Abstract
Pesticides are essential to contemporary agriculture and are required to safeguard plants from hazardous pests, diseases, and weeds. In addition to harming the environment, overusing these pesticides causes pests to become resistant over time. Alternative methods and agrochemicals are therefore required to combat resistance. A potential solution to pesticide resistance and other issues may be found in nanotechnology. Due to their small size, high surface-area-to-volume ratio, and ability to offer novel crop protection techniques, nanoformulations, primarily biopolymer-based ones, can address specific agricultural concerns. Several biopolymers can be employed to load pesticides, including starch, cellulose, chitosan, pectin, agar, and alginate. Other biopolymeric nanomaterials can load pesticides for targeted delivery, including gums, carrageenan, galactomannans, and tamarind seed polysaccharide (TSP). Aside from presenting other benefits, such as reduced toxicity, increased stability/shelf life, and improved pesticide solubility, biopolymeric systems are also cost-effective; readily available; biocompatible; biodegradable; and biosafe (i.e., releasing associated active compounds gradually, without endangering the environment) and have a low carbon footprint. Additionally, biopolymeric nanoformulations support plant growth while improving soil aeration and microbial activity, which may favor the environment. The present review provides a thorough analysis of the toxicity and release behavior of biopolymeric nanopesticides for targeted delivery in precision crop protection.
1. Introduction
Agriculture is the backbone of any economy, and food is a vital resource for living beings. Crops face many issues, including insects, pests, diseases, pesticides, and the toxicity associated with these agrochemicals. Pests cause a total loss of 50% in wheat and 80% in cotton worldwide. Other crops that experience considerable output losses include soybeans (26–29%), maize (31%), rice (37%), and potatoes (40%) [1]. Pest insects account for about 30% of crop losses [2], and weeds also cause substantial losses of about 34%. Pesticides have been used to combat pests; however, the widespread application of pesticides has had a devastating effect on humans and other living organisms, with an increasing incidence of human poisoning. In addition to pests, fungal diseases also affect crops worldwide. In the 19th century in Ireland, a fungal disease destroyed the potato crops to such an extent that it was considered one of the greatest European famines. Likewise, an annual loss of about USD 60 billion in the five most important food crops (wheat, rice, maize, soybean, and potato) as a result of fungi has been reported. Thus, it is important to control fungal diseases in these crops. Enough food to feed about 600 million people each year could be saved by effectively controlling fungal diseases alone [3,4].
In order to expand production to feed the increasing population, the excessive usage of chemical fertilizers and pesticides was carried out during the green revolution of the late 20th century in India, which caused a great loss in soil biodiversity and soil health. The overuse of pesticides has also led to the development of resistance among pests, a major agricultural problem [5]. Nanotechnology can be used to tackle such problems. Active ingredients in conventional formulations are generally mixed with inert materials. However, immediate release leads to the quick loss of these chemicals in the field by various degradation processes, which causes leaching, evaporation, and volatilization. As a result, the active ingredient concentration declines to the minimum level required to maintain biological efficacy. Hence, these conventional pesticides must be applied again and again, which causes soil, human health, and environmental problems. However, this adds chemicals to the soil that persist for a long time or do not degrade easily, deteriorating the soil’s health. Later on, polymeric nanoformulations were developed, which achieved the slow, controlled, and sustained release of active ingredients with less impact on the environment [6]. Biological entities such as bacteria, fungi, actinomycetes, viruses, diatoms, and higher plants are used for environmentally friendly, greener, and safer methods of synthesizing nanoparticles (NPs) that directly inhibit the growth of pathogens [7]. The authors of [8] applied microwave-assisted synthesis to produce silver (Ag) NPs using the hybrid citrus fruit “kinnow” and found them effective against the early blight of tomatoes. Similarly, the authors of [9] managed chickpea (Cicer arietinum) wilting disease in vivo by synthesizing silver NPs from the rhizospheric microflora of chickpeas.
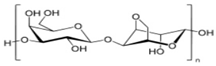
Recently, biopolymers as nanoparticulate materials have attracted attention due to their eco-friendly nature and ability to offer the persistent release of the associated active components of pesticides. Natural polymers and gums are biocompatible, sustainable, biodegradable, economical, and non-toxic and are considered an alternative source of raw materials. These natural gums (guar gum and gum acacia) and biopolymers (starch, cellulose, pectin, galactomannan, and lignin) occur in seaweed extracts (carrageenan); fungi (chitosan and chitin); bacteria (polyhydroxybutyrate, xanthan gum); algae (agar, alginate); and plant seeds and stems and their exudates [10,11,12]. The largest portion of biopolymeric materials are derived from plants and animals. The application of NP synthesis based on biopolymers can solve the specific agricultural problem of plant–pathogen interactions, thus presenting a means of crop protection [13,14]. NPs have great potential as ‘magic bullets’ that can be loaded with herbicides, weedicides, fungicides, and fertilizers for the targeted delivery of these agrochemicals to crops. Biopolymeric NPs can release the desired active components at a prescribed rate with a constant dose to the target pest and thus are more effective. Developing controlled-release formulations (CRFs) is highly desirable from the perspective of compliance with international environmental and biodiversity laws. The use of polymers for sustained release started in the early 1970s, due to the higher efficacy of their encapsulated components compared to commercial formulations. To upgrade the value of traditional pesticides, many natural biopolymers such as carrageenan, galactomannans, chitosan, alginate, pectin, cellulose, gum acacia, guar gum, cashew gum, chitin, agar, tamarind seed polysaccharide (TSP), and starch are processed into nanomaterials for the slow and targeted delivery of agrochemicals [15,16,17,18,19,20].
Biopolymeric nanopesticides have various benefits compared to their bulk counterparts, which can be summarized as follows: they cause less environmental contamination due to their low application doses and reduced leaching losses and are thus safe for non-target organisms; they present better efficacy due to their controlled release; and they are resistant to photo-degradation, thus achieving the maximum impact on their target organisms [21]. In addition to the above, biopolymeric nanopesticides are biocompatible, biodegradable, and biosafe, with minimal adverse effects on non-target organisms. They can be degraded easily to form compost, which supports plant growth and performance. Biopolymers increase soil permeability and aeration, ultimately increasing soil microorganism activity. All these properties make biopolymeric nanoformulations the prime choice for encapsulating active components. Their efficacy against different target organisms has been confirmed in many greenhouse and field studies [14,15,22]. The economic cost of biopolymeric nanoparticles and their carbon footprint are also low due to their small size, high surface-area-to-volume ratio, and a very low dose (of both biopolymer and encapsulated pesticide) requirements as compared to their bulk counterparts. Thus, keeping in mind this knowledge gap and their low dose requirements, this review presents a comprehensive overview of biopolymeric NPs as carriers of insecticides, fungicides, herbicides, molluscicides, and acaricides for plant disease management, taking into account their lower toxicity and release behavior.
2. Pesticides
Until the end of the 15th century, several toxic chemicals such as arsenic, mercury and lead were used to kill pests threatening staple agricultural crops because of a lack of domain knowledge and the scarcity of resources. In the 17th century, nicotine sulfate was isolated from tobacco leaves and used for the first time as an insecticide. Later, in the 19th century, two more natural pesticides, pyrethrum (obtained from chrysanthemums) and rotenone (extracted from tropical vegetable roots), were introduced [23]. Paul Muller discovered DDT, the first synthetic pesticide, in the 1940s. Subsequently, the growth of synthetic pesticides accelerated with the discovery of BHC, aldrin, 2,4-D, endrin, chlordane, parathion, and dieldrin. All of these pesticides were effective, inexpensive, and used by people all over the world. DDT emerged as the most popular, due to its broad-spectrum activity [24].
Generally, insecticides are grouped into two classes, i.e., systemic or contact insecticides. Based on the physiological functions affected, they can be further classified into different chemical groups. Additionally, to prevent pesticide resistance, modes of action (MoA) must be used in rotation [25]. Fungicides function through contact or penetration. Because systemic mobility throughout a plant is rare, fungicide spraying is quite significant. There are 14 separate MoA groups and 49 Fungicide Resistance Action Committee (FRAC) codes for fungicides [26]. Selective or non-selective herbicides can be sprayed on weeds before they sprout or emerge. Rotation within these groups is necessary to avoid pesticide resistance.
The worldwide human population Is increasing at an annual rate of 1.2%, i.e., approximately 77 million people are added yearly to the existing inhabitants. Asian countries such as China, India, Pakistan, Indonesia, and Bangladesh, as well as Nigeria, are responsible for half of this global annual increment. By 2050, the planet’s population will be around 9.1 billion [27]. Humans and other animals need daily food to maintain their metabolic rate and survival. Cereal grain production worldwide is estimated to total approximately 3300 metric tons by 2050 [28], 60% more than today. Since 1960, noteworthy progress has been made toward improving the nutritional value and ensuring the security of food for living beings. Gross agricultural production has increased more rapidly than the world population, evidenced by the increased availability of food per capita. However, the gap between the quantity of food produced and the global population needing to be feed is likely to increase until the year 2050 [29]. Thus, the need to increase cereal production is inevitable and could be met by disease and pest control methods based on modern nanotechnology in addition to the modern molecular biology methods of increasing the productivity of staple crops.
3. Food Loss Due to Insects, Pests, and Diseases
Today, crop growers are facing numerous challenges. The yield and productivity of crops grown for human consumption are in danger because of the prevalence of pests, especially weeds, animal pests, pathogens, and insects. These organisms account for a significant portion crop loss that may be avoided or diminished by modern crop protection measures. Worldwide, USD 2000 billion in economic loss per year has been observed in food production, caused by plant disease (13%), insects (14%), and weeds (3%) [30]. Fungi are responsible for about 70% of diseases in all major crops [31], such as wheat, potato, cotton, tomato, groundnut, cotton, and grapevine [32]. The gravity of the situation can be understood by the fact that in the agricultural industry, annual crop losses due to fungal diseases in the field and after harvest exceed USD 200 billion. In contrast, the USA spends over USD 600 million annually on fungicides [33]. Globally, approximately one fourth of food crops are damaged by fungal toxins such as aflatoxins, ergot toxins, Fusarium toxins, patulin, and tenuazonic acid. Worldwide losses due to pests vary from approximately 50% in wheat to more than 80% in cotton production. For other crops, the estimated losses are 26–29% for soybean and 31, 37, and 40% for maize, rice, and tomatoes, respectively [34].
4. Biopolymers as Nanoparticulate Materials
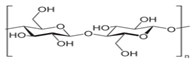
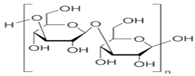
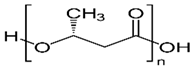
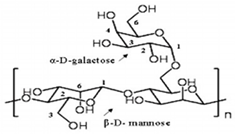
The European Commission defined a NPs as “a natural, incidental or manufactured material containing particles, in an unbound state or as an aggregate where, for 50% or more of the particles in the number size distribution, one or more external dimensions is in size range 1 nm–100 nm” [35]. Biopolymers synthesized from naturally occurring sources have several advantageous properties (e.g., biodegradability, biocompatibility, ready availability, and inexpensiveness), which make these biopolymers suitable for numerous research-based applications. The largest portion of biopolymeric materials are derived from plants, such as gum acacia [36], galactomannan [37], pectin, lignin [38], starch [39], and cellulose [40]; animals, such as chitosan and chitin [41,42]; and algae, such as agar [43], alginates [44], and xanthan gum [41]. The polysaccharide cashew gum (CG), a plant exudate, is produced due to the plant’s defense mechanisms against stress. This gum production takes place in all parts of the tree and depends on the maturity of the tree and the environmental conditions. It is partially or sparsely soluble in water and swells, producing a highly viscous solution. It is made of a branched framework of D-galactose units and D-glucuronic acid, L-arabinose, and L-rhamnose. Numerous applications of modified CG have been described. It can be used as an alternative for liquid glue in the paper industry, in the cosmetic industry, as an edible coating for application on apples [45] and mangoes [46], and as an agglutinant for capsules and pills in the pharma sector [47]. It can also be a protective, edible coating on fruits and vegetables. For example, the authors of [48] described CG and carboxymethylcellulose-based formulations that could be used as protective edible coatings on whole red guavas and fruits cut by birds to enhance their shelf life and defense mechanisms. CG is used in many industries.
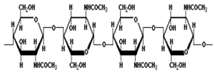
Chitosan is a non-toxic, biodegradable polymer. Chitin is the primary structural element of crustaceans and some fungal cell walls. It keeps the immune system of plants functioning, secretes enzymes, and enhances plants’ ability to withstand illnesses and insects. The authors of [49] formulated nisin-loaded chitosan/carrageenan nanocapsules using an ionic complexation method and tested their antibacterial efficacy. The concentration of polymer and surfactant affected the particle size and encapsulation efficiency. The release study conducted in vitro indicated slow and sustained release, while the assessment of the antibacterial activity against Micrococcus luteus (MTCC 1809), Pseudomonas aeruginosa (MTCC 424), Salmonella enteric (MTCC 1253), and Enterobactor aerogenes (MTCC 2823) indicated that the encapsulated nanocapsules exhibited a better antibacterial effect on the microbes both in vitro and in vivo for prolonged periods of six months, contrary to the components evaluated separately [49].
Similarly, using this biopolymer (chitosan), the authors of [11] synthesized acetamiprid-loaded controlled-release nanocapsules by polyelectrolyte complexation with another natural polymer, sodium alginate. As observed by TEM, the zeta potential results revealed that the nanocapsules formed were stable with a spherical shape. The encapsulation efficiency was 62%, as computed by ultra-high-pressure liquid chromatography (UHPLC). The in vitro release experiment showed maximum release at pH 10.0, followed by pH 7.0 and 4.0, respectively, with a non-Fickian release pattern that was more effective than that of a commercial formulation in soil. The formulation of these nanocapsules could help to cut down the frequency and dose of pesticides by controlling the release and subsequent leaching side effects of conventional pesticides.
Similarly, the authors of [50] prepared chitosan NPs functionalized with β-cyclodextrin and containing carvacrol and linalool. High encapsulation efficiencies (<90%) were shown for both carvacrol and linalool. The synthesized NPs demonstrated acaricidal, repellency, and anti-oviposition activity against mites (Tetranychus urticae). The nanoforms were efficient in their acaricidal and anti-oviposition activity, while the unencapsulated compounds showed improved repellency. Table 1 shows examples of biopolymers used as nanoparticulate materials, their characteristics, and their uses in agriculture and other fields.

Table 1.
Biopolymers used as nanoparticulate materials in agriculture and other fields: sources, structure, characteristics, and uses.
5. Biopolymeric Nanopesticides
The term “nanopesticides” is used to describe any pesticide formulation that “involves either very small particles of an active pesticide ingredient or other small engineered structures with useful pesticidal properties” [70,71]. Nano-formulations for pesticide products offer several benefits, such as increased solubility, kinesis, and durability; significantly fewer active ingredients; less harm caused to non-target organisms, thereby reducing the development of resistance; and protection against premature degradation [72,73].
Nowadays, polymers have become the main components of NP and nanocapsule synthesis, being firmly held together by covalent bonds that make the polymer steadier and more robust than liposomes [74]. Their sizes, morphology, and shapes can be managed more proficiently using polymers by varying their concentration and pH, making them more effective against chemical compounds or metals. Additionally, polymeric nanocapsules can easily be made operational with different substances, increasing their value for achieving certain goals. Natural biopolymers such as chitosan and chitin derivatives are popular objects of research in the field of nanocapsules containing natural polymers [75]. These polysaccharides are biodegradable, made up of linear β-1, 4 linkages of glucose residues, and are the second most common in nature after cellulose. Chitosan can be used to encapsulate and deliver drugs by transforming them into thin membranous films and thus can be used in the biomedical or pharma industry. The application of their mucoadhesive properties in drug transport across cellular membranes supports their possible use in the agrochemical industry [76]. In agriculture, chitosan is well-known for its antimicrobial properties against various microorganisms. In addition to producing defense responses in plants, it also shows antibacterial and insecticidal activity [77,78]. Experimentation on NPs as antifungal agents has been reported by many researchers [79,80]. Natural polymers have been used to encapsulate conventional fungicides to produce nanofungicides [81], nanoinsecticides [82], or nanoherbicides [83] for the slow release of active ingredients and for the enhancement of their disease-prevention efficacy. Figure 1 summarizes the use of biopolymeric NPs as carriers of fungicides, insecticides, and herbicides.
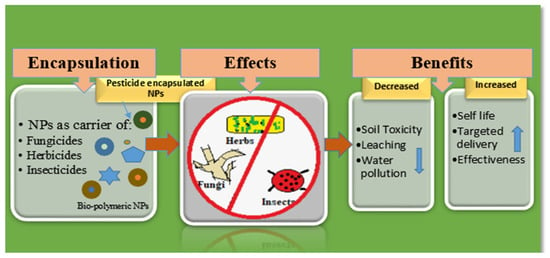
Figure 1.
Biopolymeric NPs as carriers of herbicides, fungicides, and insecticides and their benefits.
5.1. Biopolymeric Nanofungicides
About 20–40% of crop products are lost annually because of pests and pathogens. Traditionally, plant disease management mainly relies on toxic pesticides that are potentially dangerous to the environment and humans. Nanotechnology offers benefits such as reduced environmental and cell toxicity, increased shelf life, and the improved solubility of water-soluble synthetic pesticides. All these factors could have positive impacts on the environment [84]. Fungicides are mainly used to control the diseases caused by fungi in the agricultural industry, which reduce the quality and quantity of produce. The constant use of fungicides is particularly harmful to the ecosystem. Residual fungicides in soil and produce have detrimental effects on the soil properties, microbial community, and animal and human health [11]. Thus, there is a need to find other ways to increase the efficacy and reduce the dose of fungicides. Using nano-encapsulated pesticides reduces their phytotoxicity and improves their diffusion through the cuticle and the sustained release of their active components in the target site. Using biocompatible polymers for the nano-entrapment of agrochemicals can further reduce toxicity by achieving the targeted and controlled release of the fungicides [10]. Nowadays, there is an increasing interest in non-toxic and biocompatible polymers such as chitosan, carboxymethyl, sodium alginate, starch, cellulose, gelatin, and pectin [85].
Different amounts of the low-water-soluble fungicide pyraclostrobin were added to chitosan–lactide copolymer NPs. Three days post-application, it was discovered that the nanofungicide was either equally as effective as commercial pyraclostrobin or less effective in preventing C. gossypii inhibition. However, compared to acting alone, an increase in inhibition was observed five days after application [86]. Similarly, another research group [12] employed polymeric and solid lipid NPs (SLNPs) for the persistent release of the antifungal drugs tebuconazole and carbendazim, which are common fungicides. Compared to the SLNPs, these polymeric NPs released around 47% of the fungicides over six days. The high association efficiency (>99%) of both formulations demonstrated a strong ionic interaction. When the effect of commercial and NP formulations on plant emergence was assessed using P. vulgaris seeds, the germination index of the seeds (92%) was not modified by either formulation (with or without fungicides).
In another trial, kaempferol (another low-soluble fungicide) loaded onto lecithin/chitosan displayed 67% inhibition efficacy after 60 days against Fusarium oxysporum infected petri dish [87]. The effect of carbendazim-loaded biopolymeric NPs of chitosan and pectin compared to carbendazim alone on Aspergillus parasiticus and Fusarium oxysporum, the authors of [88] discovered an enhanced fungal inhibition rate. According to phytotoxicity studies, maize, cucumber, and tomato seeds did not experience any negative effects from the nanoformulated carbendazim when planted and allowed to grow roots. Similarly, the authors of [9] incorporated copper-oxy-chloride into chitosan–CuO/ZnO nanoformulations and managed Fusarium oxysporum f. sp.ciceri (FOC) in chickpeas. Similar process was used by the authors of [89] to create chitosan NPs (CNPs) from chitosan (CS). The phytopathogenic fungi Phytophthora capsici, Colletotrichum gelosporidies, Fusarium oxysporum, Sclerotinia sclerotiorum, and Gibberella fujikuori were used as test subjects to assess the NPs’ in vitro antimicrobial activity. Following closely behind P. capsici, the CNPs revealed the greatest growth-inhibitory effects on F. oxysporum mycelial growth. Their antibacterial activity against Xanthomonas and Erwinia bacterial strains were also tested. The study showed that both CS and CNPs have tremendous potential as nanofungicides, as they markedly inhibited the growth of fungal and bacterial strains in tomatoes and could be used as crop protectants. Similarly, the authors of [90] found that the encapsulation of the fungicide spinosad in chitosan–isoleucine NPs protected the fungicide against photo-degradation, thereby increasing the effectiveness of this commercial fungicide. Likewise, the authors of [91] demonstrated a 74.5% reduction in basal stem rot disease caused by G. boninense in palm trees by applying chitosan hexaconazole-dazomet. When encapsulated in lignin NPs, the fungicides azoxystrobin, pyraclostrobin, tebuconazole, and boscalid azoxystrobin inhibited the growth of test fungi (Phaeomoniella chlamydospora and Phaeoacremonium minimum) under in vitro conditions after 96 h. These fungi cause grapevine trunk disease [92]. Table 2 lists some biopolymeric nanoformulations that have been used as nanofungicides.

Table 2.
Biopolymeric nanoparticle-based carriers of fungicides used to target pathogenic fungi.
5.2. Biopolymeric Nanoinsecticides
Insects represent the largest populations of animals and are found in all possible environments throughout the globe. Several evolutionary aspects are responsible for their success, including their habit diversification, reproductive potential, desiccation-resistant eggs, ability to metamorphosize, and development of wings and exoskeletons. Approximately 500,000 species of identified insects feed on green leaves. The majority of them eat from a small range of plant species, and some of them are even species-specific [108]. These insects are tiny organisms that attack plants en masse and contribute significantly to crop losses, mainly during the period of crop flowering. They mainly feed on crop leaves, flowers, and fruits, making them unusable. Some insects also damage fruits post-harvest. The authors of [10] synthesized sodium alginate nanocapsules loaded with the insecticide imidacloprid (IMI) by the water-in-oil-in-water (W/O/W) double-emulsion method. An aqueous solution of sodium alginate was supplemented with a solid form of IMI and then sonicated and emulsified by methylene chloride and dioctyl sodium sulfosuccinate. The formed NPs were harvested by ultracentrifugation and tested on a leafhopper, an insect that consumes plant sap, to evaluate the efficacy of the IMI nanoformulations. Leaf infestation was assessed by counting the leafhopper population on three leaves in a scientific manner. The results showed that blank NPs had no positive effect; however, the nanoencapsulated IMI was more effective than the free insecticide or commercial form of IMI. Toxicity studies also discovered that the nanoformulated IMI was considerably less toxic than the trade pesticide.
Similarly, the authors of [109] encapsulated the hydrophilic carbamate insecticide methomyl in polymers. This is a broad-spectrum insecticide, but when applied via water it undergoes rapid decomposition in the air or sunlight, so nanoencapsulation is vital to avoid its premature degradation. An amphiphilic, photo-crosslinkable carboxymethyl-chitosan (Az-CMCS) biopolymer was formulated by reacting a previously prepared ethanolic solution of azidobenzaldehyde (Az) with an aqueous solution of carboxymethyl chitosan (CMCS). Methomyl was dissolved in an aqueous solution of Az-CMCS at pH 4.0 under sonication. Under these conditions, the amphiphilic polymer self-assembled into methomyl-loaded nanocapsules with an aqueous core, resulting in a more stable and slow-releasing nanoinsecticide with premature-degradation-resistant properties.
Similarly, Helicoverpa armigera larvae were managed using carboxymethyl chitosan and sodium alginate nanoformulations with methomyl and pyridalyl insecticides [110]. The low-water-solubility insecticide acetamiprid was transformed into a controlled-release nanoformulation [11]. In order to create acetamiprid-loaded alginate–chitosan nanocapsules, ionic pregelation and polyelectrolyte complexation were used. A polyionic complex was created with a loading efficiency of 62% due to ionic interactions between the positively charged ammonium groups of chitosan and the negatively charged carboxylate groups of alginate. Studies on its controlled release in various soil mediums showed that it was superior to commercial acetamiprid. Acidic soils achieved the best results. Essential oils have also been encapsulated in chitosan and other natural biopolymers to increase the insecticidal activity of biopolymers. The authors of [50,111,112,113] synthesized chitosan/gum arabic, chitosan/zinc oxide, and chitosan NPs encapsulating geraniol, azadirachtin, carvacrol, and linalool to control whitefly (B. tabaei), groundnut bruchid (C. serratus), and mite (T. urticae), respectively. The authors of [17] formed spinosad- and permethrin-loaded chitosan NPs to control Drosophila melanogaster. The insecticides emamectin benzoate and thiamethoxam were encapsulated into carboxymethyl chitosan and cellulose NPs to control the insect pests Mythimna separate and Phenacoccus solenopsis by the authors of [20,113], respectively. In a comprehensive review, the authors of [114] described the use of 15 polymers as nanocarriers of insecticides. Table 3 summarizes some of the biopolymeric NPs that have been tested as carriers of insecticides and active compounds.

Table 3.
Biopolymeric nanoparticles tested as carriers of insecticides.
5.3. Biopolymeric Nanoherbicides/Nanoweedicides
Herbs, or weeds, are unwanted plants that grow alongside cultivated crops. Herbs are becoming a new challenge for modern agriculture. With the advent of modern agrochemicals, agricultural productivity and disease resistance have increased. This has also produced new types of herbs that must be eradicated to improve productivity and soil maintenance.
The herbicide paraquat is a fast-performing, non-selective, and widely used contact herbicide. However, its water solubility and soil sorption can cause toxicity problems in non-target organisms and humans via groundwater and rivers. To combat this toxicity problem, 635 ± 12 nm alginate/chitosan conjugated NPs were prepared as a green carrier system for the herbicide paraquat [134] with a zeta potential of −22.8 ± 2.3 mV and association efficiency of 74.2%. The conjugated NPs with encapsulated paraquat improved the release profile of the herbicide and its interaction with the soil, demonstrating that this formulation can effectively reduce the adverse impacts of paraquat. Grillo and others [135] performed similar work with paraquat-loaded chitosan/tripolyphosphate (CSTPP) NPs. An encapsulation efficiency of 62.66 ± 0.77% was achieved, representing good affinity between the CSTPP NPs and the active component of the herbicide. The herbicidal activity was investigated in maize (Zea mays) and mustard (Brassica sp.). Both free and encapsulated forms of paraquat caused limp leaf necrosis within 48 h, a distinctive effect of this herbicide on both plants. In Z. mays, the use of the nanoherbicide caused more significant necrosis, possibly due to the better adhesion of the NPs to the leaf, as NPs have an increased surface-area-to-volume ratio. It is likely that the greater sensitivity of the plants to the herbicide had a more negligible effect in Brassica. NPs increase particle adhesion to surfaces due to their increased surface area, enhancing the effectiveness of the associated active agents.
In comparison to the commercial herbicide MTT, testing revealed that nanoherbicides had reduced cytotoxicity to Chinese hamster ovary (CHO) cells, whereas nanoparaquat induced modest chromosome damage in A. cepa [135]. The authors of [19] embedded paraquat in pectin, chitosan, and sodium tripolyphosphate (PEC/CS/TPP) NPs for sustained pesticide release. Alveolar and oral cell lines were less hazardous to the encapsulated herbicide. The nanoformulation mutagenicity of a model using the Salmonella typhimurium strain was markedly lower than that of paraquat in its commercial forms. The soil sorption of paraquat and the deep soil penetration of the NP-linked herbicides decreased. After encapsulation, paraquat’s herbicidal efficacy against maize and mustard was sustained and substantially improved. The authors of [83] assessed the herbicidal effect of glyphosate in chitosan nanoformulations on three weed species: gallant soldier (G. parviflora Cav), white goosefoot (C. album L.), and common sorrel (R. acetosa L.), among the most glyphosate-resistant weeds. The advantage of the above formulation was that chitosan dissolved in a water solution of glyphosate may play a double role as an environmentally friendly adjuvant (sticker) and as a biopolymeric carrier for the prolonged release of glyphosate. Some of the biopolymeric NPs that have been used as carriers of herbicides are listed in Table 4.

Table 4.
Biopolymeric nanoparticles assessed as carriers of herbicides.
5.4. Nanonematicides
Nematodes are common soilborne organisms, and more than 4100 plant-parasitic species cause significant damage to crops in sandy soil and water-stressed conditions, representing approximately USD 80–118 billion in losses worldwide [147,148,149]. Nematodes mainly feed on roots and can lead to the entry of other disease-causing microbes, such as bacteria and fungi, further worsening crop damage. Conventional nematicides have been found to be environmental toxins, and new approaches in the form of NP applications are being explored. The microemulsion polymerization process was used to create nanocapsules of lansiumamide B (N-methyl-N-cis-styrylcinnamamide) with nematicidal activity against Bursaphelenehus xylophilus and Meloidogyne incognita (LC50 values of 2.14 and 19.36 mg/L, respectively, after 24 h). Additionally, treatment with nanocapsules, regular polymers, and ethoprophos (an insecticide and nematicide) saw 68.42, 36.84, and 26.32% reductions in the disease’s progression, respectively. The average number of root knots in Ipomoea aquatica decreased by 83.94, 78.03, and 63.66%, respectively, showing that the nematicide nanoformulation worked more effectively and maintained its effectiveness against plant parasitic nematodes for a longer period [150].
The effectiveness of starch-stabilized silver NPs against the root-knot nematode M. incognita was investigated in [151]. Under in vitro conditions, this nanoformulation inactivated > 99% of the root nematodes in 6 h. The population of nematodes in the soil samples treated with this nanoformulation (150 g/mL) in the in vivo experiment was reduced by 92 and 82% after 4 and 2 days of exposure, respectively, compared to the nontreated soil samples. In a related study, the authors of [152] discovered that administering silver NPs (AgNPs) with particle sizes of 200, 400, and 800 mg/mL to M. incognita resulted in 100% immobility and mortality, with a determined LC50 value of 100 mg/mL. The same nanoformulation at 0.02, 0.01, 0.005, 0.0025, 0.00125, and 0.0007% concentrations (w/w) controlled M. incognita; however, treatment with 0.02, 0.01, and 0.005% AgNPs was lethal to tomato plants and reduced tomato root and stem length and fresh weight significantly in comparison with the control. The authors of [153] applied the flash nanoprecipitation technique to produce abamectin-loaded NPs with a loading capacity > 40% and encapsulation efficiency > 95% using the amphiphilic copolymers poly(lactic-co-glycolic acid)-b-poly (ethylene glycol) (PLGA-b-PEG), poly(d,l-lactide)-b-poly(ethylene glycol) (PLA-b-PEG), and poly(caprolactone)-b-poly(ethylene glycol) (PCL-b-PEG); these were tested against the southern root-knot nematode Meloidogyne incognita. There are no reports regarding the use of biopolymers as carriers of nematicides. Table 5 lists some of the polymeric nanonematicides that have been developed.

Table 5.
Polymeric nanoparticles developed as carriers of nanonematicides.
5.5. Nanomolluscicides and Nanomiticides
Mollusks are soft-bodied animals. They also cause significant damage to the crops in many regions of the world, besides being the reason for many livestock and human diseases. Table 6 summarizes several of the studies that have been performed using polymeric NPs to target mollusks.

Table 6.
Biopolymeric nanoparticles studied as carriers of nanomolluscicides.
Similarly, metallic nanocomposites have been used extensively to control termites in woody species. However, little work has been carried out using polymeric NPs for this purpose, as shown in Table 7.

Table 7.
Polymeric nanoparticles tested as carriers of nanomiticides.
6. Antimicrobial Activity of Biopolymeric Nanoparticles Alone
NPs have been studied comprehensively in regard to their antimicrobial properties to determine their efficacy against fungal and bacterial diseases in plants and animals. The efficacy of NPs against antibiotic-resistant strains of bacteria and fungi lies in their small size. On the nanoscale, particles act as molecules when interacting with a cell, allowing them to penetrate the cell membrane easily and positively affecting the central molecular pathways. Their high surface-area-to-volume ratio increases contact with the target, making NPs a magic bullet for drug delivery. They may be synthesized from biological entities such as bacteria, fungi, biopolymers, and lipids, making them useful in various fields. NPs’ interactions with fungal pathogens rely on the size and shape of the NPs; thus, disease control and toxicity are governed by these factors.
Several experiments have been performed to determine the antimicrobial activity of NPs. The authors of [157] prepared chitosan NPs from diverse concentrations of low-molecular-weight (LMW) and high-molecular-weight (HMW) chitosan. These were found to show superior inhibitory activity, i.e., a low MIC (minimum inhibitory concentration) for F. solani (MICLMW = 0.86–1.2 mg/mL and MICHMW = 0.5–1.2 mg/mL) and C. albicans (MICLMW = 0.25–0.86 mg/mL and MICHMW = 0.6–1.0 mg/mL) compared to the solution form (MIC = 3 mg/mL for both molecular weights and fungal strains). The particle size and zeta potential of the chitosan NPs also impacted their inhibitory effect. Additionally, Aspergillus niger was unaffected by the chitosan NPs, except for the HMW NPs at higher concentrations. Trimethyl chitosan (TMC) NPs showed insignificant antifungal activity. Therefore, the parent compound, i.e., chitosan, can be used as a natural antifungal agent in its NP form to enhance its antifungal activity.
The authors of [158] used low-molecular-weight chitosan as a reducing and stabilizing agent to create silver NPs between 10 and 15 nm in size. The production of NPs was confirmed by the UV–Vis spectrum, EDS (energy-dispersive X-ray spectroscopy), and FESEM (field emission scanning electron microscopy), and the structure of the composite of chitosan and AgNPs was made clear. The conidial germination of C. gloeosporioides was significantly reduced by the chitosan–Ag nanocomposite (treated with 30 mM AgNO3) compared to chitosan alone. The chitosan–Ag nanocomposite suppressed conidial germination by 44, 70, and 78% at concentrations of 0.1 (0.00001%), 1.0 (0.0001%), and 10 g/mL (0.001%). The concentration of 100 g/mL (0.01%) hindered spore germination. This effectively reduced the frequency of anthracnose in mango and inhibited the conidial germination of C. gloeosporioides. This nanocomposite could find applications in preventing dormant infections of Colletotrichum in mango, thus avoiding huge crop losses and promoting the export of high-quality fruits and vegetables.
Fusarium wilt is a fungal disease of chickpea (Cicer arietinum) caused by Fusarium oxysporum f. sp. ciceri (FOC) and has seed- and soil-borne origins. The authors of [9] performed in vitro and in vivo studies of chitosan (CS) and its nanocomposites as antifungal agents against FOC. Chitosan–copper oxide nanocomposites (CS-CuO) and chitosan–zinc oxide nanocomposites (CS-ZnO) were the most effective against various FOC concentrations, i.e., 50, 100, and 200 µg/mL. The CS NPs and chitosan–silver (CS-Ag) nanocomposites were effective and more efficient than standard fungicides, i.e., copper oxy-chloride (CuOCl). Based on the in vitro results, a 100 µg/mL concentration of all nanoformulations was selected for in vivo plant studies under pot conditions. The highest wilt disease reduction (46.67%) was observed in CS-CuO nanoformulations, followed by CS-ZnO (40%). In contrast, CS-Ag and CS caused only a 33.33% reduction in wilt incidence, representing the lowest impact. All synthesized nanoformulations showed excellent antifungal efficacy, inhibited the pathogens, and promoted the growth of the chickpea plants compared to the untreated plants.
The authors of [159] reported on the applications of chitin- and chitosan-based polymers in controlling plant diseases. Similarly, the authors of [160] found that compared to untreated plants, adding a chitosan nanoformulation to the soil marginally increased the growth of tomato plants and decreased the occurrence of Fusarium wilt disease. Utilizing chitosan nanoformulations complexed with both proteins and CaCO3 in protein/CaCO3/chitin nanofibers proved more successful at preventing disease than protein alone or protein/chitin nanofibers. The authors of [161] employed oligochitosansilica/carboxymethyl cellulose NPs to control Phytophthora infestans at a concentration of 800 mg/L (the lowest concentration that inhibited fungal growth). The antimicrobial activity of some biopolymeric nanocomposites is summarized in Table 8.

Table 8.
Antimicrobial activity of some biopolymeric nanocomposites.
7. Release Dynamics of Nanopesticides
Modern pesticide formulations are a burden to farming systems because they build up in the soil and ecosystems and can have detrimental impacts on humans and other living things. Nanotechnology makes it easy to control the release of agrochemicals and deliver certain macromolecules to specific locations for improved plant disease resistance, enhanced plant growth, and effective nutrient use. With reduced contact with the environment, nanoencapsulation provides the advantage of safer handling and more effective pesticide usage, which ensures environmental protection. In plant entomology, nanotechnology focuses on particular agricultural issues in the interactions between plant pests and provides novel crop security strategies. In their study, the authors of [10] delivered the pesticide imidacloprid (admire) to plants in a nanoformulation and examined its impacts. Their findings may offer some guidance for the safe application of this cutting-edge technology to increase crop output and safety.
The authors of [172] developed a nanoparticulate system based on ionic gelation between chitosan and arabic gum for loading insulin. The release profile of insulin in phosphate-buffered solutions (pH 6.5 and pH 7.2) was found to be entirely different from that in an acidic medium (pH 1.2). The increased solubility of chitosan in an acidic medium and the improved swelling of arabic gum chains at pH 6.5 resulted in lower insulin release at pH 6.5 as compared to other pH values. A non-Fickian transport pattern was observed, possibly indicating that release was controlled by the diffusion or relaxation of the biopolymer chains.
The authors of [173] prepared a spherical-shaped, nisin-loaded tripolymeric nanoformulation using chitosan, sodium alginate, and pluronic F68, with a mean particle size of 208.2 nm. The Fourier transform infrared study did not show any ionic interaction among the components of the composite NPs. Th in vitro release experiment showed an initial burst release in the first week, followed by the sustained release of nisin from the formulation in the second week. This confirmed the experiment’s success and provided favorable prospects for the use of these biopolymers in agriculture.
Similarly, guar gum was successfully acrylated by the authors of [11] to create guar-gum-grafted polyacrylic acid. The grafted biopolymer displayed enhanced stimulus responsiveness, low viscosity, and improved heat stability. During grafting, the pesticide chlorpyrifos was captured as a model bioactive molecule to examine the effectiveness of its entrapment and release behavior. The sizable release of chlorpyrifos was noticed when a methanolic buffer (pH 7.4, 25:75 v/v) was utilized. The calibration curve determined that the formulation’s entrapment efficiency was 60%. The goodness-of-fit model-dependent technique and other kinetic models showed that the release was concentration-dependent and followed first-order kinetics for a slow and prolonged period. The site-directed delivery of biopolymeric NPs is represented schematically in Figure 2.
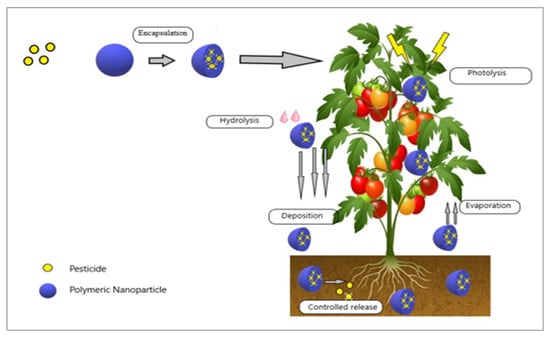
Figure 2.
Encapsulation of pesticides into NPs for site-directed delivery and to avoid premature degradation.
The partial release of carbendazim from chitosan-pectin NPs into the media at various pH values was noted [88]. After 48 h, the average cumulative percent release of carbendazim from the nanoformulation was 61.9 ± 0.1% at pH 4.0, 50.4 ± 0.13% at pH 7.4, and 62.8 ± 0.13% at pH 10.0. In contrast, the percentage release of pure carbendazim was recorded to be 82.4 ± 17% at pH 4.0, 67.7 ± 0.1% at pH 7.4, and 86.8 ± 0.2% at pH 10.0. These data supported the persistent release of NPs, because the active ingredient was contained between the core of the biopolymeric nanoformulation, which shielded it from environmental variables such as light, water, oxygen, and hydrolysis. This release pattern was observed in the experiments. Polybutylene succinate (PBS) and poly lactic acid (PLA) were used together in [174] to improve encapsulation effectiveness and ionic interaction. The resulting 7.2 μm (micrometer) microsphere demonstrated a high degree of encapsulation efficiency. Comparative studies showed that the microsphere had a longer period of sustained release than the difenoconazole-azoxystrobin (5:8, 32.5% w/v) suspension concentrate due to its complex stoichiometry, which was optimal for refining the disease efficacy of the pesticides. These results verified that such a pesticide microsphere delivery structure could be a promising target for further exploration.
Similarly, the authors of [82] applied sodium alginate, polyacrylamide (PAM), and montmorillonite (MMT) to construct several stretchable double-network nanocomposite hydrogels for controlling the release of λ-cyhalothrin. Adopting sodium alginate with the appropriate incorporation of MMT significantly improved the system’s tensile properties, loading efficiency, and slow-release behavior. The hydrogels’ maximum fracture strain and loading efficiency reached 2000% and 81.30%, respectively. The hydrogel with 5% MMT content showed the least cumulative release percentage of 6.68% over 87 h, and the pesticide release curves fit into the Weibull model.
The authors of [175] applied the oil/water emulsion solvent evaporation technique to polylactic acid and fungicide azoxystrobin and demonstrated that the cumulative release percentage was inversely related to particle size. Another study [81] used ionic gelation to encapsulate commercial hexaconazole in chitosan NPs, resulting in a continuous release of 99.91% over a protracted period of 86 h. Using a similar synthesis process and the same biopolymer but different agricultural chemicals (spinosad and permethrin), the authors of [143] formulated a N-hexanoyl-O-glycol chitosan–atrazine micelle, which enhanced the water solubility of atrazine three-fold. The successful release of dicamba dimethylamine in aqueous and agricultural soils was described by [176] using Cu–chitosan NPs synthesized by a chemical reduction method. The release mechanism was tested using the Korsmeyer–Peppas model (KP) for a calcium-alginate–sulfentrazone (a herbicide) nanoformulation [18]. The high-molecular-weight chitosan biopolymer matrix caused a slower release of the herbicide glyphosate than the low-molecular-weight chitosan system [83]. The time taken for 50% of the herbicide imazethapyr to be released increased from 11.30 days to 43.73 days using nanoformulations of alginate–cellulose synthesized via the ionotropic gelation method [177]. The research on active ingredients encapsulated in biopolymer-based controlled-release matrices and their use in agriculture is summarized in Table 9.

Table 9.
Research on controlled-release matrices of active ingredients encapsulated in biopolymers and their use in agriculture.
8. Toxicity Profile of Nanopesticides
The indirect or direct exposure of humans to pesticides causes several health issues, such as obesity, endocrine disorders [195], cancer [196], and neurological illness [197]. The authors of [198] found organochlorine-pesticide-induced carcinogenic effects in some patients. Similarly, the authors of [199] found organophosphate and organochlorine pesticide exposure in children of farm workers aged 6 to 11 years. The case group’s serum levels of beta-HCH, 4,4 DDE, and 4,4 DDT were considerably higher than those of the control group.
For nanoparticulate systems, it is not sufficient to characterize the particles and demonstrate their good association with the active compound of the pesticide or other drug. It is also essential to assess their toxicity to target and non-target organisms to further the holistic and sustainable development of the environment. Toxicity profiling is a prerequisite for any drug or agrochemical before it is released on the market. A group of microbiologists [200] studied the influence of nanohexaconazole on soil nitrifiers for 28 days in three replicates with NP doses of 10, 25, and 50 g/ha in 98% humidity at 25 °C. Soil nitrification activity was observed, and it was found that the ammonium, nitrogen, and nitrate-N curve was the same for both the nano- and commercial pesticides. No adverse effects of nanohexaconazole were observed on the two key microorganisms, Nitrosomonas and Nitrobacter species.
Similarly, cytotoxicity and genotoxicity assays of the herbicide paraquat on Chinese hamster ovary (CHO) cells and A. cepa seeds were conducted in [136]. The IC50 value for free paraquat was about 0.12 mg/mL, while the NPs (with or without paraquat) showed almost no toxicity at the same concentrations tested for 24 h. The genotoxicity data revealed that there was increased DNA damage for all the treatments as compared to the negative control. However, the damage was insignificant when blank NPs were used, as greater damage was observed when free paraquat was used. Figure 3 summarizes the impacts of biopolymeric NP-encapsulated pesticides on the environment.
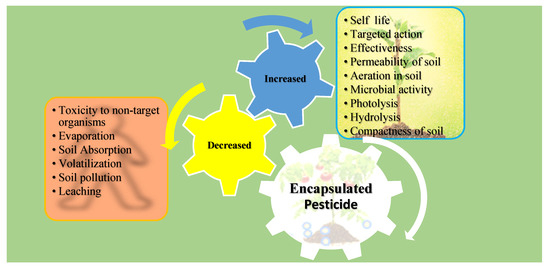
Figure 3.
Impacts of pesticides encapsulated in biopolymeric NPs on soil, organisms, and the environment.
When carbendazim-loaded biopolymeric NPs were tested against Aspergillus parasiticus and Fusarium oxysporum and compared to carbendazim alone, the authors of [88] discovered an increase in the fungal inhibition rate. Phytotoxicity studies confirmed that maize, cucumber, and tomato seeds germinated and grew roots more efficiently when carbendazim was nanoformulated. The impact of carbendazim and tebuconazole on mouse fibroblast cells was studied in [12] using polymeric and solid lipid NPs. The cellular viability assays performed on normal cells (3T3 and MC3T3) demonstrated that, regardless of the cell types used, the NPs were less toxic than the commercial fungicides. Cellular viability levels below 25% and 60% at the maximum concentration for the cell lines 3T3 and MC3T3, respectively, indicated the cytotoxicity of the fungicides. The effect of exposing the cells to the commercial formulation was dose-dependent. However, the adenocarcinoma cell (HeLa) results revealed that the fungicide-loaded solid lipid NPs had higher cytotoxicity than the commercial formulations. This could be ascribed to the variation in how well the cells absorbed these NPs. In its commercial and nanoformulation, hexaconazole was more hazardous to Vero cell lines when the pesticide concentration was increased from 10 to 20 ppm [98]. The pesticide-free blank nanoformulation exhibited no cytotoxicity, which could be attributed to the biocompatibility of the polymers used to create the nanocapsules.
In contrast to seeds treated with pure carbendazim, which exhibited a decrease (up to 60%) in germination, seeds treated with nanoformulations had a 96% germination rate, according to a study in 2017 [88]. During the phytotoxicity investigation, a significant decrease in root length and germination % was seen in seeds treated with carbendazim compared to those treated with nanoformulations. The authors of [201] found that the fungicide prochloraz encapsulated in chitosan/silica reduced zebrafish toxicity six-fold compared to commercial prochloraz. In their study, Dong et al. [90] successfully demonstrated that the encapsulation of the fungicide spinosad in chitosan–isoleucine NPs protected the fungicide against photo-degradation.
The authors of [138], in their study on soil biota under the application of the herbicides imazapic (IMC) and imazapyr (IMR) encapsulated in alginate/chitosan (ALG/CS) and chitosan/TPP (CS/TPP) NPs, found that after 30 days, higher numbers of bacteria (compared to the negative control) were observed in the soils treated with the NPs alone (CS/ALG and CS/TPP), while the treatment with CS/ALG/IMC + IMR showed the greatest similarity to the negative control. The Allium cepa assay demonstrated that the encapsulation of the herbicides could reduce the extent of damage compared to the free compounds when applied to black-jack (B. pilosa). Similarly, the authors of [19] found that the encapsulated herbicide paraquat was less toxic to alveolar and mouth cell lines than its trade form. Additionally, the toxicity of NPs for the A549 cell line was lower than for the KB cell line for most doses. While applying sodium alginate NPs to cucumber plants, the authors of [145] observed that the leaching of the commercial herbicide tebuthiuron in the soil reached 40–50 cm deep, while for the nanoformulations, it reached 20–30 cm deep, suggesting that they were a safe alternative to the commercial herbicide. The authors of [47] assessed the cytotoxicity of metsulfuron-methyl-loaded pectin NPs using healthy cell lines (Vero cell lines) and compared it to that of the commercial herbicide. They also used a pectin nanocarrier to conduct an in-field evaluation using Chenopodium album plants. The findings suggested that using herbicide-loaded NPs could minimize the dose requirements of herbicides while improving their effectiveness and environmental safety. Mancozeb-loaded chitosan–gum-acacia NPs at 0.25 mg/mL showed the least cytotoxicity (25.92%), which was considerably lower than the cytotoxicity of commercial mancozeb (33.72%), according to [106]. The cytotoxicity of all three mancozeb-loaded nanoformulations at 2.0 mg/mL was also much lower than that of commercial mancozeb (87.61%), showing values of 61.40, 69.99, and 77.78%, respectively. The very low toxicity of the blank NCs at all concentrations demonstrated that mancozeb, not the nanocomposites, was the cause of the cytotoxicity.
Thus, NPs provide a more environmentally friendly method of battling human infections and fungal crop diseases by reducing the cytotoxicity and genotoxicity of pesticides for non-target species [202,203,204]. The authors of [83], while working to eliminate the deadliest weeds, common sorrel (R. acetosa L.) and white goosefoot (C. album L.), found that a high concentration of chitosan NPs with encapsulated glyphosate caused a reduction in toxic effects against the roots of Avena sativa and Raphanus sativus.
9. Conclusions, Challenges, and Future Prospects
The application of nanotechnology in agriculture throughout the globe is at its embryonic stage. Modern agrochemicals such as nanopesticides and nanofungicides are being developed for enhanced plant growth, nutrition, and protection against diseases to meet the food demands of the ever-increasing world population. Biopolymeric NPs such as chitosan, carrageenan, guar gum, gum acacia, and sodium alginate efficiently dispense pesticides and nutrients precisely and with high site-specificity. From the perspective of plant–pathogen interactions and the delivery of systemic agrochemicals to specific sites, they provide innovative solutions to problems such as resistance to synthetic pesticides, disease outbreaks, and leaching, thus reducing environmental pollution. Functionalized NPs can pass through a plant’s vascular system and guide agrochemicals (fungicides, herbicides, and insecticides) or other substances (plant hormones, elicitors, and nucleic acids) into targeted, localized areas of plant tissues with sustained release. Natural polymer-mediated NP synthesis provides a benign synthesis route with better control over NP morphology, which ultimately decides the toxicity of the synthesized NPs. Toxicity profiling is essential to achieve sustainable agriculture goals. Biopolymeric NPs are a promising technology, representing a greener approach to target-specific disease control and the sustained release of pesticides in modern agricultural practices without compromising the ecosystem.
Nanotechnology has progressed rapidly in other industries but lags in agriculture. Despite their advantages, many biopolymeric NP-based products have not been commercialized yet for practical implementation in agriculture. This is due to the lack of sufficient field trials, sophisticated labs/instruments, a regulatory framework, and guidelines. Consistent field trials are a prerequisite for the massive adoption or commercialization of nanopesticides, as results under laboratory or greenhouse conditions do not always reflect those in the field. Material scientists and biologists need to understand the fundamental questions related to nanotechnology research and address the research gap to facilitate the production of biocompatible, biodegradable, and safe commercial biopolymeric nanoproducts that can be used without disturbing the ecological balance. Multidisciplinary and collaborative research projects in this field will provide a platform for making plant protection a reality.
Author Contributions
Conceptualization, R.K. and J.S.D.; methodology, N.K.; software, R.K., B.S.S. and D.K.; validation, V.D.R. and J.S.D.; formal analysis, T.M.; investigation, J.S.D.; resources, P.K.S.; data curation, R.K.; writing—original draft preparation, S.M. and R.K.; writing—review and editing, V.D.R. and T.M.; visualization, R.K.; supervision, J.S.D., B.S.S., S.M. and D.K. project administration, J.S.D. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge the support of the “Soil Health” laboratory of the Southern Federal University and the financial support of the Ministry of Science and Higher Education of the Russian Federation, agreement No. 075-15-2022-1122.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Vinutha, J.S.; Bhagat, D.; Bakthavatsalam, N. Nanotechnology in the management of polyphagous pest Helicoverpa armigera. J. Acad. Ind. Res. 2013, 1, 606–608. [Google Scholar]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186. [Google Scholar] [CrossRef] [PubMed]
- EXTOXNET-The Extension Toxicology Network. Available online: http://extoxnet.orst.edu/ (accessed on 16 November 2017).
- Duhan, J.S.; Kumar, R.; Kumar, N.; Kaur, P.; Nehra, K.; Duhan, S. Nanotechnology: The new perspective in precision agriculture. Biotechnol. Rep. 2017, 15, 11–23. [Google Scholar] [CrossRef]
- Campos, E.V.R.; de Oliveira, J.L.; Fraceto, L.F. Applications of controlled release systems for fungicides, herbicides, acaricides, nutrients, and plant growth hormones: A review. Adv. Sci. Eng. Med. 2014, 6, 373–387. [Google Scholar] [CrossRef]
- Rajput, V.D.; Singh, A.; Minkina, T.; Rawat, S.; Mandzhieva, S.; Sushkova, S.; Shuvaeva, V.; Nazarenko, O.; Rajput, P.; Komariah; et al. Nano-enabled products: Challenges and opportunities for sustainable agriculture. Plants 2021, 10, 2727. [Google Scholar] [CrossRef]
- Bansal, P.; Kaur, P.; Kumar, A.; & Duhan, J.S. Microwave-assisted quick synthesis method of silver nanoparticles using a citrus hybrid “Kinnow” and its potential against the early blight of tomato. Res. Crops 2017, 18, 650–655. [Google Scholar] [CrossRef]
- Kaur, P.; Duhan, J.S.; Thakur, R. Comparative pot studies of chitosan and chitosan-metal nanocomposites as nano-agrochemicals against fusarium wilt of chickpea (Cicer arietinum L.). Biocatal. Agric. Biotechnol. 2018, 14, 466–471. [Google Scholar] [CrossRef]
- Kumar, S.; Bhanjana, G.; Sharma, A.; Sidhu, M.C.; Dilbaghi, N. Synthesis, characterization and on-field evaluation of pesticide-loaded sodium alginate nanoparticles. Carbohydr. Polym. 2014, 101, 1061–1067. [Google Scholar] [CrossRef]
- Faizan, M.; Rajput, V.D.; Al-Khuraif, A.A.; Arshad, M.; Minkina, T.; Sushkova, S.; Yu, F. Effect of Foliar Fertigation of Chitosan Nanoparticles on Cadmium Accumulation and Toxicity in Solanum lycopersicum. Biology 2021, 10, 666. [Google Scholar] [CrossRef]
- Campos, E.V.R.; De Oliveira, J.L.; Da Silva, C.M.G.; Pascoli, M.; Pasquoto, T.; Lima, R.; Abhilash, P.C.; Fraceto, L.F.U. Polymeric and solid lipid nanoparticles for sustained release of carbendazim and tebuconazole in agricultural applications. Sci. Rep. 2015, 5, 13809. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.; Varghese, S.H.; Nair, B.G.; Maekawa, T.; Yoshida, Y.; Kumar, D.S. Nanoparticulate material delivery to plants. Plant Sci. 2010, 179, 154–163. [Google Scholar] [CrossRef]
- Kumar, A.; De, A.; Mozumdar, S. Synthesis of acrylate guar-gum for delivery of bio-active molecules. Bull. Mater. Sci. 2015, 38, 1025–1032. [Google Scholar] [CrossRef]
- Salgueiro, A.M.; Daniel-da-Silva, A.L.; Fateixa, S.; Trindade, T. κ-Carrageenan hydrogel nanocomposites with release behaviour mediated by morphological distinct Au nanofillers. Carbohydr. Polym. 2013, 91, 100–109. [Google Scholar] [CrossRef]
- Albuquerque, P.; Coelho, L.C.; Teixeira, J.A.; Carneiro-da-Cunha, M.G. Approaches in biotechnological applications of natural polymers. AIMS Mol. Sci. 2016, 3, 386–425. [Google Scholar] [CrossRef]
- Sharma, A.; Sood, K.; Kaur, J.; Khatri, M. Agrochemical-loaded biocompatible chitosan nanoparticles for insect pest management. Biocatal. Agric. Biotechnol. 2019, 18, 101079. [Google Scholar] [CrossRef]
- Slade, G.G.; Dourado, S.M., Jr.; Oliveira, R.J.D.; Moreto, J.A. Developing a mathematical model for the controlled release over time of sulfentrazone herbicide from a biodegradable polymer. Mater. Res. 2019, 22. [Google Scholar] [CrossRef]
- Rashidipour, M.; Maleki, A.; Kordi, S.; Birjandi, M.; Pajouhi, N.; Mohammadi, E.; Rasoulian, R.; Davari, B. Pectin/chitosan/tripolyphosphate nanoparticles: Efficient carriers for reducing soil sorption, cytotoxicity, and mutagenicity of paraquat and enhancing its herbicide activity. J. Agric. Food Chem. 2019, 67, 5736–5745. [Google Scholar] [CrossRef]
- Elabasy, A.; Shoaib, A.; Waqas, M.; Shi, Z.; Jiang, M. Cellulose nanocrystals loaded with thiamethoxam: Fabrication, characterization, and evaluation of insecticidal activity against Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae). Nanomaterials 2020, 10, 788. [Google Scholar] [CrossRef]
- Das, R.K.; Sarma, S.J.; Brar, S.K.; Verma, M. Nanoformulation of Insecticides-Novel Products. J. Biofertil. Biopestic. 2014, 5, e120. [Google Scholar]
- Sathiyabama, M.; Manikandan, A. Application of copper-chitosan nanoparticles stimulates growth and induces resistance in finger millet (Eleusine coracana Gaertn.) plants against blast disease. J. Agric. Food Chem. 2018, 66, 1784–1790. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.T. Living in the Environment; Wadsworth/Thomson Learning: Belmont, CA, USA, 2002; ISBN 9781133940135 o-534-37697-5. [Google Scholar]
- Unsworth, J. History of Pesticide Use; IUPAC-International Union of Pure and Applied Chemistry: Research Triangle Park, NC, USA, 2010; Available online: https://agrochemicals.iupac.org/index.php?option=com_sobi2&sobi2Task=sobi2Details&catid=3&sobi2Id=31 (accessed on 3 April 2018).
- Sparks, T.C.; Nauen, R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 2015, 121, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Kuck, K.H.; Leadbeater, A.; Gisi, U. FRAC Mode of Action Classification and Resistance Risk of Fungicides. In Modern Crop Protection Compounds; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; pp. 539–557. [Google Scholar]
- Carvalho, F.P. Agriculture, pesticides, food security and food safety. Environ. Sci. Policy 2006, 9, 685–692. [Google Scholar] [CrossRef]
- Gilland, B. World population and food supply: Can food production keep pace with population growth in the next half-century? Food Policy 2002, 27, 47–63. [Google Scholar] [CrossRef]
- Klassen, W. World food security up to 2010 and the global pesticide situation. In Option 2000, Proceedings of the Eighth International Congress of Pesticide Chemistry; American Chemical Society: Washington, DC, USA, 1995. [Google Scholar]
- Peshin, R.; Bandral, R.S.; Zhang, W.; Wilson, L.; Dhawan, A.K. Integrated pest management: A global overview of history, programs and adoption. In Integrated Pest Management: Innovation-Development Process; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–49. [Google Scholar]
- Agrios, G.N. Introduction to Plant Pathology, 5th ed.; Elsevier Academic Press Publication: New York, NY, USA, 2005; p. 922. [Google Scholar]
- Dhekney, S.A.; Li, Z.T.; Van Aman, M.; Dutt, M.; Tattersall, J.; Kelley, K.T.; Gray, D.J. Genetic transformation of embryogenic cultures and recovery of transgenic plants in Vitis vinifera, Vitis rotundifolia and Vitis hybrids. In Proceedings of the International Symposium on Biotechnology of Temperate Fruit Crops and Tropical Species, Daytona Beach, FL, USA, 10–14 October 2005; Volume 738, pp. 743–748. [Google Scholar]
- Fernandez-Perez, M.; González-Pradas, E.; Villafranca-Sánchez, M.; Flores-Céspedes, F. Mobility of isoproturon from an alginate–bentonite controlled release formulation in layered soil. Chemosphere 2000, 41, 1495–1501. [Google Scholar] [CrossRef]
- Schneider, S.; Ullrich, W.R. Differential induction of resistance and enhanced enzyme activities in cucumber and tobacco is caused by treatment with various abiotic and biotic inducers. Physiol. Mol. Plant Pathol. 1994, 45, 291–304. [Google Scholar] [CrossRef]
- European Commission. Commission Recommendation of 18 October 2011 on the Definition of Nanomaterial (2011/696/EU). 2011. Available online: http://eur-lex.europa.eu/legalcontent/EN/TXT/PDF/?uri=CELEX:32011H0696&from=EN (accessed on 5 March 2019).
- Nasir, O.; Artunc, F.; Saeed, A.; Kambal, M.A.; Kalbacher, H.; Sandulache, D.; Boini, K.M.; Jahovic, N.; Lang, F. Effects of gum arabic (Acacia senegal) on water and electrolyte balance in healthy mice. J. Ren. Nutr. 2008, 18, 230–238. [Google Scholar] [CrossRef]
- Albuquerque, P.B.; Coelho, L.C.; Correia, M.T.; Teixeira, J.; Carneiro-da-Cunha, M.G. Biotechnological applications of galactomannan matrices: Emphasis on immobilization of biomolecules. Adv. Res. 2016, 6, 1–17. [Google Scholar] [CrossRef]
- Kameshwar, A.K.S.; Qin, W. Structural and functional properties of pectin and lignin–carbohydrate complexes de-esterases: A review. Bioresour. Bioprocess. 2018, 5, 43. [Google Scholar] [CrossRef]
- Fatokun, O.T. Micrometric and Morphological Properties of Starch. In Chemical Properties of Starch; IntechOpen: London, UK, 2019. [Google Scholar]
- Gupta, P.K.; Raghunath, S.S.; Prasanna, D.V.; Venkat, P.; Shree, V.; Chithananthan, C.; Choudhary, S.; Surender, K.; Geetha, K. An update on overview of cellulose, its structure and applications. In Cellulose; IntechOpen: London, UK, 2019. [Google Scholar]
- Kim, D.; Petrisor, I.G.; Yen, T.F. Evaluation of biopolymer-modified concrete systems for disposal of cathode ray tube glass. J. Air Waste Manag. Assoc. 2005, 55, 961–969. [Google Scholar] [CrossRef]
- Pighinelli, L.; Broquá, J.; Zanin, B.G.; Flach, A.M.; Mallmann, C.; Taborda, F.G.D.; Machado, L.E.L.; Alves, S.M.L.; Silva, M.M.; Dias, R.J.S.P.; et al. Methods of Chitin Production a Short Review. Am. J. Biomed. Sci. Res. 2019, 3, 307–314. [Google Scholar] [CrossRef]
- Kaushik, K.; Sharma, R.B.; Agarwal, S. Natural polymers and their applications. Int. J. Pharm. Sci. Rev. Res. 2016, 37, 30–36. [Google Scholar]
- Pereira, L.; Cotas, J. Introductory Chapter: Alginates-A General Overview. In Alginates-Recent Uses of This Natural Polymer; IntechOpen: London, UK, 2020. [Google Scholar]
- Carneiro-da-Cunha, M.G.; Cerqueira, M.A.; Souza, B.W.; Souza, M.P.; Teixeira, J.A.; Vicente, A.A. Physical properties of edible coatings and films made with a polysaccharide from Anacardium occidentale L. J. Food Eng. 2009, 95, 379–385. [Google Scholar] [CrossRef]
- Souza, M.; Cerqueira, M.; Souza, B.; Teixeira, J.; Porto, A.; Vicente, A.; Carneiro-da-Cunha, M. Polysaccharide from Anacardium occidentale L. tree gum (Policaju) as a coating for Tommy Atkins mangoes. Chem. Pap. 2010, 64, 475–481. [Google Scholar] [CrossRef]
- Kumar, A.; Moin, A.; Ahmed, A.; Shivakumar, H.G. Cashew gum a versatile hydrophilic polymer: A review. Curr. Drug Ther. 2012, 7, 2–12. [Google Scholar] [CrossRef]
- Forato, L.A.; de Britto, D.; de Rizzo, J.S.; Gastaldi, T.A.; Assis, O.B. Effect of cashew gum-carboxymethylcellulose edible coatings in extending the shelf-life of fresh and cut guavas. Food Pack. Shelf Life 2015, 5, 68–74. [Google Scholar] [CrossRef]
- Chopra, M.; Kaur, P.; Bernela, M.; Thakur, R. Surfactant-assisted nisin-loaded chitosan-carrageenan nanocapsule synthesis for controlling food pathogens. Food Control 2014, 37, 158–164. [Google Scholar] [CrossRef]
- Campos, E.V.; Proença, P.L.; Oliveira, J.L.; Melville, C.C.; Vechia, J.F.; Andrade, D.J.; Fraceto, L.F. Chitosan nanoparticles functionalized with β-cyclodextrin: A promising carrier for botanical pesticides. Sci. Rep. 2018, 8, 2067. [Google Scholar] [CrossRef]
- Bourbon, A.I.; Pinheiro, A.C.; Ribeiro, C.; Miranda, C.; Maia, J.M.; Teixeira, J.A.; Vicente, A.A. Characterization of galactomannans extracted from seeds of Gleditsia triacanthos and Sophora japonica through shear and extensional rheology: Comparison with guar gum and locust bean gum. Food Hydrocoll. 2010, 24, 184–192. [Google Scholar] [CrossRef]
- Kumar, M.N.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Jana, S.; Saha, A.; Nayak, A.K.; Sen, K.K.; Basu, S.K. Aceclofenac-loaded chitosan-tamarind seed polysaccharide interpenetrating polymeric network microparticles. Colloids Surf. B 2013, 105, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.K.; Bishnoi, R.S.; Kumar, M.; Fenin, V.; Jain, C.P. Applications of tamarind seeds polysaccharide-based copolymers in controlled drug delivery: An overview. Asian J. Pharm. Pharmacol. 2018, 4, 23–30. [Google Scholar] [CrossRef]
- Vamadevan, V.; Bertoft, E. Structure-function relationships of starch components. Starch-Stärke 2015, 67, 55–68. [Google Scholar] [CrossRef]
- Mahmoud, M.G.; El Kady, E.M.; Asker, M.S. Chitin, chitosan and glucan, properties and applications. World J. Agric. Sci. 2019, 3, 1–19. [Google Scholar]
- Kulkarni Vishakha, S.; Butte Kishor, D.; Rathod Sudha, S. Natural polymers—A comprehensive review. Int. J. Pharm. Biomed. Res. 2012, 3, 1597–1613. [Google Scholar]
- Abd El-Rehim, H.A. Characterization and possible agricultural application of polyacrylamide/sodium alginate crosslinked hydrogels prepared by ionizing radiation. J. Appl. Polym. Sci. 2006, 101, 3572–3580. [Google Scholar] [CrossRef]
- Sian, H.K.; Said, M.; Hassan, O.; Kamaruddin, K.; Ismail, A.F.; Rahman, R.A.; Mahmood, N.A.N.; Illias, R. Purification and characterization of cyclodextrin glucanotransferase from alkalophilic Bacillus sp. G1. Process Biochem. 2005, 40, 1101–1111. [Google Scholar] [CrossRef]
- Du, H.; Bandara, S.; Carson, L.E.; Kommalapati, R.R. Association of polyethylene glycol solubility with emerging membrane technologies, wastewater treatment, and desalination. In Polyethylene Glycol; IntechOpen: London, UK, 2019. [Google Scholar]
- Heinze, T.; Liebert, T.; Heublein, B.; Hornig, S. Functional Polymers Based on Dextran. Adv. Polym. Sci. 2006, 205, 199–291. [Google Scholar]
- Kaur, R.; Sharma, M.; Ji, D.; Xu, M.; Agyei, D. Structural features, modification, and functionalities of beta-glucan. Fibers 2020, 8, 1. [Google Scholar] [CrossRef]
- Deng, Y.; Song, X.; Ma, Z.; Zhang, X.; Shu, D.; Nan, J. Al2O3/PVdF-HFP-CMC/PE separator prepared using aqueous slurry and post-hot-pressing method for polymer lithium-ion batteries with enhanced safety. Electrochim. Acta 2016, 212, 416–425. [Google Scholar] [CrossRef]
- Yang, W.; Wang, J.; Yang, Q.; Pei, H.; Hu, N.; Suo, Y.; Suo, Y.; Li, Z.; Zhang, D.; Wang, J. Facile fabrication of robust MOF membranes on cloth via a CMC macromolecule bridge for highly efficient Pb (II) removal. Chem. Eng. J. 2018, 339, 230–239. [Google Scholar] [CrossRef]
- Galia, M.B. Isolation and analysis of storage compounds. In Handbook of Hydrocarbon and Lipid Microbiology; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Bui, T.N.T.V. Structure, Rheological Properties and Connectivity of Gels Formed by Carrageenan Extracted from Different Red Algae Species. Ph.D. Thesis, Université du Maine, Le Mans, France, 2019. [Google Scholar]
- Shirwaikar, A.; Prabu, S.L.; Kumar, G.A. Herbal excipients in novel drug delivery systems. Indian J. Pharm. Sci. 2008, 70, 415. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.P.D.S.; Holanda e Silva, K.G.D.; Mansur, C.R.E. Evaluation of the application of cashew gum as an excipient to produce tablets. Polímeros 2018, 28, 302–308. [Google Scholar] [CrossRef]
- Garcıa-Ochoa, F.; Santos, V.E.; Casas, J.A.; Gómez, E. Xanthan gum: Production, recovery, and properties. Biotechnol. Adv. 2000, 18, 549–579. [Google Scholar] [CrossRef]
- Bergeson, L.L. Nanosilver: US EPA’s pesticide office considers how best to proceed. Environ. Qual. Manag. 2010, 19, 79–85. [Google Scholar] [CrossRef]
- Kookana, R.S.; Boxall, A.B.; Reeves, P.T.; Ashauer, R.; Beulke, S.; Chaudhry, Q.; Cornelis, G.; Fernandes, T.F.; Gan, J.; Kah, M.; et al. Nanopesticides: Guiding principles for regulatory evaluation of environmental risks. J. Agric. Food Chem. 2014, 62, 4227–4240. [Google Scholar] [CrossRef]
- Kah, M.; Beulke, S.; Tiede, K.; Hofmann, T. Nanopesticides: State of knowledge, environmental fate, and exposure modelling. Crit. Rev. Environ. Sci. Technol. 2013, 43, 1823–1867. [Google Scholar] [CrossRef]
- Kah, M.; Hofmann, T. Nanopesticide research: Current trends and future priorities. Environ. Int. 2014, 63, 224–235. [Google Scholar] [CrossRef]
- Holister, P.; Harper, T.E.; Vas, C.R. Nanotubes white paper. CMP Cientìfica Apdo. Correos 2003, 20, 28230. [Google Scholar]
- Prashanth, K.H.; Tharanathan, R.N. Chitin/chitosan: Modifications and their unlimited application potential—An overview. Trends Food Sci. Technol. 2007, 18, 117–131. [Google Scholar] [CrossRef]
- Pérez-de-Luque, A.; Rubiales, D. Nanotechnology for parasitic plant control. Pest Manag. Sci. Formerly Pest. Sci. 2009, 65, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Scrinis, G.; Lyons, K. The emerging nano-corporate paradigm: Nanotechnology and the transformation of nature, food and agri-food systems. Int. J. Sociol. Agric. Food Chem. 2007, 15, 22–44. [Google Scholar]
- Torney, F. Nanoparticle mediated plant transformation: Emerging technologies in plant science research. In The Interdepartmental Plant Physiology Major Fall Seminar Series; IPPM’09; Iowa State University: Ames, IA, USA, 1990. [Google Scholar]
- Kumar, S.S. Customizing Nanoparticles for the Maintenance of Seed Vigour and Viability in Black Gram (Vigna mungo) cv. Ph.D. Thesis, Tamil Nadu Agricultural University, Coimbatore, India, 2011. VBN 4. [Google Scholar]
- Sridhar, C. Effect of Nanoparticles on the Maintenance of Tomato Seed Vigour and Viability. Master’s Thesis, TNAU, Coimbatore, India, 2012. [Google Scholar]
- Maluin, F.N.; Hussein, M.Z.; Yusof, N.A.; Fakurazi, S.; Idris, A.S.; Hilmi, N.H.Z.; Daim, L.D.J. Preparation of Chitosan–Hexaconazole Nanoparticles as Fungicide Nanodelivery System for Combating Ganoderma Disease in Oil Palm. Molecules 2019, 24, 2498. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, G.; Li, J.; Feng, Y.; Peng, Y.; Zhao, X.; Tang, Y.; Zhang, Q. Stretchable hydrophobic modified alginate double-network nanocomposite hydrogels for sustained release of water-insoluble pesticides. J. Clean. Prod. 2019, 226, 122–132. [Google Scholar] [CrossRef]
- Rychter, P. Chitosan/glyphosate formulation is a potential, environmental friendly herbicide with prolonged activity. J. Environ. Sci. Health B 2019, 54, 681–692. [Google Scholar] [CrossRef]
- Worrall, E.; Hamid, A.; Mody, K.; Mitter, N.; Pappu, H. Nanotechnology for plant disease management. Agronomy 2018, 8, 285. [Google Scholar] [CrossRef]
- Yearla, S.R.; Padmasree, K. Exploitation of subabul stem lignin as a matrix in controlled release agrochemical nanoformulations: A case study with herbicide diuron. Environ. Sci. Pollut. Res. 2016, 23, 18085–18098. [Google Scholar] [CrossRef]
- Xu, L.; Cao, L.D.; Li, F.M.; Wang, X.J.; Huang, Q.L. Utilization of chitosan-lactide copolymer nanoparticles as controlled release pesticide carrier for pyraclostrobin against Colletotrichum gossypii Southw. J. Dispers. Sci. Technol. 2014, 35, 544–550. [Google Scholar] [CrossRef]
- Ilk, S.; Saglam, N.; Özgen, M. Kaempferol loaded lecithin/chitosan nanoparticles: Preparation, characterization, and their potential applications as a sustainable antifungal agent. Artif. Cells Nanomed. Biotechnol. 2017, 45, 907–916. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, D.; Dilbaghi, N. Preparation, characterization, and bio-efficacy evaluation of controlled release carbendazim-loaded polymeric nanoparticles. Environ. Sci. Pollut. Res. 2017, 24, 926–937. [Google Scholar]
- Oh, J.W.; Chun, S.; Chandrasekaran, M. Preparation and in vitro characterization of chitosan nanoparticles and their broad-spectrum antifungal action compared to antibacterial activities against phytopathogens of tomato. Agronomy 2019, 9, 21. [Google Scholar] [CrossRef]
- Dong, E.; Yang, Z.; Zhou, C.; Wang, C.; Li, S.; Ouyang, Q.; Kong, L.; He, Z.; Xie, J.; Li, P.; et al. pH-responsive ultrasonic self-assembly spinosad-loaded nanomicelles and their antifungal activity to Fusarium oxysporum. React. Funct. Polym. 2019, 141, 123–132. [Google Scholar] [CrossRef]
- Maluin, F.N.; Hussein, M.Z.; Azah Yusof, N.; Fakurazi, S.; Idris, A.S.; Zainol Hilmi, N.H.; Jeffery Daim, L.D. Chitosan-based agronanofungicides as a sustainable alternative in the basal stem rot disease management. J. Agric. Food Chem. 2020, 68, 4305–4314. [Google Scholar] [CrossRef] [PubMed]
- Machado, T.O.; Beckers, S.J.; Fischer, J.; Müller, B.; Sayer, C.; de Araújo, P.H.H.; Landfester, K.; Wurm, F.R. Bio-based lignin nanocarriers loaded with fungicides as a versatile platform for drug delivery in plants. Biomacromolecules 2020, 21, 2755–2763. [Google Scholar] [CrossRef]
- Zhang, W.; He, S.; Liu, Y.; Geng, Q.; Ding, G.; Guo, M.; Guo, M.; Deng, Y.; Zhu, J.; Li, J.; et al. Preparation and characterization of novel functionalized prochloraz microcapsules using silica–alginate–elements as controlled release carrier materials. ACS Appl. Mater. Interfaces 2014, 6, 11783–11790. [Google Scholar] [CrossRef]
- Kheiri, A.; Jorf, S.M.; Malihipour, A.; Saremi, H.; Nikkhah, M. Application of chitosan and chitosan nanoparticles for the control of Fusarium head blight of wheat (Fusarium graminearum) in vitro and greenhouse. Int. J. Biol. Macromol. 2016, 93, 1261–1272. [Google Scholar] [CrossRef]
- Manikandan, A.; Sathiyabama, M. Preparation of chitosan nanoparticles and its effect on detached rice leaves infected with Pyricularia grisea. Int. J. Biol. Macromol. 2016, 84, 58–61. [Google Scholar] [CrossRef]
- Kumar, S.; Kaur, P.; Bernela, M.; Rani, R.; Thakur, R. Ketoconazole encapsulated in chitosan-gellan gum nanocomplexes exhibits prolonged antifungal activity. Int. J. Biol. Macromol. 2016, 93, 988–994. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, H.; Cao, C.; Zhang, J.; Li, F.; Huang, Q. Quaternized chitosan-capped mesoporous silica nanoparticles as nanocarriers for controlled pesticide release. Nanomaterials 2016, 6, 126. [Google Scholar] [CrossRef]
- Chauhan, N.; Dilbaghi, N.; Gopal, M.; Kumar, R.; Kim, K.H.; Kumar, S. Development of chitosan nanocapsules for the controlled release of hexaconazole. Int. J. Biol. Macromol. 2017, 97, 616–624. [Google Scholar] [CrossRef]
- Kumar, R.; Nain, V.; Duhan, J.S. An Ecological Approach to Control Pathogens of Lycopersicon esculentum L. by Slow Release of Mancozeb from Biopolymeric Conjugated Nanoparticles. J. Xenobiot. 2022, 12, 297–323. [Google Scholar]
- Rubina, M.S.; Vasil’kov, A.Y.; Naumkin, A.V.; Shtykova, E.V.; Abramchuk, S.S.; Alghuthaymi, M.A.; Abd-Elsalam, K.A. Synthesis and characterization of chitosan–copper nanocomposites and their fungicidal activity against two sclerotia-forming plant pathogenic fungi. J. Nanostruct. Chem. 2017, 7, 249–258. [Google Scholar] [CrossRef]
- Kalagatur, N.K.; Ghosh, O.S.N.; Sundararaj, N.; Mudili, V. Antifungal activity of chitosan nanoparticles encapsulated with Cymbopogon martinii essential oil on plant pathogenic fungi Fusarium graminearum. Front. Pharmacol. 2018, 6, 610. [Google Scholar] [CrossRef] [PubMed]
- Siddaiah, C.N.; Prasanth, K.V.H.; Satyanarayana, N.R.; Mudili, V.; Gupta, V.K.; Kalagatur, N.K.; Satyavati, T.; Dai, X.-F.; Chen, J.-Y.; Mocan, A.; et al. Chitosan nanoparticles having a higher degree of acetylation induce resistance against pearl millet downy mildew through nitric oxide generation. Sci. Rep. 2018, 8, 2485. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aliem, H.A.; Gibriel, A.Y.; Rasmy, N.M.; Sahab, A.F.; El-Nekeety, A.A.; Abdel-Wahhab, M.A. Antifungal efficacy of chitosan nanoparticles against phytopathogenic fungi and inhibition of zearalenone production by Fusarium graminearum. Comun. Sci. 2019, 10, 338–345. [Google Scholar] [CrossRef]
- Hasheminejad, N.; Khodaiyan, F.; Safari, M. Improving the antifungal activity of clove essential oil encapsulated by chitosan nanoparticles. Food Chem. 2019, 275, 113–122. [Google Scholar] [CrossRef]
- Kumar, R.; Najda, A.; Duhan, J.S.; Kumar, B.; Chawla, P.; Klepacka, J.; Malawski, S.; Kumar Sadh, P.; Poonia, A.K. Assessment of antifungal efficacy and release behaviour of fungicide-loaded chitosan-carrageenan nanoparticles against phytopathogenic fungi. Polymers 2021, 14, 41. [Google Scholar] [CrossRef]
- Kumar, R.; Duhan, J.S.; Manuja, A.; Kaur, P.; Kumar, B.; Sadh, P.K. Toxicity Assessment and control of early blight and stem rot of Solanum tuberosum L. by mancozeb-loaded chitosan-gum acacia nanocomposites. J. Xenobiot. 2022, 12, 74–90. [Google Scholar] [CrossRef]
- Kumar, R.; Poonia, A.K.; Duhan, J.S. Synthesis, characterization and antimicrobial potential of mancozeb loaded chitosan nanoparticles against Fusarium pallidoroseum. Ann. Biol. 2022, 38, 138–144. [Google Scholar]
- Perlatti, B.; de Souza Bergo, P.L.; Fernandes, J.B.; Forim, M.R. Polymeric nanoparticle-based insecticides: A controlled release purpose for agrochemicals. In Insecticides-Development of Safer and More Effective Technologies; IntechOpen: London, UK, 2013. [Google Scholar]
- Sun, C.; Shu, K.; Wang, W.; Ye, Z.; Liu, T.; Gao, Y.; Zheng, H.; He, G.; Yin, Y. Encapsulation and controlled release of hydrophilic pesticide in shell cross-linked nanocapsules containing aqueous core. Int. J. Pharm. 2014, 463, 108–114. [Google Scholar] [CrossRef]
- Saini, P.; Gopal, M.; Kumar, R.; Gogoi, R.; Srivastava, C. Bioefficacy evaluation and dissipation pattern of nanoformulation versus commercial formulation of pyridalyl in tomato (Solanum lycopersicum). Environ. Monit. Assess. 2015, 187, 541. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.L.; Campos, E.V.R.; Pereira, A.E.S.; Pasquoto, T.; Lima, R.; Grillo, R.; de Andrade, D.J.; dos Santos, F.A.; Fraceto, L.F. Zein nanoparticles as eco-friendly carrier systems for botanical repellents aiming for sustainable agriculture. J. Agric. Food Chem. 2018, 66, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- Jenne, M.; Kambham, M.; Tollamadugu, N.P.; Karanam, H.P.; Tirupati, M.K.; Balam, R.R.; Shameer, S.; Yagireddy, M. The use of slow-releasing nanoparticle encapsulated Azadirachtin formulations for the management of Caryedon serratus O. (groundnut bruchid). IET Nanobiotechnol. 2018, 12, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Wang, Y.; Xie, J.; Sun, B.; Zhou, N.; Shen, H.; Shen, J. Carboxymethyl chitosan modified carbon nanoparticle for controlled emamectin benzoate delivery: Improved solubility, pH-responsive release, and sustainable pest control. ACS Appl. Mater. Interfaces 2019, 11, 34258–34267. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Verheggen, F.; Zeng, Z.; Cui, H. Polymer-based nanoinsecticides: Current developments, environmental risks and future challenges—A Review. Biotechnol. Agron. Soc. Environ. 2019, 24, 59–69. [Google Scholar] [CrossRef]
- Guan, H.; Chi, D.; Yu, J.; Li, X. A novel photodegradable insecticide: Preparation, characterization and properties evaluation of nano-imidacloprid pesticide. Pestic. Biochem. Physiol. 2008, 92, 83–91. [Google Scholar] [CrossRef]
- Shakil, N.A.; Singh, M.K.; Pandey, A.; Kumar, J.; Pankaj; Parmar, V.S.; Singh, M.K.; Pandey, R.P.; Watterson, A.C. Development of poly (ethylene glycol) based amphiphilic copolymers for controlled release delivery of carbofuran. J. Macromol. Sci.-Pure Appl. Chem. 2010, 47, 241–247. [Google Scholar] [CrossRef]
- Hwang, I.; Kim, T.; Bang, S.; Kim, K.; Kwon, H.; Seo, M.; Youn, Y.N.; Park, H.J.; Yasunaga-Aoki, C.; Yu, Y.M. IInsecticidal effect of controlled release formulations of etofenprox based on nano-bio technique. J. Fac. Agric. Kyushu Univ. 2011, 56, 33–40. [Google Scholar] [CrossRef]
- Kang, M.A.; Seo, M.J.; Hwang, I.C.; Jang, C.; Park, H.J.; Yu, Y.M.; Youn, Y.N. Insecticidal activity and feeding behaviour of the green peach aphid, Myzus persicae, after treatment with nano types of pyrifluquinazon. J. Asia Pac. Entomol. 2012, 15, 533–541. [Google Scholar] [CrossRef]
- Flores-Céspedes, F.; Figueredo-Flores, C.I.; Daza-Fernández, I.; Vidal-Peña, F.; Villafranca-Sánchez, M.; Fernández-Pérez, M. Preparation and characterization of imidacloprid lignin–polyethylene glycol matrices coated with ethylcellulose. J. Agric. Food Chem. 2012, 60, 1042–1051. [Google Scholar] [CrossRef]
- Feng, B.H.; Peng, L.F. Synthesis and characterization of carboxymethyl chitosan carrying ricinoleic functions as an emulsifier for Azadirachtin. Carbohydr. Polym. 2012, 88, 576–582. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Hwang, I.C.; Park, J.W.; Park, H.J. Photoprotection for deltamethrin using chitosan-coated beeswax solid lipid nanoparticles. Pest Manag. Sci. 2012, 68, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Lu, M.L.; Zhang, Q.P.; Tian, Y.Q.; Zhang, Z.X.; Xu, H.H. Octahydrogenated retinoic acid-conjugated glycol chitosan nanoparticles as a novel carrier of azadirachtin: Synthesis, characterization, and in vitro evaluation. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 3932–3940. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Fan, T.; Xu, Q.; Wu, Y.; Chen, C.; Huang, Q. Construction of novel amphiphilic chitosan copolymer nanoparticles for chlorpyrifos delivery. J. Polym. Res. 2013, 20, 107. [Google Scholar] [CrossRef]
- Xiang, C.; Taylor, A.G.; Hinestroza, J.P.; Frey, M.W. Controlled release of nonionic compounds from poly (lactic acid)/cellulose nanocrystal nanocomposite fibers. J. Appl. Polym. Sci. 2013, 127, 79–86. [Google Scholar] [CrossRef]
- Huayao, C.; Yueshun, L.; Hongjun, Z.; Xinhua, Z.; Sheng, G.; Hua, X. Highly efficient alginate sodium encapsulated chlorpyrifos/copper (II) Schiff base mesoporous silica sustained release system with pH and ion response for pesticide delivery. RSC Adv. 2016, 6, 114714–114721. [Google Scholar] [CrossRef]
- Badawy, M.E.; Taktak, N.; Awad, O.M.; Elfiki, S.A.; Abou El-Ela, N.E. Evaluation of released malathion and spinosad from chitosan/alginate/gelatin capsules against Culex pipiens larvae. Res. Rep. Trop. Med. 2016, 7, 23–38. [Google Scholar] [PubMed]
- Paulraj, M.G.; Ignacimuthu, S.; Gandhi, M.R.; Shajahan, A.; Ganesan, P.; Packiam, S.M.; Al-Dhabi, N.A. Comparative studies of tripolyphosphate and glutaraldehyde cross-linked chitosan-botanical pesticide nanoparticles and their agricultural applications. Int. J. Biol. Macromol. 2017, 104, 1813–1819. [Google Scholar] [CrossRef]
- Xiang, Y.; Zhang, G.; Chi, Y.; Cai, D.; Wu, Z. Fabrication of a controllable nanopesticide system with magnetic collectability. Chem. Eng. J. 2017, 328, 320–330. [Google Scholar] [CrossRef]
- Shoaib, A.; Waqas, M.; Elabasy, A.; Cheng, X.; Zhang, Q.; Shi, Z. Preparation and characterization of emamectin benzoate nanoformulations based on colloidal delivery systems and use in controlling Plutella xylostella (L.) (Lepidoptera: Plutellidae). RSC Adv. 2018, 8, 15687–15697. [Google Scholar] [CrossRef]
- Namasivayam, S.K.R.; Bharani, R.A.; Karunamoorthy, K. Insecticidal fungal metabolites fabricated chitosan nanocomposite (IM-CNC) preparation for the enhanced larvicidal activity-An effective strategy for green pesticide against economic important insect pests. Int. J. Biol. Macromol. 2018, 120, 921–944. [Google Scholar] [CrossRef]
- Ahmadi, Z.; Saber, M.; Akbari, A.; Mahdavinia, G.R. Encapsulation of Satureja hortensis L.(Lamiaceae) in chitosan/TPP nanoparticles with enhanced acaricide activity against Tetranychus urticae Koch (Acari: Tetranychidae). Ecotoxicol. Environ. Saf. 2018, 161, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cheng, J.; Garamus, V.M.; Li, N.; Zou, A. Preparation of an environmentally friendly formulation of the insecticide nicotine hydrochloride through encapsulation in chitosan/tripolyphosphate nanoparticles. J. Agric. Food Chem. 2018, 66, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Del Prado-Audelo, M.L.; Bernal-Chávez, S.A.; Gutiérrez-Ruíz, S.C.; Hernández-Parra, H.; Kerdan, I.G.; Reyna-González, J.M.; Sharifi-Rad, J.; Leyva-Gómez, G. Stability phenomena associated with the development of polymer-based nanopesticides. Oxidative Med. Cell. Longev. 2022, 2022, 1–15. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Silva, M.; Cocenza, D.S.; Grillo, R.; de Melo, N.F.S.; Tonello, P.S.; de Oliveira, L.C.; Cassimiro, D.L.; Rosa, A.H.; Fraceto, L.F. Paraquat-loaded alginate/chitosan nanoparticles: Preparation, characterization and soil sorption studies. J. Hazard. Mater. 2011, 190, 366–374. [Google Scholar] [CrossRef]
- Grillo, R.; Pereira, A.E.; Nishisaka, C.S.; de Lima, R.; Oehlke, K.; Greiner, R.; Fraceto, L.F. Chitosan/tripolyphosphate nanoparticles loaded with paraquat herbicide: An environmentally safer alternative for weed control. J. Hazard. Mater. 2014, 278, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Grillo, R.; Santos, N.Z.P.; Maruyama, C.R.; Rosa, A.H.; Lima, R.; Fraceto, L.F. Poly(-ɛ-caprolactone) nanocapsules as carrier systems for herbicides: Physico-chemical characterization and genotoxicity evaluation. J. Hazard. Mater. 2012, 232, 1–9. [Google Scholar] [CrossRef]
- Yu, Z.; Sun, X.; Song, H.; Wang, W.; Ye, Z.; Shi, L.; Ding, K. Glutathione-responsive carboxymethyl chitosan nanoparticles for controlled release of herbicides. Mater. Sci. Appl. 2015, 6, 591. [Google Scholar] [CrossRef]
- Maruyama, C.R.; Guilger, M.; Pascoli, M.; Bileshy-José, N.; Abhilash, P.C.; Fraceto, L.F.; De Lima, R. Corrigendum: Nanoparticles based on chitosan as carriers for the combined herbicides imazapic and imazapyr. Sci. Rep. 2016, 6, 23854. [Google Scholar] [CrossRef]
- de Jacques, M.T.; Oliveira, J.L.; Campos, E.V.; Fraceto, L.F.; Ávila, D.S. Safety assessment of nanopesticides using the roundworm Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2017, 139, 245–253. [Google Scholar] [CrossRef]
- Oliveira, C.R.; Fraceto, L.F.; Rizzi, G.M.; Salla, R.F.; Abdalla, F.C.; Costa, M.J.; Silva-Zacarin, E.C.M. Hepatic effects of the clomazone herbicide in both its free form and associated with chitosan-alginate nanoparticles in bullfrog tadpoles. Chemosphere 2016, 149, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Riyajan, S.A. Physical property testing of a novel hybrid natural rubber-graft-cassava starch/sodium alginate bead for encapsulating herbicide. Polym. Test. 2017, 58, 300–307. [Google Scholar] [CrossRef]
- Schnoor, B.; Elhendawy, A.; Joseph, S.; Putman, M.; Chacón-Cerdas, R.; Flores-Mora, D.; Bravo-Moraga, F.; Gonzalez-Nilo, F.; Salvador-Morales, C. Engineering Atrazine Loaded Poly (lactic-co-glycolic acid) Nanoparticles to Ameliorate Environmental Challenges. J. Agric. Food Chem. 2018, 66, 7889–7898. [Google Scholar] [CrossRef] [PubMed]
- Yusoff, S.N.M.; Kamari, A.; Ishak, S.; Halim, A.L.A. N-hexanoyl-O-glycol chitosan as a carrier agent for water-insoluble herbicide. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2018; Volume 1097, p. 012053. [Google Scholar]
- Chen, X.T.; Wang, T. Preparation and characterization of atrazine-loaded biodegradable PLGA nanospheres. J. Integr. Agric. 2019, 18, 1035–1041. [Google Scholar] [CrossRef]
- Macedo, D.F.; Dourado, S.M., Jr.; Nunes, E.S.; Marques, R.P.; Moreto, J.A. Controlled Release of TBH Herbicide Encapsulated on Ca-ALG Microparticles: Leaching and Phytointoxication Plants. Planta Daninha 2019, 37, e019189934. [Google Scholar] [CrossRef]
- Chaud, M.; Souto, E.B.; Zielinska, A.; Severino, P.; Batain, F.; Oliveira-Junior, J.; Alves, T. Nanopesticides in agriculture: Benefits and challenge in agricultural productivity, toxicological risks to human health and environment. Toxics 2021, 9, 131. [Google Scholar] [CrossRef]
- Sasser, J.N.; Freckman, D.W. A World Perspective on Nematology: The Role of the Society in Vistas on Nematology; Veech, J.A., Dickson, D.W., Eds.; Society of Nematologists: Hyattsville, MD, USA, 1987; pp. 7–14. [Google Scholar]
- Decraemer, W.; Hunt, D.J. Structure and classification. In Plant Nematology; Perry, R.N., Moens, M., Eds.; CABI: Wallingford, UK, 2006; pp. 3–32. [Google Scholar]
- Nicol, J.M.; Turner, S.J.; Coyne, D.L.; Nijs, L.D.; Hockland, S.; Maafi, Z.T. Current nematode threats to world agriculture. In Genomics and Molecular Genetics of Plant-Nematode Interactions; Jones, J., Gheysen, G., Fenoll, C., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 21–43. [Google Scholar]
- Yin, Y.H.; Guo, Q.M.; Yun, H.A.N.; Wang, L.J.; Wan, S.Q. Preparation, characterization and nematicidal activity of lansiumamide B nano-capsules. J. Integr. Agric. 2012, 11, 1151–1158. [Google Scholar] [CrossRef]
- Cromwell, W.A.; Yang, J.; Starr, J.L.; Jo, Y.K. Nematicidal effects of silver nanoparticles on root-knot nematode in bermudagrass. J. Nematol. 2014, 46, 261. [Google Scholar]
- Ardakani, A.S. Toxicity of silver, titanium and silicon nanoparticles on the root-knot nematode, Meloidogyne incognita, and growth parameters of tomato. Nematology 2013, 15, 671–677. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, K.; Li, L.; Zhao, F.; Wang, Y.; Wang, M.; Shen, Y.; Cui, H.; Liu, D.; Guo, X. Spherical and spindle-like abamectin-loaded nanoparticles by flash nanoprecipitation for southern root-knot nematode control: Preparation and characterization. Nanomaterials 2018, 8, 449. [Google Scholar] [CrossRef]
- Omobhude, M.E.; Morenikeji, O.A.; Oyeyemi, O.T. Molluscicidal activities of curcumin-nisin polylactic acid nanoparticle on Biomphalaria pfeifferi. PLoS Negl. Trop. Dis. 2017, 11, e0005855. [Google Scholar] [CrossRef] [PubMed]
- Omobhude, M.E.; Morenikeji, O.A.; Oyeyemi, O.T. Molluscicidal Activities of Curcumin-Nisin Polylactic Acid Nanoparticle (PLA) on Adult Snail Intermediate Hosts of Schistosomes and Fasciola spp. Adv. Res. Life Sci. 2019, 3, 28–32. [Google Scholar] [CrossRef]
- Peters, B.C.; Wibowo, D.; Yang, G.Z.; Hui, Y.; Middelberg, A.P.; Zhao, C.X. Evaluation of baiting fipronil-loaded silica nanocapsules against termite colonies in fields. Heliyon 2019, 5, e02277. [Google Scholar] [CrossRef] [PubMed]
- Yien, L.; Zin, N.M.; Sarwar, A.; Katas, H. Antifungal activity of chitosan nanoparticles and correlation with their physical properties. Int. J. Biomater. 2012, 2012, 632698. [Google Scholar] [CrossRef]
- Chowdappa, P.; Gowda, P.; Chethana, S.; Madhura, S. Antifungal activity of chitosan-silver nanoparticle composite against Colletotrichum gloeosporioides associated with mango anthracnose. Afr. J. Microbiol. Res. 2014, 8, 1803–1812. [Google Scholar]
- Malerba, M.; Cerana, R. Recent applications of chitin-and chitosan-based polymers in plants. Polymers 2019, 11, 839. [Google Scholar] [CrossRef]
- Egusa, M.; Parada, R.; Aklog, Y.F.; Ifuku, S.; Kaminaka, H. Nanofibrillation enhances the protective effect of crab shells against Fusarium wilt disease in tomato. Int. J. Biol. Macromol. 2019, 128, 22–27. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Huynh, T.N.; Hoang, D.; Nguyen, D.H.; Nguyen, Q.H.; Tran, T.H. Functional nanostructured oligochitosan-silica/carboxymethyl cellulose hybrid materials: Synthesis and investigation of their antifungal abilities. Polymers 2019, 11, 628. [Google Scholar] [CrossRef]
- Kaur, P.; Thakur, R.; Choudhary, A. An in vitro study of the antifungal activity of silver/chitosan nanoformulations against important seed-borne pathogens. Int. J. Sci. Technol. Res. 2012, 1, 83–86. [Google Scholar]
- Brunel, F.; Gueddari, N.E.E.; Moerschbacher, B.M. Complexation of copper (II) with chitosan nanogels: Toward control of microbial growth. Carbohydr. Polym. 2013, 92, 1348–1356. [Google Scholar] [CrossRef]
- Saharan, V.; Mehrotra, A.; Khatik, R.; Rawal, P.; Sharma, S.S.; Pal, A. Synthesis of chitosan-based nanoparticles and their in vitro evaluation against phytopathogenic fungi. Int. J. Biol. Macromol. 2013, 62, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Thakur, R.; Barnela, M.; Chopra, M.; Manuja, A.; Chaudhury, A. Synthesis, characterization and in vitro evaluation of cytotoxicity and antimicrobial activity of chitosan–metal nanocomposites. J. Chem. Technol. Biotechnol. 2014, 90, 867–873. [Google Scholar] [CrossRef]
- Anusuya, S.; Sathiyabama, M. Foliar application of β-D-glucan nanoparticles to control rhizome rot disease of turmeric. Int. J. Biol. Macromol. 2015, 72, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.A.; Abdel-Aziz, M.S. In vitro and in vivo antibacterial potential of chitosan-g-acrylonitrile silver nanocomposite against a pathogenic bacterium. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 5–19. [Google Scholar]
- Saharan, V.; Sharma, G.; Yadav, M.; Choudhary, M.K.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P. Synthesis and in vitro antifungal efficacy of Cu–chitosan nanoparticles against pathogenic fungi of tomato. Int. J. Biol. Macromol. 2015, 75, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elsalam, K.A.; Vasil’kov, A.Y.; Said-Galiev, E.E.; Rubina, M.S.; Khokhlov, A.R.; Naumkin, A.V.; Shtykova, E.V.; Alghuthaymi, M.A. Bimetallic blends and chitosan nanocomposites: Novel antifungal agents against cotton seedling damping-off. Eur. J. Plant Pathol. 2017, 151, 57–72. [Google Scholar] [CrossRef]
- Dananjaya, S.H.S.; Erandani, W.K.C.U.; Kim, C.H.; Nikapitiya, C.; Lee, J.; De Zoysa, M. Comparative study on antifungal activities of chitosan nanoparticles and chitosan silver nanocomposites against Fusarium oxysporum species complex. Int. J. Biol. Macromol. 2017, 105, 478–488. [Google Scholar] [CrossRef]
- Tan, W.; Li, Q.; Wei, L.; Wang, P.; Gao, Z.; Chen, Y.; Dong, F.; Guo, Z. Synthesis, characterization, and antifungal property of starch derivatives modified with quaternary phosphonium salts. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 1048–1105. [Google Scholar] [CrossRef]
- Avadi, M.R.; Sadeghi, A.M.M.; Mohammadpour, N.; Abedin, S.; Atyabi, F.; Dinarvand, R.; Rafiee-Tehrani, M. Preparation and characterization of insulin nanoparticles using chitosan and Arabic gum with ionic gelation method. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 58–63. [Google Scholar] [CrossRef]
- Bernela, M.; Kaur, P.; Chopra, M.; Thakur, R. Synthesis, characterization of nisin-loaded alginate–chitosan–pluronic composite nanoparticles and evaluation against microbes. LWT-Food Sci. Technol. 2014, 59, 1093–1099. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Wang, Y.; Zhang, Y.; Li, X. Compound pesticide controlled release system based on the mixture of poly (butylene succinate) and PLA. J. Microencapsul. 2018, 35, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Cui, B.; Zhao, X.; Zhi, H.; Zeng, Z.; Wang, Y.; Sun, C.; Liu, G.; Gao, J.; Cui, H. Antagonistic effect of azoxystrobin poly (lactic acid) microspheres with controllable particle size on Colletotrichum higginsianum Sacc. Nanomaterials 2018, 8, 857. [Google Scholar] [CrossRef] [PubMed]
- Wuana, R.A.; Nnamonu, L.A.; Sha’Ato, R.; Itodo, H.U. Characterization of formulated copper chitosan nanoparticles for the controlled release studies of dicamba dimethylamine herbicide. Chem Search J. 2019, 10, 74–87. [Google Scholar]
- Nörnberg, A.B.; Gehrke, V.R.; Mota, H.P.; Camargo, E.R.; Fajardo, A.R. Alginate-cellulose biopolymeric beads as efficient vehicles for encapsulation and slow-release of herbicide. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123970. [Google Scholar] [CrossRef]
- Teixeira, M.A.; Paterson, W.J.; Dunn, E.J.; Li, Q.; Hunter, B.K.; Goosen, M.F. Assessment of chitosan gels for the controlled release of agrochemicals. Ind. Eng. Chem. Res. 1990, 29, 1205–1209. [Google Scholar] [CrossRef]
- Yeom, C.K.; Kim, Y.H.; Lee, J.M. Microencapsulation of water-soluble herbicide by the interfacial reaction. II. Release properties of microcapsules. J. Appl. Polym. Sci. 2002, 84, 1025–1034. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, D.K.; Gupta, A. In vitro release dynamics of thiram fungicide from starch and poly (methacrylic acid)-based hydrogels. J. Hazard. Mater. 2008, 154, 278–286. [Google Scholar] [CrossRef]
- Garrido-Herrera, F.J.; Daza-Fernández, I.; González-Pradas, E.; Fernández-Pérez, M. Lignin-based formulations to prevent pesticides pollution. J. Hazard. Mater. 2009, 168, 220–225. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, D.K.; Gupta, A. A study towards release dynamics of thiram fungicide from starch–alginate beads to control environmental and health hazards. J. Hazard. Mater. 2009, 161, 208–216. [Google Scholar] [CrossRef]
- Lao, S.B.; Zhang, Z.X.; Xu, H.H.; Jiang, G.B. Novel amphiphilic chitosan derivatives: Synthesis, characterization and micellar solubilization of rotenone. Carbohydr. Polym. 2010, 82, 1136–1142. [Google Scholar] [CrossRef]
- Quiñones, J.P.; García, Y.C.; Curiel, H.; Covas, C.P. Microspheres of chitosan for controlled delivery of brassinosteroids with biological activity as agrochemicals. Carbohydr. Polym. 2010, 80, 915–921. [Google Scholar] [CrossRef]
- Flores-Céspedes, F.; Daza-Fernández, I.; Villafranca-Sánchez, M.; Fernández-Pérez, M.; Morillo, E.; Undabeytia, T. Lignin and ethylcellulose in controlled release formulations to reduce leaching of chloridazon and metribuzin in light-textured soils. J. Hazard. Mater. 2018, 343, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Grillo, R.; Pereira, A.E.S.; Melo, N.F.S.de.; Porto, R.Q.; Feitosa, L.O.; Tonello, P.S.; Fihlo, N.L.D.; Rosa, A.H.; Lima, R.; Frecito, L.F. Controlled release system for ametryn using polymer microspheres: Preparation, characterization and release kinetics in water. J. Hazard. Mater. 2011, 8, 1645–1661. [Google Scholar] [CrossRef] [PubMed]
- Saifuddin, N.; Nian, C.Y.; Zhan, L.W.; Ning, K.X. Chitosan-silver nanoparticles composite as a point-of-use drinking water filtration system for the household to remove pesticides in water. Asian J. Biochem. 2011, 6, 142–159. [Google Scholar] [CrossRef]
- Wen, Y.; Chen, H.; Yuan, Y.; Xu, D.; Kang, X. Enantioselective ecotoxicity of the herbicide dichlorprop and complexes formed with chitosan in two freshwater green algae. J. Environ. Manag. 2011, 13, 879–885. [Google Scholar]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Antimicrobial chitosan—PVA hydrogel as a nanoreactor and immobilizing matrix for silver nanoparticles. Appl. Nanosci. 2012, 2, 179–188. [Google Scholar] [CrossRef]
- Nnamonu, L.A.; Sha’Ato, R.; Onyido, I. Alginate reinforced chitosan and starch beads in slow release formulation of imazaquin herbicide preparation and characterization. Mater. Sci. Appl. 2012, 3, 566. [Google Scholar] [CrossRef]
- Bharani, R.A.; Namasivayam, S.K.R.; Shankar, S.S. Biocompatible chitosan nanoparticles incorporated pesticidal protein beauvericin (CSNp-BV) preparation for the improved pesticidal activity against major groundnut defoliator Spodoptera litura (Fab.) (Lepidoptera; Noctuidae). Int. J. ChemTech Res. 2014, 6, 5007–5012. [Google Scholar]
- Gaur, S.P.; Dilbaghi, N.; Kumar, D. Development of polymeric nanoparticles for sustained release of thiabendazole in agricultural applications. INROADS-Int. J. Jaipur Natl. Univ. 2016, 5, 358–361. [Google Scholar]
- Wang, D.; Jia, M.; Wang, L.; Song, S.; Feng, J.; Zhang, X. Chitosan and β-Cyclodextrin-epichlorohydrin polymer composite film as a plant healthcare material for carbendazim-controlled release to protect rape against Sclerotinia sclerotiorum (Lib.) de Bary. Materials 2017, 10, 343. [Google Scholar] [CrossRef]
- Kumar, R.; Ashfaq, M.; Verma, N. Synthesis of novel PVA–starch formulation-supported Cu–Zn nanoparticle carrying carbon nanofibers as a nanofertilizer: Controlled release of micronutrients. J. Mater. Sci. 2018, 53, 7150–7164. [Google Scholar] [CrossRef]
- Gore, A.C. Endocrine-disrupting chemicals and the brain. Lancet Diabetes. Endocrinol. 2018, 6, 172. [Google Scholar] [CrossRef]
- Autier, P. Increasing incidence of cancer in children and competing risks. Lancet Oncol. 2018, 19, 1136–1137. [Google Scholar] [CrossRef]
- Mostafalou, S.; Abdollahi, M. The link of organophosphorus pesticides with neurodegenerative and neurodevelopmental diseases based on evidence and mechanisms. Toxicology 2018, 409, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, F.; Asadikaram, G.; Karamouzian, S.; Abolhassani, M.; Moazed, V.; Nematollahi, M.H. MGMT methylation alterations in brain cancer following organochlorine pesticides exposure. J. Environ. Health Eng. 2021, 8, 47–53. [Google Scholar] [CrossRef]
- Abdollahdokht, D.; Asadikaram, G.; Abolhassani, M.; Pourghadamyari, H.; Abbasi-Jorjandi, M.; Faramarz, S.; Nematollahi, M.H. Pesticide exposure and related health problems among farmworkers’ children: A case-control study in southeast Iran. Environ. Sci. Pollut. Res. 2021, 28, 57216–57231. [Google Scholar] [CrossRef]
- Kumar, R.; Nair, K.K.; Alam, M.; Gogoi, R.; Singh, P.K.; Srivastava, C.; Gopal, M.; Goswami, A. Development and quality control of nanohexaconazole as an effective fungicide and its biosafety studies on soil nitrifiers. J. Nanosci. Nanotechnol. 2015, 15, 1350–1356. [Google Scholar] [CrossRef]
- Liang, Y.; Fan, C.; Dong, H.; Zhang, W.; Tang, G.; Yang, J.; Yang, J.; Jiang, N.; Cao, Y. Preparation of MSNs-chitosan@ prochloraz nanoparticles for reducing toxicity and improving release properties of prochloraz. ACS Sustain. Chem. Eng. 2018, 6, 10211–10220. [Google Scholar] [CrossRef]
- Duhan, J.S.; Bansal, P.; Sadh, P.K.; Kumar, R.; Kumar, A. Biosynthesis, characterization, toxicity assessment, and bio-efficacy of silver nanoparticles synthesized by Microbacterium mitrae in controlling early blight in tomato (Lycopersicon esculentum L.). Res. J. Biotechnol. 2022, 17, 73–81. [Google Scholar]
- Bansal, P.; Kaur, P.; Kumar, A.; Duhan, J.S. Biogenesis of silver nanoparticles using Fusarium pallidoroseum and its potential against human pathogens. Ann. Biol. 2017, 33, 180–185. [Google Scholar]
- Bansal, P.; Kaur, P.; Surekha; Kumar, A.; Duhan, J.S. Biogenesis of silver nanoparticles using Aspergillus terreus, its cytotoxicity and potential as therapeutic against human pathogens. Res. J. Pharm. Biol. Chem. Sci. 2017, 8, 898–906. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).