Toward Clean and Economic Production of Highly Efficient Perovskite Solar Module Using a Cost-Effective and Low Toxic Aqueous Lead-Nitrate Precursor
Abstract
:1. Introduction
| Year | Module Area (cm2) | FF | Module Efficiency (%) | Architecture | Process | Deposition Method | Perovskite Solvent | Laser Wavelength (nm)/Type | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 2021 | 36.6 | 0.710 | 16.06 | Inverted | 1-step | SC + DC | DMF + DMSO | 532 | [8] |
| 2021 | 2.2 | 0.699 | 14.57 | Inverted | 1-step | SDC | 2-ME + DMSO | 532 | [17] |
| 2020 | 10.6 | 0.684 | 13.03 | Inverted | 1-step | BC | GBL + DMSO | CO2 laser | [14] |
| 2019 | 47.0 | 0.650 | 14.70 | Regular | 2-step | BC | DMF/IPA | 1064 | [15] |
| 2018 | 63.7 | 0.762 | 16.90 | Inverted | 1-step | BC | DMF | 532 | [16] |
| 2018 | 10.1 | 0.699 | 13.03 | Regular | 2-step | SC + DC | DMF/IPA | - | [10] |
| 2018 | 25.2 | 0.710 | 14.19 | Regular | 2-step | SC | DMF/IPA | - | [11] |
| 2018 | 31.7 | 0.7445 | 13.98 | Regular | 2-step | TE + SC | IPA | - | [26] |
| 2018 | 149.5 | 0.706 | 11.80 | Regular | 1-step | SDC | DMF | - | [18] |
| 2017 | 36.1 | 0.715 | 12.10 | Regular | 1-step | PPM | DMF | 532 | [27] |
| 2017 | 15.3 | - | 8.82 | Regular | 1-step | SC | DMF + DMSO | 1064/532 | [9] |
| 2016 | 3.8 | 0.700 | 11.70 | Regular | 1-step | USP | DMF | Mechanically scribed | [12] |
| 2016 | 40.0 | 0.702 | 15.50 | Regular | 1-step | SP | DMF + GBL | - | [13] |
| Deposited technology: Spray-pyrolysis (SP)/Ultrasonic Spray-pyrolysis (USP)/Thermally evaporation (TE)/Spin-coating (SC)/Dip-coating (DC)/Blade coating (BC)/Pressure processing method (PPM)/Slot-die coating (SDC) Solvent: Iso-propanaol (IPA)/Dimethylformamide (DMF)/Gammabuthyrolactone (GBL)/Dimethyl sulfoxide (DMSO)/2-methoxy-ethanol (2-ME) | |||||||||
2. Experimental Section
2.1. Perovskite Module Fabrication
2.2. Characterization and Measurement
3. Results and Discussion
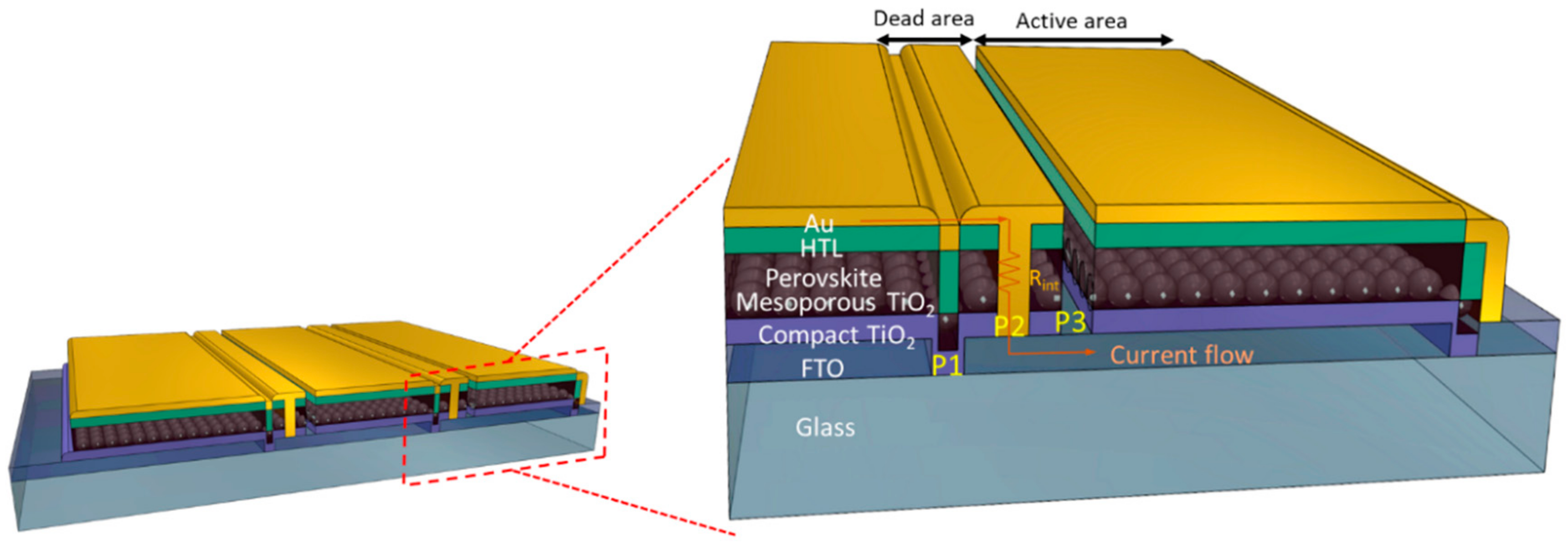
3.1. Laser Scribing (P1-P2-P3) Adjustment
3.2. PSM Architecture for Individual Cell Measurement
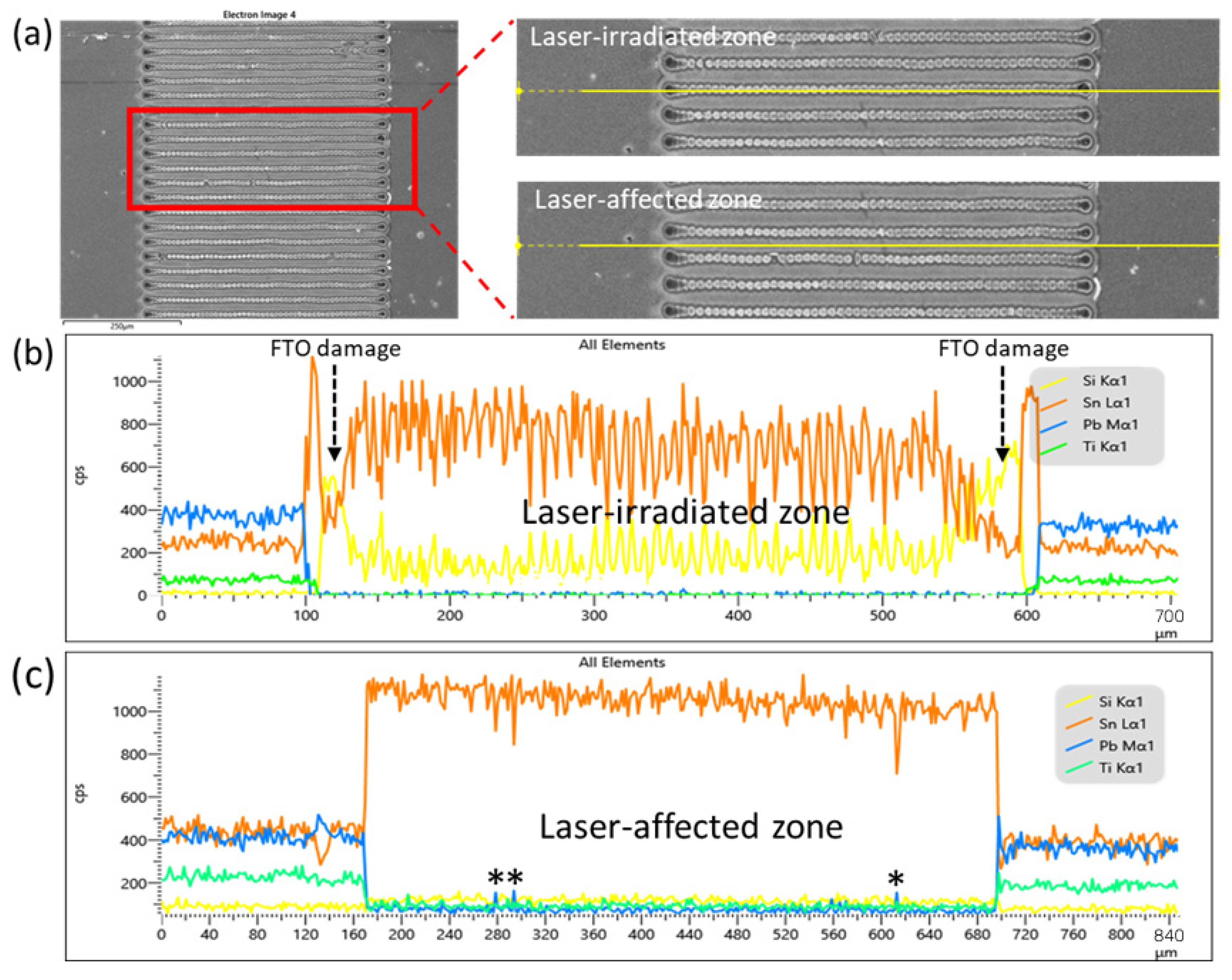
3.3. Effects of Problematic P2 Scribing and Inhomogeneous Spin-Coated Film
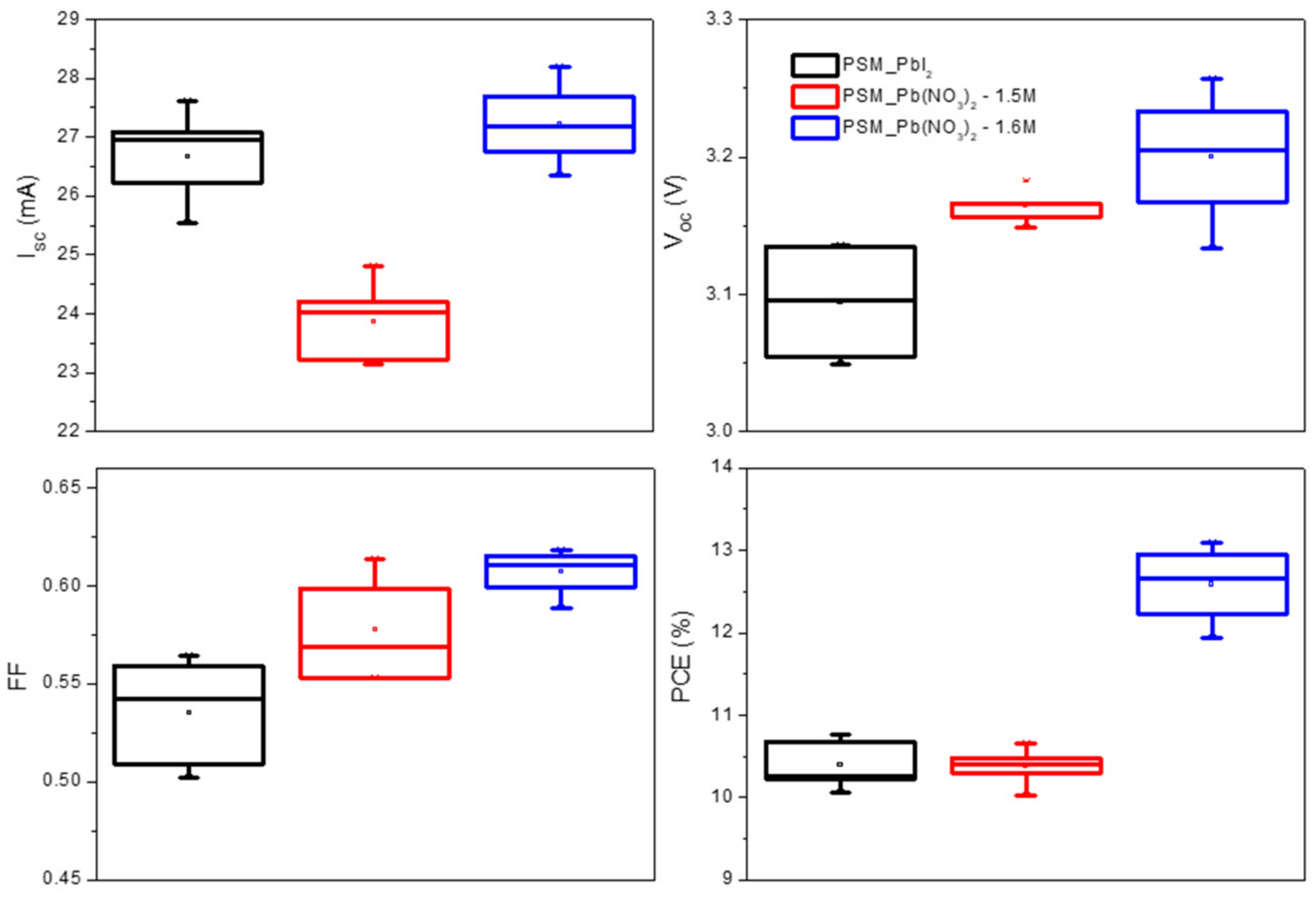
3.4. Comparison of PbI2/DMF and Pb(NO3)2/Water Two Step Dipping Process
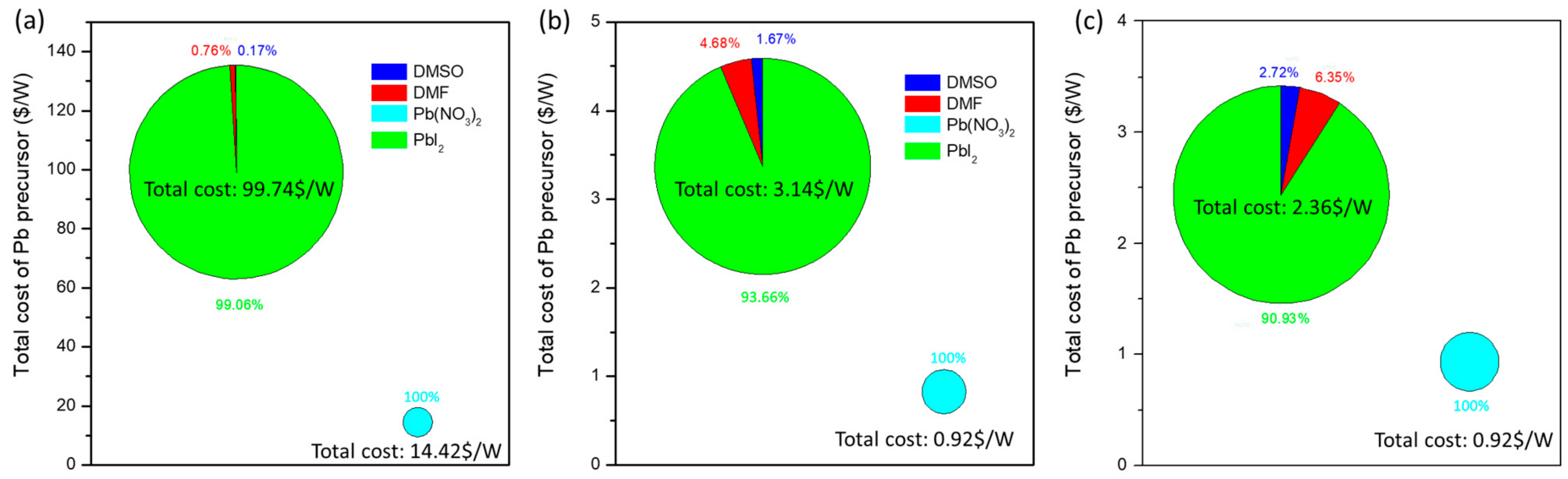
3.5. Breakdown of Materials of Pb-Precursor Film of Two-Step Fabrication
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Best Research-Cell Efficiency Chart. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 30 June 2022).
- Matteocci, F.; Razza, S.; Di Giacomo, F.; Casaluci, S.; Mincuzzi, G.; Brown, T.M.; D’Epifanio, A.; Licoccia, S.; Di Carlo, A. Solid-state solar modules based on mesoscopic organometal halide perovskite: A route towards the up-scaling process. Phys. Chem. Chem. Phys. 2014, 16, 3918–3923. [Google Scholar] [CrossRef] [PubMed]
- Razza, S.; Giacomo, F.D.; Matteocci, F.; Cinà, L.; Palma, A.; Casaluci, S.; Cameron, P.; D’Epifanio, A.; Licoccia, S.; Reale, A.; et al. Perovskite solar cells and large area modules (100 cm2) based on an air flow-assisted PbI2 blade coating deposition process. J. Power Sources 2015, 277, 286–291. [Google Scholar] [CrossRef]
- Heo, J.H.; Han, H.J.; Kim, D.; Ahn, T.K.; Im, S.H. Hysteresis-less inverted CH3NH3PbI3 planar perovskite hybrid solar cells with 18.1% power conversion efficiency. Energy Environ. Sci. 2015, 8, 1602–1608. [Google Scholar] [CrossRef]
- Priyadarshi, A.; Haur, L.J.; Murray, P.; Fu, D.; Kulkarni, S.; Xing, G.; Sum, T.C.; Mathews, N.; Mhaisalkar, S.G. A large area (70 cm2) monolithic perovskite solar module with a high efficiency and stability. Energy Environ. Sci. 2016, 9, 3687–3692. [Google Scholar] [CrossRef]
- Chen, R.; Hui, Y.; Wu, B.; Wang, Y.; Huang, X.; Xu, Z.; Ruan, P.; Zhang, W.; Cheng, F.; Zhang, W.; et al. Moisture-tolerant and high-quality α-CsPbI3 films for efficient and stable perovskite solar modules. J. Mater. Chem. A 2020, 8, 9597–9606. [Google Scholar] [CrossRef]
- Zhang, J.; Bu, T.; Li, J.; Li, H.; Mo, Y.; Wu, Z.; Liu, Y.; Zhang, X.-L.; Cheng, Y.-B.; Huang, F. Two-step sequential blade-coating of high quality perovskite layers for efficient solar cells and modules. J. Mater. Chem. A 2020, 8, 8447–8454. [Google Scholar] [CrossRef]
- Huang, H.-H.; Liu, Q.-H.; Tsai, H.; Shrestha, S.; Su, L.-Y.; Chen, P.-T.; Chen, Y.-T.; Yang, T.-A.; Lu, H.; Chuang, C.-H.; et al. A simple one-step method with wide processing window for high-quality perovskite mini-module fabrication. Joule 2021, 5, 958–974. [Google Scholar] [CrossRef]
- Palma, A.L.; Matteocci, F.; Agresti, A.; Pescetelli, S.; Calabrò, E.; Vesce, L.; Christiansen, S.; Schmidt, M.; Di Carlo, A. Laser-patterning engineering for perovskite solar modules with 95% aperture ratio. IEEE J. Photovolt. 2017, 7, 1674–1680. [Google Scholar] [CrossRef]
- Nia, N.Y.; Zendehdel, M.; Cinà, L.; Matteocci, F.; Di Carlo, A. A crystal engineering approach for scalable perovskite solar cells and module fabrication: A full out of glove box procedure. J. Mater. Chem. A 2018, 6, 659–671. [Google Scholar]
- Singh, M.; Chiang, C.-H.; Boopathi, K.M.; Hanmandlu, C.; Li, G.; Wu, C.-G.; Lin, H.-C.; Chu, C.-W. A novel ball milling technique for room temperature processing of TiO2 nanoparticles employed as the electron transport layer in perovskite solar cells and modules. J. Mater. Chem. A 2018, 6, 7114–7122. [Google Scholar] [CrossRef]
- Tait, J.G.; Manghooli, S.; Qiu, W.; Rakocevic, L.; Kootstra, L.; Jaysankar, M.; Masse de la Huerta, C.A.; Paetzold, U.W.; Gehlhaar, R.; Cheyns, D.; et al. Rapid composition screening for perovskite photovoltaics via concurrently pumped ultrasonic spray coating. J. Mater. Chem. A 2016, 4, 3792–3797. [Google Scholar] [CrossRef]
- Heo, J.H.; Lee, M.H.; Jang, M.H.; Im, S.H. Highly efficient CH3NH3PbI3−xClx mixed halide perovskite solar cells prepared by re-dissolution and crystal grain growth via spray coating. J. Mater. Chem. A 2016, 4, 17636–17642. [Google Scholar] [CrossRef]
- Lee, K.-M.; Lai, C.-H.; Chu, W.-C.; Chan, S.-H.; Suryanarayanan, V. Thermal assisted blade coating methylammonium lead iodide films with non-toxic solvent precursors for efficient perovskite solar cells and sub-module. Sol. Energy 2020, 204, 337–345. [Google Scholar] [CrossRef]
- Matteocci, F.; Vesce, L.; Kosasih, F.U.; Castriotta, L.A.; Cacovich, S.; Palma, A.L.; Divitini, G.; Ducati, C.; Di Carlo, A. Fabrication and Morphological Characterization of High-Efficiency Blade-Coated Perovskite Solar Modules. ACS Appl. Mater. Interfaces 2019, 11, 25195–25204. [Google Scholar] [CrossRef]
- Deng, Y.; Zheng, X.; Bai, Y.; Wang, Q.; Zhao, J.; Huang, J. Surfactant-controlled ink drying enables high-speed deposition of perovskite films for efficient photovoltaic modules. Nat. Energy 2018, 3, 560–566. [Google Scholar] [CrossRef]
- Li, J.; Dagar, J.; Shargaieva, O.; Flatken, M.A.; Köbler, H.; Fenske, M.; Schultz, C.; Stegemann, B.; Just, J.; Többens, D.M.; et al. 20.8% Slot-Die Coated MAPbI3 Perovskite Solar Cells by Optimal DMSO-Content and Age of 2-ME Based Precursor Inks. Adv. Energy Mater. 2021, 11, 2003460. [Google Scholar] [CrossRef]
- Di Giacomo, F.; Shanmugam, S.; Fledderus, H.; Bruijnaers, B.J.; Verhees, W.J.; Dorenkamper, M.S.; Veenstra, S.C.; Qiu, W.; Gehlhaar, R.; Merckx, T. Up-scalable sheet-to-sheet production of high efficiency perovskite module and solar cells on 6-in. substrate using slot die coating. Sol. Energy Mater. Sol. Cells 2018, 181, 53–59. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Chin, X.Y.; Dewi, H.A.; Vergeer, K.; Goh, T.W.; Lim, J.W.M.; Lew, J.H.; Loh, K.P.; Soci, C.; et al. Highly Efficient Thermally Co-evaporated Perovskite Solar Cells and Mini-modules. Joule 2020, 4, 1035–1053. [Google Scholar] [CrossRef]
- Vaynzof, Y. The Future of Perovskite Photovoltaics—Thermal Evaporation or Solution Processing? Adv. Energy Mater. 2020, 10, 2003073. [Google Scholar] [CrossRef]
- Gardner, K.L.; Tait, J.G.; Merckx, T.; Qiu, W.; Paetzold, U.W.; Kootstra, L.; Jaysankar, M.; Gehlhaar, R.; Cheyns, D.; Heremans, P.; et al. Nonhazardous Solvent Systems for Processing Perovskite Photovoltaics. Adv. Energy Mater. 2016, 6, 1600386. [Google Scholar] [CrossRef]
- Galvao, J.; Davis, B.; Tilley, M.; Normando, E.; Duchen, M.R.; Cordeiro, M.F. Unexpected low-dose toxicity of the universal solvent DMSO. FASEB J. 2014, 28, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.-Y.; Wei, T.-C.; Wu, K.-L.; Ikegami, M.; Miyasaka, T. Efficient perovskite solar cells fabricated using an aqueous lead nitrate precursor. Chem. Commun. 2015, 51, 13294–13297. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.-Y.; Pylnev, M.; Palomares, E.; Wei, T.-C. Exceptional Long Electron Lifetime in Methylammonium Lead Iodide Perovskite Solar Cell Made from Aqueous Lead Nitrate Precursor. Adv. Funct. Mater. 2020, 30, 1909644. [Google Scholar] [CrossRef]
- Hsieh, T.-Y.; Su, T.-S.; Ikegami, M.; Wei, T.-C.; Miyasaka, T. Stable and efficient perovskite solar cells fabricated using aqueous lead nitrate precursor: Interpretation of the conversion mechanism and renovation of the sequential deposition. Mater. Today Energy 2019, 14, 100125. [Google Scholar] [CrossRef]
- Li, K.; Xiao, J.; Yu, X.; Bu, T.; Li, T.; Deng, X.; Liu, S.; Wang, J.; Ku, Z.; Zhong, J. Influence of Hot Spot Heating on Stability of Large Size Perovskite Solar Module with a Power Conversion Efficiency of ∼14%. ACS Appl. Energy Mater. 2018, 1, 3565–3570. [Google Scholar] [CrossRef]
- Chen, H.; Ye, F.; Tang, W.; He, J.; Yin, M.; Wang, Y.; Xie, F.; Bi, E.; Yang, X.; Grätzel, M.; et al. A solvent- and vacuum-free route to large-area perovskite films for efficient solar modules. Nature 2017, 550, 92–95. [Google Scholar] [CrossRef]
- Brecl, K.; Topič, M.; Smole, F. A detailed study of monolithic contacts and electrical losses in a large-area thin-film module. Prog. Photovolt. Res. Appl. 2005, 13, 297–310. [Google Scholar] [CrossRef]
- Probst, V.; Stetter, W.; Riedl, W.; Vogt, H.; Wendl, M.; Calwer, H.; Zweigart, S.; Ufert, K.D.; Freienstein, B.; Cerva, H.; et al. Rapid CIS-process for high efficiency PV-modules: Development towards large area processing. Thin Solid Film. 2001, 387, 262–267. [Google Scholar] [CrossRef]
- Rakocevic, L.; Gehlhaar, R.; Merckx, T.; Qiu, W.; Paetzold, U.W.; Fledderus, H.; Poortmans, J. Interconnection Optimization for Highly Efficient Perovskite Modules. IEEE J. Photovolt. 2017, 7, 404–408. [Google Scholar] [CrossRef]
- Turan, B.; Huuskonen, A.; Kühn, I.; Kirchartz, T.; Haas, S. Cost-Effective Absorber Patterning of Perovskite Solar Cells by Nanosecond Laser Processing. Sol. RRL 2017, 1, 1700003. [Google Scholar] [CrossRef]
- Bayer, L.; Ye, X.; Lorenz, P.; Zimmer, K. Studies on perovskite film ablation and scribing with ns-, ps- and fs-laser pulses. Appl. Phys. A 2017, 123, 619. [Google Scholar] [CrossRef]
- Bado, P.; Clark, W.; Said, A. Ultrafast Laser Micromachining Handbook; Clark-MXR: Dexter, MI, USA, 2007; Available online: https://www.cmxr.com (accessed on 30 June 2022).
- Schultz, C.; Fenske, M.; Dagar, J.; Zeiser, A.; Bartelt, A.; Schlatmann, R.; Unger, E.; Stegemann, B. Ablation mechanisms of nanosecond and picosecond laser scribing for metal halide perovskite module interconnection—An experimental and numerical analysis. Sol. Energy 2020, 198, 410–418. [Google Scholar] [CrossRef]
- Fenske, M.; Schultz, C.; Dagar, J.; Kosasih, F.U.; Zeiser, A.; Junghans, C.; Bartelt, A.; Ducati, C.; Schlatmann, R.; Unger, E.; et al. Improved Electrical Performance of Perovskite Photovoltaic Mini-Modules through Controlled PbI2 Formation Using Nanosecond Laser Pulses for P3 Patterning. Energy Technol. 2021, 9, 2000969. [Google Scholar] [CrossRef]
- Kosasih, F.U.; Rakocevic, L.; Aernouts, T.; Poortmans, J.; Ducati, C. Electron Microscopy Characterization of P3 Lines and Laser Scribing-Induced Perovskite Decomposition in Perovskite Solar Modules. ACS Appl. Mater. Interfaces 2019, 11, 45646–45655. [Google Scholar] [CrossRef]
- Wang, K.-T.; Wang, W.-Y.; Wei, T.-C. Photomask-Free, Direct Selective Electroless Deposition on Glass by Controlling Surface Hydrophilicity. ACS Omega 2019, 4, 7706–7710. [Google Scholar] [CrossRef]
- Ma, C.; Park, N.-G. A Realistic Methodology for 30% Efficient Perovskite Solar Cells. Chem 2020, 6, 1254–1264. [Google Scholar] [CrossRef]
- Chen, B.; Wang, P.; Ren, N.; Li, R.; Zhao, Y.; Zhang, X. Tin dioxide buffer layer-assisted efficiency and stability of wide-bandgap inverted perovskite solar cells. J. Semicond. 2022, 43, 052201. [Google Scholar] [CrossRef]
- Zhai, P.; Su, T.-S.; Hsieh, T.-Y.; Wang, W.-Y.; Ren, L.; Guo, J.; Wei, T.-C. Toward clean production of plastic perovskite solar cell: Composition-tailored perovskite absorber made from aqueous lead nitrate precursor. Nano Energy 2019, 65, 104036. [Google Scholar] [CrossRef]

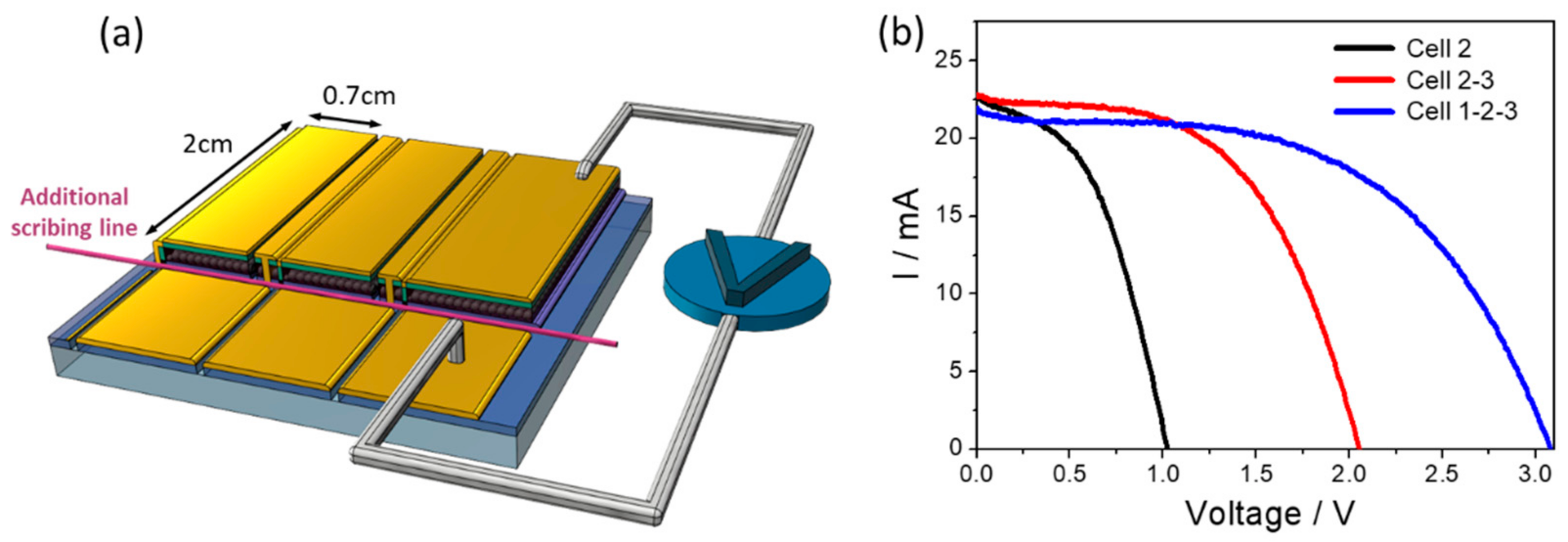

| Cell | ISC (mA) | JSC (mA/cm2) | VOC (V) | FF | Pmax (mW) | PCE (%) | Active Area (cm2) |
|---|---|---|---|---|---|---|---|
| 1 | 21.8 | 15.6 | 1.04 | 0.38 | 8.8 | 6.2 | 1.4 |
| 2 | 22.8 | 16.3 | 1.02 | 0.46 | 10.8 | 7.7 | 1.4 |
| 3 | 23.1 | 16.5 | 1.03 | 0.43 | 10.3 | 7.4 | 1.4 |
| 1-2 | 21.9 | 15.6 | 2.00 | 0.50 | 21.8 | 7.8 | 2.8 |
| 2-3 | 22.9 | 16.4 | 2.05 | 0.54 | 25.3 | 9.1 | 2.8 |
| 1-2-3 | 22.0 | 15.7 | 3.08 | 0.54 | 36.5 | 8.7 | 4.2 |
| Sample | Type | ISC (mA) | JSC (mA/cm2) | VOC (V) | FF | PCE (%) |
|---|---|---|---|---|---|---|
| PSM with inhomogeneous perovskite film (~450 nm) | 1 | 22.8 | 16.3 | 1.07 | 0.41 | 7.1 |
| 2 | 25.6 | 18.3 | 1.07 | 0.48 | 9.4 | |
| 3 | 24.2 | 17.3 | 1.01 | 0.50 | 8.8 | |
| 1-2 | 22.3 | 15.9 | 2.09 | 0.55 | 9.1 | |
| 2-3 | 24.4 | 17.3 | 2.06 | 0.57 | 10.3 | |
| 1-2-3 | 22.8 | 16.3 | 3.06 | 0.57 | 9.4 | |
| PSM contains problematic P2 structure between Cell 2 and Cell 3 (~600) | 1 | 29.6 | 21.1 | 1.01 | 0.31 | 6.6 |
| 2 | 30.0 | 21.4 | 0.99 | 0.39 | 8.3 | |
| 3 | 29.8 | 21.3 | 0.98 | 0.40 | 8.4 | |
| 1-2 | 29.9 | 21.4 | 1.98 | 0.42 | 9.0 | |
| 2-3 | 29.1 | 20.8 | 2.03 | 0.24 | 5.1 | |
| 1-2-3 | 29.3 | 20.9 | 2.99 | 0.30 | 6.2 |
| Type | ISC (mA) | JSC (mA/cm2) | VOC (V) | FF | PCE (%) | |
|---|---|---|---|---|---|---|
| PSM_PbI2 | Average | 26.7 ± 0.72 | 19.05 ± 0.72 | 3.10 ± 0.034 | 0.54 ± 0.026 | 10.4 ± 0.27 |
| Champion | 25.5 | 18.2 | 3.14 | 0.56 | 10.8 | |
| 1.5 M PSM_Pb(NO3)2 | Average | 23.9 ± 0.63 | 17.1 ± 0.63 | 3.16 ± 0.012 | 0.58 ± 0.025 | 10.4 ± 0.21 |
| Champion | 23.1 | 16.5 | 3.16 | 0.61 | 10.7 | |
| 1.6 M PSM_Pb(NO3)2 | Average | 27.2 ± 0.65 | 19.4 ± 0.65 | 3.20 ± 0.044 | 0.61 ± 0.011 | 12.6 ± 0.43 |
| Champion | 28.2 | 20.1 | 3.20 | 0.61 | 13.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, Y.-C.; Su, T.-S.; Lan, S.; Musa, A.F.; Wei, T.-C. Toward Clean and Economic Production of Highly Efficient Perovskite Solar Module Using a Cost-Effective and Low Toxic Aqueous Lead-Nitrate Precursor. Nanomaterials 2022, 12, 3783. https://doi.org/10.3390/nano12213783
Teng Y-C, Su T-S, Lan S, Musa AF, Wei T-C. Toward Clean and Economic Production of Highly Efficient Perovskite Solar Module Using a Cost-Effective and Low Toxic Aqueous Lead-Nitrate Precursor. Nanomaterials. 2022; 12(21):3783. https://doi.org/10.3390/nano12213783
Chicago/Turabian StyleTeng, Yi-Chen, Tzu-Sen Su, Shiang Lan, Ahmed Fouad Musa, and Tzu-Chien Wei. 2022. "Toward Clean and Economic Production of Highly Efficient Perovskite Solar Module Using a Cost-Effective and Low Toxic Aqueous Lead-Nitrate Precursor" Nanomaterials 12, no. 21: 3783. https://doi.org/10.3390/nano12213783
APA StyleTeng, Y.-C., Su, T.-S., Lan, S., Musa, A. F., & Wei, T.-C. (2022). Toward Clean and Economic Production of Highly Efficient Perovskite Solar Module Using a Cost-Effective and Low Toxic Aqueous Lead-Nitrate Precursor. Nanomaterials, 12(21), 3783. https://doi.org/10.3390/nano12213783









