Hygroscopic Behavior of Polypropylene Nanocomposites Filled with Graphene Functionalized by Alkylated Chains
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
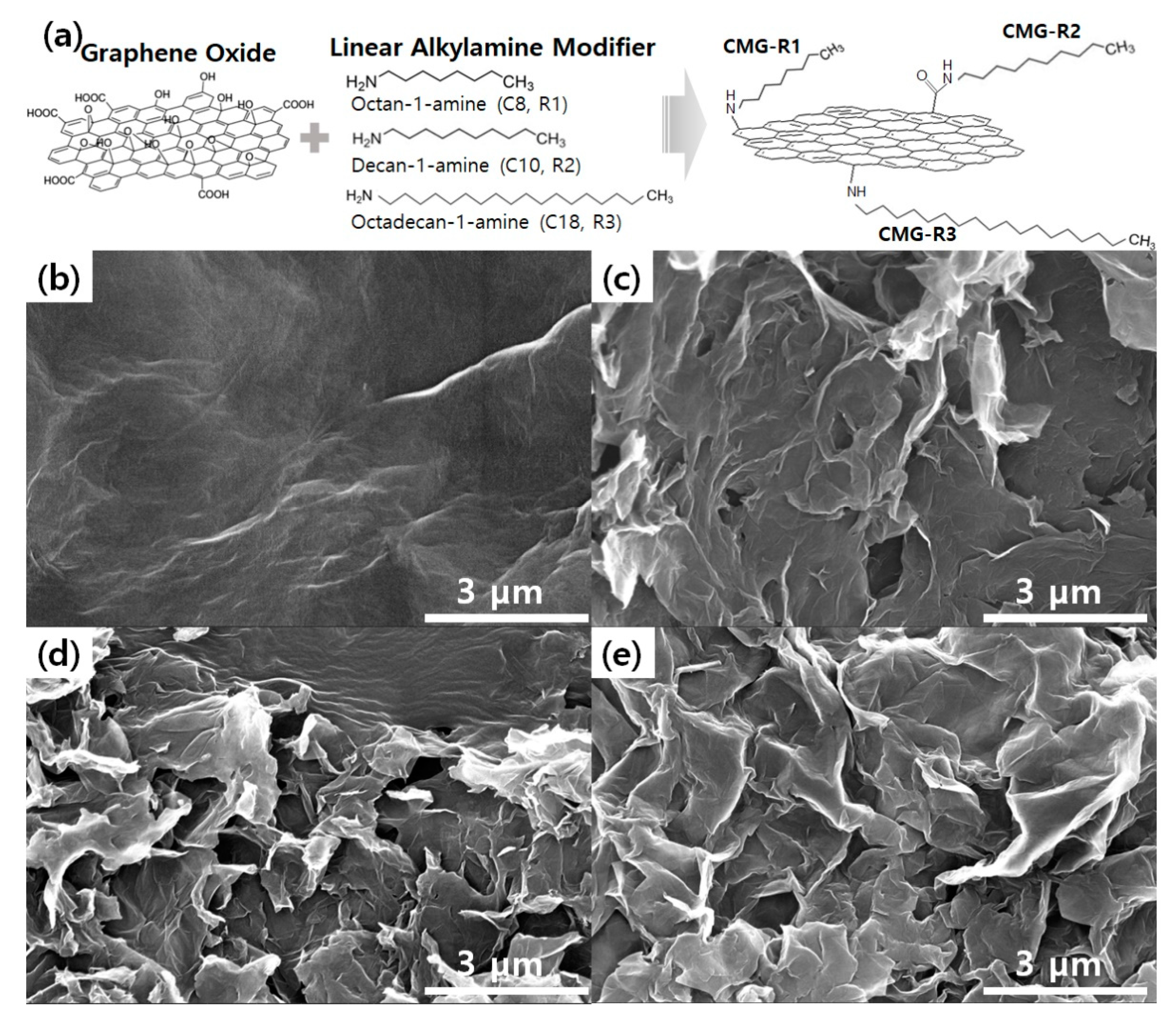
2.2. Preparation of Alkylated CMG (CMG-R)
2.3. Preparation of Nanocomposites
2.4. Characterization and Instruments
3. Results
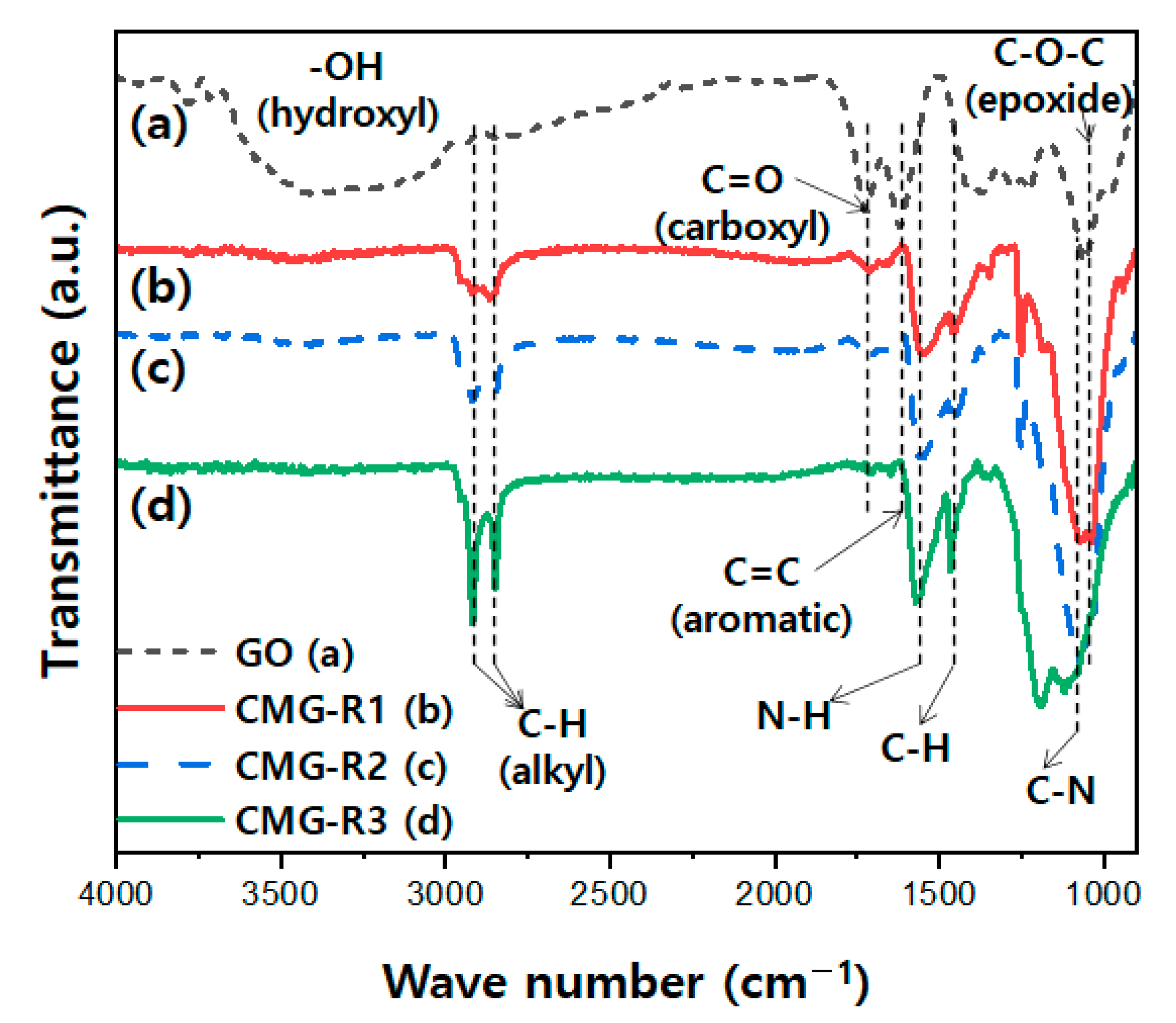
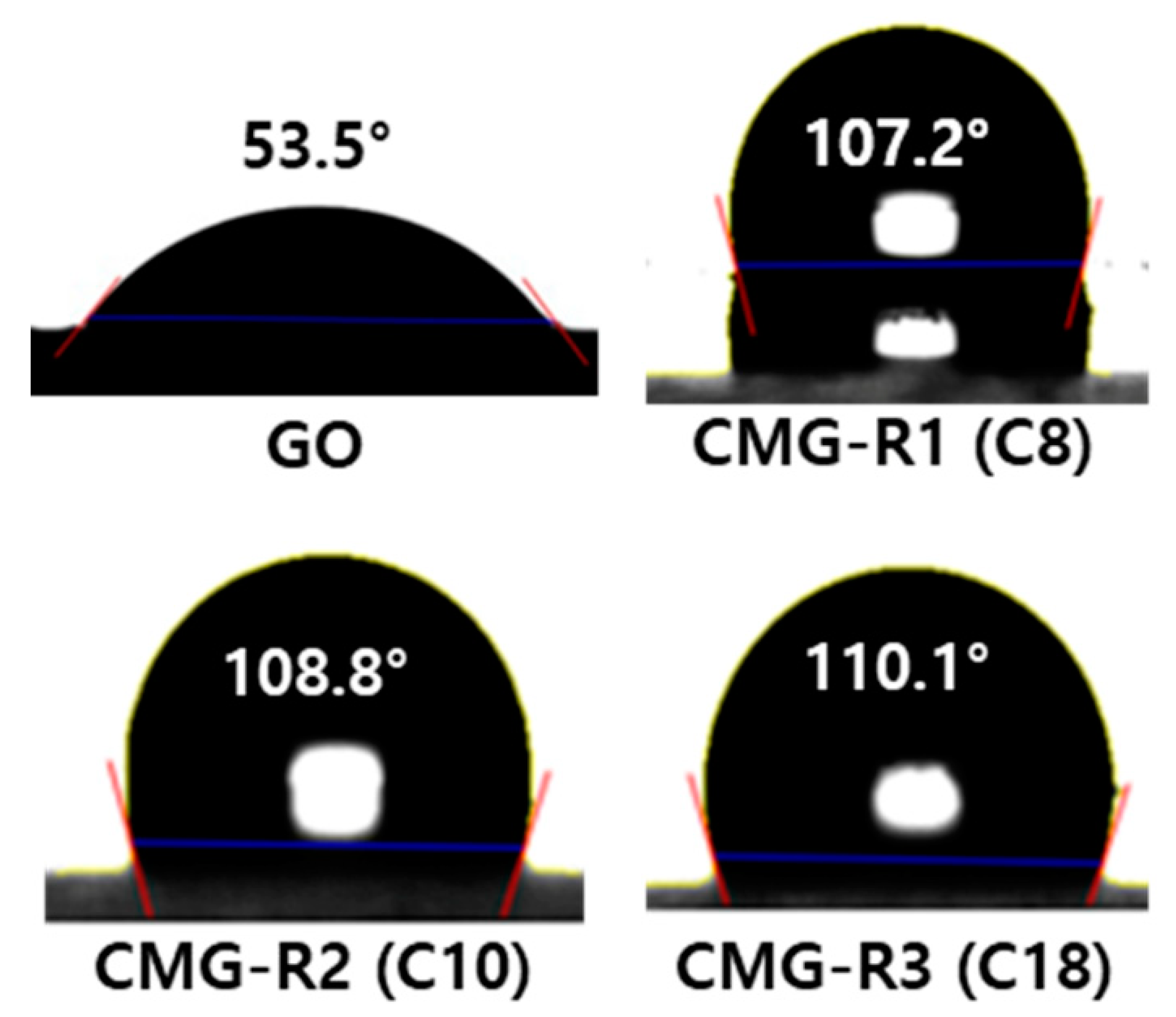
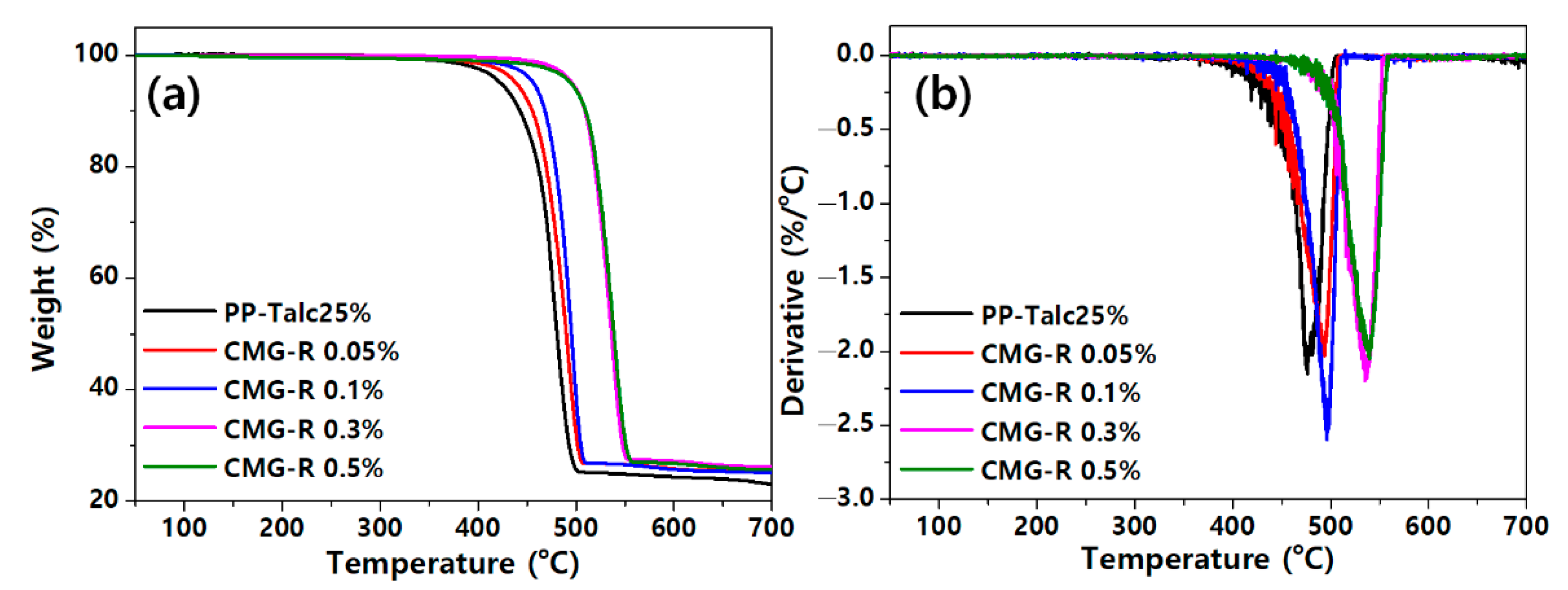
3.1. Characterization of CMG-R
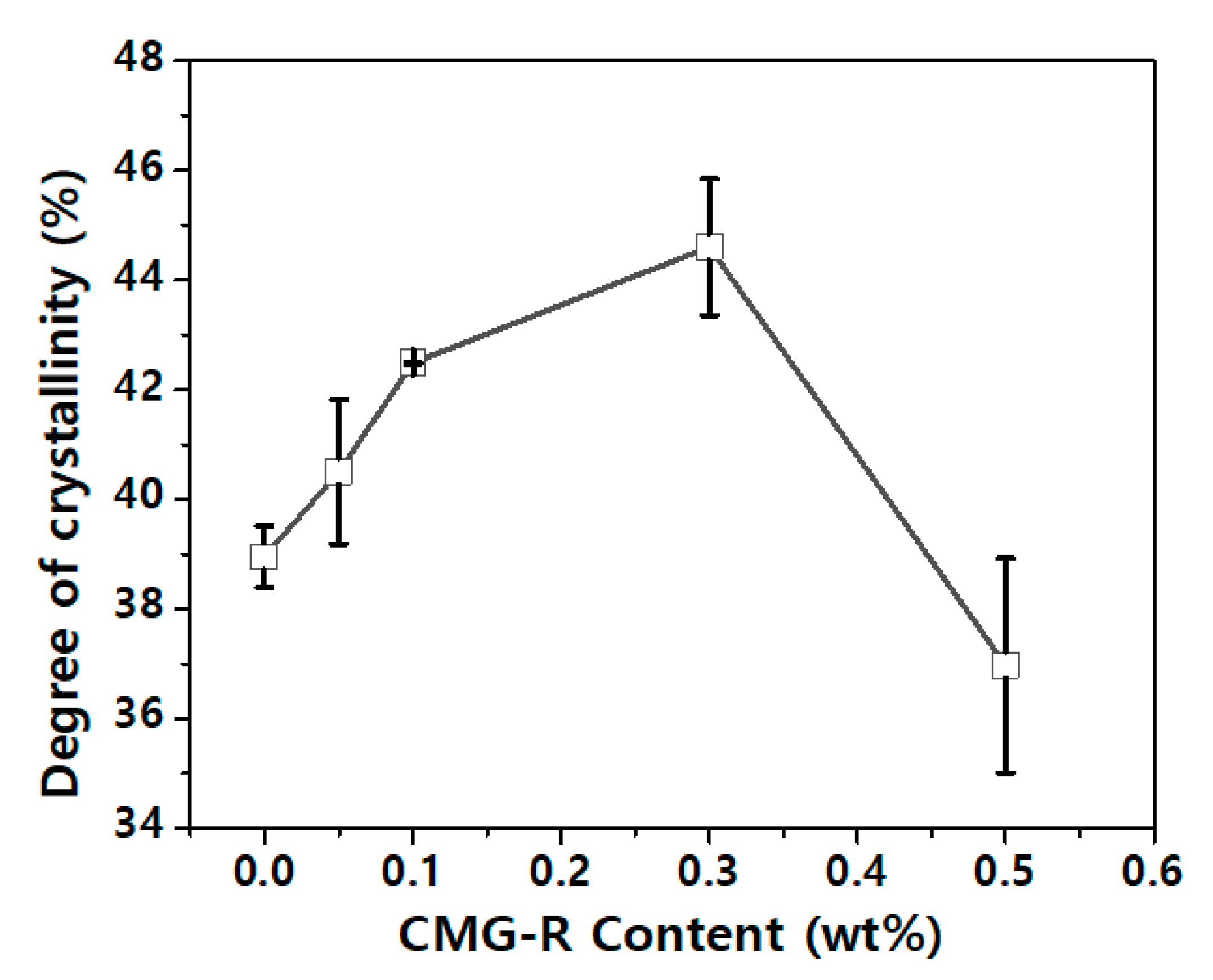
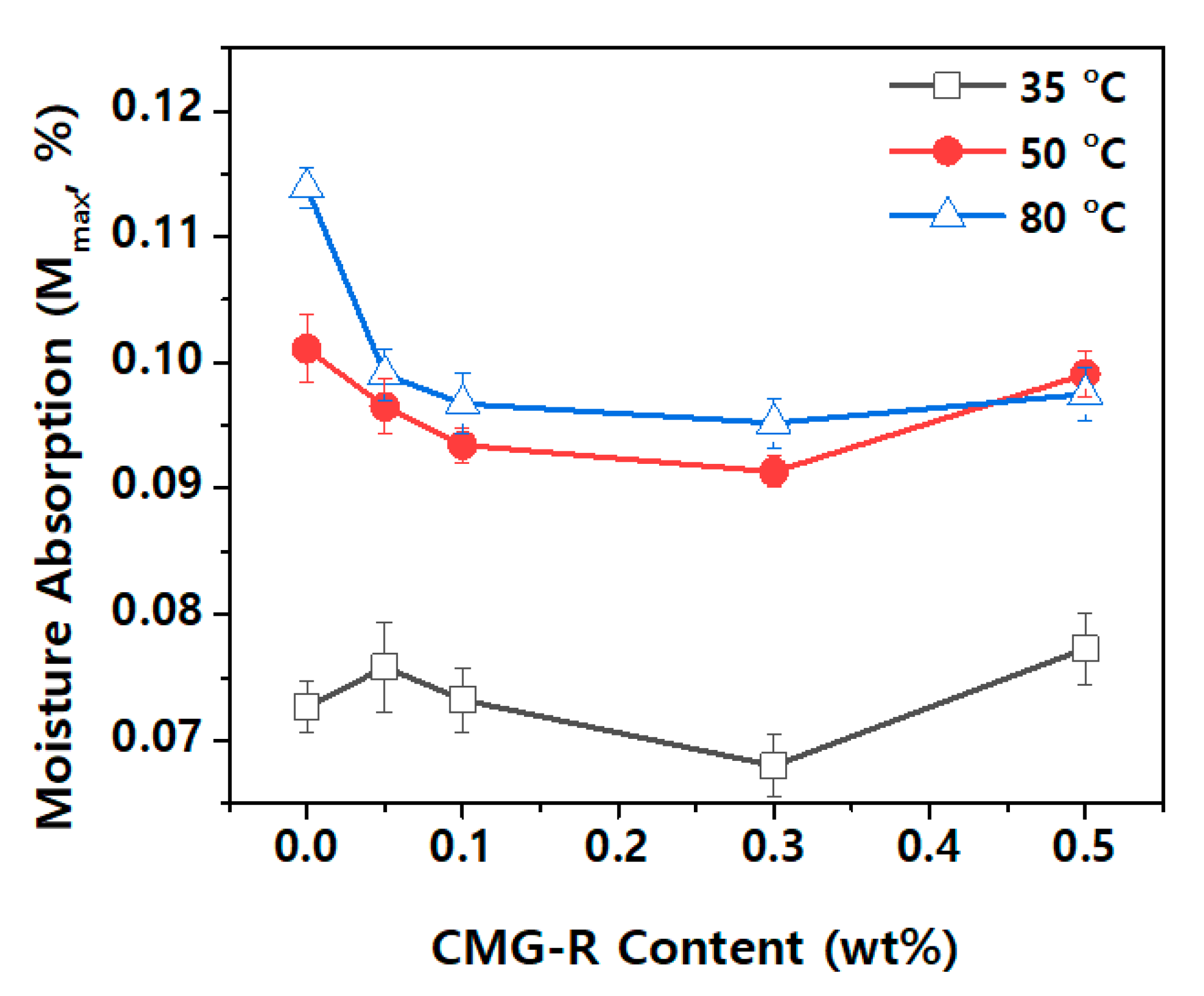
3.2. Characterization of Nanocomposites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Li, D.; Yang, W.; Xiao, C. Enhancement of mechanical, thermal and tribological properties of AAPS-modified graphene oxide/ polyamide 6 nanocomposites. Compos. Part B Eng. 2018, 138, 55–65. [Google Scholar] [CrossRef]
- Raji, M.; Mekhzouma, M.E.M.; Rodriguec, D.; Qaissa, A.E.K.; Bouhfid, R. Effect of silane functionalization on properties of polypropylene/clay nanocomposites. Compos. Part B Eng. 2018, 146, 106–115. [Google Scholar] [CrossRef]
- Bee, S.-L.; Abdullah, M.A.A.; Mamat, M.; Bee, S.-T.; Sin, L.T.; Hui, D.; Rahmat, A.R. Characterization of silylated modified clay nanoparticles and its functionality in PMMA. Compos. Part B Eng. 2017, 110, 83–95. [Google Scholar] [CrossRef]
- Gates, W.P.; Aldridge, L.P.; Carnero-Guzman, G.G.; Mole, R.A.; Yu, D.; Iles, G.N.; Klapproth, A.; Bordallo, H.N. Water desorption and absorption isotherms of sodium montmorillonite: A QENS study. Appl. Clay Sci. 2017, 147, 97–104. [Google Scholar] [CrossRef]
- Vlasveld, D.P.N.; Groenewold, J.; Bersee, H.E.N.; Picken, S.J. Moisture absorption in polyamide-6 silicate nanocomposites and its influence on the mechanical properties. Polymer 2005, 46, 12567–12576. [Google Scholar] [CrossRef]
- Saravanan, S.; Ramamurthy, P.C.; Madras, G. Effects of temperature and clay content on water absorption characteristics of modified MMT clay/cyclic olefin copolymer nanocomposite films: Permeability, dynamic mechanical properties and the encapsulated organic device performance. Compos. Part B Eng. 2015, 73, 1–9. [Google Scholar] [CrossRef]
- Mekhzoum, M.E.M.; Essabir, H.; Rodrigue, D.; Qaiss, A.K.; Bouhfid, R. Graphene/montmorillonite hybrid nanocomposites based on polypropylene: Morphological, mechanical, and rheological properties. Polym. Compos. 2016, 39, 2046–2053. [Google Scholar] [CrossRef]
- Yun, S.-S.; Shin, D.-H.; Jang, K.-S. Influence of Ionomer and Cyanuric Acid on Antistatic, Mechanical, Thermal, and Rheological Properties of Extruded Carbon Nanotube (CNT)/Polyoxymethylene (POM) Nanocomposites. Polymers 2022, 14, 1849. [Google Scholar] [CrossRef]
- Shangguan, Q.; Chen, Z.; Yang, H.; Cheng, S.; Yang, W.; Yi, Z.; Wu, X.; Wang, S.; Yi, Y.; Wu, P. Design of Ultra-Narrow Band Graphene Refractive Index Sensor. Sensors 2022, 22, 6483. [Google Scholar] [CrossRef]
- Cheng, Z.; Liao, J.; He, B.; Zhang, F.; Zhang, F.; Huang, X.; Zhou, L. One-Step Fabrication of Graphene Oxide Enhanced Magnetic Composite Gel for Highly Efficient Dye Adsorption and Catalysis. ACS Sustain. Chem. Eng. 2015, 3, 1677–1685. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Z.; Yang, H.; Wen, L.; Yi, Z.; Zhou, Z.; Dai, B.; Zhang, J.; Wu, X.; Wu, P. Multi-mode surface plasmon resonance absorber based on dart-type single-layer graphene. RSC Adv. 2022, 12, 7821–7829. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cai, R.; Long, F.; Wang, J. Development and application of tetrabromobisphenol A imprinted electrochemical sensor based on graphene/carbon nanotubes three-dimensional nanocomposites modified carbon electrode. Talanta 2015, 134, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-C.; Kim, S.Y.; Ko, Y.K.; Ha, J.U.; Jeoung, S.K.; Shin, D.; Kim, J.H.; Kim, M.-G. Tribological Properties of Polyamide 46/Graphene Nanocomposites. Polymers 2022, 14, 1139. [Google Scholar] [CrossRef] [PubMed]
- Gahleitner, M.; Paulik, C. Polypropylene. In ULLMANN’S Polymers and Plastics, 1st ed.; Elvers, B., Ed.; Wiley-VCH: Hamburg, Germany, 2016; pp. 937–979. [Google Scholar]
- Guzej, M.; Zachr, M. CFD Simulation of Defogging Effectivity in Automotive Headlamp. Energies 2019, 12, 2609. [Google Scholar] [CrossRef]
- Drapala, E. Experimental Study on Water Condensation in Automotive Headlamp; SAE Technical Paper 2010; SAE International: Warrendale, PA, USA, 2010. [Google Scholar] [CrossRef]
- Singh, R.; Kuzhikkali, R.; Shet, N.; Natarajan, S.; Kizhedath, G.; Arumugam, M. Automotive LED Headlamp Defogging: Experimental and Numerical Investigation; SAE Technical Paper 2016; SAE International: Warrendale, PA, USA, 2016. [Google Scholar] [CrossRef]
- Paul, D.R.; Robeson, L.M. Polymer nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef]
- Lee, P.-C.; Ha, J.U.; Kim, S.Y.; Um, C.; Kim, S.H.; Jeoung, S.K.; Shin, D.; Jung, W. Effects of Temperature and Nano-filler Content on Water Uptake in Nanocomposites. Polym. Korea 2019, 43, 584–588. [Google Scholar] [CrossRef]
- Ren, P.-G.; Wang, H.; Huang, H.-D.; Yan, D.-X.; Li, Z.-M. Characterization and performance of dodecyl amine functionalized graphene oxide and dodecyl amine functionalized graphene/high-density polyethylene nanocomposites: A comparative study. J. Appl. Polym. Sci. 2014, 131, 39803. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Shanmugharaj, A.M.; Yoon, J.H.; Yang, W.J.; Ryu, S.H. Synthesis, characterization, and surface wettability properties of amine functionalized graphene oxide films with varying amine chain lengths. J. Colloid Interface Sci. 2013, 401, 148–154. [Google Scholar] [CrossRef]
- Jebaranjitham, J.N.; Mageshwari, C.; Saravanan, R.; Mu, N. Fabrication of amine functionalized graphene oxide-AgNPs nanocomposite with improved dispersibility for reduction of 4-nitrophenol. Compos. Part B Eng. 2019, 171, 302–309. [Google Scholar] [CrossRef]
- Daud, N.A.; Cheng, B.W.; Ibrahim, N.A.; Talib, Z.A.; Muhamad, E.N.; Abidin, Z.Z. Functionalizing Graphene Oxide with Alkylamine by Gamma-ray Irradiation Method. Nanomaterials 2017, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Faiz, M.S.A.; Azurahanim, C.A.C.; Raba’ah, S.A.; Ruzniza, M.Z. Low cost and green approach in the reduction of graphene oxide (GO) using palm oil leaves extract for potential in industrial applications. Results Phys. 2020, 16, 102954. [Google Scholar] [CrossRef]
- Shi, T.; Chen, F.; Yang, J.; Zhong, M. Crystallinity and thermal stability of LDH/polypropylene nanocomposites. Appl. Clay Sci. 2010, 50, 87–91. [Google Scholar] [CrossRef]
- Polyák, P.; Szemerszki, D.; Benke, H.C.; Pukánszky, B. A novel method for the determination of diffusion coefficients in amorphous poly(3-hydroxybutyrate). Polym. Test. 2017, 63, 342–348. [Google Scholar] [CrossRef][Green Version]
- Joannès, S.; Mazé, L.; Bunsell, A.R. A concentration-dependent diffusion coefficient model for water sorption in composite. Compos. Struct. 2014, 108, 111–118. [Google Scholar] [CrossRef]
- Joannès, S.; Mazé, L.; Bunsell, A.R. A simple method for modeling the concentration-dependent water sorption in reinforced polymeric materials. Compos. Part B Eng. 2014, 57, 219–227. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, D.; Kim, S.H.; Shin, D.; Oh, J.T.; Kim, M.-G.; Lee, P.-C. Hygroscopic Behavior of Polypropylene Nanocomposites Filled with Graphene Functionalized by Alkylated Chains. Nanomaterials 2022, 12, 4130. https://doi.org/10.3390/nano12234130
Kang D, Kim SH, Shin D, Oh JT, Kim M-G, Lee P-C. Hygroscopic Behavior of Polypropylene Nanocomposites Filled with Graphene Functionalized by Alkylated Chains. Nanomaterials. 2022; 12(23):4130. https://doi.org/10.3390/nano12234130
Chicago/Turabian StyleKang, Dongwoo, Sung Hee Kim, Donghyeok Shin, Ji Taek Oh, Myeong-Gi Kim, and Pyoung-Chan Lee. 2022. "Hygroscopic Behavior of Polypropylene Nanocomposites Filled with Graphene Functionalized by Alkylated Chains" Nanomaterials 12, no. 23: 4130. https://doi.org/10.3390/nano12234130
APA StyleKang, D., Kim, S. H., Shin, D., Oh, J. T., Kim, M.-G., & Lee, P.-C. (2022). Hygroscopic Behavior of Polypropylene Nanocomposites Filled with Graphene Functionalized by Alkylated Chains. Nanomaterials, 12(23), 4130. https://doi.org/10.3390/nano12234130






