Abstract
Removing toxic metal ions arising from contaminated wastewaters caused by industrial effluents with a cost-effective method tackles a serious concern worldwide. The adsorption process onto metal oxide and carbon-based materials offers one of the most efficient technologies adopted for metal ion removal. In this study, mesoporous MgO/g-C3N4 sorbent is fabricated by ultrasonication method for the uptake Pb (II) and Cd (II) heavy metal ions from an aqueous solution. The optimum conditions for maximum uptake: initial concentration of metal ions 250 mg g−1, pH = 5 and pH = 3 for Pb++ and Cd++, and a 60 mg dose of adsorbent. In less than 50 min, the equilibrium is reached with a good adsorption capacity of 114 and 90 mg g−1 corresponding to Pb++ and Cd++, respectively. Moreover, the adsorption isotherm models fit well with the Langmuir isotherm, while the kinetics model fitting study manifest a perfect fit with the pseudo-second order. The as fabricated mesoporous MgO/g-C3N4 sorbent exhibit excellent Pb++ and Cd++ ions uptake and can be utilized as a potential adsorbent in wastewater purification.
1. Introduction
Today, heavy metals, such as Lead (Pb++) and Cadmium (Cd++), exist in the natural environment and are identified as presenting a serious worldwide challenge since they cause severe impacts on public health, environment, and economy [1,2]. Both Pb++ and Cd++ are ranked among the metals that raise the most significant concerns for human health due to their persistent nature, non-biodegradability, and high toxicity even at lower exposure levels [3,4]. Furthermore, they are designated as probably carcinogens to human beings by the U.S. Environmental Protection Agency (USEPA) and the International Agency for Research on Cancer (IARC) [5]. Industrialization and sewage dumping are the main cause of the discharge of highly toxic heavy metals into natural water resources [6].
In recent years, different technologies have been utilized for wastewater purification from heavy metals, such as oxidation and reduction processes, membrane filtration, chemical precipitation, electrodialysis, biological methods, photocatalysis and adsorption, and ions exchange [7,8,9,10,11,12,13]. Of these techniques, the adsorption process has been strongly recommended in wastewater remediation due to several advantages and features, such as cost-effectiveness, simple operation, flexibility in design, and the facile regeneration of the adsorbents [14].
Among the standard materials available for the adsorption process in heavy metal uptake from contaminated water, such as activated carbon (AC), inorganic materials, modified silica gel, metal oxides, and biomaterials; the most widely used are porous metal oxides [1]. Today, metal oxide nanostructures are considered suitable adsorbents because of their inherent surface reactivity due to the presence of numerous active sites and enhanced surface area. Aside from these exceptional capabilities, the bulk form of g-C3N4 has inadequate adsorption effectiveness due to its low specific surface area [15,16], which hinders its practical use in environmental applications. Numerous techniques, such as doping [17], metal deposition [18], and heterojunctions with other semiconductors [19], have been used to overcome the disadvantages and further improve the surface properties of g-C3N4. A simple and more cost-efficient strategy is necessary for large-scale applications, even though these methods are extremely effective. In this regard, the exfoliation of g-C3N4 has shown to increase its adsorption efficacy [20]. The sonochemical method has been found to produce extraordinarily stable dispersions of g-C3N4 with high surface characteristics [21], which can then be used in a variety of cutting-edge techniques. However, metal oxides usually have a large bandgap and hence absorb only UV light, which represents 3–5% of the whole solar energy spectrum. Moreover, the purification efficiency was found to be insufficient because of the recombination probability of photoinduced electron-hole pairs [22]. Notwithstanding, researchers immobilized metal oxide nanostructures onto several substrates, including active carbon (AC) [23], carbon nanotubes [24], graphene [25], and graphitic carbon nitrate g-C3N4 [26]. As a result, the combination of metal oxides and carbon-based materials has gained significant attention in recent years since the overall efficiency was significantly enhanced for both water and air purification [1].
Graphitic carbon nitrate (g-C3N4) is a 2D carbon material with a moderate bandgap (about 2.7 eV) that recently attracted scientist’s attention with its numerous advantages, including excellent photo-degradation efficiency in visible light, high chemical and thermal stabilities, low cost, easy preparation, non-toxicity, and reliability [26,27]. Additionally, in the literature, new nanocomposites with tailored properties were developed, resulting in high photocatalytic activity, and consequently successfully used for energy production and storage, as well as the degradation of organic pollutants [22,28,29].
Magnesium oxide (MgO) nanoparticles (NPs) are ecofriendly and odorless white powders, and possess good stability under severe process conditions alongside high surface characteristics due to the evolution of edge/corner Frenkel or Schottky defects and their polyhedral nature [30]. MgO NPs exhibit interesting properties, such as high corrosion resistance, optical transmittance, low cost, and non-toxicity [31,32,33,34], and can thereby be potentially utilized in water and air purification. MgO NPs can be synthesized by several chemical and physical routes, such as co-precipitation, sol-gel, solvo-/hydro-thermal, and combustion, as well as green synthesis [35].
Herein, MgO was loaded onto 2D g-C3N4 (MGCN) to enhance the adsorption efficiency of heavy metals, specifically Pb++ and Cd++. The mesoporous MgO/g-C3N4 was fabricated by ultrasonication method and characterized using spectroscopic and analytical techniques. The adsorption capacity towards Pb++ and Cd++ ions from synthetic aqueous solution at various operational conditions, i.e., the initial concentration at different pH and contact time, was investigated.
2. Experimental
2.1. Fabrication of MGCN
The sample was prepared by using a procedure reported before (A. Modwi et al., 2022).
2.2. Characterization
The particles morphology of MCGN was analyzed using scanning/transmitted electron microscopy (SEM-EDX and TEM) using Jeol S-3400 (Japan) and Tecnai G20 (USA) for TEM. X-ray diffraction (XRD) patterns were recorded to identify the crystalline phase structure by using the Bruker AXS (German) diffractometer (λ = 1.5418 Å) in the 2θ range 5–80°. The surface area and pore size of MGCN were determined by measuring N2 adsorption/desorption isotherms using ASAP 2020HD 88 apparatus. The surface characteristics were characterized by X-ray photoelectron spectroscopy (XPS) using VG ESCALAB 220i-XL (UK). Finally, to illustrate the adsorption mechanism, Fourier transform infrared spectroscopy (FT-IR) spectra were recorded before and after metal ions adsorption by MGCN by using Nicolet 5700 FT-IR spectrophotometer (USA). Raman spectroscopy analysis was performed using Thermo Nicolet Dispersive with a spectral resolution of 4 cm−1 and a spectral range of 50–500 cm−1.
2.3. Adsorption Measurements
For adsorption experiments, the 10 mg of MGCN were introduced in contact with the 25 mL Pb++/Cd++ solution at different initial concentrations (5–200 mg/L). A magnetic stirrer was employed to stirring the mixture at 400 rpm for 24 h to attain equilibrium.
For kinetics study, the experiments were conducted at fixed volume of 150 mL, initial concentration of 250 mg g−1, and 60 mg of MGCN. All measurements were run in the dark under magnetic stirring. At a planned timing, 5mL of the suspension was removed, centrifuged, and tested by spectrophotometer to estimate the residual concentration of Pb++/Cd++ metal ions. The Cd++ and Pb++ ions concentration was estimated using ICP Spectro Genesis Spectrometer. The adsorption equilibrium capacity ( in mg g−1) was computed by:
where (m in g) is the adsorbate mass, (V in L) is the solution volume, and (Ci and Ce in mg L−1) are the initial and equilibrium concentrations of metal ions.
For the pH-dependence experiments, the metal ions concentration with an initial pH was set in solution at various pH values ranging from 1 to 8 by adding HCl (0.1 mole/L) or NaOH (0.1 mole/L).
3. Results and Discussion
3.1. MGCN Nanomaterial’s Surface Properties
The textural properties of the prepared MGCN, including specific surface area and pore diameter, are analyzed by nitrogen gas sorption analysis. Figure 1a depicts N2 adsorption–desorption isotherms. The results manifest the presence of mesopores as it displays a type IV isotherm of the IUPAC with a clear hysteresis loop (H2) within the range 0.0–1.0 of relative pressure. The pore size distribution is displayed Figure 1b and calculated adopting the procedure of Barret–Joyner–Halender (BJH). The as prepared MGCN exhibits 84.4 m2 g−1 surface area and 0.87 m3 g−1 average pore volume. Higher surface area and large pore volume are beneficial to improving the adsorption efficiency of metal ions from water due to the increase of the active sites at nanoparticle’ surface [26,36].
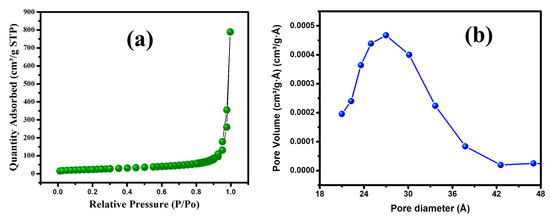
Figure 1.
(a) Adsorption isotherm and (b) pore size distribution of MGCN sorbent.
The Raman spectrum ranging from 0–2000 cm−1 is shown in Figure 2a. The peak below 400 cm−1 belongs to MgO [37]. The inset in Figure 2a presents three main peaks located at cm−1 belonging to the C–N, and C=N stretching vibration modes for g-C3N4 [38,39].
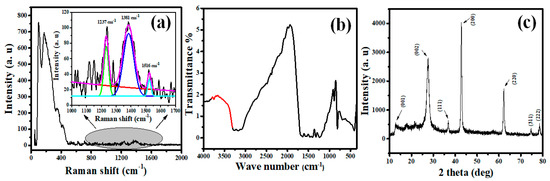
Figure 2.
(a) Raman spectrum, (b) FTIR spectrum, and (c) XRD pattern of MGCN sorbent.
The bonding and functional groups of MGCN sorbent are determined by the FTIR spectrum recorded in the range 400–4000 cm−1, see Figure 2b. The intense band at 810 cm−1 is relevant to heptazine units’ vibration, which agrees with the literature [31,40]. Moreover, the bands 1414/1486 cm−1 are assigned to Mg–O–Mg/Mg–O stretching, thereby confirming the formation of MgO phase. Additionally, the band at 1640 cm−1 is assigned to MgO. In fact, the vibrational band observed at the wavenumber at 1384 cm−1 corresponding to the C–O–Mg bonding, manifests the existence of an interaction between MgO and g-C3N4. This result corroborates with the previous study reported in the literature [41]. Additionally, other peaks between 3000–3500 cm−1 contribute to the aromatic groups’ symmetric and asymmetric vibrations of O–H and N–H bonds, implying the absorbance of water molecules [31,42]. Based on the results obtained above, the existence of g-C3N4 and the presence of its crystal structure was proven by the similarity of the distinct peaks before and after adding MgO.
The crystalline structure of the fabricated MgO/g-C3N4 sorbent is depicted in Figure 2c. The results obviously show the characteristic peaks for g-C3N4 and MgO, indicating that the loading of MgO onto g-C3N4 has been successfully achieved. For g-C3N4, the strong peak at 27.6° alongside a the small peak at 13.2◦ correspond to the (002) and (001) planes, consecutively [43]. The remaining peaks observed in the XRD pattern at 2θ = 36.8°, 42.7°, 62.12°, 74.6°, and 78.6° are indexed as (111), (200), (220), (311), and (222) reflections, belonging to the cubic structure of MgO phase in agreement with JCPDS card No. 78–0430 [31], which is consistent with Raman and FTIR analyses. The Scherrer formula (D = Kλ/βcosθ where β is the full-width at full maximum) has been utilized to estimate the mean crystallite size (D) of MGCN sorbent using the main peak (002) of g-C3N4 and (200) of MgO. The calculated crystallite size is found to be 7.83 nm g-C3N4 and 23.91 nm for MgO, which corroborates with previous studies [26]. The XRD pattern does not reveal any additional peaks, indicating the absence of impurities and confirms the high purity of the prepared MGCN sorbent.
TEM images of MGCN shows 2D irregular sheet or flakes-like morphology corrugated around 30 nm of thickness, as depicted in Figure 3a–c. The MgO particle size in MGCN nanocomposite is around 25–50 nm, in agreement with the crystallite size estimated by XRD analysis. Moreover, it can be observed that MgO nanoparticles are evenly and highly dispersed onto sheet-like g-C3N4, which form self-active sides through the MGCN surface. Figure 3d presents the elemental composition as obtained by EDX analysis. The EDX spectrum reveals the absence of any impurities other than C, N, O, and Mg.
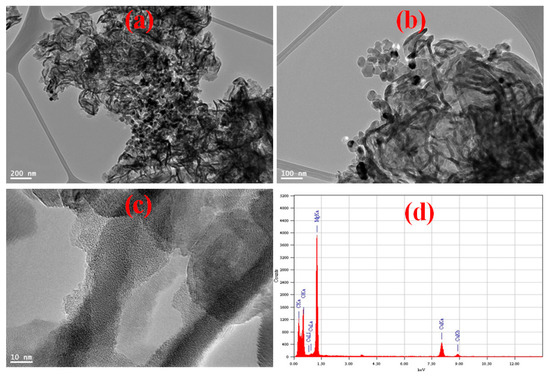
Figure 3.
(a–c) TEM micrographs, (d) EDX spectrum with the corresponding chemical composition of MGCN sorbent.
High-resolution X-ray photoelectron spectroscopy (HR-XPS) has been utilized to evaluate the chemical constituents of the mesoporous MGCN nanocomposite. The individual peaks of Mg, O, C, and N elements are depicted in Figure 4 to confirm the physical binding of MgO onto the g-C3N4 surface. The peak at 289.3 eV is assigned to C-1s, as illustrated in Figure 4c, and corresponds to the sp2 hybridized C = N species covalent bond (A. Modwi et al., 2022; Toghan and Modwi, 2021). The peak at 51.56 eV, suggesting the characteristic Mg-2p peak for MgO (Figure 4a); likewise, the signals of O-1s at 534 eV and O-2s at 42.75 eV are attributed to Mg-O bonding. Furthermore, the two signals N-1s observed in Figure 4d at 399.3 eV and 401.32 eV are ascribed to C–N–C of sp2 hybridized nitrogen and N–(C)3 tertiary nitrogen, respectively. The O-1s depicted in Figure 4b reveals a supplementary signal at 531.98 eV, corresponding to C-O bonding. The XPS analysis of the prepared MGCN nanocomposite also confirms its high purity, since only the constituents of the composite C, N, Mg, and O are detected, which corroborates with the results reported in the literature [40,43].
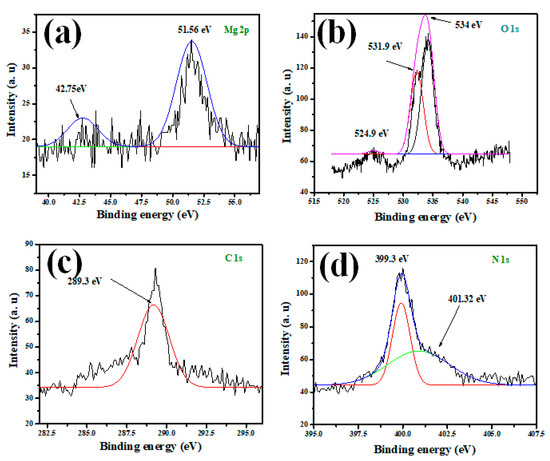
Figure 4.
XPS spectra of (a) Mg-2p, (b) O-1s, (c) C-1s, and (d) N-1s for MGCN sorbent.
3.2. MGCN Adsorption Study
3.2.1. Comparative Analysis of the Adsorption Capabilities of MgO, g-C3N4, and MGCN
To compare the Pb++ and Cd++ adsorption capacities of MgO, g-C3N4, and MGCN, a series of adsorption experiments were conducted at a fixed initial heavy metals concentration of 45 mg g−1 and a pH value 5, and the obtained results are given in Figure 5. It is interesting to note that MGCN demonstrates selective adsorption towards Cd++ compared to Pb++, i.e., the adsorbed amount is more than three times higher (90.83 mg g−1 for Pb++ and 367.73 mg g−1 for Cd++). Furthermore, the adsorption capacity of MGCN towards Cd++ is substantially higher than the respective capacities of pure g-C3N4 (42.50 mg g−1) and MgO (112.43 mg g−1). For Pb++, the adsorption capacity increases slightly, reaching 62.13 mg g−1 for MgO followed by 67.13 mg g−1 for g-C3N4 then up to 90.83 mg g−1 for MGCN. The obtained results highlight the synergistic effect as consequence of combining of 3D MgO NPs with 2D g-C3N4 nanosheets.
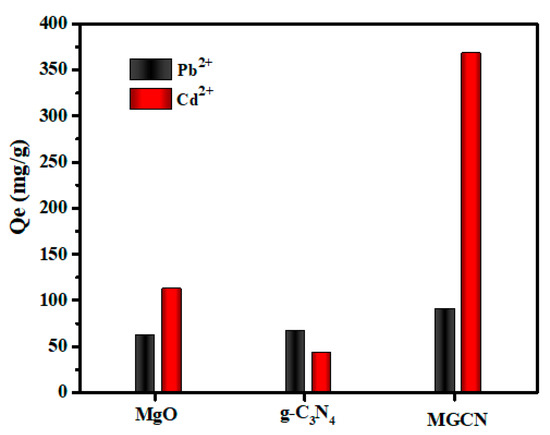
Figure 5.
Pb++ and Cd++ adsorption capacities of MGCN, g-C3N4, and MgO with an initial Pb++ and Cd++ concentration of 45 ppm and initial pH = 5.
3.2.2. Impact of pH on the Uptake Process
The pH is a well-known factor affecting the heavy metal uptake from an aqueous medium because it influences the charge transfer onto solid/liquid interface. The pH effect on Pb++/Cd++ ions uptake has been investigated within the range 1.0–9.0. It can clearly be observed from Figure 6 that Pb++/Cd++ adsorption onto MGCN sorbent is pH-dependent. There is a noticeable increase in the adsorption of Pb++ and Cd++ uptake as the solution pH increases to reach its maximum capacity at pH 5 and 3, respectively, then followed by a gradual reduction in the adsorption for greater pH values. The reduction in the adsorption rate at lower pH elucidates the competitive adsorption between H+ available in the solution and cations Pb++/Cd++ [41,44,45]. The solubility of metal ions like Pb++ and Cd++ varies with the pH value of the solution. At lower pH values, Pb++ and Cd++ are extensively soluble as Pb++ and Cd++ free ions, as well as Cd(OH)+ and Pb(OH)+ [46]. Additionally, at higher pH values, the cations of Pb++ and Cd++ precipitate as metal hydroxides Pb(OH)2 and Cd(OH), respectively [47]. At higher pH, a reduction of the adsorption is noted, corresponding to the formation of metal hydroxides [48]. Therefore, pH 3 and 5 are chosen as the optimum pH conditions for the consequent experiments’ Pb++ and Cd++ uptake.
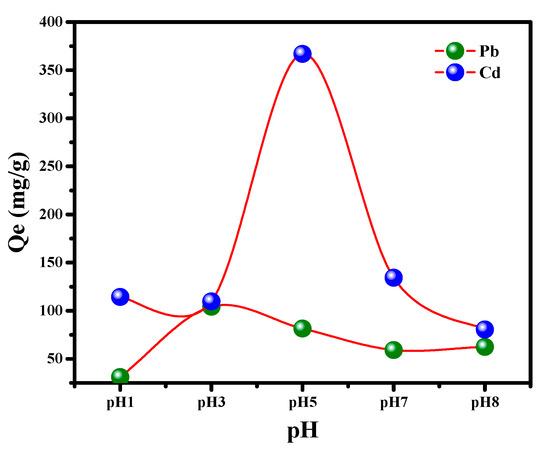
Figure 6.
Effect of pH on the removal of Pb++/Cd++ by MGCN nanosorbent.
3.2.3. Adsorption Kinetics
The study of kinetics is essential to better elucidate the adsorption process. The adsorption rate is crucial in the metal ions’ adsorption onto the adsorbent. Hence, the time-dependent adsorption experiment has been carried out to estimate the adsorption rate, and the obtained results are illustrated in Figure 7a. A contact time ranging from 0 min to 24 h is adopted during the adsorption experiments. It is clearly observed that the adsorption capacity increases sharply with the contact time, and in less than 50 min, the equilibrium is attained.
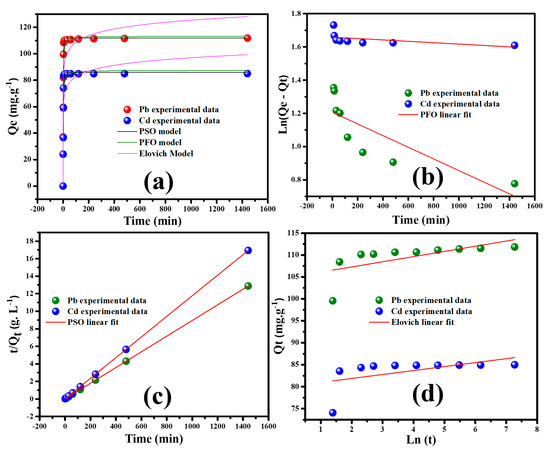
Figure 7.
(a) Effect of contact time, linear fit of (b) pseudo–first order, (c) pseudo–second order, and (d) Elovich on the sorption of Pb++/Cd++ onto MGCN sorbent.
The presence of active metal sites and facile accessibility without obstacles mainly affects the sorbent’s kinetic characteristic. In other words, the adsorption mechanism depends on the mass transfer process and the chemical reaction [6]. Therefore, Lagergren pseudo-first/-second order and Elovich are the three general models employed for the adsorption kinetics of Pb++/Cd++ ions from water by MGCN nanocomposite, as shown in Figure 7b–d. The models’ kinetic linear equations are listed in Table 1 while the obtained parameters are given in Table 2. The fit of the experimental data with the theoretical models is validated by expressing the correlation coefficient r2, i.e., the higher r2 value, the more suitable the model for Pb++/Cd++ uptake onto MGCN sorbent.

Table 1.
Kinetics models for the adsorption of Pb++/Cd++ by MGCN sorbent.

Table 2.
Kinetic models parameters for the adsorption of Pb and Cd by MGCN sorbent.
Figure 7b illustrates the kinetic plots of the pseudo-first order model in the form of vs. . This linear plot is used to determine the first order rate constant and , corresponding to the slope and intercept, respectively. The failure of the first order model to describe the adsorption process is evidenced from the obtained remarkably poor correlation coefficient values: 0.7880 and 0.8649 for Pb++ and Cd++ ions, respectively.
The kinetic graphs of the pseudo-second order model are shown in Figure 6c. From the plots vs., straight lines are obtained for both Pb++ and Cd++ with a high r2 value of 0.999 is found for both Pb++/Cd++. Subsequently, the values of k2 and from the slope and the intercept, respectively, are evaluated. These obtained results, as given in Table 2, indicate that the adsorption of Pb++/Cd++ ions onto MGCN sorbent is better suited to the pseudo-second order kinetics rather than pseudo-first order kinetics.
Furthermore, the Elovich model has been also tested by plotting qt versus Ln t, as illustrated in Figure 7d. The correlation coefficient r2 = 0.9864 for Pb++ uptake onto MGCN sorbent is like the value acquired by the pseudo-second order model and more significant than the value taken from the pseudo-first order model.
The obtained results, as shown in Figure 7a–d and Table 2, indicate that the chemisorption mainly controls the adsorption process based on valence force by electron participating between heavy metal ions and the adsorbents. On the contrary, the low value of r2 = 0.6744 obtained for Cd++ uptake onto MGCN sorbent signifies the bad fit of the adsorption process.
3.2.4. Intra-Particle Diffusion Study
Weber and Morris’s diffusion theory has been examined to investigate the intra-particle diffusion model, defined as a restrictive step for some adsorption processes. Based on this theory (equation listed in Table 1), the kinetic parameters and fitting parameters can be determined from the plot of . (Figure 8), where the intra-particle diffusion constants and acquired from the plot’s slope and intercept, respectively, are presented in Table 2. It is apparent from the linearity of plots that Pb++ and Cd++ ions uptake onto the MGCN surface exhibit a high efficiency. The intra-particle diffusion model is an important mode of diffusion due to the superior values of the correlation coefficient, i.e., r2 = 0.9941 and 0.9818 for Pb++ and Cd++, respectively. The C factor is the parameter indicating the boundary layer diameter, and its higher values demonstrate the effect of the solution boundary layer on Pb++/Cd++ uptake. A substantial value of C in the second step is obtained compared to the first step, which manifests the occurrence of heavy metal ion uptake by MGCN through the intraparticle diffusion phenomenon [26].
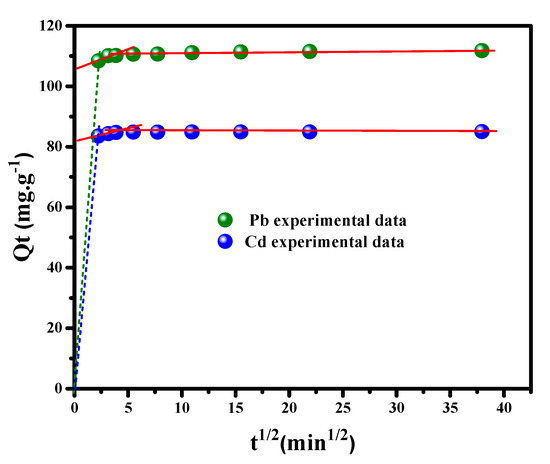
Figure 8.
Intra-particle-diffusion (qt vs. t1/2) for Pb++/Cd++ removal by MGCN sorbent.
3.2.5. Adsorption Isotherms
The adsorption isotherms describe the equilibrium relationship between the heavy metal ions and the sorbent under given conditions. Figure 9 shows the adsorption isotherms of Pb++/Cd++ ions adsorption by MGCN.
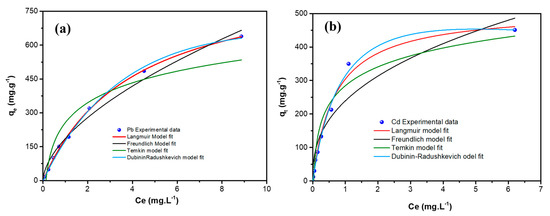
Figure 9.
Non-linear Adsorption equilibrium isotherms of (a) Pb++ and (b) Cd++ metal ions onto MGCN sorbent.
The Langmuir, Freundlich, Temkin, and Dubinin–Radushkevich models are the four different isotherms’ models used to fit the obtained experimental data, as illustrated in Figure 9 and Figure 10. Both linear and non-linear equations of the isotherm are listed in Table 3, and the obtained parameters with the corresponding regression coefficient r2 are given in Table 4 for both metal ions. Obviously, the Langmuir adsorption isotherm model manifests the highest regression coefficient, i.e., r2 values are 0.9989 and 0.9909, for the adsorption of Pb++ and Cd++ onto MGCN. More it is a favorable adsorption process since the RL value is positive for both metal ions (Table 4). In contrast, Freundlich and Temkin’s models are found to be unsatisfactory, and the values of r2 are below 0.99. These findings demonstrate that the Langmuir adsorption isotherms model is a well-fitting model.
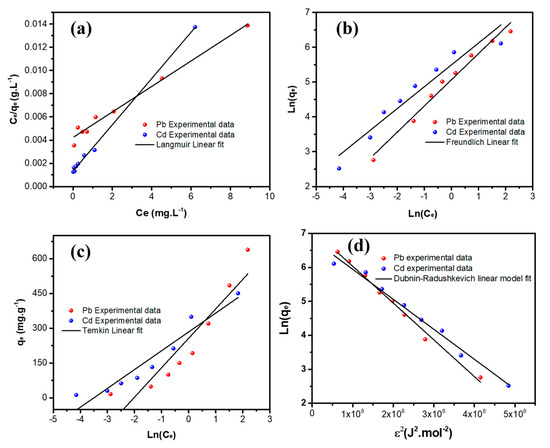
Figure 10.
Linear adsorption equilibrium isotherms of Pb++/Cd++ onto MGCN sorbent fitted with the (a) Langmuir, (b) Freundlich, (c) Temkin, and (d) Dubinin–Radushkevich models.
Moreover, it should be pointed out that the D-R equilibrium model can also be regarded as suitable to describe the experimental adsorption data of Pb++ and Cd++ by MGCN, considering the same criterion, i.e., R2 = 0.9924 and 0.9852, for Pb and Cd metal ions, respectively (Table 4). Depending on the D-R isothermal model’s energy value (E), the adsorption process is generally communed to physical or chemical [51]. Physisorption ensures whether the E value is less than 8 kJ.mol−1 [52]. In contrast, chemisorption occurs for energy values ranging from 8 to 16 kJ.mol−1 [52]. The obtained D-R isothermal model’s energy values are 6.795 and 7.525 kJ.mol−1 for Pb++ and Cd++ removal, respectively, manifesting that the metal ion adsorption mechanism onto MGCN occurs by physisorption.

Table 3.
Equilibrium models.
Table 3.
Equilibrium models.
| Equilibrium Model | Linear Form | Non-Linear Form |
|---|---|---|
| Langmuir [53] | ||
| Freundlich [54] | = | |
| Temkin [55] | ||
| Dubnin-Radushkevich [53] | , |

Table 4.
Isotherm parameters for the adsorption of Pb++/Cd++ ions onto MGCN sorbent.
Table 4.
Isotherm parameters for the adsorption of Pb++/Cd++ ions onto MGCN sorbent.
| Equilibrium Model | Parameters | Pb++ | Cd++ |
| Langmuir | qm (mg g−1) | 927.81 | 511.55 |
| KL (mg g−1) | 0.247 | 1.467 | |
| RL (L mg−1) | 0.0043 | 0.0013 | |
| r2 | 0.9989 | 0.9909 | |
| Freundlich | n | 1.71 | 2.57 |
| KF (L mg−1) | 186.34 | 239.24 | |
| R2 | 0.9867 | 0.9168 | |
| Temkin | B (J mol−1) | 19.51 | 30.51 |
| KT (L mg−1) | 7.55 | 33.14 | |
| r2 | 0.8879 | 0.9367 | |
| Dubnin–Radishkevich | qm (mg g−1) | 1234 | 922 |
| K (mol kJ−1)2 | 1.08 × 10−8 | 8.83 × 10−9 | |
| E (kJ mol−1) | 6.795 | 7.525 | |
| r2 | 0.9924 | 0.9852 |
Khezami et al. [56,57] proved by studying the Cd (II) ions adsorption onto pure and Al-doped ZnO nanoparticles, is a spontaneous physisorption process. Similarly, Mustapha et al. [58] confirmed the physisorption nature of the Pb2+ adsorption onto a RuO2–ZnO nanocomposite.
3.3. Adsorption Mechanism
The adsorption mechanism of Pb and Cd metal ions onto the MGCN surface is clarified by recording the FTIR spectra of MGCN before and after the adsorption process, as depicted in Figure 11. The spectra depict different changes and shifts in the peaks. A shift in the peak at 3750 cm−1 to 3697 cm−1 after Pb++ adsorption and into 3693 cm−1 after Cd++ adsorption, signifying that (O–H) contributes to the adsorption of metal ions [59]. The displacement of the OH stretching vibration band towards a higher/lower wavenumber confirms the interaction of Pb++ and Cd++ ions with oxygen atoms present at the surface of MGCN nanosorbent through surface complexation [60,61]. Moreover, the apparition of the (Pb–O) vibration band at 715 cm−1 and (Cd–O) stretching vibration at 862 cm−1 in the FTIR spectra of MGCN after adsorption [62,63], confirms the interaction of Pb++ and Cd++ ions with the oxygen atoms of MgO. Additionally, a broadened and shift after adsorption is noticeable within the range 3000–3500 cm−1 to a higher wavenumber, indicating that amino group stretching bands are involved during the adsorption through cation-bonding ([61,64].
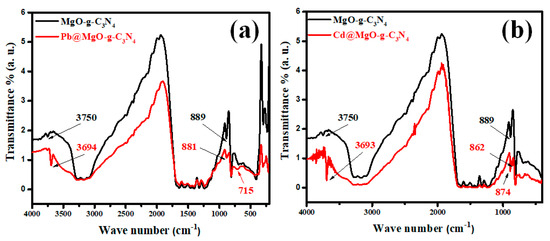
Figure 11.
FTIR spectra of MGCN sorbent before and after adsorption of (a) Pb++ and (b) Cd++.
Moreover, the peak at 889 cm−1 ascribed triazine ring (C3N3) mode also slightly shifted to 881 cm−1 for Pb++ and 874 cm−1 for Cd++. The delocalized electron of the tri-s-triazine ring (C3N3) acts as Lewis’s base, as mentioned by Xiao et al., while the metal ions act as Lewis’s acid [41]. Accordingly, the electrostatic interaction involved during adsorption of heavy metal ions onto MGCN occurs through the Lewis base–acid interaction. Figure 12 illustrates the main sorption mechanism of Pb++ and Cd++ ions onto the MGCN sorbent.
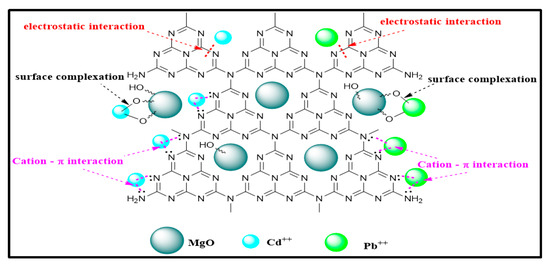
Figure 12.
The main sorption mechanism of Pb++ and Cd++ ions onto MGCN sorbent.
The adsorption optimum experimental conditions for removing Pb and Cd metal ions through adsorption onto MGCN alongside other previously investigated nanocomposites are listed in Table 5. The MGCN prepared by sonification demonstrates excellent potential for treating wastewater from Pb++ and Cd++ compared to other sorbents reported in the literature. Although the decreased size of MgO has a high surface area, it unavoidably reduces the stability. According to van der Waals force/other interactions, the aggregation of particles with a small diameter is likely to occur, consequently causing a decrease in MgO adsorption performance with smaller diameters/large surface area [65]. Furthermore, the formation aggregates result in the reduction of active sites, and hence a decline in the adsorption rate. In 2021, Fouda et al. investigated the adsorption of several heavy metal ions onto MgO-NPs and the results showed a moderate removal percentage for Cd and Pb metal ions around 74.1% ± 1.8% and 72.7% ± 1.3%, respectively. Nonetheless, although the achieved efficiency is relatively good, the adsorption reached equilibrium after a longer contact time of around 180 min using a 100 mg dosage of MgO-NPs, which are considered unsatisfactory optimal adsorption conditions [66]. While in this study, the as fabricated composite MGCN showed a better adsorption performance toward Cd and Pb metal ions with a removal efficiency around 97% and 95.6%, respectively, at 60 mg of MGCN within less than 50 min. This is attributed to the improvement in the number of active sites with much lower tendency to aggregation of MGCN nanostructured composite. Therefore, mesopores MGCN can be considered as a promising adsorbent in wastewater purification. The present MGCN nanostructured composite with high surface area and large pore size shown as a promising sorbent to eliminate other heavy metal ions from aqueous solutions.

Table 5.
Adsorption characteristics of MGCN and other nanosorbents for the removal of Pb++ and Cd++.
4. Conclusions
The fabricated mesoporous MGCN using the ultrasonication method are utilized for Pb++ and Cd++ metal ion removal from aqueous solutions. The adsorption of both metal ions onto MGCN sorbent is affected by the operating factors, namely solution pH, initial metal ion concentration, and sorbent dose. The results demonstrated that the MGCN sorbent has a high surface area 84.4 m2/g and pore volume of 1.22 cm3/g, fast adsorption equilibrium for Pb++/Cd++ in less than 50 min, and a high adsorption capacity of 114 and 90 mg g−1 for Pb++ and Cd++, respectively. Moreover, the adsorption isotherm/kinetics models perfectly fit with the Langmuir isotherm/pseudo-second order models. A plausible mechanism of Pb++/Cd++ adsorption onto mesoporous MGCN is elucidated. The Pb++/Cd++ adsorption mechanism was associated with surface complexation, cation-bonding, and electrostatic interaction, as indicated by FTIR analysis. The mesoporous MGCN sorbent exhibits excellent Pb++/Cd++ uptake and can be potentially utilized in wastewater purification.
Author Contributions
Conceptualization, L.K. and M.B.; methodology, A.M. and M.A.B.A.; software, M.B., A.M. and M.A.B.A.; validation, A.M., M.A.B.A. and M.B.; formal analysis, A.M., M.A.B.A. and M.B.; investigation, M.I. and M.B.; resources, L.K., A.M. and M.A.B.A.; data curation, A.M., M.A.B.A. and L.K.; writing—original draft preparation, R.A.A., M.B. and L.K.; writing—review and editing, M.B., A.M., M.A.B.A., L.K. and R.A.A.; visualization, R.A.A. and M.B.; supervision, M.B. and A.M.; project administration, M.B. and R.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The Authors would like to acknowledge the support of Prince Sultan University for paying the article processing charge of this publication.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vajedi, F.; Dehghani, H. The characterization of TiO2-reduced graphene oxide nanocomposites and their performance in electrochemical determination for removing heavy metals ions of cadmium(II), lead(II) and copper(II). Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2019, 243, 189–198. [Google Scholar] [CrossRef]
- Naiya, T.K.; Bhattacharya, A.K.; Das, S.K. Adsorption of Cd(II) and Pb(II) from aqueous solutions on activated alumina. J. Colloid Interface Sci. 2009, 333, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, T.O.; Oyewo, O.A.; Onwudiwe, D.C. Conventional and Current Methods of Toxic Metals Removal from Water Using g-C3N4-Based Materials. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1419–1442. [Google Scholar] [CrossRef]
- Jiang, M.Q.; Jin, X.Y.; Lu, X.Q.; Chen, Z.L. Adsorption of Pb(II), Cd(II), Ni(II) and Cu(II) onto natural kaolinite clay. Desalination 2010, 252, 33–39. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Agents classified by the IARC monographs. Igarss 2012, 1–105, 1–5. [Google Scholar]
- Lasheen, M.R.; Ammar, N.S.; Ibrahim, H.S. Adsorption/desorption of Cd(II), Cu(II) and Pb(II) using chemically modified orange peel: Equilibrium and kinetic studies. Solid State Sci. 2012, 14, 202–210. [Google Scholar] [CrossRef]
- Skyllberg, U.; Drott, A. Competition between disordered iron sulfide and natural organic matter associated thiols for mercury(II)—An EXAFS study. Environ. Sci. Technol. 2010, 44, 1254–1259. [Google Scholar] [CrossRef]
- Melita, L.; Popescu, M. Removal of Cr (VI) from industrial water effluents and surface waters using activated composite membranes. J. Memb. Sci. 2008, 312, 157–162. [Google Scholar] [CrossRef]
- Lu, X.; Huangfu, X.; Zhang, X.; Wang, Y.; Ma, J. Removal of trace mercury (II) from aqueous solution by in situ MnOx combined with poly-aluminum chloride. J. Water Health 2015, 13, 383–393. [Google Scholar] [CrossRef]
- Farrukh, A.; Akram, A.; Ghaffar, A.; Hanif, S.; Hamid, A.; Duran, H.; Yameen, B. Design of polymer-brush-grafted magnetic nanoparticles for highly efficient water remediation. ACS Appl. Mater. Interfaces 2013, 5, 3784–3793. [Google Scholar] [CrossRef]
- Teekayuttasakul, P.; Annachhatre, A.P. Lead removal and toxicity reduction from industrial wastewater through biological sulfate reduction process. J. Environ. Sci. Health—Part A Toxic/Hazardous Subst. Environ. Eng. 2008, 43, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Duan, X. Chemical precipitation of heavy metals from wastewater by using the synthetical magnesium hydroxy carbonate. Water Sci. Technol. 2020, 81, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Efome, J.E.; Rana, D.; Matsuura, T.; Lan, C.Q. Effects of operating parameters and coexisting ions on the efficiency of heavy metal ions removal by nano-fibrous metal-organic framework membrane filtration process. Sci. Total Environ. 2019, 674, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Baruah, A.; Mondal, S.; Sahoo, L.; Gautam, U.K. Ni-Fe-layered double hydroxide/N-doped graphene oxide nanocomposite for the highly efficient removal of Pb(II) and Cd(II) ions from water. J. Solid State Chem. 2019, 280, 120963. [Google Scholar] [CrossRef]
- Papailias, I.; Todorova, N.; Giannakopoulou, T.; Ioannidis, N.; Dallas, P.; Dimotikali, D.; Trapalis, C. Novel torus shaped g-C3N4 photocatalysts. Appl. Catal. B Environ. 2020, 268, 118733. [Google Scholar] [CrossRef]
- Schaber, P.M.; Colson, J.; Higgins, S.; Thielen, D.; Anspach, B.; Brauer, J. Thermal decomposition (pyrolysis) of urea in an open reaction vessel. Thermochim. Acta 2004, 424, 131–142. [Google Scholar] [CrossRef]
- Hu, S.; Ma, L.; You, J.; Li, F.; Fan, Z.; Lu, G.; Liu, D.; Gui, J. Enhanced visible light photocatalytic performance of g-C3N4 photocatalysts co-doped with iron and phosphorus. Appl. Surf. Sci. 2014, 311, 164–171. [Google Scholar] [CrossRef]
- Marom, O.; Nakhoul, F.; Tisch, U.; Shiban, A.; Abassi, Z.; Haick, H. Gold nanoparticle sensors for detecting chronic kidney disease and disease progression. Nanomedicine 2012, 7, 639–650. [Google Scholar] [CrossRef]
- Li, J.; Liu, E.; Ma, Y.; Hu, X.; Wan, J.; Sun, L.; Fan, J. Synthesis of MoS2/g-C3N4 nanosheets as 2D heterojunction photocatalysts with enhanced visible light activity. Appl. Surf. Sci. 2016, 364, 694–702. [Google Scholar] [CrossRef]
- Ali, M.K.M.; Modwi, A.; Idriss, H.; Aldaghri, O.; Ismail, M.; Ibnaouf, K.H. Detoxification of Pb (II) from aquatic media via CaMgO2@g-C3N4 nanocomposite. Mater. Lett. 2022, 322, 132501. [Google Scholar] [CrossRef]
- Toghan, A.; El-Lateef, H.M.A.; Taha, K.K.; Modwi, A. Mesoporous TiO2@g-C3N4 composite: Construction, characterization, and boosting indigo carmine dye destruction. Diam. Relat. Mater. 2021, 118, 108491. [Google Scholar] [CrossRef]
- Yin, R.; Luo, Q.; Wang, D.; Sun, H.; Li, Y.; Li, X.; An, J. SnO2/g-C3N4 photocatalyst with enhanced visible-light photocatalytic activity. J. Mater. Sci. 2014, 49, 6067–6073. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Qian, Q.; Machida, M.; Tatsumoto, H. Effect of ZnO loading to activated carbon on Pb(II) adsorption from aqueous solution. Carbon N. Y. 2006, 44, 195–202. [Google Scholar] [CrossRef]
- Di, Z.C.; Ding, J.; Peng, X.J.; Li, Y.H.; Luan, Z.K.; Liang, J. Chromium adsorption by aligned carbon nanotubes supported ceria nanoparticles. Chemosphere 2006, 62, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Tahir, M.N.; Adil, S.F.; Khan, H.U.; Siddiqui, M.R.H.; Al-Warthan, A.A.; Tremel, W. Graphene based metal and metal oxide nanocomposites: Synthesis, properties and their applications. J. Mater. Chem. A 2015, 3, 18753–18808. [Google Scholar] [CrossRef]
- Modwi, A.; Khezami, L.; Ghoniem, M.; Nguyen-Tri, P.; Baaloudj, O.; Guesmi, A.; AlGethami, F.; Amer, M.; Assadi, A. Superior removal of dyes by mesoporous MgO/g-C3N4 fabricated through ultrasound method: Adsorption mechanism and process modeling. Environ. Res. 2022, 205, 112543. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wu, K.; Xu, W.; Chen, D.; Fang, J.; Zhu, X.; Sun, J.; Liang, Y.; Hu, X.; Li, R.; et al. Adsorption-photocatalysis synergistic removal of contaminants under antibiotic and Cr(VI) coexistence environment using non-metal g-C3N4 based nanomaterial obtained by supramolecular self-assembly method. J. Hazard. Mater. 2021, 404, 124171. [Google Scholar] [CrossRef]
- Rajalakshmi, N.; Barathi, D.; Meyvel, S.; Sathya, P. S-scheme Ag2CrO4/g-C3N4 photocatalyst for effective degradation of organic pollutants under visible light. Inorg. Chem. Commun. 2021, 132, 108849. [Google Scholar] [CrossRef]
- Vuggili, S.B.; Khanth, S.K.; Kadiya, K.; Gaur, U.K.; Sharma, M. Improvement in visible light stimulated photocatalysis by the inducement of magnesium dopant inside graphitic carbon nitride frameworks. J. Environ. Chem. Eng. 2019, 7, 103440. [Google Scholar] [CrossRef]
- Modwi, A.; Khezami, L.; Taha, K.K.; Idriss, H. Flower Buds Like MgO Nanoparticles: From Characterisation to Indigo Carmine Elimination. Zeitschrift fur Naturforsch.—Sect. A J. Phys. Sci. 2018, 73, 975–983. [Google Scholar] [CrossRef]
- Vesali-Kermani, E.; Habibi-Yangjeh, A.; Ghosh, S. Visible-light-induced nitrogen photofixation ability of g-C3N4 nanosheets decorated with MgO nanoparticles. J. Ind. Eng. Chem. 2020, 84, 185–195. [Google Scholar] [CrossRef]
- Suresh, J.; Yuvakkumar, R.; Sundrarajan, M.; Hong, S.I. Green synthesis of magnesium oxide nanoparticles. Adv. Mater. Res. 2014, 952, 141–144. [Google Scholar] [CrossRef]
- Imani, M.M.; Safaei, M. Optimized Synthesis of Magnesium Oxide Nanoparticles as Bactericidal Agents. J. Nanotechnol. 2019, 2019, 6063832. [Google Scholar] [CrossRef]
- Abinaya, S.; Kavitha, H.P.; Prakash, M.; Muthukrishnaraj, A. Green synthesis of magnesium oxide nanoparticles and its applications: A review. Sustain. Chem. Pharm. 2021, 19, 100368. [Google Scholar] [CrossRef]
- Hornak, J. Synthesis, Properties, and Selected Technical Applications of Magnesium Oxide Nanoparticles: A Review. Int. J. Mol. Sci. 2021, 22, 12752. [Google Scholar] [CrossRef]
- Talapaneni, S.N.; Ramadass, K.; Benzigar, M.R.; Lakhi, K.S.; Yang, J.-H.; Ravon, U.; Albahily, K.; Vinu, A. Controlled synthesis of three dimensional mesoporous C3N4 with ordered porous structure for room temperature Suzuki coupling reaction. ScienceDirect 2019, 477, 110548. [Google Scholar] [CrossRef]
- Jiang, J.; Ou-Yang, L.; Zhu, L.; Zheng, A.; Zou, J.; Yi, X.; Tang, H. Dependence of electronic structure of g-C3N4 on the layer number of its nanosheets: A study by Raman spectroscopy coupled with first-principles calculations. Carbon N. Y. 2014, 80, 213–221. [Google Scholar] [CrossRef]
- Yang, N.-N.; Chen, Z.-G.; Zhao, Z.-G.; Cui, Y. Electrochemical fabrication of ultrafine g-C3N4 quantum dots as a catalyst for the hydrogen evolution reaction. New Carbon Mater. 2022, 37, 392–401. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Pham, M.-T.; Tran, D.P.H.; Bui, X.-T.; You, S.-J. Rapid Fabrication of MgO@g-C3N4 Heterojunction for Photocatalytic Nitric Oxide degradation under Visible Light. Beilstein Arch. 2022, 2022, 30. [Google Scholar] [CrossRef]
- Xiao, G.; Wang, Y.; Xu, S.; Li, P.; Yang, C.; Jin, Y.; Sun, Q.; Su, H. Superior adsorption performance of graphitic carbon nitride nanosheets for both cationic and anionic heavy metals from wastewater. Chin. J. Chem. Eng. 2019, 27, 305–313. [Google Scholar] [CrossRef]
- Kumar, M.R.A.; Nagaswarupa, H.P.; Ravikumar, C.R.; Prashantha, S.C.; Nagabhushana, H.; Bhatt, A.S. Green engineered nano MgO and ZnO doped with Sm3+: Synthesis and a comparison study on their characterization, PC activity and electrochemical properties. J. Phys. Chem. Solids 2019, 127, 127–139. [Google Scholar] [CrossRef]
- Mao, N.; Jiang, J.X. MgO/g-C3N4 nanocomposites as efficient water splitting photocatalysts under visible light irradiation. Appl. Surf. Sci. 2019, 476, 144–150. [Google Scholar] [CrossRef]
- Toghan, A.; Modwi, A. Boosting unprecedented indigo carmine dye photodegradation via mesoporous MgO@g-C3N4 nanocomposite. J. Photochem. Photobiol. A Chem. 2021, 419, 113467. [Google Scholar] [CrossRef]
- Sharma, M.; Poddar, M.; Gupta, Y.; Nigam, S.; Avasthi, D.; Adelung, R.; Abolhassani, R.; Fiutowski, J.; Joshi, M.; Mishra, Y. Solar light assisted degradation of dyes and adsorption of heavy metal ions from water by CuO–ZnO tetrapodal hybrid nanocomposite. Mater. Today Chem. 2020, 17, 100336. [Google Scholar] [CrossRef]
- Uchimiya, M.; Lima, I.M.; Klasson, K.T.; Chang, S.; Wartelle, L.H.; Rodgers, J.E. Immobilization of heavy metal ions (CuII, CdII, NiII, and PbII) by broiler litter-derived biochars in water and soil. J. Agric. Food Chem. 2010, 58, 5538–5544. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Rafatullah, M.; Sulaiman, O.; Ibrahim, M.H.; Chii, Y.Y.; Siddique, B.M. Removal of Cu(II) and Pb(II) ions from aqueous solutions by adsorption on sawdust of Meranti wood. Desalination 2009, 247, 636–646. [Google Scholar] [CrossRef]
- Adarakatti, P.S.; Gangaiah, V.K.; Banks, C.E.; Siddaramanna, A. One-pot synthesis of Mn3O4/graphitic carbon nanoparticles for simultaneous nanomolar detection of Pb(II), Cd(II) and Hg(II). J. Mater. Sci. 2018, 53, 4961–4973. [Google Scholar] [CrossRef]
- Baaloudj, O.; Nasrallah, N.; Kebir, M.; Khezami, L.; Amrane, A.; Assadi, A.A. A comparative study of ceramic nanoparticles synthesized for antibiotic removal: Catalysis characterization and photocatalytic performance modeling. Environ. Sci. Pollut. Res. 2020, 28, 13900–13912. [Google Scholar] [CrossRef]
- Chien, S.H.; Clayton, W.R. Application of Elovich Equation to the Kinetics of Phosphate Release and Sorption in Soils. Soil Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
- Mondal, N.K.; Basu, S. Potentiality of waste human hair towards removal of chromium(VI) from solution: Kinetic and equilibrium studies. Appl. Water Sci. 2019, 9, 49. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, J.; Xie, J.; Wang, S.; Cao, Y. A solid-state chemical method for synthesizing MgO nanoparticles with superior adsorption properties. RSC Adv. 2019, 9, 2011–2017. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghouti, M.A.; Razavi, M.M. Water reuse: Brackish water desalination using Prosopis juliflora. Environ. Technol. Innov. 2020, 17, 100614. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Günay, A.; Arslankaya, E.; Tosun, I. Lead removal from aqueous solution by natural and pretreated clinoptilolite: Adsorption equilibrium and kinetics. J. Hazard. Mater. 2007, 146, 362–371. [Google Scholar] [CrossRef]
- Khezami, L.; Modwi, A.; Ghiloufi, I.; Taha, K.K.; Bououdina, M.; ElJery, A.; El Mir, L. Effect of aluminum loading on structural and morphological characteristics of ZnO nanoparticles for heavy metal ion elimination. Environ. Sci. Pollut. Res. 2020, 27, 3086–3099. [Google Scholar] [CrossRef]
- Khezami, L.; Taha, K.K.; Amami, E.; Ghiloufi, I.; El Mir, L. Removal of cadmium (II) from aqueous solution by zinc oxide nanoparticles: Kinetic and thermodynamic studies. Desalin. Water Treat. 2017, 62, 346–354. [Google Scholar] [CrossRef]
- Mustafa, B.; Modwi, A.; Ismail, M.; Makawi, S.; Hussein, T.; Abaker, Z.; Khezami, L. Adsorption performance and Kinetics study of Pb(II) by RuO2–ZnO nanocomposite: Construction and Recyclability. Int. J. Environ. Sci. Technol. 2021, 19, 327–340. [Google Scholar] [CrossRef]
- Li, C.; Yan, W.; Xin, Q. Interaction of methane with surface of alumina studied by FT-IR spectroscopy. Catal. Lett. 1994, 24, 249–256. [Google Scholar] [CrossRef]
- Wadhawan, S.; Jain, A.; Nayyar, J.; Mehta, S.K. Role of nanomaterials as adsorbents in heavy metal ion removal from waste water: A review. J. Water Process Eng. 2020, 33, 101038. [Google Scholar] [CrossRef]
- Fdez-Sanromán, A.; Pazos, M.; Rosales, E.; Sanromán, M.A. Unravelling the Environmental Application of Biochar as Low-Cost Biosorbent: A Review. Appl. Sci. 2020, 10, 7810. [Google Scholar] [CrossRef]
- Bhukkal, C.; Ahlawat, R. Cu2+–Mn2+-Co-doped CdO nanocrystallites: Comprehensive research on phase, morphology and optoelectronic properties. Res. Chem. Intermed. 2020, 46, 4211–4232. [Google Scholar] [CrossRef]
- Arulmozhi, K.T.; Mythili, N. Studies on the chemical synthesis and characterization of lead oxide nanoparticles with different organic capping agents. AIP Adv. 2013, 3, 122122. [Google Scholar] [CrossRef]
- Bartoli, M.; Giorcelli, M.; Jagdale, P.; Rovere, M.; Tagliaferro, A. A Review of Non-Soil Biochar Applications. Materials 2020, 13, 261. [Google Scholar] [CrossRef]
- Xu, Y.; Xia, H.; Zhang, Q.; Jiang, G.; Cai, W.; Hu, W. Adsorption of cadmium(II) in wastewater by magnesium oxide modified biochar. Arab. J. Chem. 2022, 15, 104059. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.D.; Saied, E.; Hamza, M.F. Photocatalytic degradation of real textile and tannery effluent using biosynthesized magnesium oxide nanoparticles (MgO-NPs), heavy metal adsorption, phytotoxicity, and antimicrobial activity. J. Environ. Chem. Eng. 2021, 9, 105346. [Google Scholar] [CrossRef]
- Ianăşi, C.; Picioruş, M.; Nicola, R.; Ciopec, M.; Negrea, A.; Nižňanský, D.; Len, A.; Almásy, L.; Putz, A.-M. Removal of cadmium from aqueous solutions using inorganic porous nanocomposites. Korean J. Chem. Eng. 2019, 36, 688–700. [Google Scholar] [CrossRef]
- Baniamerian, H.; Teimoori, M.; Saberi, M. Fe2O3/TiO2/Activated Carbon Nanocomposite with Synergistic Effect of Adsorption and Photocatalysis. Chem. Eng. Technol. 2021, 44, 130–139. [Google Scholar] [CrossRef]
- Naushad, M.; Ahamad, T.; Al-Sheetan, K.M. Development of a polymeric nanocomposite as a high performance adsorbent for Pb(II) removal from water medium: Equilibrium, kinetic and antimicrobial activity. J. Hazard. Mater. 2021, 407, 124816. [Google Scholar] [CrossRef]
- Nicola, R.; Costişor, O.; Ciopec, M.; Negrea, A.; Lazău, R.; Ianăşi, C.; Picioruş, E.-M.; Len, A.; Almásy, L.; Szerb, E.I.; et al. Silica-coated magnetic nanocomposites for Pb2+ removal from aqueous solution. Appl. Sci. 2020, 10, 2726. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, R.; Sun, Y.; Zhou, X.; Baig, S.A.; Xu, X. Multifunctional nanocomposites Fe3O4@SiO2-EDTA for Pb(II) and Cu(II) removal from aqueous solutions. Appl. Surf. Sci. 2016, 369, 267–276. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).