Antibacterial Activity of Two Zn-MOFs Containing a Tricarboxylate Linker
Abstract
1. Introduction
2. Materials and Methods
2.1. Physicochemical Characterization
2.2. Antibacterial Activity
2.3. Crystallographic Refinement and Structure Solution
3. Results and Discussion
3.1. Synthesis and Crystal Structure Description of GR-MOF-8 and 9
3.2. Physicochemical Characterization
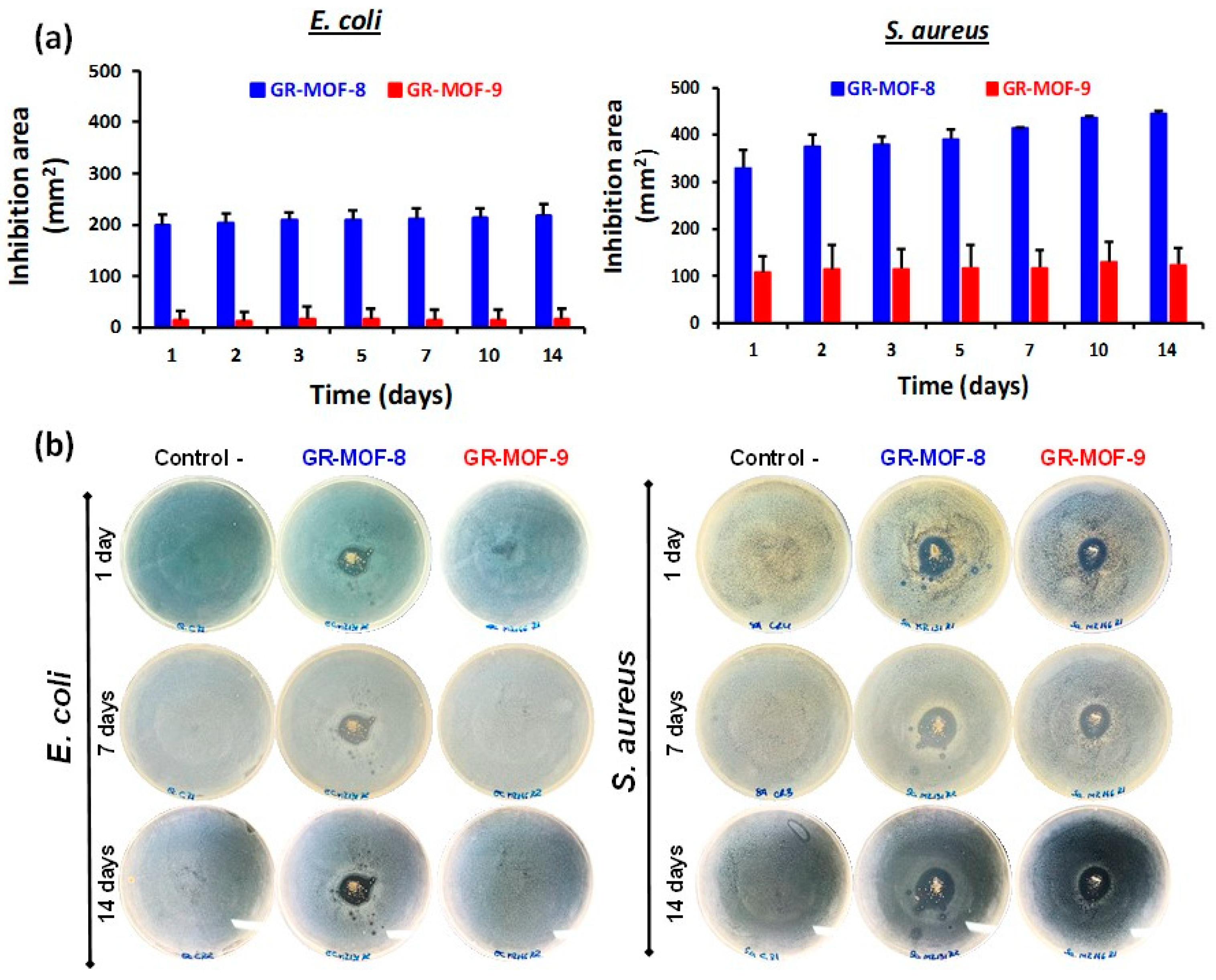
3.3. Antibacterial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Geisinger, E.; Isberg, R.R. Interplay between antibiotic resistance and virulence during Disease promoted by multidrug-resistant bacteria. J. Infect. Dis. 2017, 215, S9–S17. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Antimicrobial Resistance in the EU/EEA (EARS-Net); European Centre for Disease Prevention and Control: Solna, Sweden, 2020. [Google Scholar]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A Mini Review of Antibacterial Properties of ZnO Nanoparticles. Front. Phys. 2021, 9, 641481. [Google Scholar] [CrossRef]
- Prasad, S. Discovery of zinc for human health and biomarkers of zinc deficiency. In Molecular, Genetic, and Nutritional Aspects of Major and Trace Minerals; Elsevier: Amsterdam, The Netherlands, 2017; pp. 241–260. [Google Scholar]
- Bishop, G.M.; Dringen, R.; Robinson, S.R. Zinc stimulates the production of toxic reactive oxygen species (ROS) and inhibits glutathione reductase in astrocytes. Free Radic. Biol. Med. 2007, 42, 1222–1230. [Google Scholar] [CrossRef]
- Ryu, J.; Shin, C.; Choi, J.W.; Min, H.; Ryu, J.; Choi, C.R.; Ko, K. Depletion of intracellular glutathione mediates zinc-induced cell death in rat primary astrocytes. Exp. Brain Res. 2002, 143, 257–263. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Xia, Q.; Yuan, G.; He, Q.; Cui, Y. Multiple topological isomerism of three-connected networks in silver-based metal-organoboron frameworks. Chem. Commun. 2010, 46, 2608–2610. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic frameworks. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Y.; Gong, Q.; Li, Z.; Li, J. MOFs for CO2 capture and separation from flue gas mixtures: The effect of multifunctional sites on their adsorption capacity and selectivity. Chem. Commun. 2013, 49, 653–661. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Li, D.S.; Bu, X.; Feng, P. Metal–Organic Frameworks for Separation. Adv. Mater. 2018, 30, 1705189. [Google Scholar] [CrossRef] [PubMed]
- García-Valdivia, A.A.; Pérez-Yáñez, S.; García, J.A.; Fernández, B.; Cepeda, J.; Rodríguez-Diéguez, A. Magnetic and Photoluminescent Sensors Based on Metal-Organic Frameworks Built up from 2-aminoisonicotinate. Sci. Rep. 2020, 10, 8843. [Google Scholar] [CrossRef] [PubMed]
- Echenique-Errandonea, E.; Pérez, J.M.; Rojas, S.; Cepeda, J.; Seco, J.M.; Fernández, I.; Rodríguez-Diéguez, A. A novel yttrium-based metal-organic framework for the efficient solvent-free catalytic synthesis of cyanohydrin silyl ethers. Dalt. Trans. 2021, 50, 11720–11724. [Google Scholar] [CrossRef] [PubMed]
- Vilela, S.; Devic, T.; Varez, A.; Salles, F.; Horcajada, P. A new proton-conducting Bi-carboxylate framework. Dalt. Trans. 2019, 48, 11181–11185. [Google Scholar] [CrossRef]
- Rojas, S.; Arenas-Vivo, A.; Horcajada, P. Metal-organic frameworks: A novel platform for combined advanced therapies. Coord. Chem. Rev. 2019, 388, 202–226. [Google Scholar] [CrossRef]
- Lee, C.Y.; Farha, O.K.; Hong, B.J.; Sarjeant, A.A.; Nguyen, S.T.; Hupp, J.T. Light-harvesting metal-organic frameworks (MOFs): Efficient strut-to-strut energy transfer in bodipy and porphyrin-based MOFs. J. Am. Chem. Soc. 2011, 133, 15858–15861. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Zhang, X.; Feng, Y.; Zhang, X.; Wang, H.; Yao, J. Modified metal-organic frameworks as photocatalysts. Appl. Catal. B Environ. 2018, 231, 317–342. [Google Scholar] [CrossRef]
- Bilal, M.; Adeel, M.; Rasheed, T.; Iqbal, H.M.N. Multifunctional metal-organic frameworks-based biocatalytic platforms: Recent developments and future prospects. J. Mater. Res. Technol. 2019, 8, 2359–2371. [Google Scholar] [CrossRef]
- Restrepo, J.; Serroukh, Z.; Santiago-Morales, J.; Aguado, S.; Gómez-Sal, P.; Mosquera, M.E.G.; Rosal, R. An Antibacterial Zn–MOF with Hydrazinebenzoate Linkers. Eur. J. Inorg. Chem. 2017, 2017, 574–580. [Google Scholar] [CrossRef]
- Wang, K.; Yin, Y.; Li, C.; Geng, Z.; Wang, Z. Facile synthesis of zinc(II)-carboxylate coordination polymer particles and their luminescent, biocompatible and antibacterial properties. CrystEngComm 2011, 13, 6231–6236. [Google Scholar] [CrossRef]
- Gwon, K.; Han, I.; Lee, S.; Kim, Y.; Lee, D.N. Novel Metal—Organic Framework-Based Photocrosslinked Hydrogel System for E ffi cient Antibacterial Applications. ACS Appl. Mater. Interfaces 2020, 12, 20234–20242. [Google Scholar] [CrossRef] [PubMed]
- Tamames-Tabar, C.; Imbuluzqueta, E.; Guillou, N.; Serre, C.; Miller, S.R.; Elkaïm, E.; Horcajada, P.; Blanco-Prieto, M.J. A Zn azelate MOF: Combining antibacterial effect. CrystEngComm 2015, 17, 456–462. [Google Scholar] [CrossRef]
- Taheri, M.; Ashok, D.; Sen, T.; Enge, T.G.; Verma, N.K.; Tricoli, A.; Lowe, A.R.; Nisbet, D.; Tsuzuki, T. Stability of ZIF-8 nanopowders in bacterial culture media and its implication for antibacterial properties. Chem. Eng. J. 2021, 413, 127511. [Google Scholar] [CrossRef]
- Pérez, J.M.; Rojas, S.; García-García, A.; Montes-Andrés, H.; Ruiz Martínez, C.; Romero-Cano, M.S.; Choquesillo-Lazarte, D.; Abdelkader-Fernández, V.K.; Pérez-Mendoza, M.; Cepeda, J.; et al. Catalytic Performance and Electrophoretic Behavior of an Yttrium-Organic Framework Based on a Tricarboxylic Asymmetric Alkyne. Inorg. Chem. 2022, 61, 1377–1384. [Google Scholar] [CrossRef]
- Bruker-AXS. Bruker APEX4, APEX4 V2021.1 2021; Bruker-AXS: Madison, WI, USA, 2021. [Google Scholar]
- Krause, L.S.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Cheng, X.; Guo, L.; Wang, H.; Gu, J.; Yang, Y.; Kirillova, M.V.; Kirillov, A.M. Coordination Polymers from Biphenyl-Dicarboxylate Linkers: Synthesis, Structural Diversity, Interpenetration, and Catalytic Properties. Inorg. Chem. 2022, 61, 12577–12590. [Google Scholar] [CrossRef]
- García-Valdivia, A.A.; Jannus, F.; García-García, A.; Choquesillo-Lazarte, D.; Fernández, B.; Medina-O’donnell, M.; Lupiáñez, J.A.; Cepeda, J.; Reyes-Zurita, F.J.; Rodríguez-Diéguez, A. Anti-cancer and anti-inflammatory activities of a new family of coordination compounds based on divalent transition metal ions and indazole-3-carboxylic acid. J. Inorg. Biochem. 2021, 215, 111308. [Google Scholar] [CrossRef] [PubMed]
- Nakhaei, M.; Akhbari, K.; Kalati, M.; Phuruangrat, A. Antibacterial activity of three zinc-terephthalate MOFs and its relation to their structural features. Inorg. Chim. Acta 2021, 522, 120353. [Google Scholar] [CrossRef]
- Kobayashi, S.D.; Malachowa, N.; Deleo, F.R. Pathogenesis of Staphylococcus aureus abscesses. Am. J. Pathol. 2015, 185, 1518–1527. [Google Scholar] [CrossRef]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Teplitski, M.; George, A.; Hochmuth, G. Salmonella and Pathogenic E. coli in the Crop Production Environment: Potential Sources, Survival, and Management. Virulence 2012, 5, 573–579. [Google Scholar] [CrossRef]
- David, M.Z.; Daum, R.S. Treatment of Staphylococcus aureus Infections. Curr. Top. Microbiol. Immunol. 2017, 409, 325–383. [Google Scholar] [PubMed]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The minimum inhibitory concentration of antibiotics: Methods, interpretation, clinical relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef] [PubMed]


| GR-MOF-8 | GR-MOF-9 | |
|---|---|---|
| Formula | C35H14O15Zn4 | C34H16O14Zn4 |
| Mr | 935.94 | 909.95 |
| Crystal system | Monoclinic | Tetragonal |
| Space group (no.) | P21/c | I-4 |
| a(Å) | 16.3308(17) | 22.1989(13) |
| b(Å) | 31.267(3) | 22.1989(13) |
| c(Å) | 16.7781(17) | 42.516(3) |
| α(°) | 90 | 90 |
| β(°) | 106.443(3) | 90 |
| γ(°) | 90 | 90 |
| V (Å3) | 8216.8(14) | 20,952(3) |
| Z | 4 | 8 |
| T (K) | 150(2) | 200(2) |
| ρcalc (g/cm3) | 0.757 | 0.577 |
| μ (mm−1) | 1.185 | 0.928 |
| F(000) | 1856 | 3616 |
| Radiation | MoKα (0.71073 λ) | MoKα (0.71073 λ) |
| Index ranges | −13 ≤ h ≤ 13, −26 ≤ k ≤ 26, −13 ≤ l ≤ 13 | −18 ≤ h ≤ 18, −18 ≤ k ≤ 18, −35 ≤ l ≤ 35 |
| GoF on F2 | 1.073 | 2.541 |
| Final R indices (I >= 2σ (I)) | R1 = 0.0776; wR2 = 0.2169 | R1 = 0.1753; wR2 = 0.4279 |
| Final R indices (all data) | R1 = 0.0926; wR2 = 0.2304 | R1 = 0.2030; wR2 = 0.4922 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas, S.; García-García, A.; Hidalgo, T.; Rosales, M.; Ruiz-Camino, D.; Salcedo-Abraira, P.; Montes-Andrés, H.; Choquesillo-Lazarte, D.; Rosal, R.; Horcajada, P.; et al. Antibacterial Activity of Two Zn-MOFs Containing a Tricarboxylate Linker. Nanomaterials 2022, 12, 4139. https://doi.org/10.3390/nano12234139
Rojas S, García-García A, Hidalgo T, Rosales M, Ruiz-Camino D, Salcedo-Abraira P, Montes-Andrés H, Choquesillo-Lazarte D, Rosal R, Horcajada P, et al. Antibacterial Activity of Two Zn-MOFs Containing a Tricarboxylate Linker. Nanomaterials. 2022; 12(23):4139. https://doi.org/10.3390/nano12234139
Chicago/Turabian StyleRojas, Sara, Amalia García-García, Tania Hidalgo, María Rosales, Daniel Ruiz-Camino, Pablo Salcedo-Abraira, Helena Montes-Andrés, Duane Choquesillo-Lazarte, Roberto Rosal, Patricia Horcajada, and et al. 2022. "Antibacterial Activity of Two Zn-MOFs Containing a Tricarboxylate Linker" Nanomaterials 12, no. 23: 4139. https://doi.org/10.3390/nano12234139
APA StyleRojas, S., García-García, A., Hidalgo, T., Rosales, M., Ruiz-Camino, D., Salcedo-Abraira, P., Montes-Andrés, H., Choquesillo-Lazarte, D., Rosal, R., Horcajada, P., & Rodríguez-Diéguez, A. (2022). Antibacterial Activity of Two Zn-MOFs Containing a Tricarboxylate Linker. Nanomaterials, 12(23), 4139. https://doi.org/10.3390/nano12234139