Green Synthesis of Silver Nanoparticles Using Randia aculeata L. Cell Culture Extracts, Characterization, and Evaluation of Antibacterial and Antiproliferative Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and In Vitro Culture Conditions
2.2. Extracts Preparation for Synthesis of AgNPs
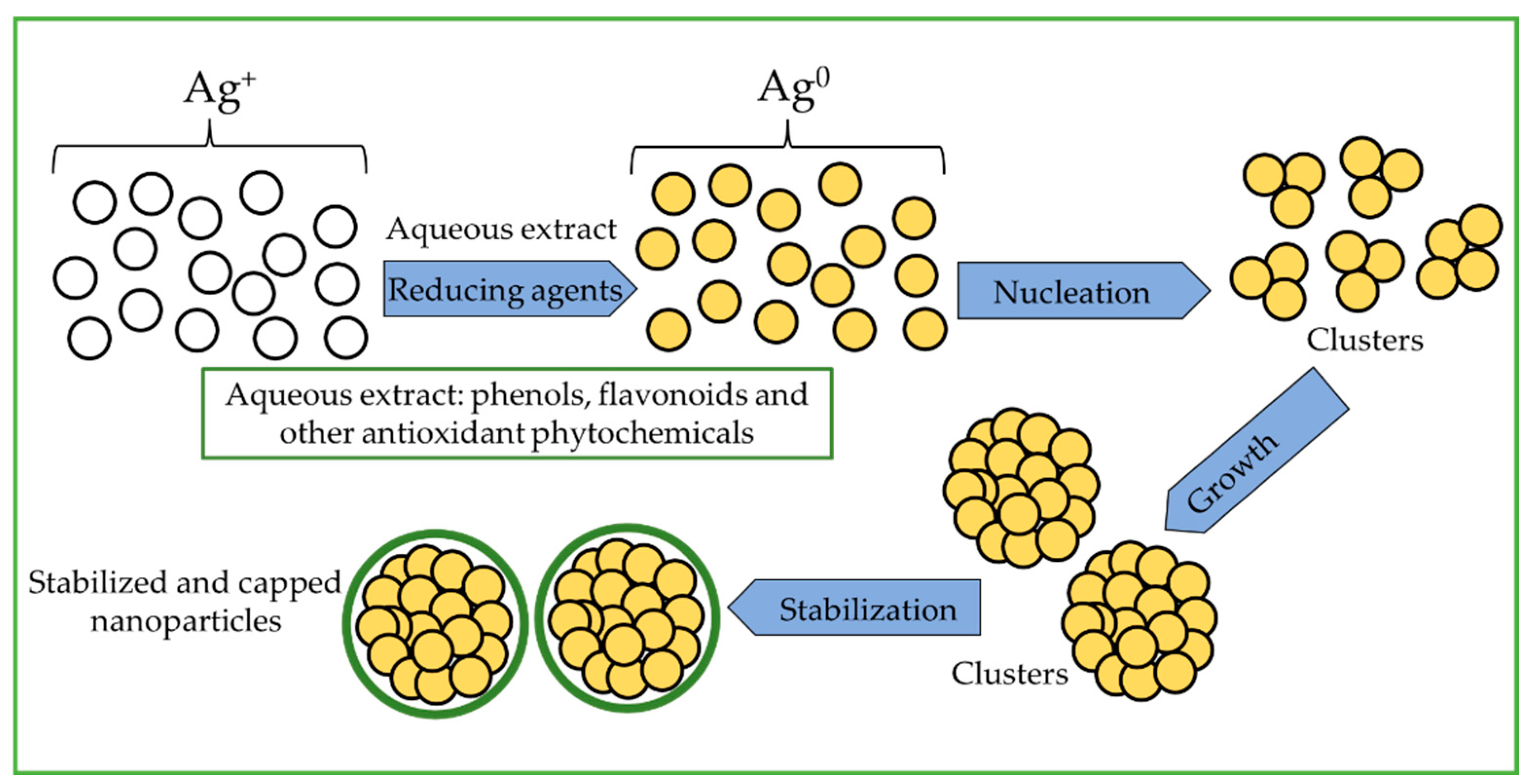
2.3. AgNP Synthesis
2.4. Characterization of AgNPs
2.5. Estimation of AgNP Concentration by UV–Vis Spectroscopy
2.6. Estimation of Total Phenol and Flavonoid Contents
2.6.1. Preparation of Extracts
2.6.2. Estimation of the Total Phenolic and Flavonoid Content
2.7. AgNP Antibacterial Activity
2.8. Antiproliferative Activity of AgNPs
3. Results and Discussion
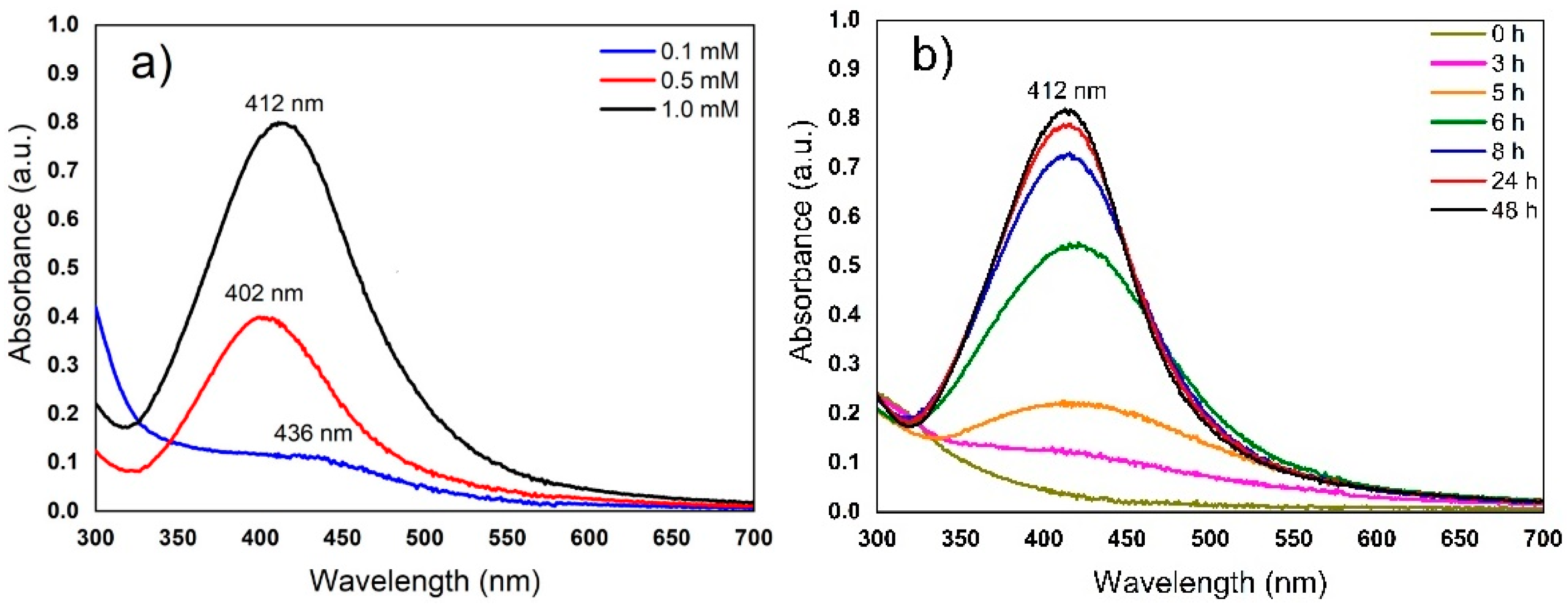
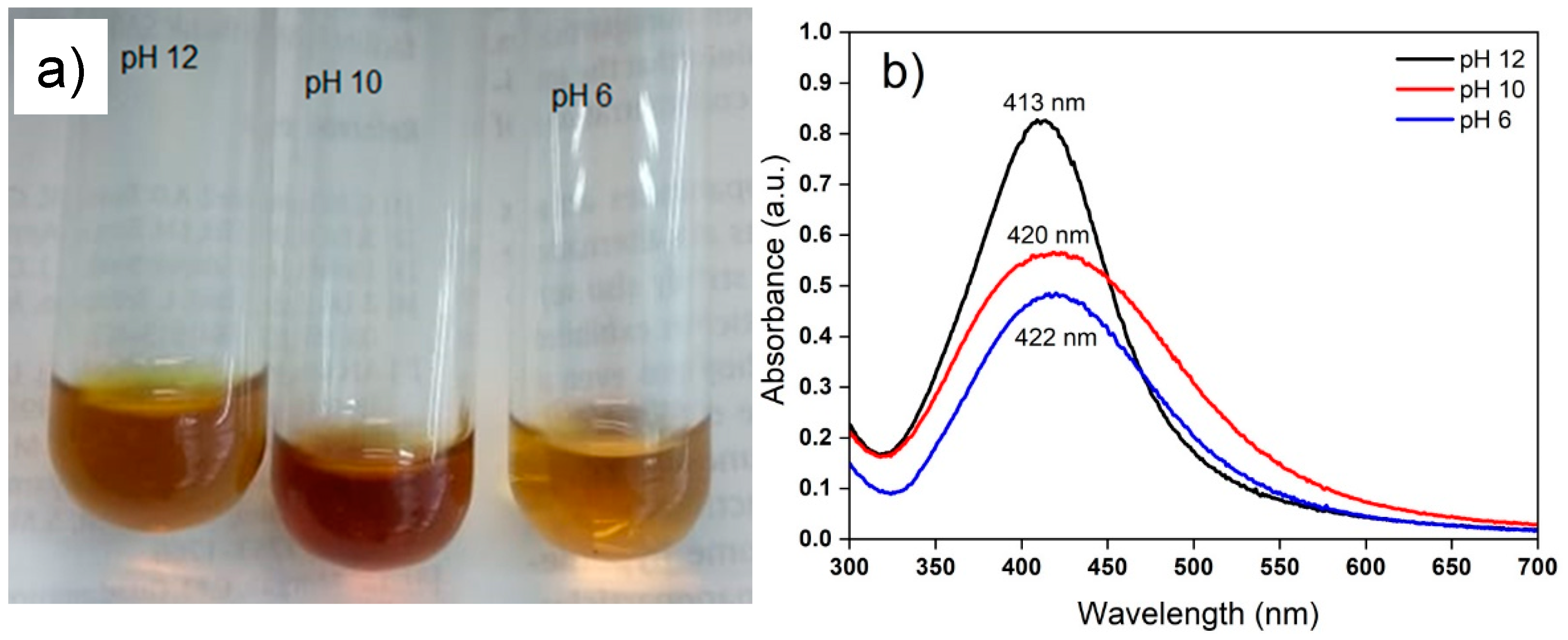
3.1. Extraction Condition and AgNP Synthesis
3.2. Characterization of AgNPs
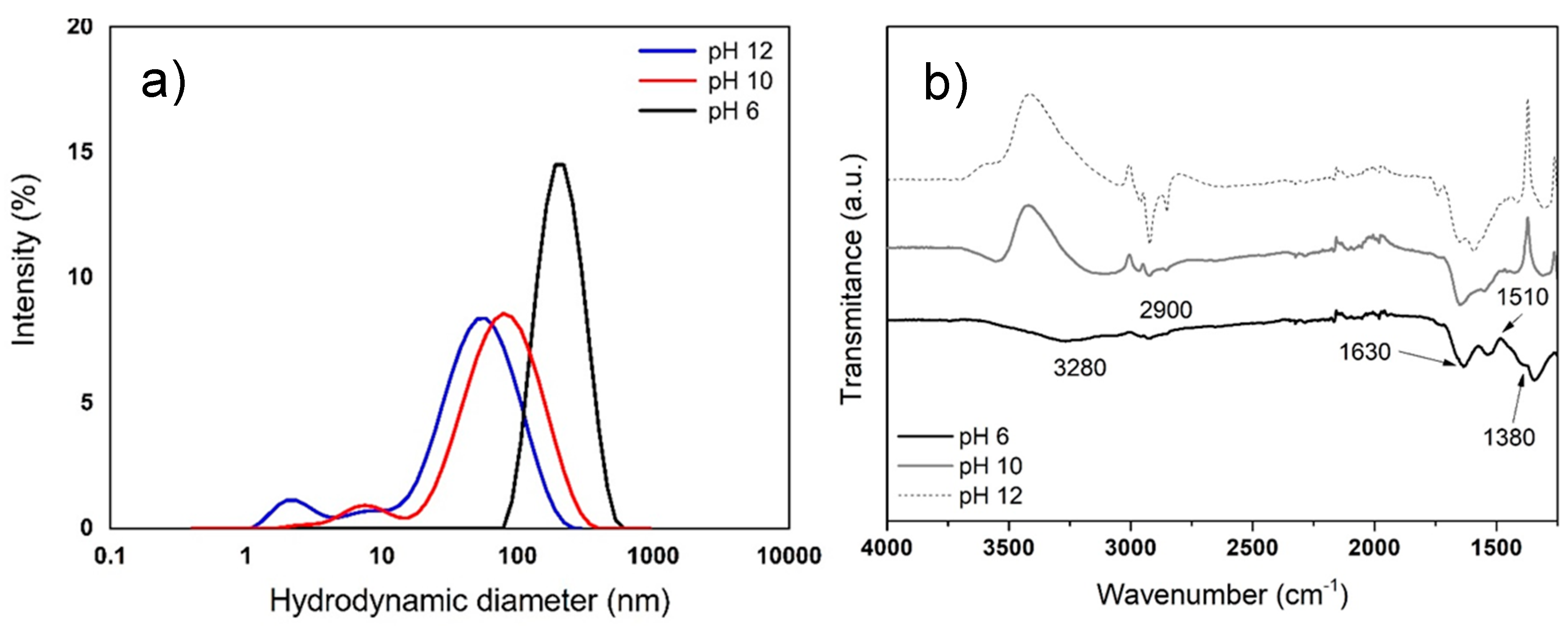
3.2.1. Average Size of AgNPs by DLS
3.2.2. Characterization of AgNPs by FTIR
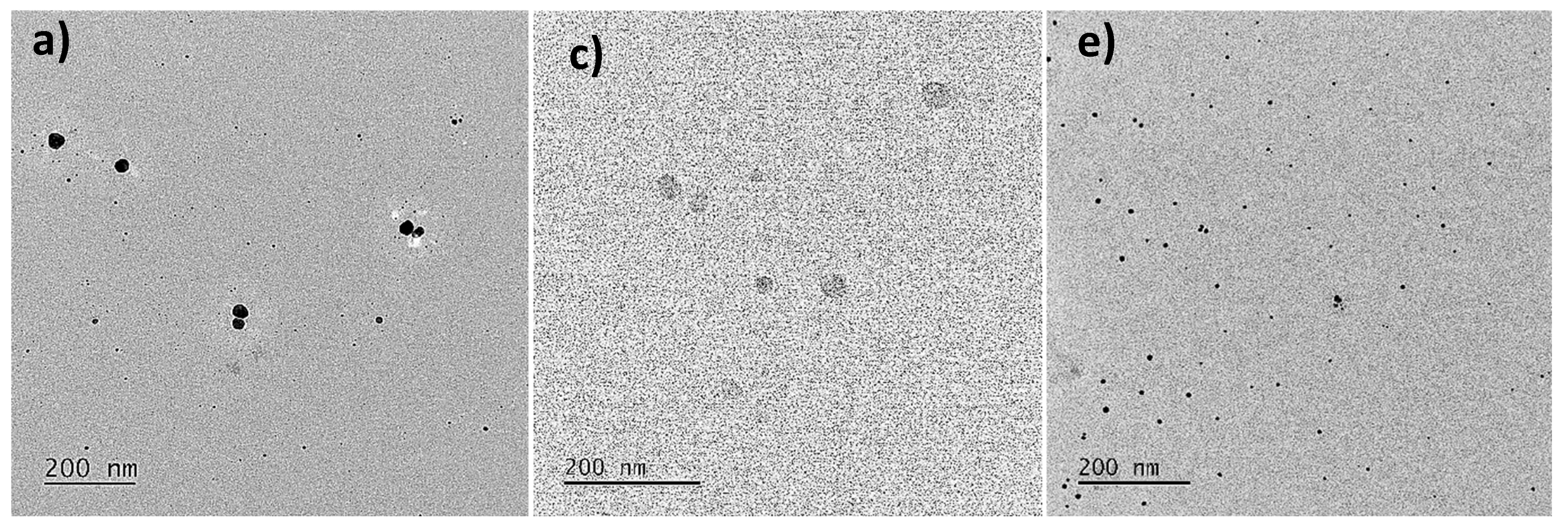
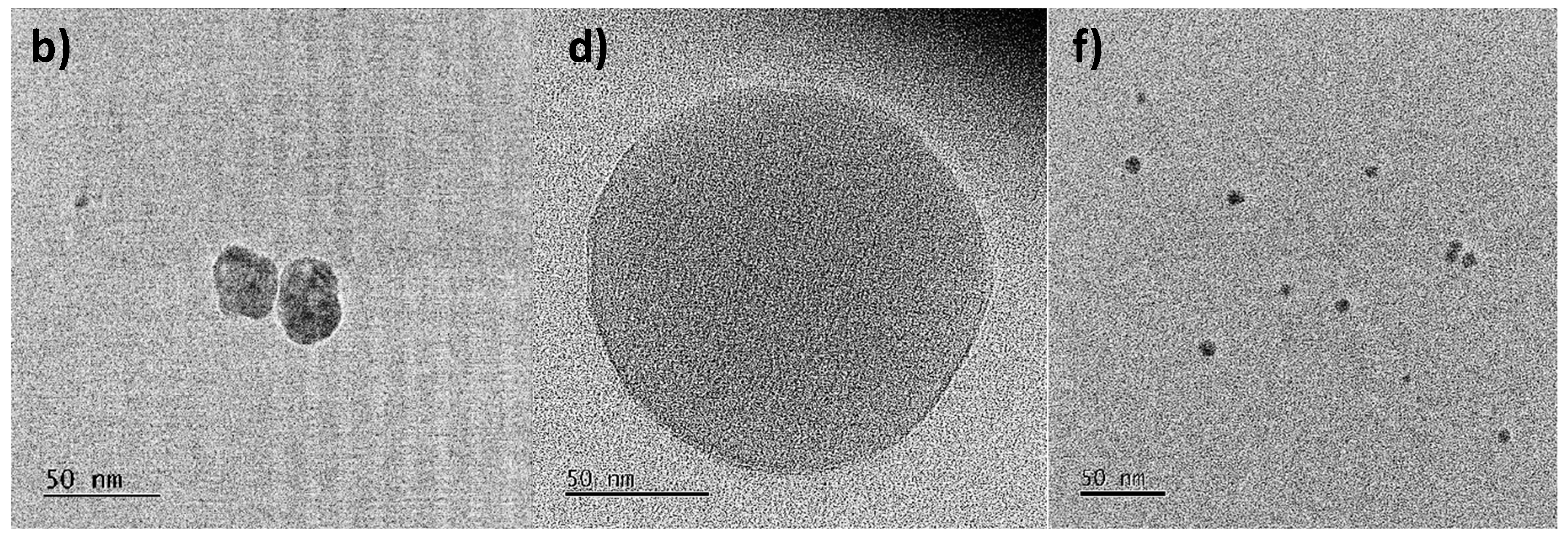
3.2.3. Morphology and Size Analysis of AgNPs by TEM
3.3. Estimation of the Concentration of AgNPs by UV–Vis
3.4. Total Phenolic and Flavonoid Content of the Extracts
3.5. Antibacterial Activity of AgNPs
3.6. Antiproliferative Activity of AgNPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sampath, G.; Chen, Y.Y.; Rameshkumar, N.; Krishnan, M.; Nagarajan, K.; Shyu, D.J.H. Biologically synthesized silver nanoparticles and their diverse applications. Nanomaterials 2022, 12, 3126. [Google Scholar] [CrossRef]
- Farooq, S.; Mehmood, Z.; Qais, F.A.; Khan, M.S.; Ahmad, I. Nanoparticles in ayurvedic medicine: Potential and prospects. In New Look to Phytomedicine; Khan, M.S.A., Ahmad, I., Chattopadhyay, D., Eds.; Academic Press: Cambridge, MA, USA, 2019; Chapter 22. [Google Scholar]
- Lu, P.J.; Huang, S.C.; Chen, Y.P.; Chiueh, L.C.; Shih, D.Y.C. Analysis of titanium dioxide and zinc oxide nanoparticles in cosmetics. J. Food Drug Anal. 2015, 23, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Angulo-Pineda, C.; Palma, P.; Bejarano, J.; Riveros, A.; Kogan, M.; Palza, H. Antibacterial silver nanoparticles supported on graphene oxide with reduced cytotoxicity. JOM 2019, 71, 3698–3705. [Google Scholar] [CrossRef]
- Foldbjerg, R.; Dang, D.A.; Autrup, H. Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Arch. Toxicol. 2011, 85, 743–750. [Google Scholar] [CrossRef]
- Mori, Y.; Ono, T.; Miyahira, Y.; Nguyen, V.Q.; Matsui, T.; Ishihara, M. Antiviral activity of silver nanoparticle/chitosan composites against H1N1 influenza A virus. Nanoscale Res. Lett. 2013, 8, 93. [Google Scholar] [CrossRef]
- Khan, F.; Shariq, M.; Asif, M.; Siddiqui, M.A.; Malan, P.; Ahmad, F. Green nanotechnology: Plant-mediated nanoparticle synthesis and application. Nanomaterials 2022, 12, 673. [Google Scholar] [CrossRef]
- Natsuki, J.; Natsuki, T.; Hashimoto, Y. A review of silver nanoparticles: Synthesis methods, properties and applications. Int. J. Mater. Sci. Appl. 2015, 4, 325–332. [Google Scholar] [CrossRef]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Parveen, K.; Banse, V.; Ledwani, L. Green synthesis of nanoparticles: Their advantages and disadvantages. AIP Conf. Proc. 2016, 1, 020048. [Google Scholar]
- Zhang, X.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Synthesis of nanoparticles by microorganisms and their application in enhancing microbiological reaction rates. Chemosphere 2011, 82, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Demissie, A.G.; Lele, S.S. Phytosynthesis and characterization of silver nanoparticles using callus of Jatropha curcas: A biotechnological approach. Int. J. Nanosci. 2013, 12, 1350012. [Google Scholar] [CrossRef]
- Malik, M.; Iqbal, M.A.; Malik, M.; Raza, M.A.; Shahid, W.; Choi, J.R.; Pham, P.V. Biosynthesis and characterizations of silver nanoparticles from Annona squamosa leaf and fruit extracts for size-dependent biomedical applications. Nanomaterials 2022, 12, 616. [Google Scholar] [CrossRef]
- Vanaja, M.; Rajeshkumar, S.; Paulkumar, K.; Gnanajobitha, G.; Malarkodi, C.; Annadurai, G. Phytosynthesis and characterization of silver nanoparticles using stem extract of Coleus aromaticus. Int. J. Mater. Biomaters Appl. 2013, 3, 1–4. [Google Scholar]
- Khane, Y.; Benouis, K.; Albukhaty, S.; Sulaiman, G.M.; Abomughaid, M.M.; Al Ali, A.; Aouf, D.; Fenniche, F.; Khane, S.; Chaibi, W.; et al. Green synthesis of silver nanoparticles using aqueous Citrus limon Zest extract: Characterization and evaluation of their antioxidant and antimicrobial properties. Nanomaterials 2022, 12, 2013. [Google Scholar] [CrossRef]
- Aref, M.S.; Salem, S.S. Bio-callus synthesis of silver nanoparticles, characterization, and antibacterial activities via Cinnamomum camphora callus culture. Biocatal. Agric. Biotechnol. 2020, 27, 101689. [Google Scholar] [CrossRef]
- Pant, B. Application of plant cell and tissue culture for the production of phytochemicals in medicinal plants. In Infectious Diseases and Nanomedicine II, Advances in Experimental Medicine and Biology 808; Adhikari, R., Thapa, S., Eds.; Springer: New Delhi, India, 2014. [Google Scholar]
- Hegazy, H.S.; Rabie, G.H.; Shaaban, L.D.; Raie, S.D. Extracellular synthesis of silver nanoparticles by callus of Medicago sativa. Life Sci. J. 2014, 11, 1211–1214. [Google Scholar]
- Nabikhan, A.; Kandasamy, K.; Raj, A.; Alikunhi, N.M. Synthesis of antimicrobial silver nanoparticles by callus and leaf extracts from saltmarsh plant, Sesuvium portulacastrum L. Colloids Surf. B-Biointerefaces 2010, 79, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Iyer, R.I.; Panda, T. Biosynthesis of gold and silver nanoparticles using extracts of callus cultures of pumpkin (Cucurbita maxima). J. Nanosci. Nanotechnol. 2018, 18, 5341–5353. [Google Scholar] [CrossRef] [PubMed]
- Asmathunisha, N.; Kathiresan, K. Rapid biosynthesis of antimicrobial silver and gold nanoparticles by in vitro callus and leaf extracts from Lycopersicon esculentum Mill. Int. J. Pharma Bio Sci. 2013, 4, 334–344. [Google Scholar]
- Osibe, D.A.; Chiejina, N.V.; Ogawa, K.; Aoyagi, H. Stable antibacterial silver nanoparticles produced with seed-derived callus extract of Catharanthus roseus. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.A. Flora of the Lesser Antilles-Dicotyledoneae; Arnold Arboretum of Harvard University: Jamaica Plain, MA, USA, 1989; Volume 5, p. 604. [Google Scholar]
- Gallardo-Casas, C.A.; Guevara-Balcázar, G.; Morales-Ramos, E.; Tadeo-Jiménez, Y.; Gutiérrez-Flores, O.; Jiménez-Sánchez, N.; Castillo-Hernández, M.C. Ethnobotanic study of Randia aculeata (Rubiaceae) in Jamapa, Veracruz, Mexico, and its anti-snake venom effects on mouse tissue. J. Venom Anim. Toxins Incl. Trop. Dis. 2012, 18, 287–294. [Google Scholar] [CrossRef]
- Martínez-Ceja, A.; Romero-Estrada, A.; Columba-Palomares, M.C.; Hurtado-Díaz, I.; Alvarez, L.; Teta-Talixtacta, R.; Cruz-Sosa, F.; Bernabé-Antonio, A. Anti-inflammatory, antibacterial and antioxidant activity of leaf and cell cultures extracts of Randia aculeata L. and its chemical components by GC-MS. S. Afr. J. Bot. 2022, 144, 206–218. [Google Scholar] [CrossRef]
- Baker, C.J.; Mock, N.M. An improved method for monitoring cell death in cell suspension and leaf disc assays using Evans blue. Plant Cell Tissue Organ Cult. 1994, 39, 7–12. [Google Scholar] [CrossRef]
- Zia, F.; Ghafoor, N.; Iqbal, M.; Mehboob, S. Green synthesis and characterization of silver nanoparticles using Cydonia oblong seed extract. App. Nanosci. 2016, 6, 1023–1029. [Google Scholar] [CrossRef]
- Ponarulselvam, S.; Panneerselvam, C.; Murugan, K.; Aarthi, N.; Kalimuthu, K.; Thangamani, S. Synthesis of silver nanoparticles using leaves of Catharanthus roseus Linn. G. Don and their antiplasmodial activities. Asian Pac. J. Trop. Biomed. 2012, 2, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Saifullah; Ahmad, M.; Swami, B.L.; Ikram, S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. Appl. Sci. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Alim-Al-Razy, M.; Bayazid, G.A.; Rahman, R.U.; Bosu, R.; Shamma, S.S. Silver nanoparticle synthesis, UV-Vis spectroscopy to find particle size and measure resistance of colloidal solution. J. Phys. Conf. Ser. 2020, 1706, 012020. [Google Scholar] [CrossRef]
- Maity, D.; Pattanayak, S.; Mollick, M.M.R.; Rana, D.; Mondal, D.; Bhowmick, B.; Dash, S.K.; Chattopadhyay, D.; Das, B.; Roy, S.; et al. Green one step morphosynthesis of silver nanoparticles and their antibacterial and anticancerous activities. New J. Chem. 2016, 40, 2749–2762. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Ramachandran, R.; Mohan, K.; Kalaichelvan, P.T. Optimization for rapid synthesis of silver nanoparticles and its effect on phytopathogenic fungi, Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 93, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Satyavani, K.; Gurudeeban, S.; Ramanathan, T.; Balasubramanian, T. Biomedical potential of silver nanoparticles synthesized from calli cells of Citrullus colocynthis (L.) Schrad. J. Nanobiotechnol. 2011, 9, 43. [Google Scholar]
- Hung, N.D.; Nam, V.N.; ThiNhan, T.; Dung, T.T.N. Quantitative concentration determination of silver nanoparticles prepared by DC high voltage electrochemical method. Vietnam J. Chem. 2018, 56, 553–558. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.Q.; Weber, C.; Lee, C.Y.; Brown, J.; Liu, R.H. Antioxidant and antiproliferative activities of raspberries. J. Agric. Food Chem. 2002, 50, 2926–2930. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 27th ed.; CLSI Supplement M100, Clinical and Laboratory Standards Institute: Wayne, PA, USA; Fifteenth Informational Supplement M100-S15: Wayne, PA, USA, 2017; 250p. [Google Scholar]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Saito, K.; Meyer, K.; Ray, R.B. Stellate cell apoptosis by a soluble mediator from immortalized human hepatocytes. Apoptosis 2006, 11, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- Bagherzade, G.; Tavakoli, M.M.; Namaei, M.H. Green synthesis of silver nanoparticles using aqueous extract of saffron (Crocus sativus L.) wastages and its antibacterial activity against six bacteria. Asian Pac. J. Trop. Biomed. 2017, 7, 227–233. [Google Scholar] [CrossRef]
- Thomas, R.; Nair, A.P.; Mathew, S.K.J.; Ek, R. Antibacterial activity and synergistic effect of biosynthesized AgNPs with antibiotics against multidrug-resistant biofilm-forming coagulase-negative staphylococci isolated from clinical samples. Appl. Biochem. Biotechnol. 2014, 173, 449–460. [Google Scholar] [CrossRef]
- Desai, R.; Mankad, V.; Gupta, S.K.; Jha, P.K. Size distribution of silver nanoparticles: UV-visible spectroscopic assessment. Nanosci. Nanotechnol. Lett. 2012, 4, 30–34. [Google Scholar] [CrossRef]
- Vanaja, M.; Gnanajobitha, G.; Paulkumar, K.; Rajeshkumar, S.; Malarkodi, C.; Annadurai, G. Phytosynthesis of silver nanoparticles by Cissus quadrangularis: Influence of physicochemical factors. J. Nanostruct. Chem. 2013, 3, 1–8. [Google Scholar] [CrossRef]
- Soares, M.R.; Corrêa, R.O.; Stroppa, P.H.F.; Marques, F.C.; Andrade, G.F.; Corrêa, C.C.; Raposo, N.R. Biosynthesis of silver nanoparticles using Caesalpinia ferrea (Tul.) Martius extract: Physicochemical characterization, antifungal activity and cytotoxicity. PeerJ 2018, 6, e4361. [Google Scholar] [CrossRef]
- Xia, Q.; Ma, Y.; Wang, J. Biosynthesis of silver nanoparticles using Taxus yunnanensis callus and their antibacterial activity and cytotoxicity in human cancer cells. Nanomaterials 2016, 6, 160. [Google Scholar] [CrossRef] [PubMed]
- Teimuri-Mofrad, R.; Hadi, R.; Tahmasebi, B.; Farhoudian, S.; Mehravar, M.; Nasiri, R. Green synthesis of gold nanoparticles using plant extract: Mini-review. Nanochem. Res. 2017, 2, 8–19. [Google Scholar]
- Kalpana, D.; Han, J.H.; Park, W.S.; Lee, S.M.; Wahab, R.; Lee, Y.S. Green biosynthesis of silver nanoparticles using Torreya nucifera and their antibacterial activity. Arabian J. Chem. 2019, 12, 1722–1732. [Google Scholar] [CrossRef]
- Dada, A.O.; Adekola, F.A.; Adeyemi, O.S.; Bello, O.M.; Oluwaseun, A.C.; Awakan, O.J.; Grace, F.A.A. Exploring the effect of operational factors and characterization imperative to the synthesis of silver nanoparticles. In Silver Nanoparticles-Fabrication, Characterization and Applications; Maaz, K., Ed.; InTechOpen: London, UK, 2018; pp. 165–184. [Google Scholar]
- Wisam, J.A.; Haneen, A.J. A novel study of pH influence on Ag nanoparticles size with antibacterial and antifungal activity using green synthesis. World Sci. News 2018, 97, 139–152. [Google Scholar]
- Fernando, I.; Zhou, Y. Impact of pH on the stability, dissolution and aggregation kinetics of silver nanoparticles. Chemosphere 2019, 216, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Sinha, I.; Mandal, R.K. Role of pH in the green synthesis of silver nanoparticles. Mater. Lett. 2009, 63, 425–427. [Google Scholar] [CrossRef]
- Dubey, S.P.; Lahtinen, M.; Sillanpää, M. Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process Biochem. 2010, 45, 1065–1071. [Google Scholar] [CrossRef]
- Traiwatcharanon, P.; Timsorn, K.; Wongchoosuk, C. Flexible room-temperature resistive humidity sensor based on silver nanoparticles. Mater. Res. Express 2017, 4, 085038. [Google Scholar] [CrossRef]
- Heydari, R.; Rashidipour, M. Green synthesis of silver nanoparticles using extract of oak fruit hull (Jaft): Synthesis and in vitro cytotoxic effect on MCF-7 cells. Int. J. Breast Cancer 2015, 2015, 846743. [Google Scholar] [CrossRef]
- Kokila, T.; Ramesh, P.S.; Geetha, D. Biosynthesis of silver nanoparticles from Cavendish banana peel extract and its antibacterial and free radical scavenging assay: A novel biological approach. Appl. Nanosci. 2015, 5, 911–920. [Google Scholar] [CrossRef]
- Meva, F.E.A.; Ntoumba, A.A.; Kedi, P.B.E.; Tchoumbi, E.; SchmitzSchmolke, A.L.; Lehman, L.G. Silver and palladium nanoparticles produced using a plant extract as reducing agent, stabilized with an ionic liquid: Sizing by X-ray powder diffraction and dynamic light scattering. J. Mater. Res. Technol. 2019, 8, 1991–2000. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Z.; Peng, F.; Fu, L. Biosynthesis of silver nanoparticles by Plectranthus amboinicus leaf extract and their catalytic activity towards methylene blue degradation. Rev. Mex. Ing. Quím. 2017, 16, 41–45. [Google Scholar] [CrossRef]
- Muniyappan, N.; Nagarajan, N.S. Green synthesis of gold nanoparticles using Curcuma pseudomontana essential oil, its biological activity and cytotoxicity against human ductal breast carcinoma cells T47D. J. Environ. Chem. Eng. 2014, 2, 2037–2044. [Google Scholar] [CrossRef]
- Siakavella, I.K.; Lamari, F.; Papoulis, D.; Orkoula, M.; Gkolfi, P.; Lykouras, M.; Avgoustakis, K.; Hatziantoniou, S. Effect of plant extracts on the characteristics of silver nanoparticles for topical application. Pharmaceutics 2020, 12, 1244. [Google Scholar] [CrossRef]
- Mollick, M.M.R.; Bhowmick, B.; Maity, D.; Mondal, D.; Bain, M.K.; Bankura, K.; Sarkar, J.; Rana, D.; Acharya, K.; Chattopadhyay, D. Green synthesis of silver nanoparticles using Paederia foetida L. leaf extract and assessment of their antimicrobial activities. Int. J. Green Nanotechnol. 2012, 4, 230–239. [Google Scholar] [CrossRef]
- Cruz-Silva, S.C.B.D.; Matias, R.; Bono, J.A.; Santos, K.S.; Ludwig, J. Antifungal potential of extracts and fractions of Randia nitida leaves on soybean pathogens and their phytochemistry. Rev. Caatinga 2016, 29, 594–602. [Google Scholar] [CrossRef]
- Kandimalla, R.; Kalita, S.; Saikia, B.; Choudhury, B.; Singh, Y.P.; Kalita, K.; Dash, S.; Kotoky, J. Antioxidant and hepatoprotective potentiality of Randia dumetorum Lam. Leaf and bark via inhibition of oxidative stress and inflammatory cytokines. Front. Pharmacol. 2016, 205, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, J.E.; Yuriar, A.K.Y.; Pío, L.J.F.; Montes, A.J.; López, A.G.; Díaz, C.S.P.; Delgado, V.F. Antioxidant and α-glucosidase inhibitory properties of soluble melanins from the fruits of Vitex mollis Kunth, Randia echinocarpa Sessé et Mociño and Crescentia alata Kunth. J. Funct. Foods 2014, 9, 78–88. [Google Scholar] [CrossRef]
- Gobbo-Neto, L.; Lopes, N.P. Plantas medicinais: Fatores de influência no conteúdo de metabólitos secundários. Quím. Nova 2007, 30, 374–381. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Lee, W.; Kim, K.J.; Lee, D.G. A novel mechanism for the antibacterial effect of silver nanoparticles on Escherichia coli. Biometals 2014, 27, 1191–1201. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, J. Antibacterial activity of silver nanoparticles: Structural effects. Adv. Healthc. Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; He, B.; Liu, L.; Qu, G.; Shi, J.; Hu, L.; Jiang, G. Antibacterial mechanism of silver nanoparticles in Pseudomonas aeruginosa: Proteomics approach. Metallomics 2018, 10, 557–564. [Google Scholar] [CrossRef]
- Keshari, A.K.; Srivastava, R.; Singh, P.; Yadav, V.B.; Nath, G. Antioxidant and antibacterial activity of silver nanoparticles synthesized by Cestrum nocturnum. J. Ayurveda Integr. Med. 2020, 11, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Konwarh, R.; Bhattacharya, S.S.; Das, P.; Devi, K.S.P.; Maiti, T.K.; Mandal, M.; Karak, N. Non-hazardous anticancerous and antibacterial colloidal ‘green’ silver nanoparticles. Colloids Surf. B-Biointerfaces 2013, 105, 37–42. [Google Scholar] [CrossRef]
- Rudrappa, M.; Rudayni, H.A.; Assiri, R.A.; Bepari, A.; Basavarajappa, D.S.; Nagaraja, S.K.; Chakraborty, B.; Swamy, P.S.; Agadi, S.N.; Niazi, S.K.; et al. Plumeria alba-mediated green synthesis of silver nanoparticles exhibit antimicrobial effect and anti-oncogenic activity against glioblastoma U118 MG cancer cell line. Nanomaterials 2022, 12, 493. [Google Scholar] [CrossRef]
- Khatami, M.; Sharifi, I.; Nobre, M.A.; Zafarnia, N.; Aflatoonian, M.R. Waste-grass-mediated green synthesis of silver nanoparticles and evaluation of their anticancer, antifungal and antibacterial activity. Green Chem. Lett. Rev. 2018, 11, 125–134. [Google Scholar] [CrossRef]
- Ganguly, S.; Mondal, S.; Das, P.; Bhawal, P.; Kanti Das, T.; Bose, M.; Choudhary, S.; Gangopadhyay, S.; Das, A.K.; Das, N.C. Natural saponin stabilized nano-catalyst as efficient dye-degradation catalyst. Nano-Struct. Nano-Objects 2018, 16, 86–95. [Google Scholar] [CrossRef]
- Mishra, V.; Nayak, P.; Singh, M.; Tambuwala, M.M.; Aljabali, A.A.; Chellappan, D.K.; Dua, K. Pharmaceutical aspects of green synthesized silver nanoparticles: A boon to cancer treatment. Anti-Cancer Agents Med. Chem. 2021, 21, 1490–1509. [Google Scholar] [CrossRef]
- Husni, E.; Nahari, F.; Wirasti, Y.; Wahyuni Dachriyanus, F.S. Cytotoxicity study of ethanol extract of the stem bark of asam kandis (Garcinia cowa Roxb.) on T47D breast cancer cell line. Asian Pac. J. Trop. Biomed. 2015, 5, 249–252. [Google Scholar] [CrossRef]
- Hussein, H.A.; Mohamad, H.; Ghazaly, M.M.; Laith, A.A.; Abdullah, M.A. Cytotoxic effects of Tetraselmis suecica chloroform extracts with silver nanoparticle co-application on MCF-7, 4 T1, and Vero cell lines. J Appl. Phycol. 2020, 32, 127–143. [Google Scholar] [CrossRef]
- Hemlata; Meena, P.R.; Singh, A.P.; Tejavath, K.K. Biosynthesis of silver nanoparticles using Cucumis prophetarum aqueous leaf extract and their antibacterial and antiproliferative activity against cancer cell lines. ACS Omega 2020, 5, 5520–5528. [Google Scholar] [CrossRef]
- Chakraborty, B.; Kumar, R.S.; Almansour, A.I.; Kotresha, D.; Rudrappa, M.; Pallavi, S.S.; Hiremath, H.; Perumal, K.; Nayaka, S. Evaluation of antioxidant, antimicrobial and antiproliferative activity of silver nanoparticles derived from Galphimia glauca leaf extract. J. King Saud Univ. Sci. 2021, 33, 101660. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Nazir, M.; Muhammad, W.; Hashmi, S.S.; Abbasi, R.; Rahman, L.; Hano, C. A comparative evaluation of the antiproliferative activity against Hepg2 liver carcinoma cells of plant-derived silver nanoparticles from basil extracts with contrasting anthocyanin contents. Biomolecules 2019, 9, 320. [Google Scholar] [CrossRef]
| Plant Resource Extract | Total Phenolics (mg GAE/100 g DW) | Total Flavonoids (mg QE/100 g DW) |
|---|---|---|
| Cell suspension cultures | 162.19 ± 27.9 a | 122.07 ± 8.2 a |
| Leaves | 198.07 ± 32.1 a | 117.70 ± 15.3 a |
| Bacteria | MIC (µg/mL) | ||||
|---|---|---|---|---|---|
| AgNPs | Gentamicin ** | ||||
| pH 6 | pH 10 | pH 12 | AgNPs * | ||
| Escherichia coli | 3.9 | 9.7 | 14.3 | 10 | ≤0.62 |
| Streptococcus pyogenes | 3.9 | 9.7 | 14.3 | ≥10 | 5 |
| Pseudomonas aeruginosa | 1.9 | 2.4 | 3.5 | 5 | 20 |
| Staphylococcus aureus | 3.9 | 9.7 | 14.3 | ≥10 | ≤0.62 |
| Salmonella typhimurium | 3.9 | 9.7 | 14.3 | 10 | ≤0.62 |
| Staphylococcus aureus-MRSA | 3.9 | 9.7 | 14.3 | ≥10 | 20 |
| Cell Line | IC50 [µg/mL] | IC50 [ng/mL] | |||
|---|---|---|---|---|---|
| pH 6 | pH 10 | pH 12 | AgNPs * | Taxol * | |
| Hep3b | 1.2 ± 0.30 | 2.4 ± 0.20 | 4.3 ± 0.20 | 4.1 ± 0.20 | 17.1 ± 0.43 |
| HepG2 | 1.2 ± 0.06 | 2.8 ± 0.15 | 4.8 ± 0.62 | 4.6 ± 0.40 | 21.4 ± 0.34 |
| HeLa | 2.2 ± 0.13 | 3.1 ± 0.25 | 5.2 ± 0.08 | 4.7 ± 0.40 | 21.4 ± 1.71 |
| A549 | 3.0 ± 0.38 | 5.0 ± 0.23 | 7.6 ± 0.65 | 6.0 ± 0.70 | 68.3 ± 5.98 |
| IHH | 3.0 ± 0.32 | 5.7 ± 0.25 | 9.0 ± 0.71 | 8.0 ± 1.0 | 82.8 ± 6.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernabé-Antonio, A.; Martínez-Ceja, A.; Romero-Estrada, A.; Sánchez-Carranza, J.N.; Columba-Palomares, M.C.; Rodríguez-López, V.; Meza-Contreras, J.C.; Silva-Guzmán, J.A.; Gutiérrez-Hernández, J.M. Green Synthesis of Silver Nanoparticles Using Randia aculeata L. Cell Culture Extracts, Characterization, and Evaluation of Antibacterial and Antiproliferative Activity. Nanomaterials 2022, 12, 4184. https://doi.org/10.3390/nano12234184
Bernabé-Antonio A, Martínez-Ceja A, Romero-Estrada A, Sánchez-Carranza JN, Columba-Palomares MC, Rodríguez-López V, Meza-Contreras JC, Silva-Guzmán JA, Gutiérrez-Hernández JM. Green Synthesis of Silver Nanoparticles Using Randia aculeata L. Cell Culture Extracts, Characterization, and Evaluation of Antibacterial and Antiproliferative Activity. Nanomaterials. 2022; 12(23):4184. https://doi.org/10.3390/nano12234184
Chicago/Turabian StyleBernabé-Antonio, Antonio, Alejandro Martínez-Ceja, Antonio Romero-Estrada, Jessica Nayelli Sánchez-Carranza, María Crystal Columba-Palomares, Verónica Rodríguez-López, Juan Carlos Meza-Contreras, José Antonio Silva-Guzmán, and José Manuel Gutiérrez-Hernández. 2022. "Green Synthesis of Silver Nanoparticles Using Randia aculeata L. Cell Culture Extracts, Characterization, and Evaluation of Antibacterial and Antiproliferative Activity" Nanomaterials 12, no. 23: 4184. https://doi.org/10.3390/nano12234184
APA StyleBernabé-Antonio, A., Martínez-Ceja, A., Romero-Estrada, A., Sánchez-Carranza, J. N., Columba-Palomares, M. C., Rodríguez-López, V., Meza-Contreras, J. C., Silva-Guzmán, J. A., & Gutiérrez-Hernández, J. M. (2022). Green Synthesis of Silver Nanoparticles Using Randia aculeata L. Cell Culture Extracts, Characterization, and Evaluation of Antibacterial and Antiproliferative Activity. Nanomaterials, 12(23), 4184. https://doi.org/10.3390/nano12234184









