Effect of Bioactive and Antimicrobial Nanoparticles on Properties and Applicability of Dental Adhesives
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Adhesives
- (a)
- 5 wt% MA-POSS and 1 wt% SiO2@Ag core-shell (Nanoshell LLC, Willmington, DE, USA)
- (b)
- 5 wt% MA-POSS and 1 wt% BAG-Bi (ETH Zurich, Zurich, Switzerland)
- (c)
- 5 wt% MA-POSS and 1 wt% CAP (ETH Zurich, Zurich, Switzerland).
2.2. Degree of Conversion (DC)
2.3. Viscosity
2.4. Water Sorption (WS) and Sol Fraction (SF)
2.5. Linear Shrinkage (LS)
2.6. Antibacterial Properties
2.7. Mineral Precipitation and Capacity
2.8. Statistical Analysis
3. Results
3.1. Degree of Conversion
3.2. Viscosity
3.3. Water Sorption and Sol Fraction
3.4. Linear Shrinkage
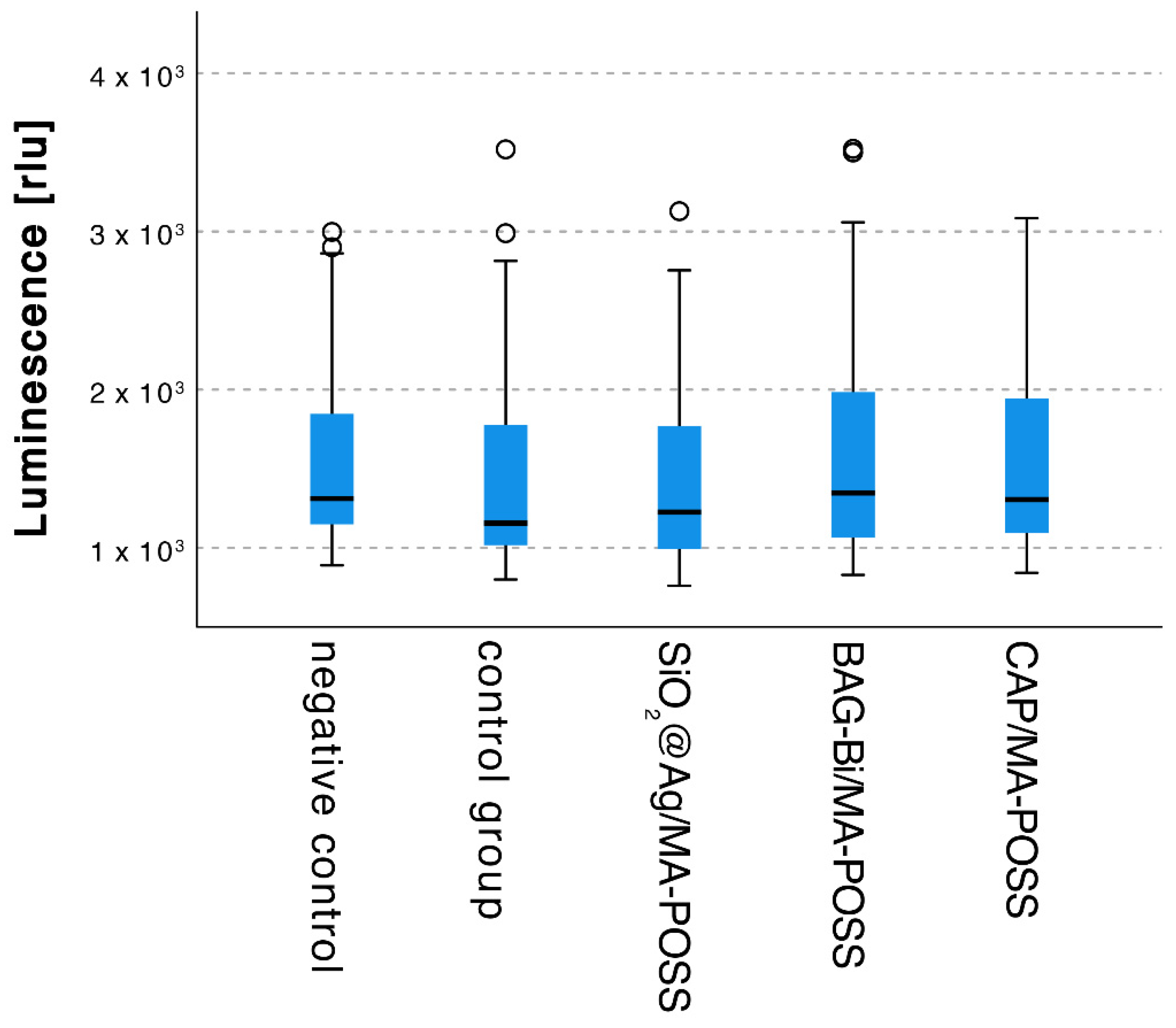
3.5. Antibacterial Properties
3.6. Mineral Precipitation Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Innes, N.P.T.; Frencken, J.E.; Bjørndal, L.; Maltz, M.; Manton, D.J.; Ricketts, D.; Van Landuyt, K.; Banerjee, A.; Campus, G.; Doméjean, S.; et al. Managing Carious Lesions: Consensus Recommendations on Terminology. Adv. Dent. Res. 2016, 28, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.A.; Hong, J.H.; Hatton, B.D.; Finer, Y. Antimicrobial Antidegradative Dental Adhesive Preserves Restoration-Tooth Bond. Dent. Mater. 2020, 36, 1666–1679. [Google Scholar] [CrossRef] [PubMed]
- Hatano, K.; Inokoshi, M.; Tamura, M.; Uo, M.; Shimizubata, M.; Tonprasong, W.; Wada, T.; Takahashi, R.; Imai, K.; Minakuchi, S. Novel Antimicrobial Denture Adhesive Containing S-PRG Filler. Dent. Mater. J. 2021, 40, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Hashem, M. Antimicrobial Capacity and Physico-Chemical Characteristics of Adhesive Resin Containing Riboflavin after Photodynamic Therapy. Photodiagn. Photodyn. Ther. 2021, 33, 102145. [Google Scholar] [CrossRef] [PubMed]
- Politano, A.D.; Campbell, K.T.; Rosenberger, L.H.; Sawyer, R.G. Use of Silver in the Prevention and Treatment of Infections: Silver Review. Surg. Infect. 2013, 14, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Klasen, H.J. A Historical Review of the Use of Silver in the Treatment of Burns. II. Renewed Interest for Silver. Burns J. Int. Soc. Burn Inj. 2000, 26, 131–138. [Google Scholar] [CrossRef]
- Melo, M.A.S.; Guedes, S.F.F.; Xu, H.H.K.; Rodrigues, L.K.A. Nanotechnology-Based Restorative Materials for Dental Caries Management. Trends Biotechnol. 2013, 31, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Xi, T.; Bai, J. Biological Effects Induced by Nanosilver Particles: In Vivo Study. Biomed. Mater. 2007, 2, S126–S128. [Google Scholar] [CrossRef]
- Kędziora, A.; Speruda, M.; Krzyżewska, E.; Rybka, J.; Łukowiak, A.; Bugla-Płoskońska, G. Similarities and Differences between Silver Ions and Silver in Nanoforms as Antibacterial Agents. Int. J. Mol. Sci. 2018, 19, 444. [Google Scholar] [CrossRef]
- Cao, W.; Zhang, Y.; Wang, X.; Chen, Y.; Li, Q.; Xing, X.; Xiao, Y.; Peng, X.; Ye, Z. Development of a Novel Resin-Based Dental Material with Dual Biocidal Modes and Sustained Release of Ag+ Ions Based on Photocurable Core-Shell AgBr/Cationic Polymer Nanocomposites. J. Mater. Sci. Mater. Med. 2017, 28, 103. [Google Scholar] [CrossRef]
- Gao, N.; Chen, Y.; Jiang, J. Ag@Fe2O3-GO Nanocomposites Prepared by a Phase Transfer Method with Long-Term Antibacterial Property. ACS Appl. Mater. Interfaces 2013, 5, 11307–11314. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.; Patil, S.D.; Jeevanandam, P.; Navani, N.K.; Singla, M.L. Synthesis, Characterization and Bactericidal Activity of Silica/Silver Core–Shell Nanoparticles. J. Mater. Sci. Mater. Med. 2014, 25, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.C.; Rolim, W.R.; Viana, M.M.; Souza, T.R.; Gonçalves, F.; Tanaka, C.J.; Bueno-Silva, B.; Seabra, A.B. Biogenic Synthesis and Antimicrobial Activity of Silica-Coated Silver Nanoparticles for Esthetic Dental Applications. J. Dent. 2020, 96, 103327. [Google Scholar] [CrossRef] [PubMed]
- Betancourt, D.E.; Baldion, P.A.; Castellanos, J.E. Resin-Dentin Bonding Interface: Mechanisms of Degradation and Strategies for Stabilization of the Hybrid Layer. Int. J. Biomater. 2019, 2019, e5268342. [Google Scholar] [CrossRef] [PubMed]
- Zanatta, R.F.; Lungova, M.; Borges, A.B.; Torres, C.; Sydow, H.-G.; Wiegand, A. Microleakage and Shear Bond Strength of Composite Restorations Under Cycling Conditions. Oper. Dent. 2017, 42, E71–E80. [Google Scholar] [CrossRef] [PubMed]
- Oudadesse, H.; Dietrich, E.; Gal, Y.L.; Pellen, P.; Bureau, B.; Mostafa, A.A.; Cathelineau, G. Apatite Forming Ability and Cytocompatibility of Pure and Zn-Doped Bioactive Glasses. Biomed. Mater. 2011, 6, 035006. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.B.; Gao, S.S.; Yu, H.Y. Effect of Nano-Hydroxyapatite Concentration on Remineralization of Initial Enamel Lesion in Vitro. Biomed. Mater. 2009, 4, 034104. [Google Scholar] [CrossRef]
- Tauböck, T.T.; Zehnder, M.; Schweizer, T.; Stark, W.J.; Attin, T.; Mohn, D. Functionalizing a Dentin Bonding Resin to Become Bioactive. Dent. Mater. 2014, 30, 868–875. [Google Scholar] [CrossRef]
- Odermatt, R.; Par, M.; Mohn, D.; Wiedemeier, D.B.; Attin, T.; Tauböck, T.T. Bioactivity and Physico-Chemical Properties of Dental Composites Functionalized with Nano- vs. Micro-Sized Bioactive Glass. J. Clin. Med. 2020, 9, 772. [Google Scholar] [CrossRef]
- Seyedlar, R.M.; Rezvani, M.; Barari, S.; Imani, M.; Nodehi, A.; Atai, M. Synthesis of Plate-like β-Tricalcium Phosphate Nanoparticles and Their Efficiency in Remineralization of Incipient Enamel Caries. Prog. Biomater. 2019, 8, 261–276. [Google Scholar] [CrossRef]
- Hu, Q.; Ji, H.; Liu, Y.; Zhang, M.; Xu, X.; Tang, R. Preparing Nano-Calcium Phosphate Particles via a Biologically Friendly Pathway. Biomed. Mater. 2010, 5, 041001. [Google Scholar] [CrossRef] [PubMed]
- Grosfeld, E.-C.; Hoekstra, J.W.M.; Herber, R.-P.; Ulrich, D.J.O.; Jansen, J.A.; van den Beucken, J.J. Long-Term Biological Performance of Injectable and Degradable Calcium Phosphate Cement. Biomed. Mater. 2016, 12, 015009. [Google Scholar] [CrossRef] [PubMed]
- Rizk, M.; Hohlfeld, L.; Thanh, L.T.; Biehl, R.; Lühmann, N.; Mohn, D.; Wiegand, A. Bioactivity and Properties of a Dental Adhesive Functionalized with Polyhedral Oligomeric Silsesquioxanes (POSS) and Bioactive Glass. Dent. Mater. 2017, 33, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Lu, Y.; Liu, S.; Jin, C.; Fang, S.; Zhou, X.; Li, Z. Tailor-Engineered POSS-Based Hybrid Gels for Bone Regeneration. Biomacromolecules 2019, 20, 3485–3493. [Google Scholar] [CrossRef]
- Tallá Ferrer, C.; Vilariño-Feltrer, G.; Rizk, M.; Sydow, H.G.; Vallés-Lluch, A. Nanocomposites Based on Poly(Glycerol Sebacate) with Silica Nanoparticles with Potential Application in Dental Tissue Engineering. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 761–772. [Google Scholar] [CrossRef]
- Wu, X.; Sun, Y.; Xie, W.; Liu, Y.; Song, X. Development of Novel Dental Nanocomposites Reinforced with Polyhedral Oligomeric Silsesquioxane (POSS). Dent. Mater. 2010, 26, 456–462. [Google Scholar] [CrossRef]
- Wang, W.; Sun, X.; Huang, L.; Gao, Y.; Ban, J.; Shen, L.; Chen, J. Structure-Property Relationships in Hybrid Dental Nanocomposite Resins Containing Monofunctional and Multifunctional Polyhedral Oligomeric Silsesquioxanes. Int. J. Nanomed. 2014, 9, 841–852. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X.; Sun, Y.; Xie, W. POSS Dental Nanocomposite Resin: Synthesis, Shrinkage, Double Bond Conversion, Hardness, and Resistance Properties. Polymers 2018, 10, 369. [Google Scholar] [CrossRef]
- Ozimek, J.; Pielichowski, K. Recent Advances in Polyurethane/POSS Hybrids for Biomedical Applications. Molecules 2021, 27, 40. [Google Scholar] [CrossRef]
- Engstrand, J.; Lopez, A.; Engqvist, H.; Persson, C. Polyhedral Oligomeric Silsesquioxane (POSS)-Poly(Ethylene Glycol) (PEG) Hybrids as Injectable Biomaterials. Biomed. Mater. 2012, 7, 035013. [Google Scholar] [CrossRef]
- Kreutz, M.; Wiegand, A.; Stawarczyk, B.; Lümkemann, N.; Rizk, M. Characterization of Methacrylate-Based Resins Containing Methacryl-Polyhedral Oligomeric Silsesquioxanes (MA-POSS-8). Materials 2021, 14, 1680. [Google Scholar] [CrossRef]
- Par, M.; Mohn, D.; Attin, T.; Tarle, Z.; Tauböck, T.T. Polymerization Shrinkage Behaviour of Resin Composites Functionalized with Unsilanized Bioactive Glass Fillers. Sci. Rep. 2020, 10, 15237. [Google Scholar] [CrossRef] [PubMed]
- Jungbluth, H.; Attin, T.; Buchalla, W. Development and Validation of an in Vitro Model for Measurements of Cervical Root Dentine Permeability. Clin. Oral Investig. 2014, 18, 2077–2086. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Klimek, J.; Hellwig, E.; Ahrens, G. Fluoride Taken up by Plaque, by the Underlying Enamel and by Clean Enamel from Three Fluoride Compounds in Vitro. Caries Res. 1982, 16, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Bair, S.; Yamaguchi, T.; Brouwer, L.; Schwarze, H.; Vergne, P.; Poll, G. Oscillatory and Steady Shear Viscosity: The Cox–Merz Rule, Superposition, and Application to EHL Friction. Tribol. Int. 2014, 79, 126–131. [Google Scholar] [CrossRef]
- Van Landuyt, K.L.; Snauwaert, J.; De Munck, J.; Peumans, M.; Yoshida, Y.; Poitevin, A.; Coutinho, E.; Suzuki, K.; Lambrechts, P.; Van Meerbeek, B. Systematic Review of the Chemical Composition of Contemporary Dental Adhesives. Biomaterials 2007, 28, 3757–3785. [Google Scholar] [CrossRef]
- Agnihotri, R.; Gaur, S.; Albin, S. Nanometals in Dentistry: Applications and Toxicological Implications-a Systematic Review. Biol. Trace Elem. Res. 2020, 197, 70–88. [Google Scholar] [CrossRef]
- Lee, Y.; An, S.-Y.; Park, Y.-J.; Yu, F.H.; Park, J.-C.; Seo, D.-G. Cytotoxic Effects of One-Step Self-Etching Adhesives on an Odontoblast Cell Line. Scanning 2016, 38, 36–42. [Google Scholar] [CrossRef]
- Rodrigues, M.C.; Natale, L.C.; Arana-Chaves, V.E.; Braga, R.R. Calcium and Phosphate Release from Resin-Based Materials Containing Different Calcium Orthophosphate Nanoparticles. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 1670–1678. [Google Scholar] [CrossRef]
- Degrazia, F.W.; Leitune, V.C.B.; Garcia, I.M.; Arthur, R.A.; Samuel, S.M.W.; Collares, F.M. Effect of Silver Nanoparticles on the Physicochemical and Antimicrobial Properties of an Orthodontic Adhesive. J. Appl. Oral Sci. 2016, 24, 404–410. [Google Scholar] [CrossRef]
- Barszczewska-Rybarek, I.; Chladek, G. Studies on the Curing Efficiency and Mechanical Properties of Bis-GMA and TEGDMA Nanocomposites Containing Silver Nanoparticles. Int. J. Mol. Sci. 2018, 19, 3937. [Google Scholar] [CrossRef] [PubMed]
- Kalathi, J.T.; Grest, G.S.; Kumar, S.K. Universal Viscosity Behavior of Polymer Nanocomposites. Phys. Rev. Lett. 2012, 109, 198301. [Google Scholar] [CrossRef]
- Lee, I.-B.; Son, H.-H.; Um, C.-M. Rheologic Properties of Flowable, Conventional Hybrid, and Condensable Composite Resins. Dent. Mater. 2003, 19, 298–307. [Google Scholar] [CrossRef]
- Dewaele, M.; Asmussen, E.; Peutzfeldt, A.; Munksgaard, E.C.; Benetti, A.R.; Finné, G.; Leloup, G.; Devaux, J. Influence of Curing Protocol on Selected Properties of Light-Curing Polymers: Degree of Conversion, Volume Contraction, Elastic Modulus, and Glass Transition Temperature. Dent. Mater. 2009, 25, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Rizk, M.; Pohle, A.; Dieckmann, P.; Tauböck, T.T.; Biehl, R.; Wiegand, A. Mineral Precipitation, Polymerization Properties and Bonding Performance of Universal Dental Adhesives Doped with Polyhedral Oligomeric Silsesquioxanes. Int. J. Adhes. Adhes. 2020, 100, 102573. [Google Scholar] [CrossRef]
- Correa Netto, L.R.; Borges, A.L.S.; Guimarães, H.B.; de Almeida, E.R.N.; Poskus, L.T.; da Silva, E.M. Marginal Integrity of Restorations Produced with a Model Composite Based on Polyhedral Oligomeric Silsesquioxane (POSS). J. Appl. Oral Sci. 2015, 23, 450–458. [Google Scholar] [CrossRef]
- Jäger, F.; Mohn, D.; Attin, T.; Tauböck, T.T. Polymerization and Shrinkage Stress Formation of Experimental Resin Composites Doped with Nano- vs. Micron-Sized Bioactive Glasses. Dent. Mater. J. 2021, 40, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Hebda, E.; Bukowczan, A.; Michałowski, S.; Wroński, S.; Urbaniak, P.; Kaczmarek, M.; Hutnik, E.; Romaniuk, A.; Wolun-Cholewa, M.; Pielichowski, K. Examining the Influence of Functionalized POSS on the Structure and Bioactivity of Flexible Polyurethane Foams. Mater. Sci. Eng. C 2020, 108, 110370. [Google Scholar] [CrossRef] [PubMed]
- Waltimo, T.; Mohn, D.; Paqué, F.; Brunner, T.J.; Stark, W.J.; Imfeld, T.; Schätzle, M.; Zehnder, M. Fine-Tuning of Bioactive Glass for Root Canal Disinfection. J. Dent. Res. 2009, 88, 235–238. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions Able to Reproduce in Vivo Surface-Structure Changes in Bioactive Glass-Ceramic A-W. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef]
- Ferraris, S.; Yamaguchi, S.; Barbani, N.; Cristallini, C.; Gautier di Confiengo, G.; Barberi, J.; Cazzola, M.; Miola, M.; Vernè, E.; Spriano, S. The Mechanical and Chemical Stability of the Interfaces in Bioactive Materials: The Substrate-Bioactive Surface Layer and Hydroxyapatite-Bioactive Surface Layer Interfaces. Mater. Sci. Eng. C 2020, 116, 111238. [Google Scholar] [CrossRef]
- Stanciu, G.; Sandulescu, I.; Savu, B.; Stanciu, S.; Paraskevopoulos, K.; Chatzistavrou, X.; Kontonasaki, E.; Koidis, P. Investigation of the Hydroxyapatite Growth on Bioactive Glass Surface. J. Biomed. Pharm. Eng. 2007, 1, 34–39. [Google Scholar]
- Li, L.; Pan, H.; Tao, J.; Xu, X.; Mao, C.; Gu, X.; Tang, R. Repair of Enamel by Using Hydroxyapatite Nanoparticles as the Building Blocks. J. Mater. Chem. 2008, 18, 4079–4084. [Google Scholar] [CrossRef]
- El-Damrawi, G.; Doweidar, H.; Kamal, H. Characterization of New Categories of Bioactive Based Tellurite and Silicate Glasses. Silicon 2017, 9, 503–509. [Google Scholar] [CrossRef]
- Zhou, X.; Sahai, N.; Qi, L.; Mankoci, S.; Zhao, W. Biomimetic and Nanostructured Hybrid Bioactive Glass. Biomaterials 2015, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Ku, S.H.; Lim, S.Y.; Kim, J.H.; Park, C.B. Graphene-Biomineral Hybrid Materials. Adv. Mater. 2011, 23, 2009–2014. [Google Scholar] [CrossRef]
- Dhanalekshmi, K.I.; Meena, K.S. Comparison of Antibacterial Activities of Ag@TiO2 and Ag@SiO2 Core-Shell Nanoparticles. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2014, 128, 887–890. [Google Scholar] [CrossRef]
- Melo, M.A.S.; Cheng, L.; Weir, M.D.; Hsia, R.-C.; Rodrigues, L.K.A.; Xu, H.H.K. Novel Dental Adhesive Containing Antibacterial Agents and Calcium Phosphate Nanoparticles. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 620–629. [Google Scholar] [CrossRef]
- Han, X.; Chen, Y.; Jiang, Q.; Liu, X.; Chen, Y. Novel Bioactive Glass-Modified Hybrid Composite Resin: Mechanical Properties, Biocompatibility, and Antibacterial and Remineralizing Activity. Front. Bioeng. Biotechnol. 2021, 9, 661734. [Google Scholar] [CrossRef]
- Begum, S.; Johnson, W.E.; Worthington, T.; Martin, R.A. The Influence of pH and Fluid Dynamics on the Antibacterial Efficacy of 45S5 Bioglass. Biomed. Mater. 2016, 11, 015006. [Google Scholar] [CrossRef]

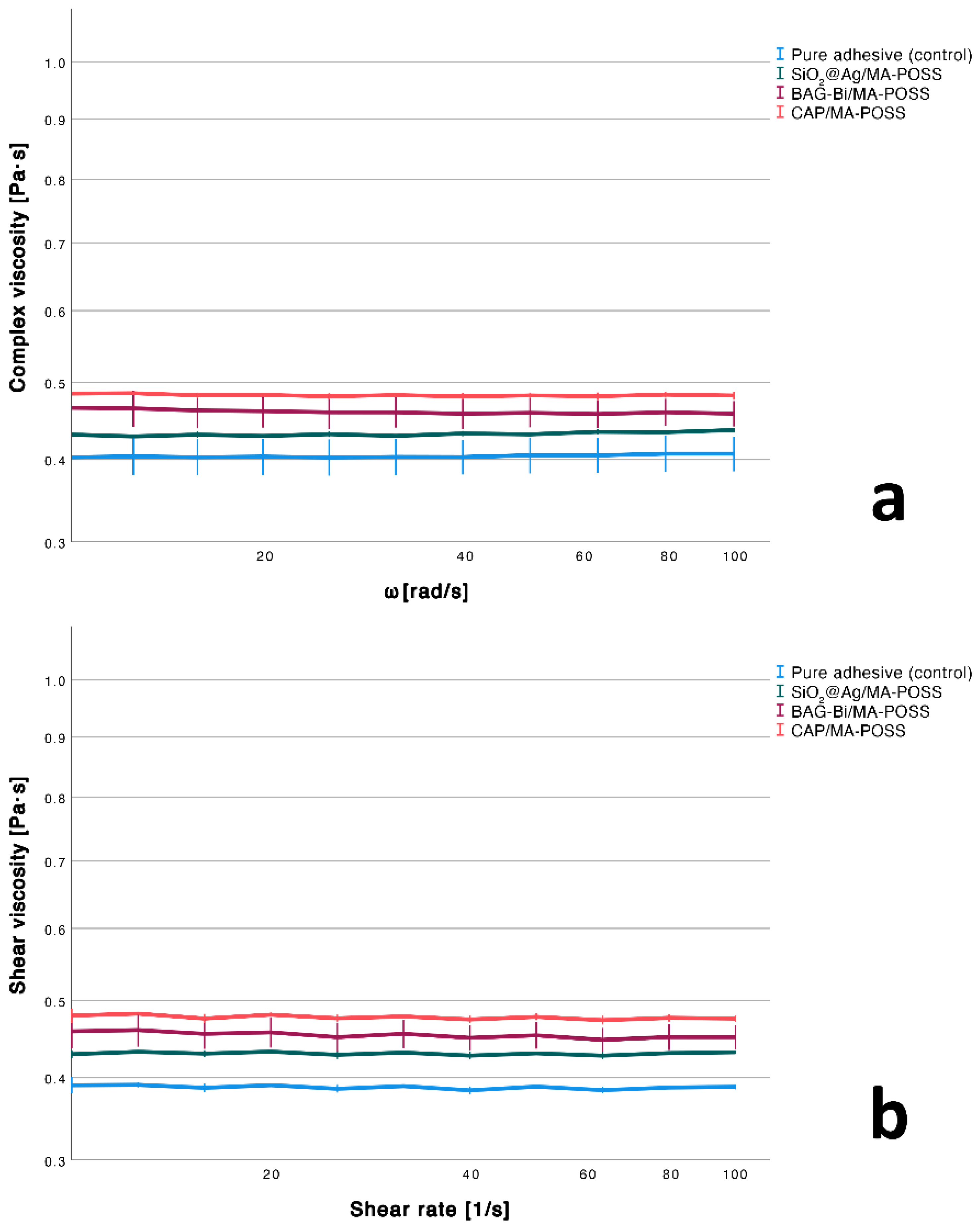
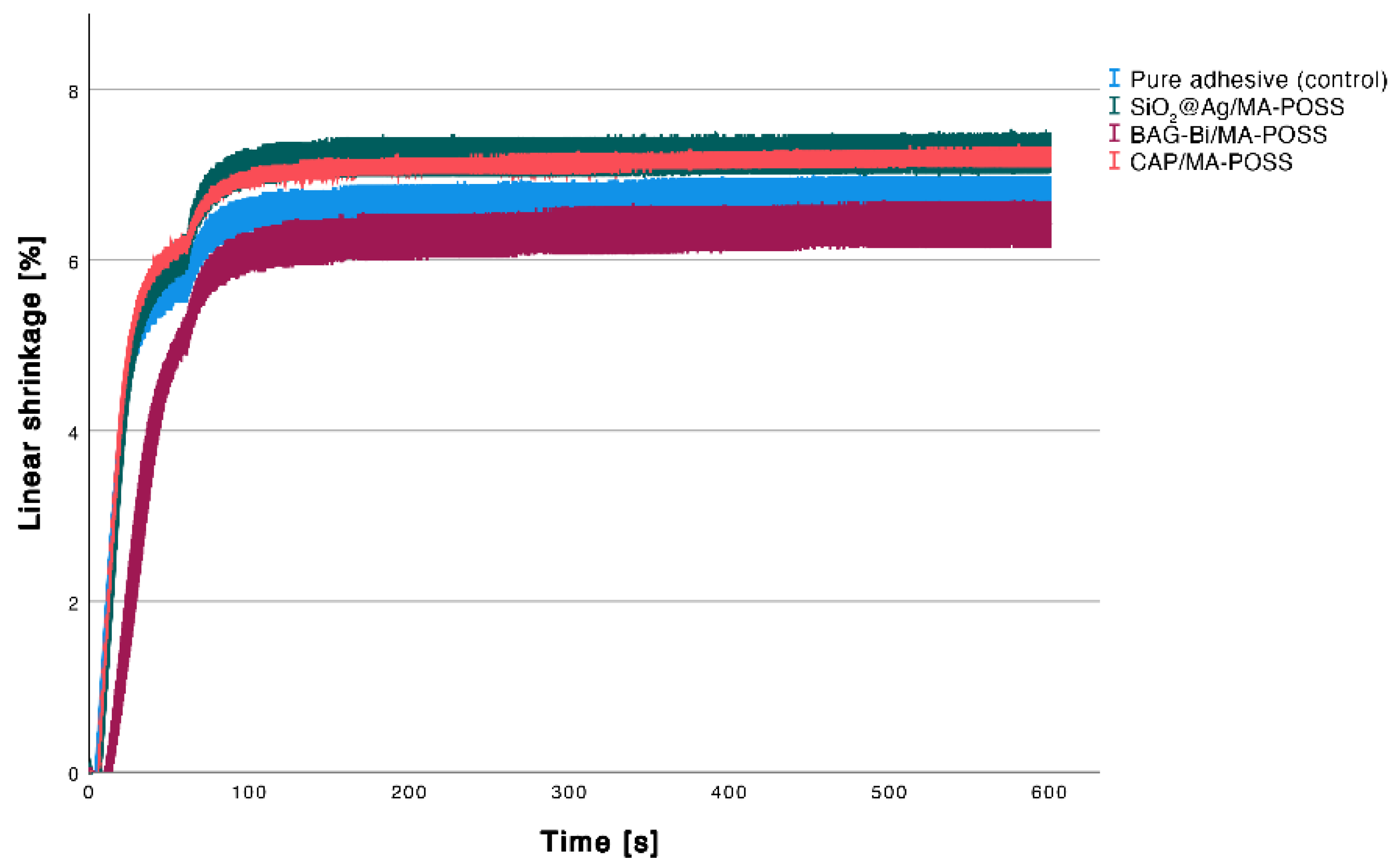

| Experimental Adhesive | Degree of Conversion [%] | Shear Viscosity [mPa·s] | Complex Viscosity [mPa·s] | Water Sorption [µg/mm3] | Sol Fraction [µg/mm3] | Linear Shrinkage [%] |
|---|---|---|---|---|---|---|
| Pure adhesive (control) | 54.4 ± 0.8 a | 386.9 ± 2.3 b | 402. 4 ± 25.8 a | 87.5 ± 6.5 b | 0.5 ± 2.8 b | 6.8 ± 0.2 ab |
| SiO2@Ag/MA-POSS | 53.1 ± 0.4 a | 430.1 ± 0.9 ab | 432.3 ± 1.4 a | 102.5 ± 11.5 ab | 8.7 ± 3.7 ab | 7.3 ± 0.2 b |
| BAG-Bi/MA-POSS | 51.7 ± 1.4 a | 450.8 ± 17.5 ab | 459.4 ± 23.4 a | 117.1 ± 3.1 a | 17.1 ± 6.6 a | 6.4 ± 0.3 a |
| CAP/MA-POSS | 53.4 ± 1.2 a | 475.7 ± 4.3 a | 481.7 ± 4.0 a | 91.8 ± 5.7 ab | 4.6 ± 6.1 ab | 7.2± 0.1 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kreutz, M.; Kreutz, C.; Kanzow, P.; Tauböck, T.T.; Burrer, P.; Noll, C.; Bader, O.; Rohland, B.; Wiegand, A.; Rizk, M. Effect of Bioactive and Antimicrobial Nanoparticles on Properties and Applicability of Dental Adhesives. Nanomaterials 2022, 12, 3862. https://doi.org/10.3390/nano12213862
Kreutz M, Kreutz C, Kanzow P, Tauböck TT, Burrer P, Noll C, Bader O, Rohland B, Wiegand A, Rizk M. Effect of Bioactive and Antimicrobial Nanoparticles on Properties and Applicability of Dental Adhesives. Nanomaterials. 2022; 12(21):3862. https://doi.org/10.3390/nano12213862
Chicago/Turabian StyleKreutz, Marietta, Christian Kreutz, Philipp Kanzow, Tobias T. Tauböck, Phoebe Burrer, Christine Noll, Oliver Bader, Bianca Rohland, Annette Wiegand, and Marta Rizk. 2022. "Effect of Bioactive and Antimicrobial Nanoparticles on Properties and Applicability of Dental Adhesives" Nanomaterials 12, no. 21: 3862. https://doi.org/10.3390/nano12213862
APA StyleKreutz, M., Kreutz, C., Kanzow, P., Tauböck, T. T., Burrer, P., Noll, C., Bader, O., Rohland, B., Wiegand, A., & Rizk, M. (2022). Effect of Bioactive and Antimicrobial Nanoparticles on Properties and Applicability of Dental Adhesives. Nanomaterials, 12(21), 3862. https://doi.org/10.3390/nano12213862









