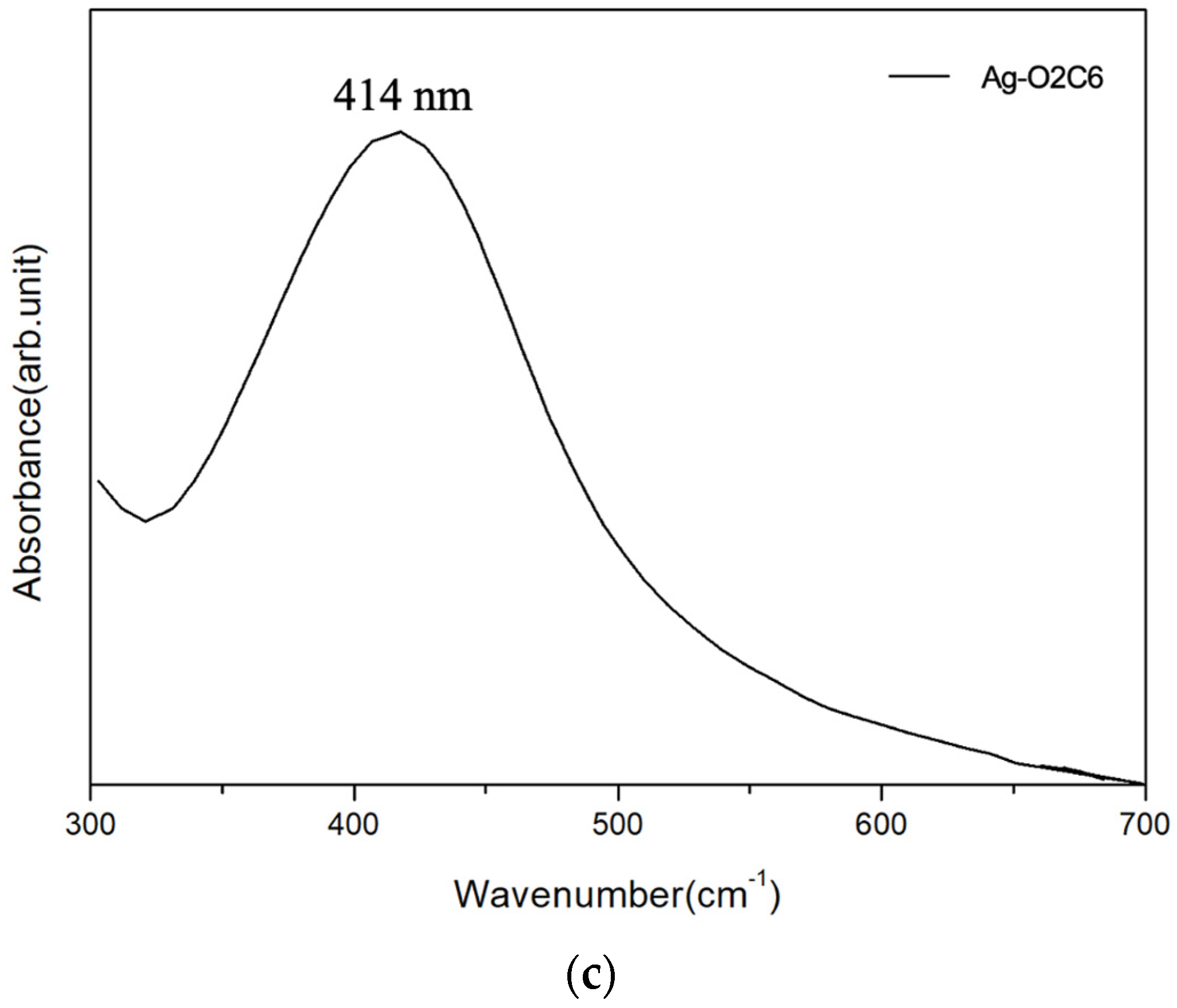
3.1. Ag Particle Characterization
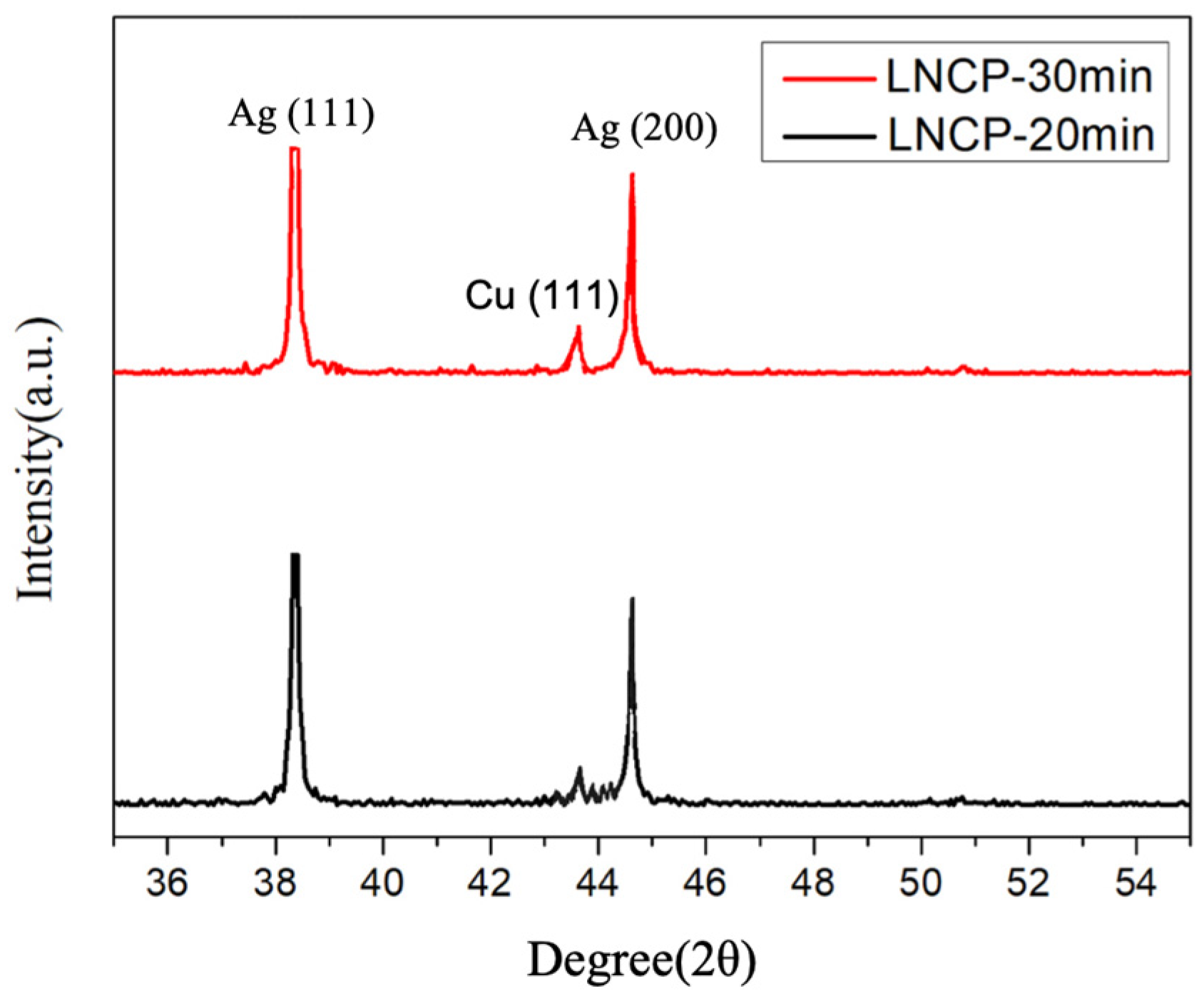
3.2. LNCP Characteristics and Sintered Structure
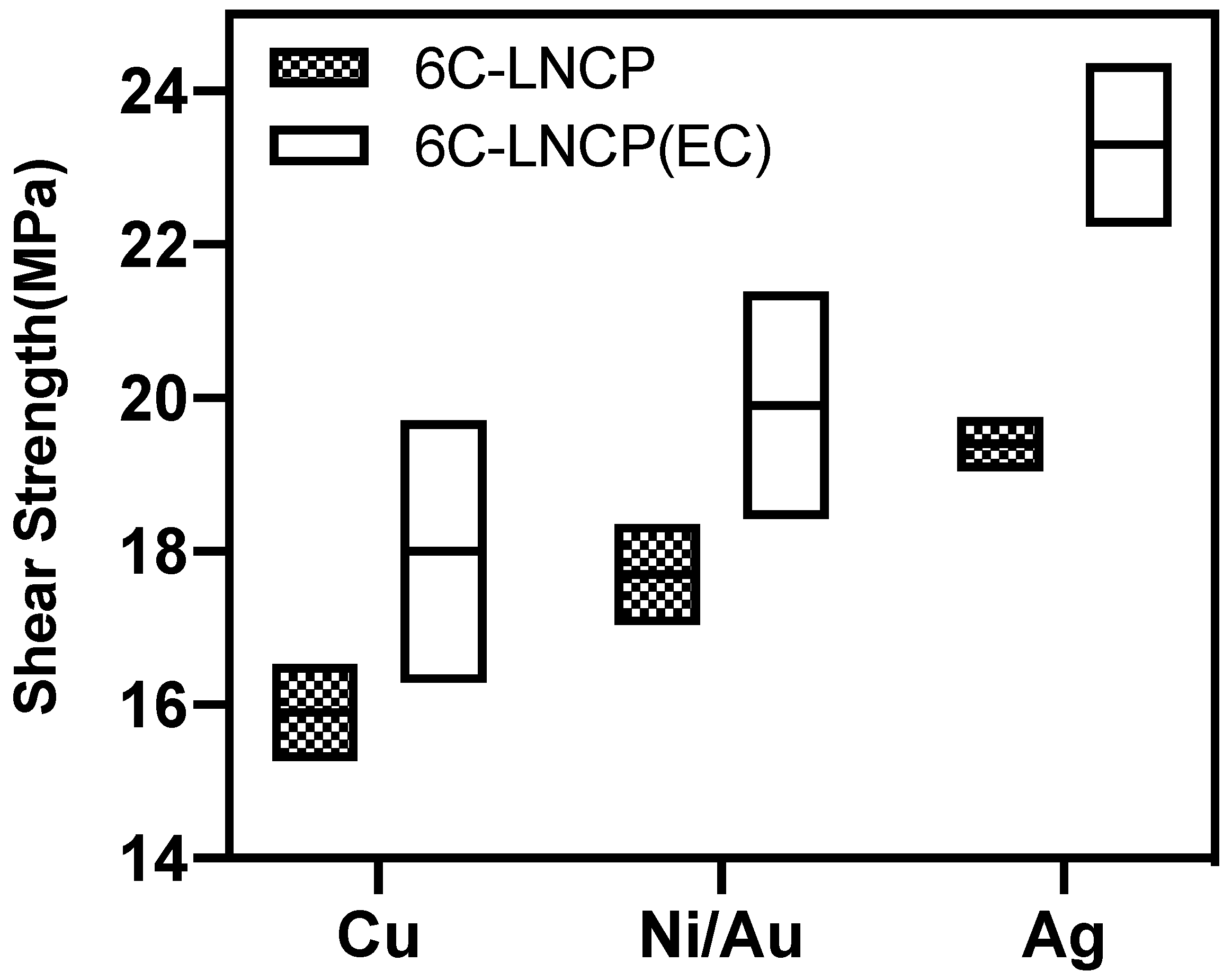
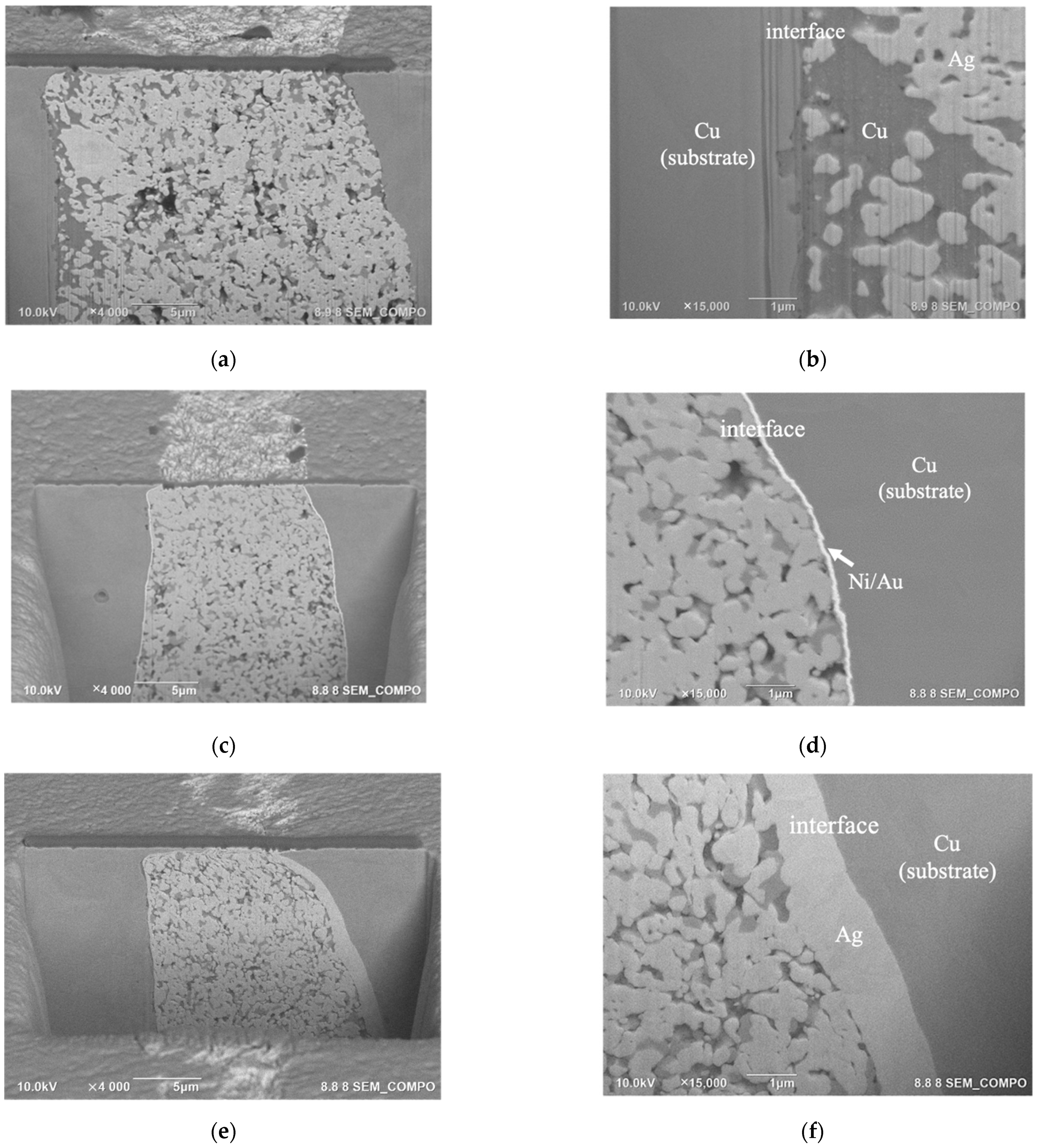
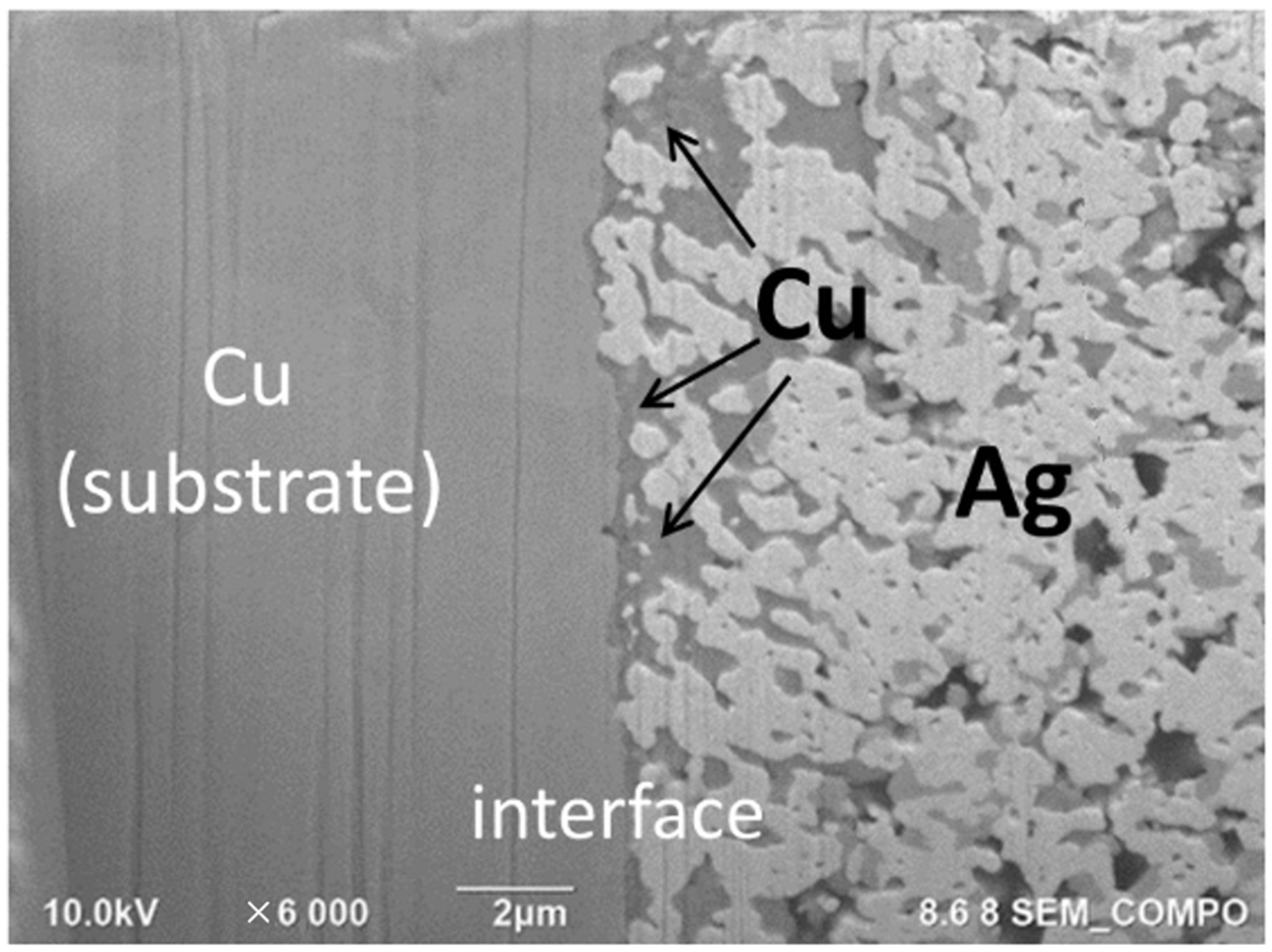
3.3. Joint Strength
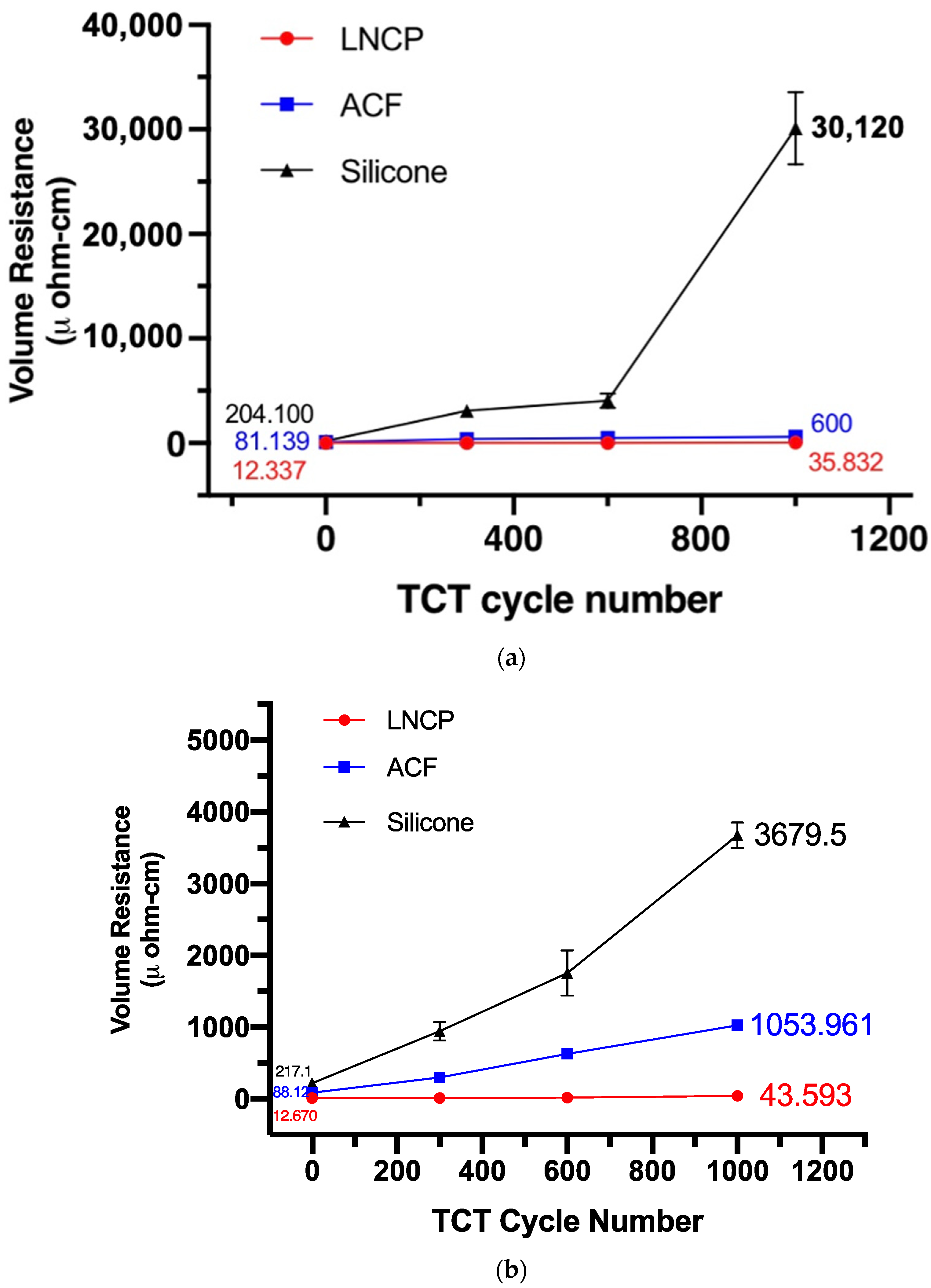
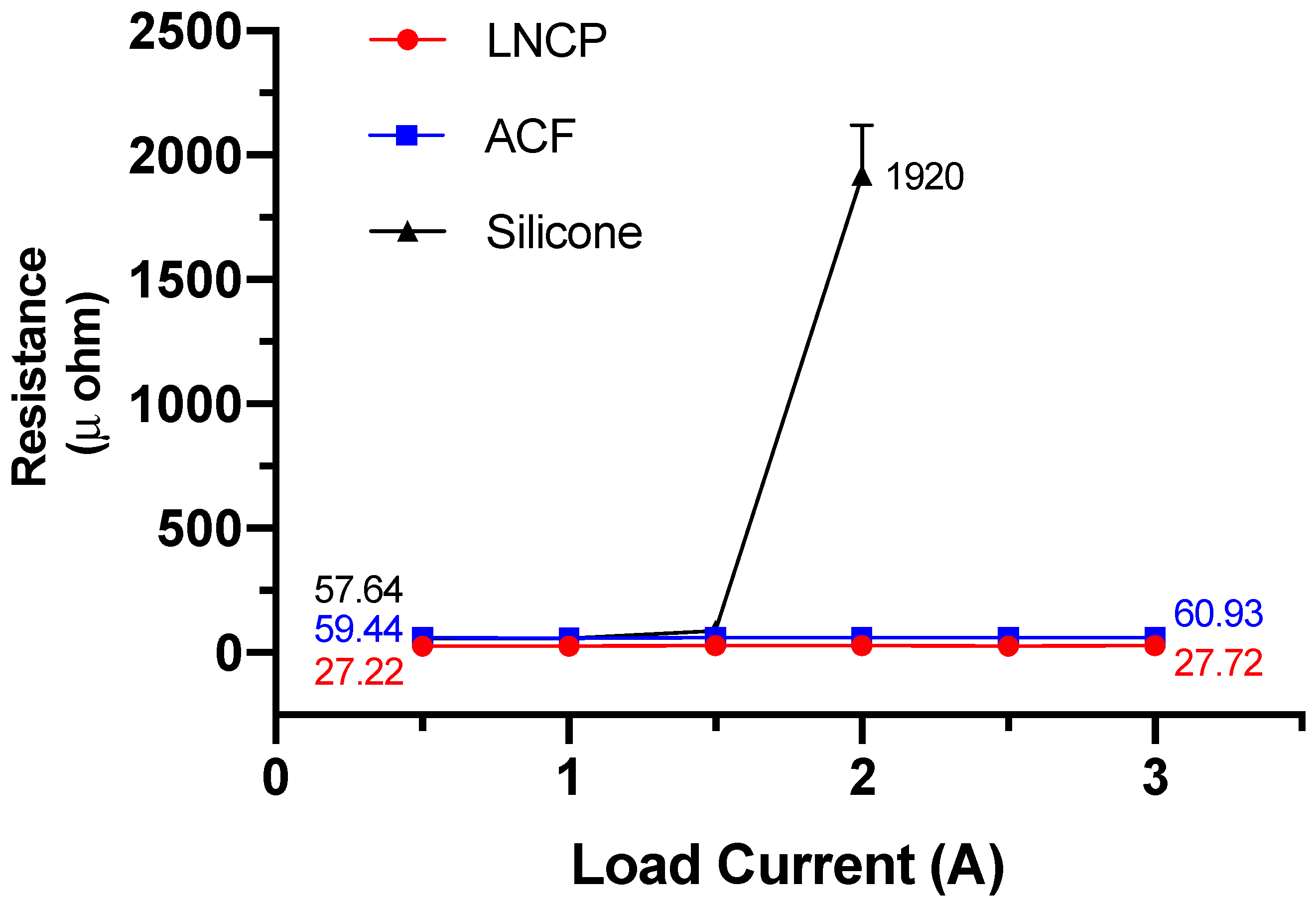
3.4. Reliability Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Moon, K.; Wong, C. Electronics Without Lead. Science 2005, 308, 1419–1420. [Google Scholar] [CrossRef] [PubMed]
- Suganuma, K. Advances in Lead-Free Electronics Soldering. Curr. Opin. Solid State Mater. Sci. 2001, 5, 55–64. [Google Scholar] [CrossRef]
- Abtew, M.; Selvaduray, G. Lead-free Solders in Microelectronics. Mater. Sci. Eng. R 2000, 27, 95–141. [Google Scholar] [CrossRef]
- Yim, B.S.; Yim, Y.; Seung, H.O.; Lim, J.; Yong, E.S.; Lei, S.H.; Lim, J.M. Characteristics of Solderable Electrically Conductive Adhesives (ECAs) for Electronic Packaging. Microelectron. Reliab. 2012, 52, 1165–1173. [Google Scholar] [CrossRef]
- Jesudoss, P.; Mathewson, A.; Wright, W.M.D.; Stam, F. Mechanical Assessment of An Anisotropic Conductive Adhesive Joint of a Direct Access Sensor on A Flexible Substrate for A Swallowable Capsule Application. Microelectron. Reliab. 2013, 53, 452–462. [Google Scholar] [CrossRef]
- Ide, E.; Angata, S.; Hirose, A.; Kobayashi, K.F. Metal–metal Bonding Process Using Ag Metallo-organic Nanoparticles. Acta Mater. 2005, 53, 23852393. [Google Scholar] [CrossRef]
- Kim, M.S.; Nishikawa, H. Effects of Bonding Temperature on Microstructure, Fracture Behavior and Joint Strength of Ag Nanoporous Bonding for High Temperature Die Attach. Mater. Sci. Eng. A 2015, 645, 264–272. [Google Scholar] [CrossRef]
- Hu, A.; Guo, J.Y.; Alarifi, H.; Patane, G.; Zhou, Y.; Compagnini, G.; Xu, C.X. Low Temperature Sintering of Ag Nanoparticles for Flexible Electronics Packaging. Appl. Phys. Lett. 2010, 97, 153117. [Google Scholar] [CrossRef]
- Yan, J.; Zou, G.; Hu, A.; Zhou, Y.N. Preparation of PVP coated Cu NPs and the Application for Low-Temperature Bonding. J. Mater. Chem. 2011, 21, 15981–15986. [Google Scholar]
- Li, W.H.; Lin, P.S.; Chen, C.N.; Dong, T.Y.; Tsai, C.H.; Kung, W.T.; Song, J.M.; Chiu, Y.T.; Yang, P.F. Low Temperature Cu-to-Cu Bonding Using Silver Nanoparticles Stabilized by Saturated Dodecanoic Acid. Mater. Sci. Eng. A 2014, 613, 372–378. [Google Scholar] [CrossRef]
- Strehle, S.; Menzel, S.; Jahn, A.; Merkel, U.; Bartha, J.W.; Wetzig, K. Electromigration in Electroplated Cu(Ag) Alloy Thin Films Investigated by Means of Single Damascene Blech Structures. Microelectron. Eng. 2009, 86, 2396–2403. [Google Scholar] [CrossRef]
- Li, Y.S.; Lu, Y.C.; Chou, K.S.; Liu, F. Synthesis and Characterization of Silver-Copper Colloidal Ink and Its Performance against Electrical Migration. Mater. Res. Bull. 2010, 45, 1837–1843. [Google Scholar] [CrossRef]
- Tsai, C.H.; Chen, S.Y.; Song, J.M.; Chen, I.G.; Lee, H.Y. Thermal Stability of Cu@Ag Core-shell Nanoparticles. Corr. Sci. 2013, 74, 123–129. [Google Scholar] [CrossRef]
- Hsiao, C.H.; Kung, W.T.; Song, J.M.; Chang, J.Y.; Chang, T.C. Development of Cu-Ag Pastes for High Temperature Sustainable Bonding. Mater. Sci. Eng. A 2017, 684, 500–509. [Google Scholar] [CrossRef]
- Liang, M.; Zhu, Y.; Sun, S. Cu@Ag Core-Shell Nanoparticle with Multiple Morphologies: A Simple Surfactant-Free Synthesis and Finite Element Simulation. Micro. Nano Lett. 2020, 15, 393–398. [Google Scholar] [CrossRef]
- Joo, S.J.; Hwang, H.J.; Kim, H.S. Highly Conductive Copper Nano/microparticles Ink via Flash Light Sintering for Printed Electronics. Nanotechnology 2014, 25, 265601. [Google Scholar] [CrossRef]
- PChiu, H.; Cheng, W.H.; Lee, M.T.; Yasuda, K.; Song, J.M. Low-Thermal-Budget Photonic Sintering of Hybrid Pastes Containing Submicron/Nano CuO/Cu2O Particles. Nanomaterials 2021, 11, 1864. [Google Scholar]
- Xu, Y.; Dai, D.; Yang, R.; Qian, J.; Wang, P.; Chen, X. Large Area Pressureless Cu-Cu bonding using Formic Acid-treated Cu Particles for Power Device Packaging. In Proceedings of the 2022 International Conference on Electronic Packaging Technology, Dalian, China, 10–13 August 2022. No. 182631. [Google Scholar]
- Dong, T.Y.; Chen, W.T.; Wang, C.W.; Chen, C.P.; Chen, C.N.; Lin, M.C.; Song, J.M.; Chen, I.G.; Kao, T.H. One-step Synthesis of Uniform Silver Nanoparticles Capped by Saturated Decanoate: Direct Spray Printing Ink to Form Metallic Silver Film. Phys. Chem. Chem. Phys. 2009, 11, 6269–6275. [Google Scholar] [CrossRef]
- Riaz, S.; Naz, S.; Younus, A.; Javd, A.; Akram, S.; Nosheen, A.; Ashraf, M. Layer by layer deposition of PEDOT, silver and copper to develop durable, flexible, and EMI shielding and antibacterial textiles. Colloids Surf. A Physicochem. Eng. 2022, 650, 129486. [Google Scholar] [CrossRef]
- Park, S.I.; Ahn, J.H.; Feng, X.; Wang, S.; Huang, Y.; Rogers, J.A. Theoretical and experimental studies of bending of inorganic electronic materials on plastic substrates. Adv. Funct. Mater. 2008, 18, 2673–2684. [Google Scholar] [CrossRef]
- Song, J.M.; Pai, T.Y.; Hsieh, K.H.; Lai, M.Y.; Cheng, C.N.; Liang, S.Y.; Lee, H.Y.; Chen, L.T. Kinetic Study on Low Temperature Coalescence of Carboxylate-Protected Ag Nanoparticles for Interconnect Applications. RSC Adv. 2016, 6, 97449. [Google Scholar] [CrossRef]
- Peng, P.; Hu, A.; Zhao, B.; Gerlich, A.P.; Zhou, Y.N. Reinforcement of Ag nanoparticle paste with nanowires for low temperature pressureless bonding. J. Mater. Sci. 2012, 47, 6801–6811. [Google Scholar] [CrossRef]
- Zou, G.; Yan, J.; Mu, F.; Wu, A.; Ren, J.; Hu, A.; Zhou, Y.N. Low Temperature Bonding of Cu Metal through Sintering of Ag Nanoparticles for High Temperature Electronic Application. Open. Surf. Sci. J. 2011, 3, 70–75. [Google Scholar] [CrossRef]
- Liu, J.D.; Chen, H.; Ji, H.; Li, M. Highly Conductive Cu−Cu Joint Formation by Low-Temperature Sintering of Formic Acid-Treated Cu Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 33289–33298. [Google Scholar] [CrossRef] [PubMed]
- Suganuma, K.; Sakamoto, S.; Kagami, N.; Wakuda, D.; Kim, K.S.; Nogi, M. Low-Temperature Low-Pressure Die Attach with Hybrid Silver Particle Paste. Microelectron. Reliab. 2012, 52, 375–380. [Google Scholar] [CrossRef]
- Yan, J.; Zou, G.; Wu, A.; Ren, J.; Yan, J.; Hu, A.; Zhou, Y. Pressureless Bonding Process Using Ag Nanoparticle Paste for Flexible Electronics Packaging. Scr. Mater. 2012, 66, 582–585. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, H.; Zhang, X.; Liu, L.; Peng, P.; Zou, G.; Zhou, Y.N. Preparation of Nanoparticle and Nanowire Mixed Pastes and Their Low Temperature Sintering. J. Alloys. Compd. 2017, 690, 86–94. [Google Scholar] [CrossRef]
- Alarifi, H.; Hu, A.; Yavuz, M.; Zhou, Y.N. Silver Nanoparticle Paste for Low-Temperature Bonding of Copper. J. Electron. Mater. 2011, 40, 1394–1402. [Google Scholar] [CrossRef]
- Hultgren, R.; Desai, P.D.; Hawkins, D.T.; Gleiser, M.; Kelley, K. Selected Values of the Thermodynamic Properties of Binary Alloys; American Society for Metals: Metals Park, OH, USA, 1973. [Google Scholar]
- Pearson, W.B. Handbook of Lattice Spacings and Structures of Metals and Alloys; Pergamon: Oxford, UK, 1967. [Google Scholar]
- Pauling, L. The Nature of the Chemical Bond, 3rd ed.; Cornell University Press: New York, NY, USA, 1960; p. 93. [Google Scholar]
- Bai, J.G.; Lu, G.Q. Thermomechanical Reliability of Low-Temperature Sintered Silver Die Attached SiC Power Device Assembly. IEEE Trans. Device Mater. Reliab. 2006, 6, 436–441. [Google Scholar] [CrossRef]





| Bonding Materials | Bonding Condition | |||||
|---|---|---|---|---|---|---|
| Temporary Bonding | Main Bonding | |||||
| Temperature | Pressure | Time | Temperature | Pressure | Time | |
| 6C-LNCP 6C-LNCP (EC) | 70 °C | 10 MPa | 600 s | 160 °C | 1.6, 10 MPa | 1200 s |
| ACF | 80 ± 10 °C | 1 MPa | 5 s | 185 ± 10 °C | 2 MPa | 20 s |
| Silicone paste | Moisture | 2 MPa | 12 h | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.-C.; Liao, W.-H.; Wu, T.-J.; Yasuda, K.; Song, J.-M. Cu-Ag Nanocomposite Pastes for Low Temperature Bonding and Flexible Interlayer-Interconnections. Nanomaterials 2022, 12, 4241. https://doi.org/10.3390/nano12234241
Lu Y-C, Liao W-H, Wu T-J, Yasuda K, Song J-M. Cu-Ag Nanocomposite Pastes for Low Temperature Bonding and Flexible Interlayer-Interconnections. Nanomaterials. 2022; 12(23):4241. https://doi.org/10.3390/nano12234241
Chicago/Turabian StyleLu, Yin-Chi, Wei-Hsun Liao, Ting-Jui Wu, Kiyokazu Yasuda, and Jenn-Ming Song. 2022. "Cu-Ag Nanocomposite Pastes for Low Temperature Bonding and Flexible Interlayer-Interconnections" Nanomaterials 12, no. 23: 4241. https://doi.org/10.3390/nano12234241
APA StyleLu, Y.-C., Liao, W.-H., Wu, T.-J., Yasuda, K., & Song, J.-M. (2022). Cu-Ag Nanocomposite Pastes for Low Temperature Bonding and Flexible Interlayer-Interconnections. Nanomaterials, 12(23), 4241. https://doi.org/10.3390/nano12234241








