Synthesis and Study of Palladium Mono- and Bimetallic (with Ag and Pt) Nanoparticles in Catalytic and Membrane Hydrogen Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanoparticles Synthesis and Morphology Investigation
2.2. Nanoparticles Investigation in Catalytic and Membrane Applications
3. Results and Discussion
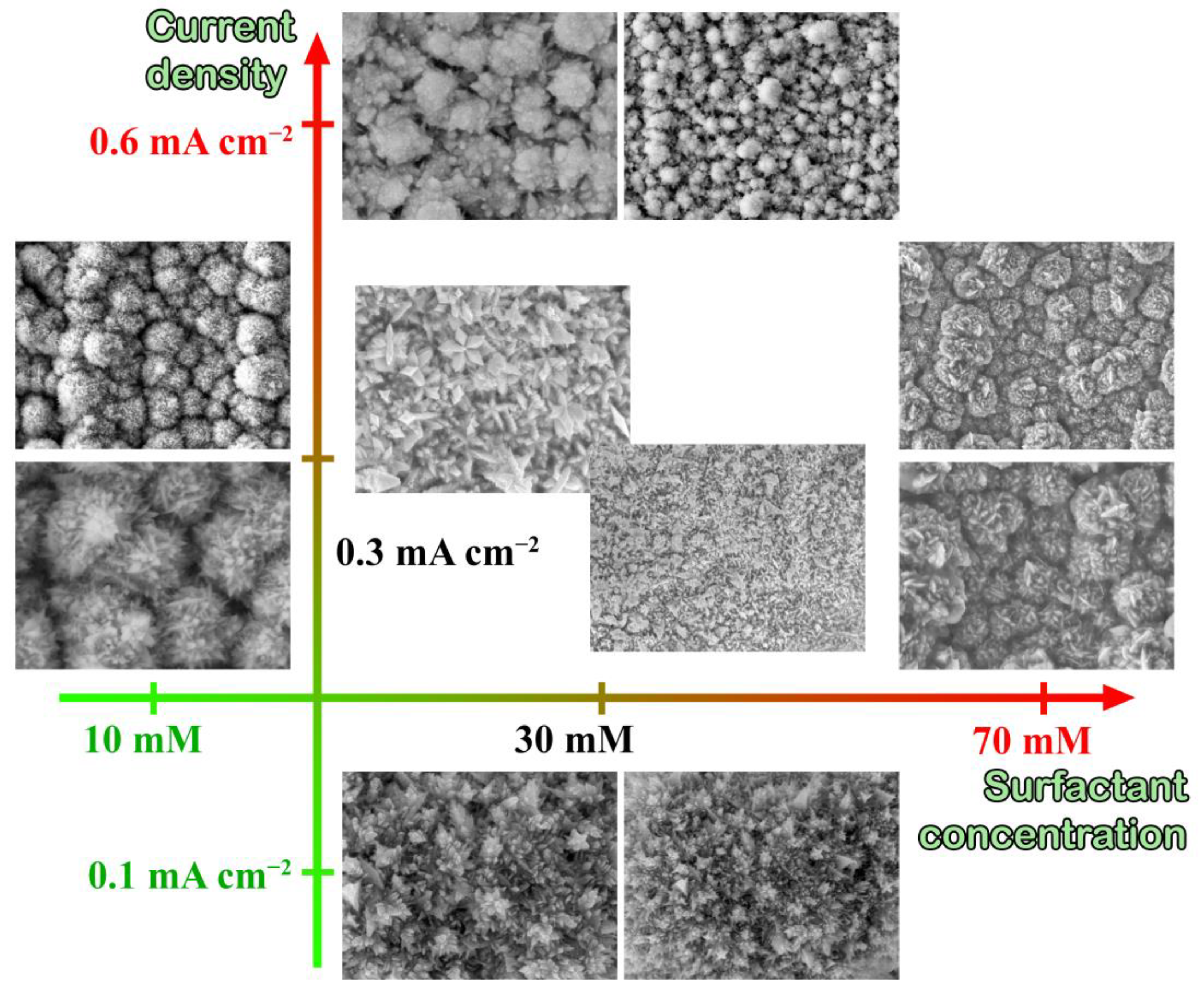
3.1. Development and Control of Metal Nanoparticles Synthetic Methods
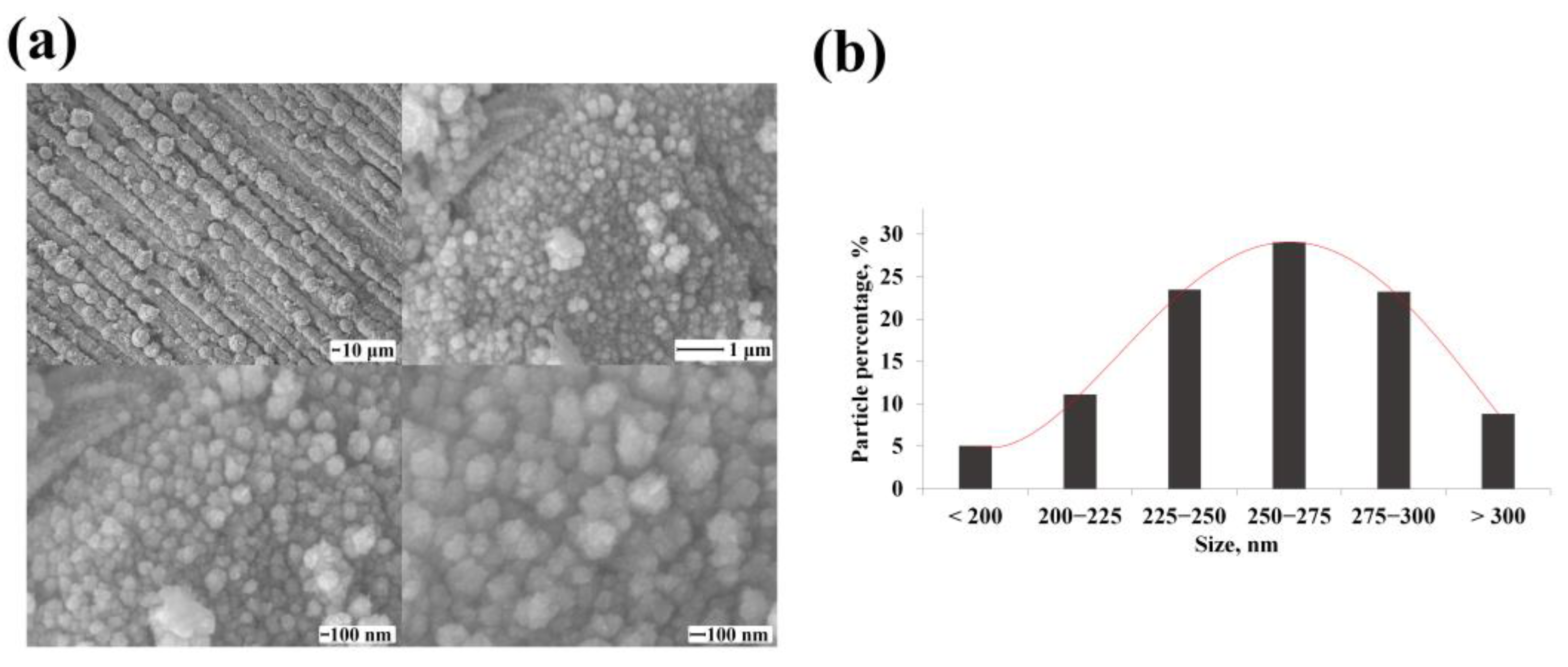
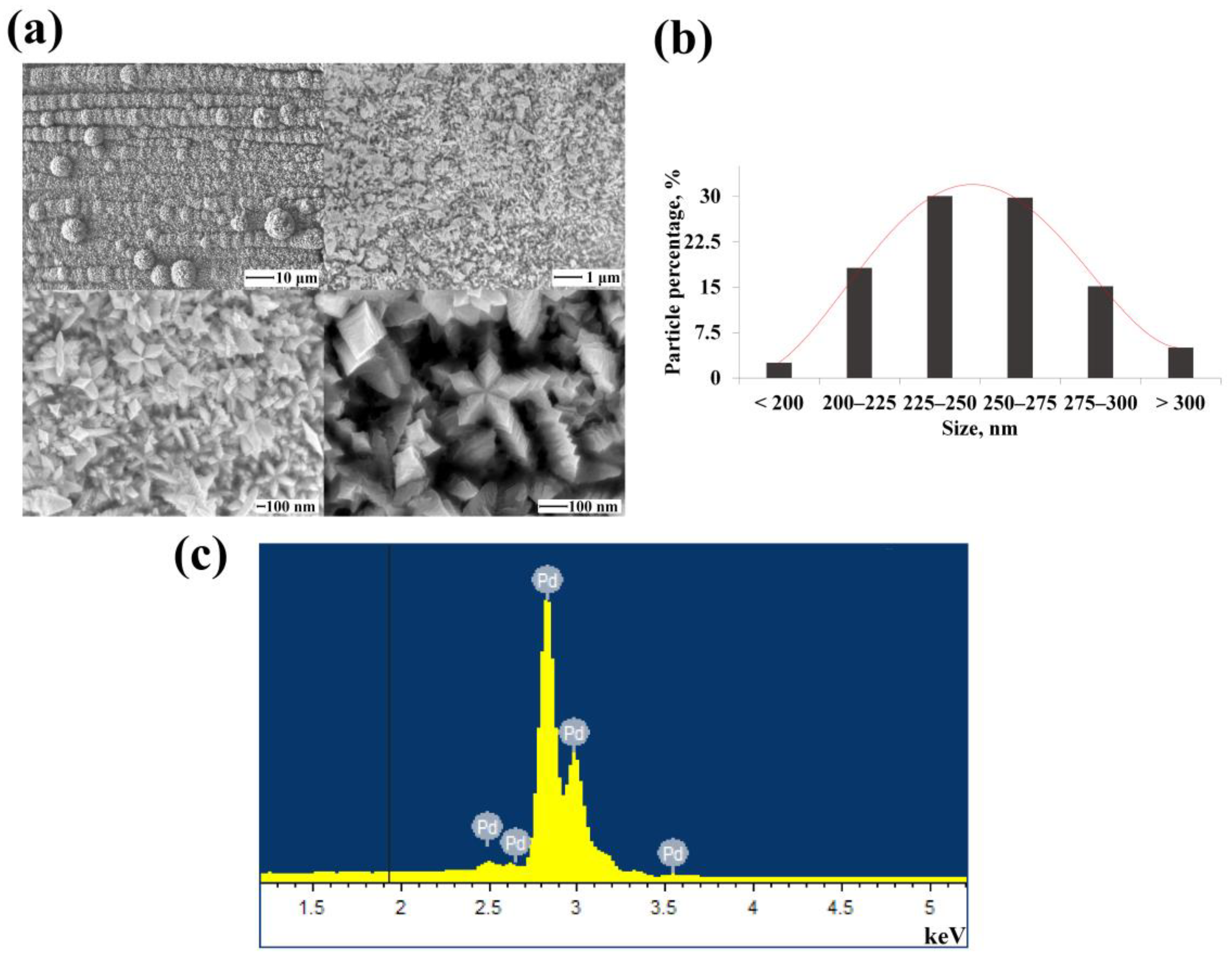
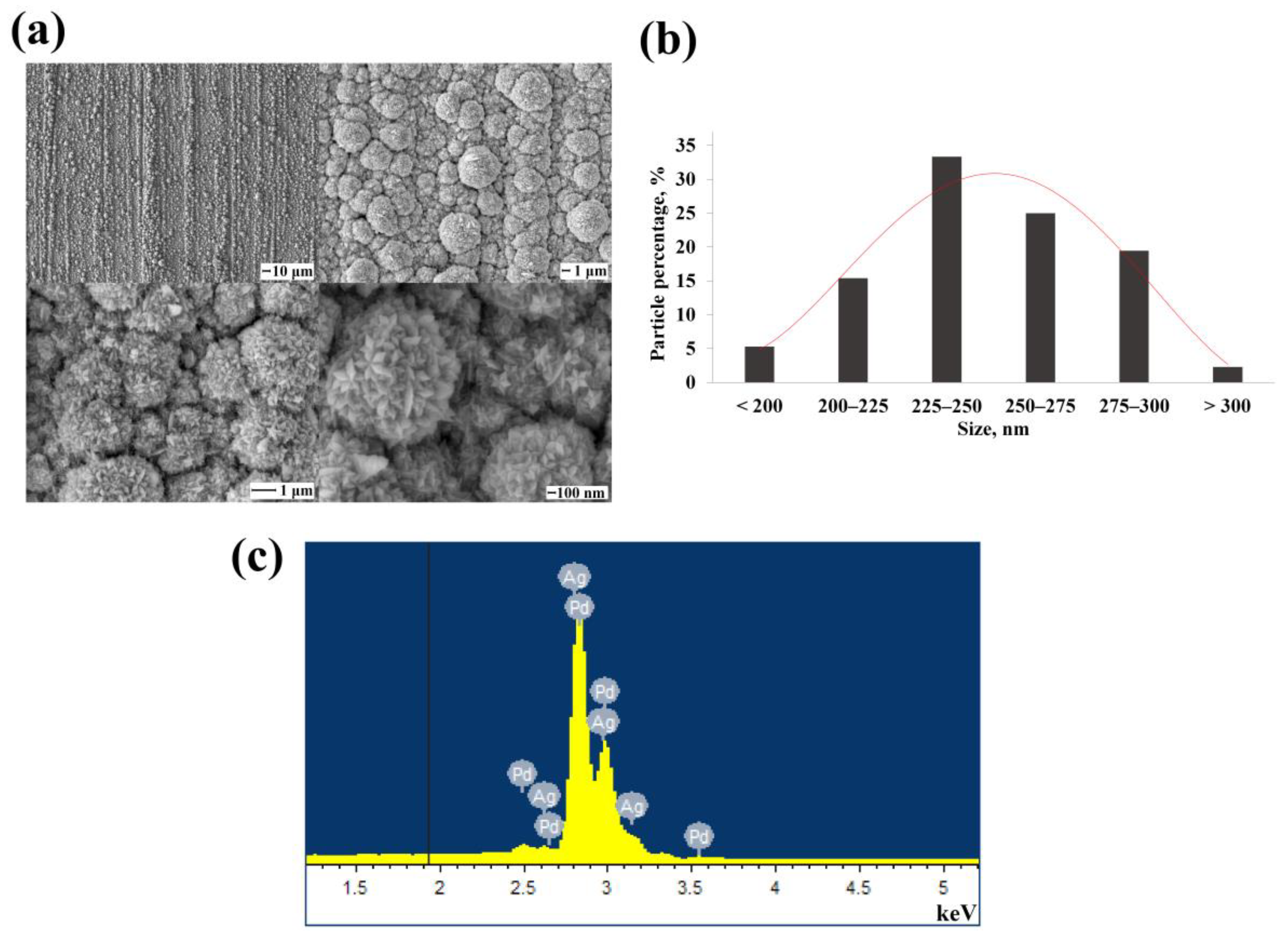
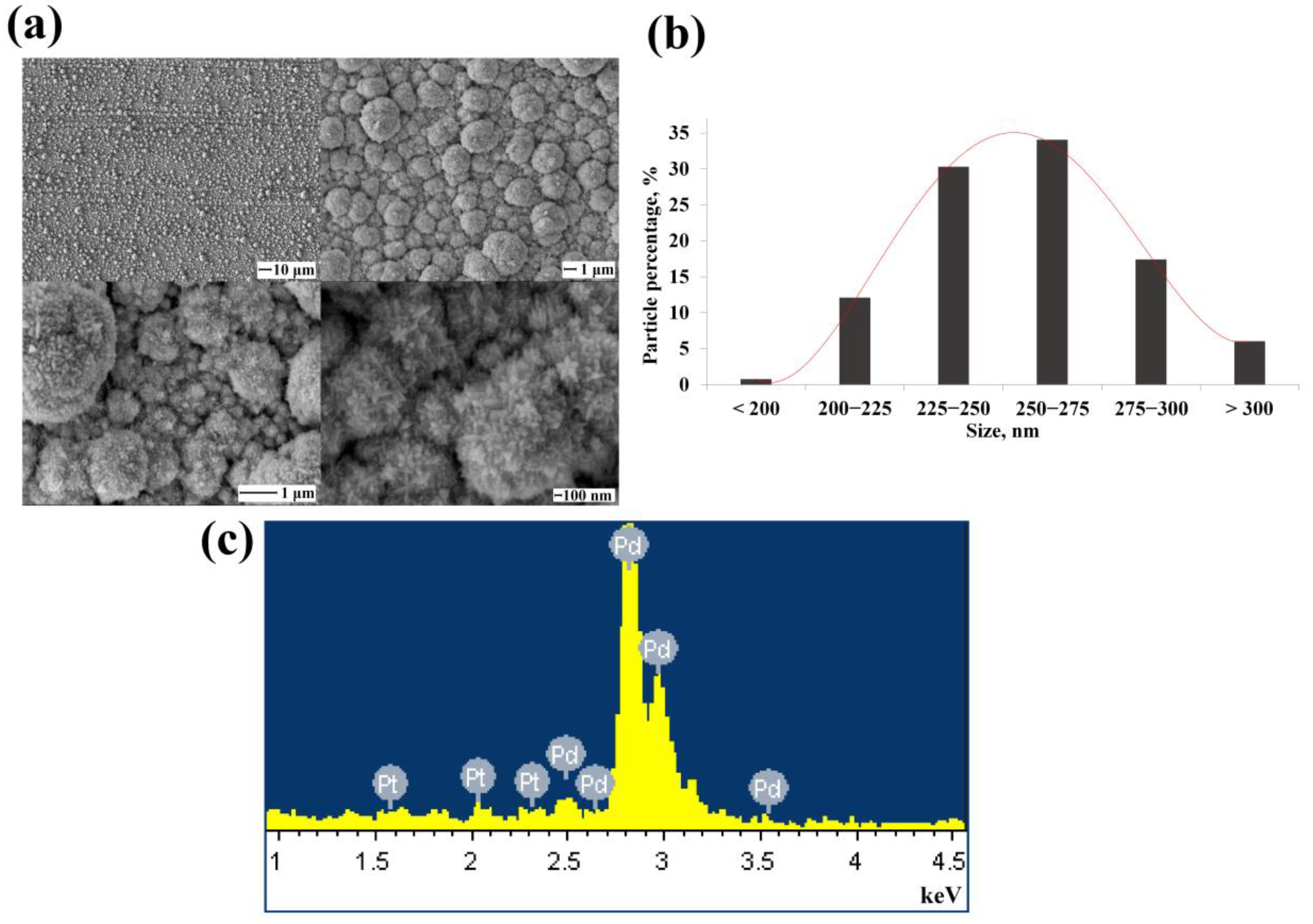
3.2. Morphology and Shaping Characteristics of the Synthesized Mono- and Bimetallic Nanoparticles Based on Pd, Pd-Ag, Pd-Pt
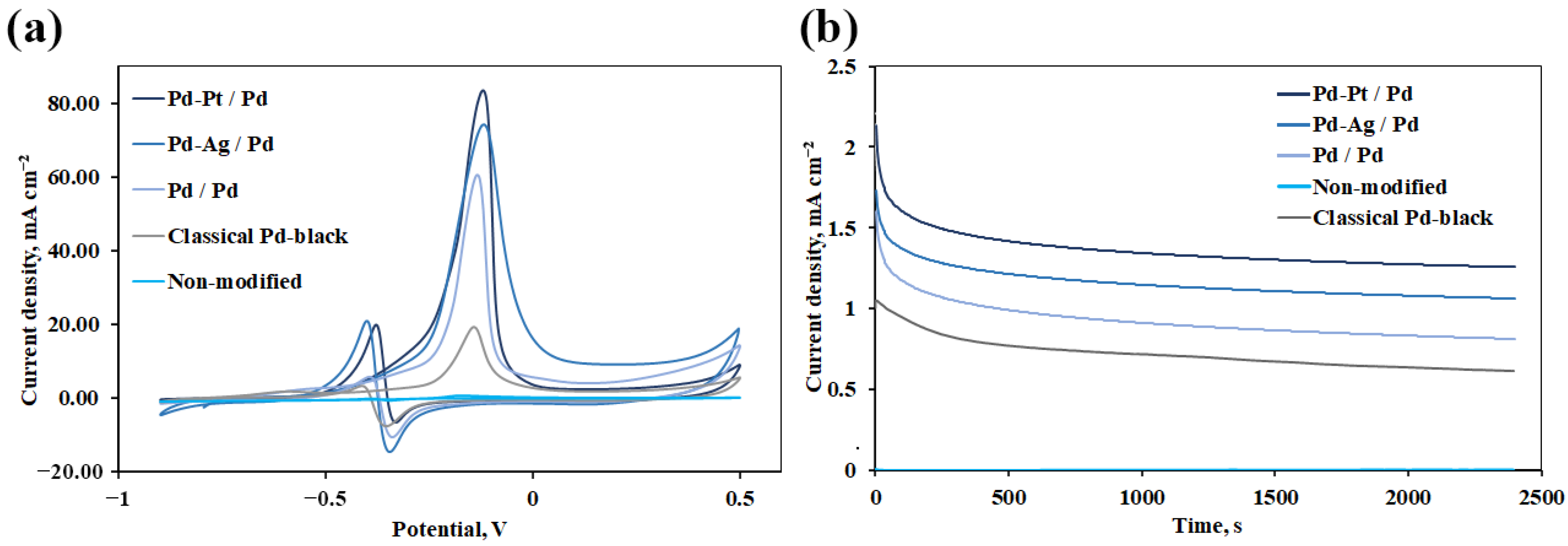
3.3. Electrochemical Study of Synthesized Mono- and Bimetallic Nanoparticles Based on Pd, Pd-Ag, Pd-Pt
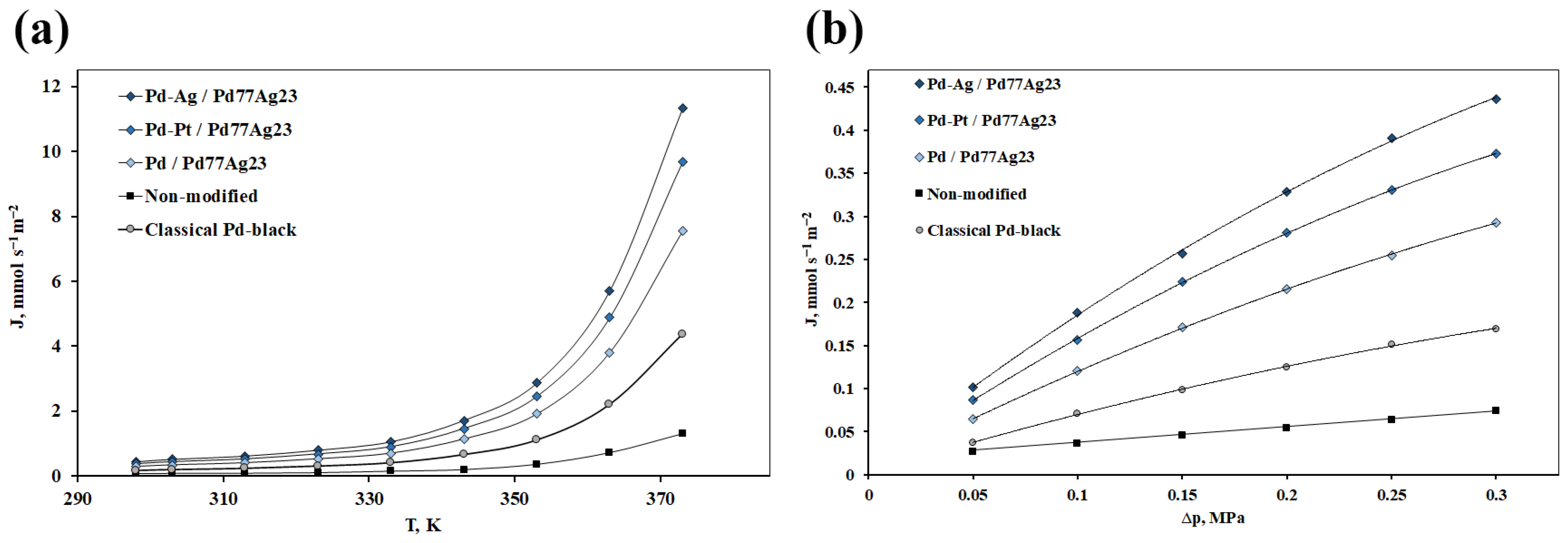
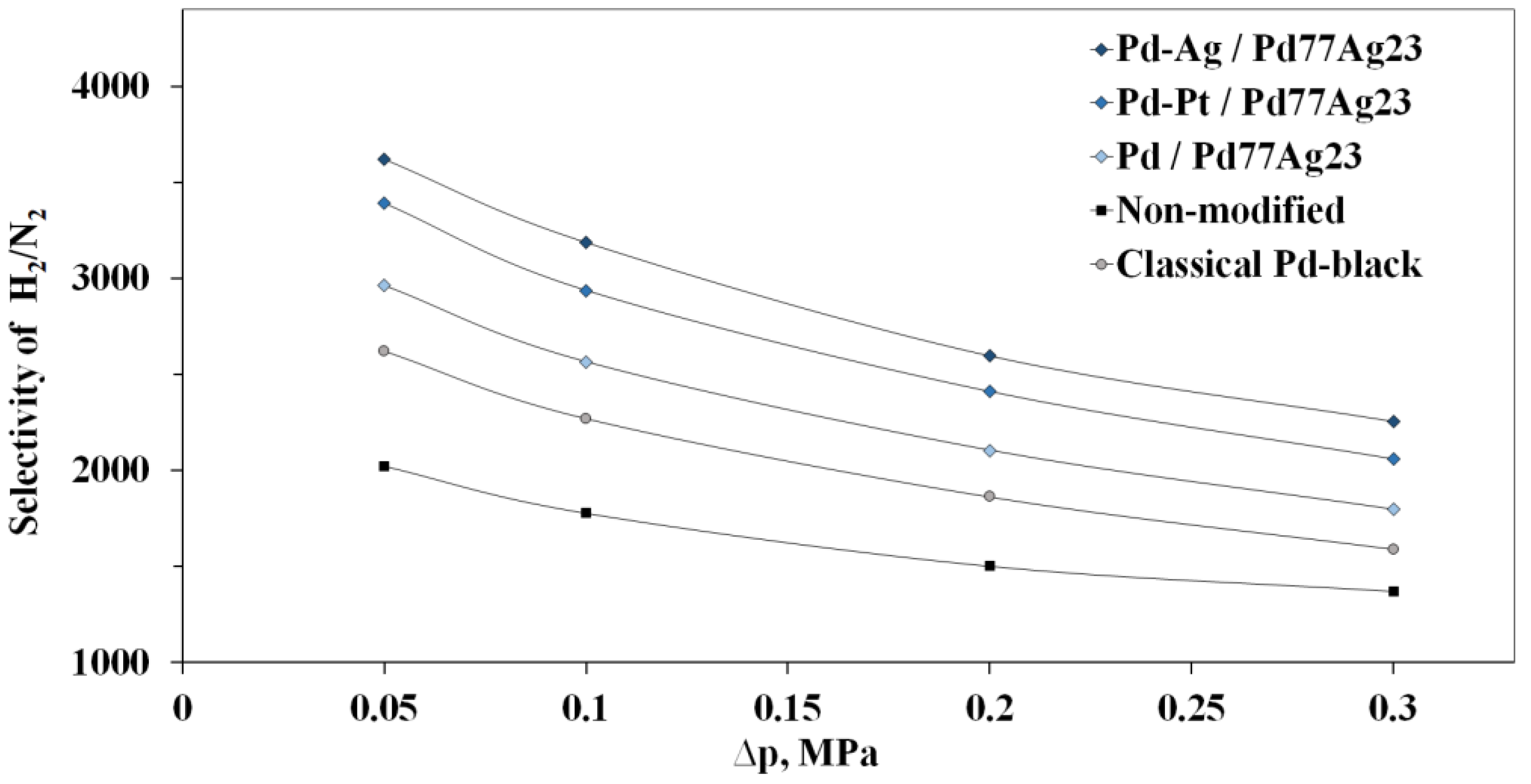
3.4. Study of Synthesized Mono- and Bimetallic Nanoparticles Based on Pd, Pd-Ag, Pd-Pt in Hydrogen Transport Processes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alafnan, A.; Rizvi, S.M.D.; Alshammari, A.S.; Faiyaz, S.S.M.; Lila, A.S.A.; Katamesh, A.A.; Khafagy, E.-S.; Alotaibi, H.F.; Ahmed, A.B.F. Gold Nanoparticle-Based Resuscitation of Cefoxitin against Clinical Pathogens: A Nano-Antibiotic Strategy to Overcome Resistance. Nanomaterials 2022, 12, 3643. [Google Scholar] [CrossRef] [PubMed]
- Jawhari, A.H.; Hasan, N.; Radini, I.A.; Narasimharao, K.; Malik, M.A. Noble Metals Deposited LaMnO3 Nanocomposites for Photocatalytic H2 Production. Nanomaterials 2022, 12, 2985. [Google Scholar] [CrossRef]
- Fernández-Arias, M.; Vilas, A.M.; Boutinguiza, M.; Rodríguez, D.; Arias-González, F.; Pou-Álvarez, P.; Riveiro, A.; Gil, J.; Pou, J. Palladium Nanoparticles Synthesized by Laser Ablation in Liquids for Antimicrobial Applications. Nanomaterials 2022, 12, 2621. [Google Scholar] [CrossRef]
- Song, L.; Tan, K.; Ye, Y.; Zhu, B.; Zhang, S.; Huang, W. Amine-Functionalized Natural Halloysite Nanotubes Supported Metallic (Pd, Au, Ag) Nanoparticles and Their Catalytic Performance for Dehydrogenation of Formic Acid. Nanomaterials 2022, 12, 2414. [Google Scholar] [CrossRef]
- Zhao, J.; Rafat, M.N.; Yoon, C.-M.; Oh, W.-C. Novel Approach to Synthesis of AgZnS and TiO2 Decorated on Reduced Graphene Oxide Ternary Nanocomposite for Hydrogen Evolution Effect of Enhanced Synergetic Factors. Nanomaterials 2022, 12, 3639. [Google Scholar] [CrossRef] [PubMed]
- Fahim, T.; Laouedj, S.; Abderrahmane, A.; Alotaibi, S.; Younis, O.; Ali, H.M. Heat Transfer Enhancement in Parabolic through Solar Receiver: A Three-Dimensional Numerical Investigation. Nanomaterials 2022, 12, 419. [Google Scholar] [CrossRef] [PubMed]
- Takai, T.; Shibatani, A.; Asakuma, Y.; Saptoro, A.; Phan, C. Microwave-assisted nanoparticle synthesis enhanced with addition of surfactant. Chem. Eng. Res. Des. 2022, 182, 714–718. [Google Scholar] [CrossRef]
- Chan, S.S.; Low, S.S.; Chew, K.W.; Ling, T.C.; Rinklebe, J.; Juan, J.C.; Ng, E.P.; Show, P.L. Prospects and environmental sustainability of phyconanotechnology: A review on algae-mediated metal nanoparticles synthesis and mechanism. Environ. Res. 2022, 212, 113140. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Liao, H.-G.; Rao, L.; Jiang, Y.-X.; Huang, R.; Zhang, B.-W.; He, C.-L.; Tian, N.; Sun, S.-G. Shape Evolution of Platinum Nanocrystals by Electrochemistry. Electrochim. Acta 2014, 140, 345–351. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Liu, C.; Rouvimov, S.; Luo, T.; Feng, S.-P. Electrochemical Synthesis to Convert a Ag Film into Ag Nanoflowers with High Electrocatalytic Activity. Chem. Commun. 2017, 53, 6752–6755. [Google Scholar] [CrossRef]
- Li, A.; Chen, Y.; Zhuo, K.; Wang, C.; Wang, C.; Wang, J. Facile and Shape-Controlled Electrochemical Synthesis of Gold Nanocrystals by Changing Water Contents in Deep Eutectic Solvents and their Electrocatalytic Activity. RSC Adv. 2016, 6, 8786–8790. [Google Scholar] [CrossRef]
- Elrouby, M.; Abdel-Mawgoud, A.M.; El-Rahman, R.A. Synthesis of iron oxides nanoparticles with very high saturation magnetization form TEA-Fe(III) complex via electrochemical deposition for supercapacitor applications. J. Mol. Struct. 2017, 1147, 84–95. [Google Scholar] [CrossRef]
- Chee, S.W.; Arce-Ramos, J.M.; Li, W.; Genest, A.; Mirsaidov, U. Structural changes in noble metal nanoparticles during CO oxidation and their impact on catalyst activity. Nat. Commun. 2020, 11, 2133. [Google Scholar] [CrossRef]
- Basov, A.; Dzhimak, S.; Sokolov, M.; Malyshko, V.; Moiseev, A.; Butina, E.; Elkina, A.; Baryshev, M. Changes in Number and Antibacterial Activity of Silver Nanoparticles on the Surface of Suture Materials during Cyclic Freezing. Nanomaterials 2022, 12, 1164. [Google Scholar] [CrossRef]
- Pan, Y.-T.; Yang, H. Design of bimetallic catalysts and electrocatalysts through the control of reactive environments. Nano Today 2020, 31, 100832. [Google Scholar] [CrossRef]
- Lytkina-Payen, A.; Tabachkova, N.; Yaroslavtsev, A. Methanol Steam Reforming on Bimetallic Catalysts Based on In and Nb Doped Titania or Zirconia: A Support Effect. Processes 2022, 10, 19. [Google Scholar] [CrossRef]
- Maier, A.; van Oossanen, R.; van Rhoon, G.C.; Pignol, J.-P.; Dugulan, I.; Denkova, A.G.; Djanashvili, K. From Structure to Function: Understanding Synthetic Conditions in Relation to Magnetic Properties of Hybrid Pd/Fe-Oxide Nanoparticles. Nanomaterials 2022, 12, 3649. [Google Scholar] [CrossRef]
- Ruditskiy, A.; Choi, S.-I.; Peng, H.-C.; Xia, Y. Shape-controlled metal nanocrystals for catalytic applications. MRS Bull. 2014, 39, 727–737. [Google Scholar] [CrossRef]
- Yang, T.; Ma, Y.; Huang, Q.; Cao, G. Palladium–iridium nanocrystals for enhancement of electrocatalytic activity toward oxygen reduction reaction. Nano Energy 2016, 19, 257–268. [Google Scholar] [CrossRef]
- Tang, J.; Seo, O.; Rocabado, D.S.R.; Koitaya, T.; Yamamoto, S.; Nanba, Y.; Song, C.; Kim, J.; Yoshigoe, A.; Koyama, M.; et al. Hydrogen absorption and diffusion behaviors in cube-shaped palladium nanoparticles revealed by ambient-pressure X-ray photoelectron spectroscopy. Appl. Surf. Sci. 2022, 587, 152797. [Google Scholar] [CrossRef]
- Alekseeva, S.; Strach, M.; Nilsson, S.; Fritzsche, J.; Zhdanov, V.P.; Langhammer, C. Grain-growth mediated hydrogen sorption kinetics and compensation effect in single Pd nanoparticles. Nat. Commun. 2021, 12, 5427. [Google Scholar] [CrossRef]
- Lao, M.; Li, P.; Jiang, Y.; Pan, H.; Dou, S.X.; Sun, W. From fundamentals and theories to heterostructured electrocatalyst design: An in-depth understanding of alkaline hydrogen evolution reaction. Nano Energy 2022, 98, 107231. [Google Scholar] [CrossRef]
- Ali, M.R.K.; Wu, Y.; El-Sayed, M.A. Gold-Nanoparticle-Assisted Plasmonic Photothermal Therapy Advances Toward Clinical Application. J. Phys. Chem. C 2019, 123, 15375–15393. [Google Scholar] [CrossRef]
- Mansoor, A.; Khurshid, Z.; Khan, M.T.; Mansoor, E.; Butt, F.A.; Jamal, A.; Palma, P.J. Medical and Dental Applications of Titania Nanoparticles: An Overview. Nanomaterials 2022, 12, 3670. [Google Scholar] [CrossRef]
- Rosales, S.A.; González, F.; Moreno, F.; Gutiérrez, Y. Non-Absorbing Dielectric Materials for Surface-Enhanced Spectroscopies and Chiral Sensing in the UV. Nanomaterials 2020, 10, 2078. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.F.; Weiner, R.G.; Skrabalak, S.E. Symmetry-Dependent Optical Properties of Stellated Nanocrystals. J. Phys. Chem. C 2016, 120, 20563–20571. [Google Scholar] [CrossRef]
- Yaldagard, M.; Shahbaz, M.; Kim, H.W.; Kim, S.S. Ethanol Electro-Oxidation on Catalysts with S-ZrO2-Decorated Graphene as Support in Fuel Cell Applications. Nanomaterials 2022, 12, 3327. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yan, X.; Zhang, M.; Dong, G.; Moro, R.; Ma, Y.; Ma, L. Real-time size tuning and measuring of silver nanoparticles by cyclic voltammetry and Raman spectroscopy. Mater. Lett. 2022, 310, 131420. [Google Scholar] [CrossRef]
- Filippov, S.P.; Yaroslavtsev, A.B. Hydrogen energy: Development prospects and materials. Russ. Chem. Rev. 2021, 90, 627–643. [Google Scholar] [CrossRef]
- Mironova, E.Y.; Ermilova, M.M.; Orekhova, N.V.; Basov, N.L.; Yaroslavtsev, A.B. Hydrogen Production by Ethanol Steam Reforming in the Presence of Pd-, Pt-, Ru-, and Ni-Containing Nanodiamonds in Conventional and Membrane Reactors. Membr. Membr. Technol. 2019, 1, 246–253. [Google Scholar] [CrossRef]
- Solla-Gullón, J.; Feliu, J.M. State of the art in the electrochemical characterization of the surface structure of shape-controlled Pt, Au, and Pd nanoparticles. Curr. Opin. Electrochem. 2020, 22, 65–71. [Google Scholar] [CrossRef]
- Petriev, I.S.; Pushankina, P.D.; Lutsenko, I.S.; Baryshev, M.G. Anomalous Kinetic Characteristics of Hydrogen Transport through Pd–Cu Membranes Modified by Pentatwinned Flower-Shaped Palladium Nanocrystallites with High-Index Facets. Tech. Phys. Lett. 2021, 47, 803–806. [Google Scholar] [CrossRef]
- Petriev, I.S.; Pushankina, P.D.; Lutsenko, I.S.; Baryshev, M.G. The Influence of a Crystallographically Atypical Pentagonal Nanostructured Coating on the Limiting Stage of Low-Temperature Hydrogen Transport through Pd–Cu Membranes. Dokl. Phys. 2021, 66, 209–213. [Google Scholar] [CrossRef]
- Vidal-Iglesias, F.J.; Solla-Gullón, J.; Herrero, E.; Montiel, V.; Aldaz, A.; Feliu, J.M. Evaluating the ozone cleaning treatment in shape-controlled Pt nanoparticles: Evidences of atomic surface disordering. Electrochem. Commun. 2011, 13, 502–505. [Google Scholar] [CrossRef]
- Petriev, I.; Pushankina, P.; Lutsenko, I.; Shostak, N.; Baryshev, M. Synthesis, Electrocatalytic and Gas Transport Characteristics of Pentagonally Structured Star-Shaped Nanocrystallites of Pd-Ag. Nanomaterials 2020, 10, 2081. [Google Scholar] [CrossRef]
- Petriev, I.; Pushankina, P.; Shostak, N.; Baryshev, M. Gas-Transport Characteristics of PdCu–Nb–PdCu Membranes Modified with Nanostructured Palladium Coating. Int. J. Mol. Sci. 2022, 23, 228. [Google Scholar] [CrossRef]
- Petriev, I.S.; Baryshev, M.G.; Voronin, K.A.; Lutsenko, I.S.; Pushankina, P.D.; Kopytov, G.F. Gas Transmission Properties of Pd–Ag Membranes Coated with Modifying Layer. Russ. Phys. J. 2020, 63, 457–461. [Google Scholar] [CrossRef]
- Garnier, E.; Vidal-Iglesias, F.J.; Feliu, J.M.; Solla-Gullón, J. Surface Structure Characterization of Shape and Size Controlled Pd Nanoparticles by Cu UPD: A Quantitative Approach. Front. Chem. 2019, 7, 527. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Xu, D.; Sun, L.; Henzie, J.; Suib, S.L.; Yamauchi, Y.; Liu, B. Ternary Palladium–Boron–Phosphorus Alloy Mesoporous Nanospheres for Highly Efficient Electrocatalysis. ACS Nano 2019, 13, 12052–12061. [Google Scholar] [CrossRef]
- Strasser, P.; Gliech, M.; Kuehl, S.; Moeller, T. Electrochemical processes on solid shaped nanoparticles with defined facets. Chem. Soc. Rev. 2018, 47, 715–735. [Google Scholar] [CrossRef]
- Petriev, I.; Pushankina, P.; Bolotin, S.; Lutsenko, I.; Kukueva, E.; Baryshev, M. The influence of modifying nanoflower and nanostar type Pd coatings on low temperature hydrogen permeability through Pd-containing membranes. J. Membr. Sci. 2021, 620, 118894. [Google Scholar] [CrossRef]
- King, M.E.; Personick, M.L. Defects by design: Synthesis of palladium nanoparticles with extended twin defects and corrugated surfaces. Nanoscale 2017, 9, 17914–17921. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ruiz, V.-F.; González-Olvera, R.; Díaz-Pardo, R.; Betancourt, I.; Zumeta-Dubé, I.; Díaz, D.; Farfán, N.; Arellano-Jiménez, M.J. Mechanochemically obtained Pd–Ag nanoalloys. Structural considerations and catalytic activity. Materialia 2018, 4, 166–174. [Google Scholar] [CrossRef]
- Vegard, L. Die Konstitution der Mischkristalle und die Raumfüllung der Atome. Z. Phys. 1921, 5, 17–26. [Google Scholar] [CrossRef]
- Bochicchio, D.; Ferrando, R.; Novakovic, R.; Panizon, E.; Rossi, G. Chemical ordering in magic-size Ag–Pd nanoparticles. Phys. Chem. Chem. Phys. 2014, 16, 26478–26484. [Google Scholar] [CrossRef]
- Guo, M.; He, H.; Zhang, Z.; Liu, Z.; Xie, F.; Shan, B.; Duan, X. Structural optimization and melting behavior investigation of Pd-Ag bimetallic nanoparticles by molecular simulations. Comput. Mater. Sci. 2020, 176, 109520. [Google Scholar] [CrossRef]
- Paz-Borbón, L.O.; Johnston, R.L.; Barcaro, G.; Fortunelli, A. Structural motifs, mixing, and segregation effects in 38-atom binary clusters. J. Chem. Phys. 2008, 128, 134517. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Lin, L.L.; Ma, D. Construction of Pd-based nanocatalysts for fuel cells: Opportunities and challenges. Catal. Sci. Technol. 2014, 4, 4116–4128. [Google Scholar] [CrossRef]
- Wang, H.; Sheng, L.; Zhao, X.; An, K.; Ou, Z.; Fang, Y. One-step synthesis of Pt-Pd catalyst nanoparticles supported on few-layer graphene for methanol oxidation. Curr. Appl. Phys. 2018, 18, 898–904. [Google Scholar] [CrossRef]
- Hanifah, M.F.R.; Jaafar, J.; Othman, M.H.D.; Ismail, A.F.; Rahman, M.A.; Yusof, N.; Aziz, F.; Rahman, N.A.A. One-pot synthesis of efficient reduced graphene oxide supported binary Pt-Pd alloy nanoparticles as superior electro-catalyst and its electro-catalytic performance toward methanol electro-oxidation reaction in direct methanol fuel cell. J. Alloys Compd. 2019, 793, 232–246. [Google Scholar] [CrossRef]
- Zhang, W.; Yao, Q.; Wu, X.; Fu, Y.; Deng, K.; Wang, X. Intimately coupled hybrid of graphitic carbon nitride nanoflakelets with reduced graphene oxide for supporting Pd nanoparticles: A stable nanocatalyst with high catalytic activity towards formic acid and methanol electrooxidation. Electrochim. Acta 2016, 200, 131–141. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pushankina, P.; Baryshev, M.; Petriev, I. Synthesis and Study of Palladium Mono- and Bimetallic (with Ag and Pt) Nanoparticles in Catalytic and Membrane Hydrogen Processes. Nanomaterials 2022, 12, 4178. https://doi.org/10.3390/nano12234178
Pushankina P, Baryshev M, Petriev I. Synthesis and Study of Palladium Mono- and Bimetallic (with Ag and Pt) Nanoparticles in Catalytic and Membrane Hydrogen Processes. Nanomaterials. 2022; 12(23):4178. https://doi.org/10.3390/nano12234178
Chicago/Turabian StylePushankina, Polina, Mikhail Baryshev, and Iliya Petriev. 2022. "Synthesis and Study of Palladium Mono- and Bimetallic (with Ag and Pt) Nanoparticles in Catalytic and Membrane Hydrogen Processes" Nanomaterials 12, no. 23: 4178. https://doi.org/10.3390/nano12234178
APA StylePushankina, P., Baryshev, M., & Petriev, I. (2022). Synthesis and Study of Palladium Mono- and Bimetallic (with Ag and Pt) Nanoparticles in Catalytic and Membrane Hydrogen Processes. Nanomaterials, 12(23), 4178. https://doi.org/10.3390/nano12234178






