ZIF-L to ZIF-8 Transformation: Morphology and Structure Controls
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. ZIF-L Crystals Synthesis
2.3. ZIF-LtoZIF-8 Transformation
- Addition of 1 M Zn
- 2.
- Addition of 0.1 M 2mIm
- 3.
- Addition of 0.01–0.05 M CTAB
- 4.
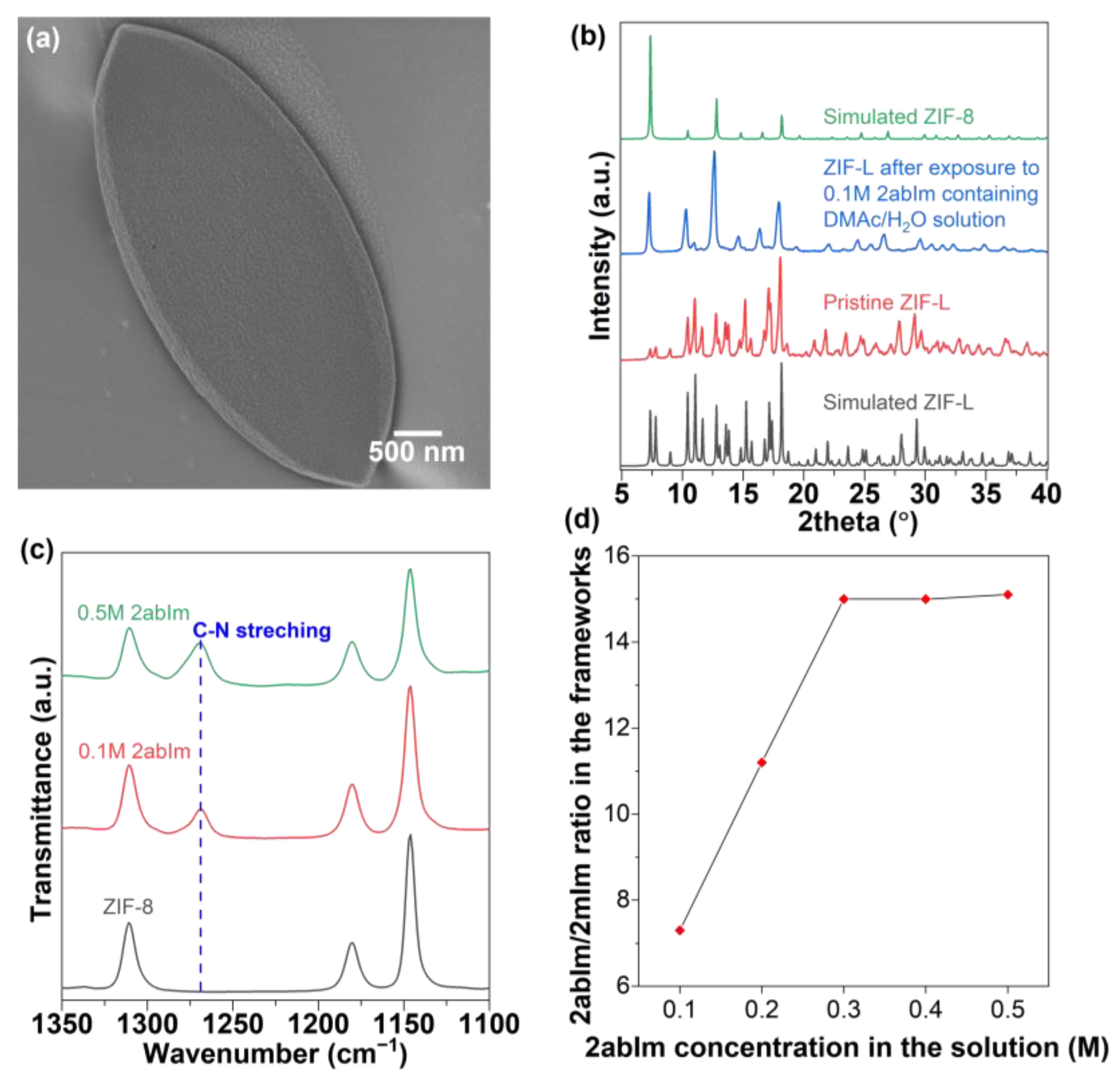
- Addition of 0.1–0.5 M 2abIm
2.4. Characterization
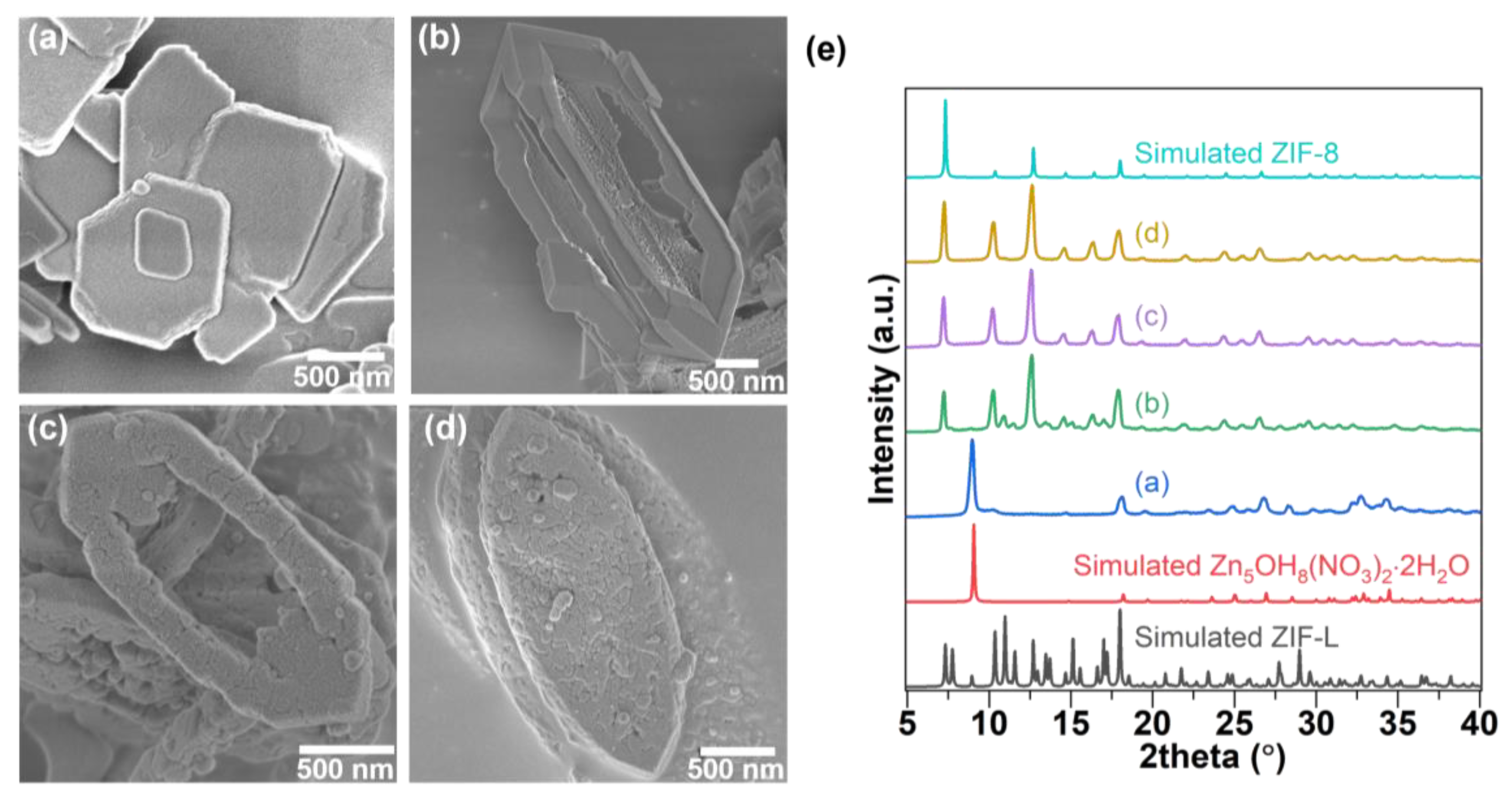
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Ji, Z.; Wuttke, S.; Yaghi, O.M. Digital Reticular Chemistry. Chem 2020, 6, 2219–2241. [Google Scholar] [CrossRef]
- Suresh, K.; Aulakh, D.; Purewal, J.; Siegel, D.J.; Veenstra, M.; Matzger, A.J. Optimizing Hydrogen Storage in MOFs through Engineering of Crystal Morphology and Control of Crystal Size. J. Am. Chem. Soc. 2021, 143, 10727–10734. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.-H.; Liu, X.-M.; He, T.; Li, J.-R. Metal-Organic Frameworks for the Capture of Trace Aromatic Volatile Organic Compounds. Chem 2018, 4, 1911–1927. [Google Scholar] [CrossRef]
- Kim, T.; Kim, Y.J.; Yu, C.; Kim, J.; Eum, K. Facile Fabrication of α-Alumina Hollow Fiber-Supported ZIF-8 Membrane Module and Impurity Effects on Propylene Separation Performance. Membranes 2022, 12, 1015. [Google Scholar] [CrossRef] [PubMed]
- Eum, K.; Yang, S.; Min, B.; Ma, C.; Drese, J.H.; Tamhankar, Y.; Nair, S. All-Nanoporous Hybrid Membranes: Incorporating Zeolite Nanoparticles and Nanosheets with Zeolitic Imidazolate Framework Matrices. ACS Appl. Mater. Interfaces 2020, 12, 27368–27377. [Google Scholar] [CrossRef]
- Kwon, O.; Kim, M.; Choi, E.; Bae, J.H.; Yoo, S.; Won, J.C.; Kim, Y.H.; Shin, J.H.; Lee, J.S.; Kim, D.W. High–Aspect Ratio Zeolitic Imidazolate Framework (ZIF) Nanoplates for Hydrocarbon Separation Membranes. Sci. Adv. 2022, 8, eabl6841. [Google Scholar] [CrossRef]
- Song, Z.; Cheng, N.; Lushington, A.; Sun, X. Recent Progress on MOF-Derived Nanomaterials as Advanced Electrocatalysts in Fuel Cells. Catalysts 2016, 6, 116. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, S.; Shin, D.; Park, J.T.; Choi, I. Sensitive and Homogeneous Surface-Enhanced Raman Scattering Detection Using Heterometallic Interfaces on Metal–Organic Framework-Derived Structure. Adv. Mater. Interfaces 2022, 9, 2102122. [Google Scholar] [CrossRef]
- Lee, C.S.; Lim, J.M.; Park, J.T.; Kim, J.H. Direct Growth of Highly Organized, 2D Ultra-Thin Nano-Accordion Ni-MOF@NiS2@C Core-Shell for High Performance Energy Storage Device. Chem. Eng. J. 2021, 406, 126810. [Google Scholar] [CrossRef]
- Windischbacher, A.; Steiner, L.; Haldar, R.; Wöll, C.; Zojer, E.; Kelterer, A.-M. Exciton Coupling and Conformational Changes Impacting the Excited State Properties of Metal Organic Frameworks. Molecules 2020, 25, 4230. [Google Scholar] [CrossRef]
- Sundriyal, S.; Kaur, H.; Bhardwaj, S.K.; Mishra, S.; Kim, K.-H.; Deep, A. Metal-Organic Frameworks and Their Composites as Efficient Electrodes for Supercapacitor Applications. Coord. Chem. Rev. 2018, 369, 15–38. [Google Scholar] [CrossRef]
- Lee, S.; Oh, S.; Oh, M. Atypical Hybrid Metal–Organic Frameworks (MOFs): A Combinative Process for MOF-on-MOF Growth, Etching, and Structure Transformation. Angew. Chem.-Int. Ed. 2020, 59, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Liu, D.; Xiao, H.; Rong, H.; Guan, S.; Xie, F.; Wang, D.; Li, Y. Facet Engineering in Metal Organic Frameworks to Improve Their Electrochemical Activity for Water Oxidation. Chem. Commun. 2020, 56, 4316–4319. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Kim, Y.J.; Kim, J.; Hayashi, M.; Kim, D.W.; Kwon, H.T.; Eum, K. A Sacrificial ZIF-L Seed Layer for Sub-100 Nm Thin Propylene-Selective ZIF-8 Membranes. J. Mater. Chem. A 2022, 10, 15390–15394. [Google Scholar] [CrossRef]
- Yang, Y.; Ren, G.; Yang, W.; Lin, D.; Li, M.; Chang, Z.; Fang, Y.; Liang, Z.; Pan, Q. Single-Crystal to Single-Crystal Transformation of Metal–Organic Framework Nanoparticles for Encapsulation and PH-Stimulated Release of Camptothecin. ACS Appl. Nano Mater. 2021, 4, 7191–7198. [Google Scholar] [CrossRef]
- Martí-Rujas, J. Thermal Reactivity in Metal Organic Materials (MOMs): From Single-Crystal-to-Single-Crystal Reactions and Beyond. Materials 2019, 12, 4088. [Google Scholar] [CrossRef]
- Low, Z.X.; Yao, J.; Liu, Q.; He, M.; Wang, Z.; Suresh, A.K.; Bellare, J.; Wang, H. Crystal Transformation in Zeolitic-Imidazolate Framework. Cryst. Growth Des. 2014, 14, 6589–6598. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, T.; Yu, D.; Xiao, K.; Hong, Y. Transition from ZIF-L-Co to ZIF-67: A New Insight into the Structural Evolution of Zeolitic Imidazolate Frameworks (ZIFs) in Aqueous Systems. CrystEngComm 2015, 17, 8212–8215. [Google Scholar] [CrossRef]
- Suresh, K.; Kalenak, A.P.; Sotuyo, A.; Matzger, A.J. Metal-Organic Framework (MOF) Morphology Control by Design. Chem.-A Eur. J. 2022, 28, e202200334. [Google Scholar] [CrossRef]
- Pérez-Miana, M.; Reséndiz-Ordóñez, J.U.; Coronas, J. Solventless Synthesis of ZIF-L and ZIF-8 with Hydraulic Press and High Temperature. Microporous Mesoporous Mater. 2021, 328, 111487. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional Chemical and Thermal Stability of Zeolitic Imidazolate Frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Yao, J.; Gu, Q.; Smeets, S.; Baerlocher, C.; Gu, H.; Zhu, D.; Morris, W.; Yaghi, O.M.; Wang, H. A Two-Dimensional Zeolitic Imidazolate Framework with a Cushion-Shaped Cavity for CO2 Adsorption. Chem. Commun. 2013, 49, 9500–9502. [Google Scholar] [CrossRef]
- Deacon, A.; Briquet, L.; Malankowska, M.; Massingberd-Mundy, F.; Rudić, S.; Hyde, T.l.; Cavaye, H.; Coronas, J.; Poulston, S.; Johnson, T. Understanding the ZIF-L to ZIF-8 Transformation from Fundamentals to Fully Costed Kilogram-Scale Production. Commun. Chem. 2022, 5, 18. [Google Scholar] [CrossRef]
- Sudha, S.; Karabacak, M.; Kurt, M.; Cinar, M.; Sundaraganesan, N. Molecular Structure, Vibrational Spectroscopic, First-Order Hyperpolarizability and HOMO, LUMO Studies of 2-Aminobenzimidazole. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2011, 84, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Casco, M.E.; Cheng, Y.Q.; Daemen, L.L.; Fairen-Jimenez, D.; Ramos-Fernández, E.V.; Ramirez-Cuesta, A.J.; Silvestre-Albero, J. Gate-Opening Effect in ZIF-8: The First Experimental Proof Using Inelastic Neutron Scattering. Chem. Commun. 2016, 52, 3639–3642. [Google Scholar] [CrossRef] [PubMed]
- Craig, G.A.; Larpent, P.; Kusaka, S.; Matsuda, R.; Kitagawa, S.; Furukawa, S. Switchable Gate-Opening Effect in Metal–Organic Polyhedra Assemblies through Solution Processing. Chem. Sci. 2018, 9, 6463–6469. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Hong, S.J.; Chen, J.; Kim, Y.J.; Choi, Y.; Kwon, O.; Eum, K.; Choi, J.I.; Jang, S.S.; Han, B.; et al. CO2-Selective Zeolitic Imidazolate Framework Membrane on Graphene Oxide Nanoribbons: Experimental and Theoretical Studies. J. Mater. Chem. A 2021, 9, 25595–25602. [Google Scholar] [CrossRef]



| DMAc:H2O Ratio | pH |
|---|---|
| 1:1 | 9.7 |
| 1:2 | 7.5 |
| 1:4 | 7.1 |
| 1:8 | 6.6 |
| 2:1 | 10.2 |
| 4:1 | 10.1 |
| 8:1 | 11.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.; Kim, Y.J.; Kim, J.; Eum, K. ZIF-L to ZIF-8 Transformation: Morphology and Structure Controls. Nanomaterials 2022, 12, 4224. https://doi.org/10.3390/nano12234224
Yu C, Kim YJ, Kim J, Eum K. ZIF-L to ZIF-8 Transformation: Morphology and Structure Controls. Nanomaterials. 2022; 12(23):4224. https://doi.org/10.3390/nano12234224
Chicago/Turabian StyleYu, Chanjong, Young Jae Kim, Jongbum Kim, and Kiwon Eum. 2022. "ZIF-L to ZIF-8 Transformation: Morphology and Structure Controls" Nanomaterials 12, no. 23: 4224. https://doi.org/10.3390/nano12234224
APA StyleYu, C., Kim, Y. J., Kim, J., & Eum, K. (2022). ZIF-L to ZIF-8 Transformation: Morphology and Structure Controls. Nanomaterials, 12(23), 4224. https://doi.org/10.3390/nano12234224






