Glucose Hydrogenolysis into 1,2-Propanediol Using a Pt/deAl@Mg(OH)2 Catalyst: Expanding the Application of a Core–Shell Structured Catalyst
Abstract
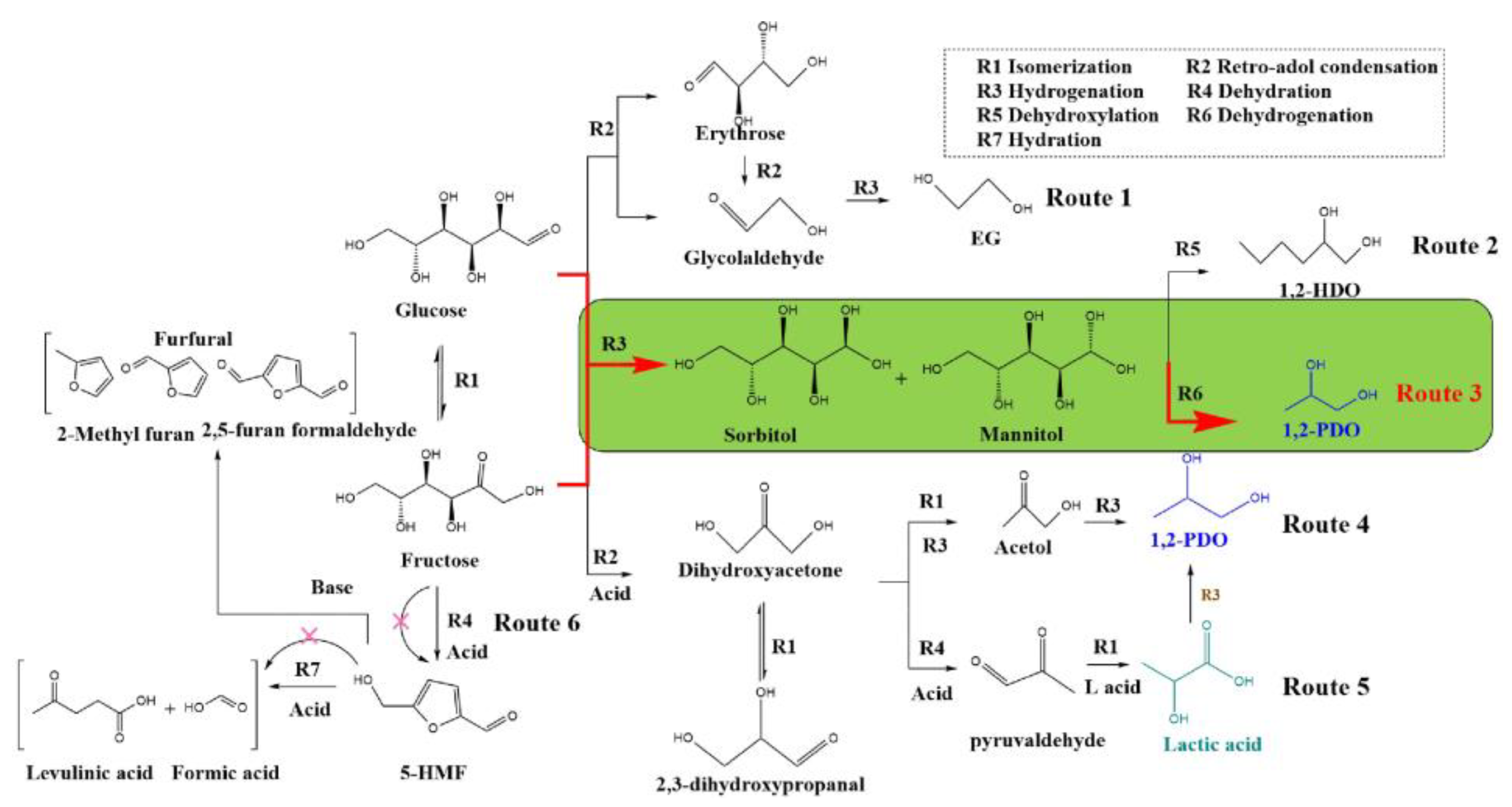
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Catalyst Preparation
2.2.1. deAl-Beta Preparation
2.2.2. Pt/deAl-Beta Preparation
2.2.3. Pt/deAl-Beta@Mg(OH)2 Preparation
2.3. Catalyst Characterization
2.4. Catalytic Reaction
2.4.1. GC Operating Condition
2.4.2. HPLC Operating Condition
2.5. Calculation
3. Results
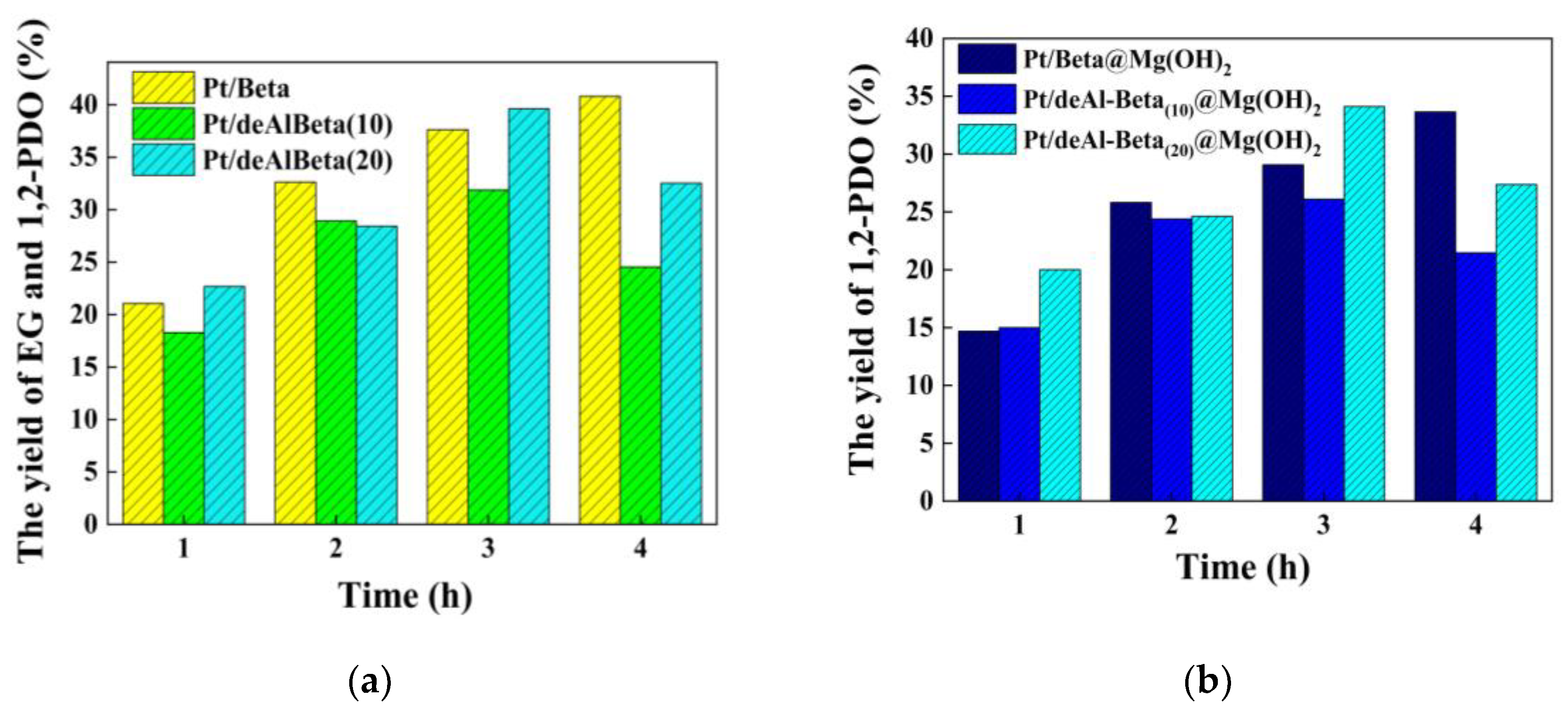
3.1. Effect of Dealumination Time
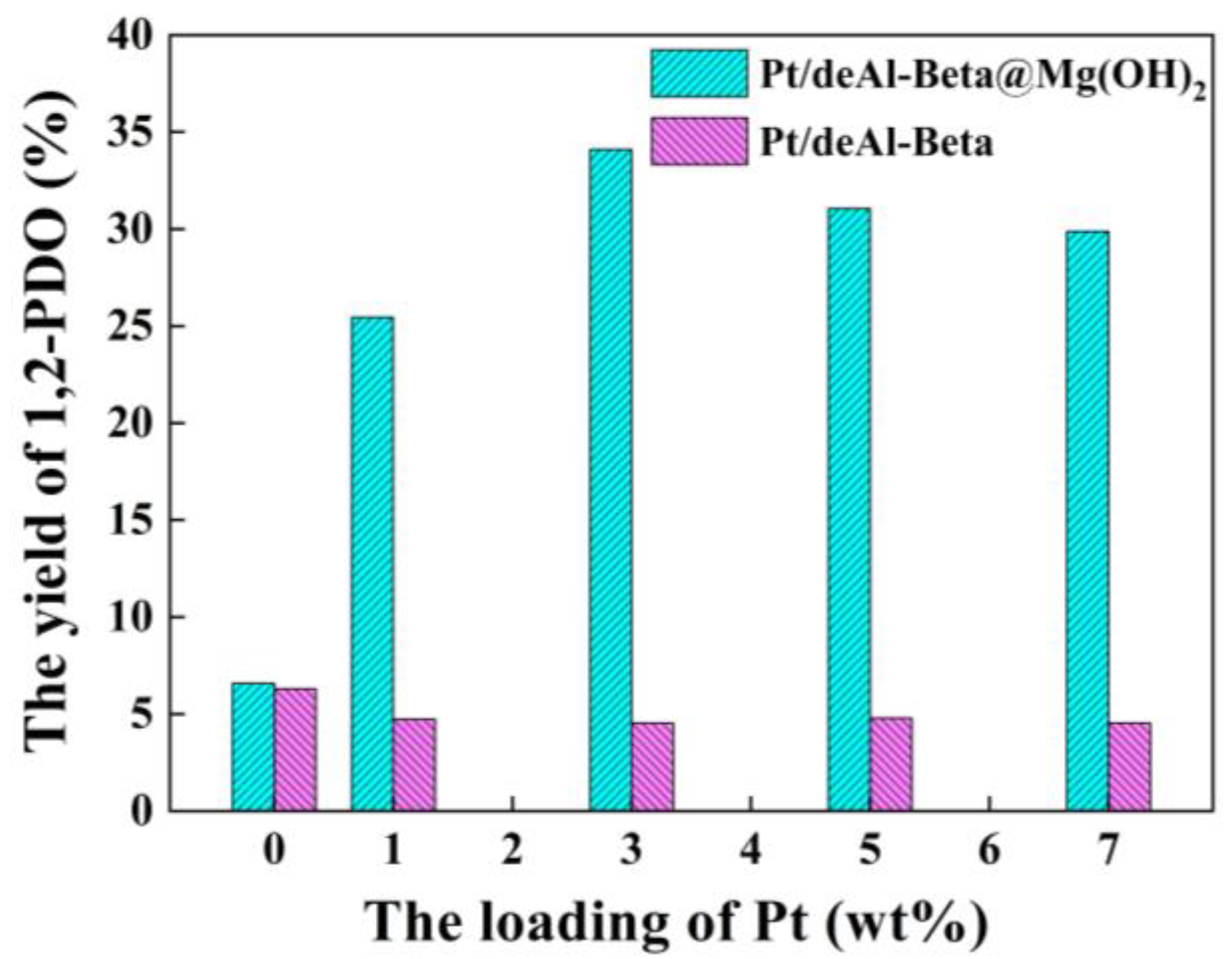
3.2. Effect of Pt Loading
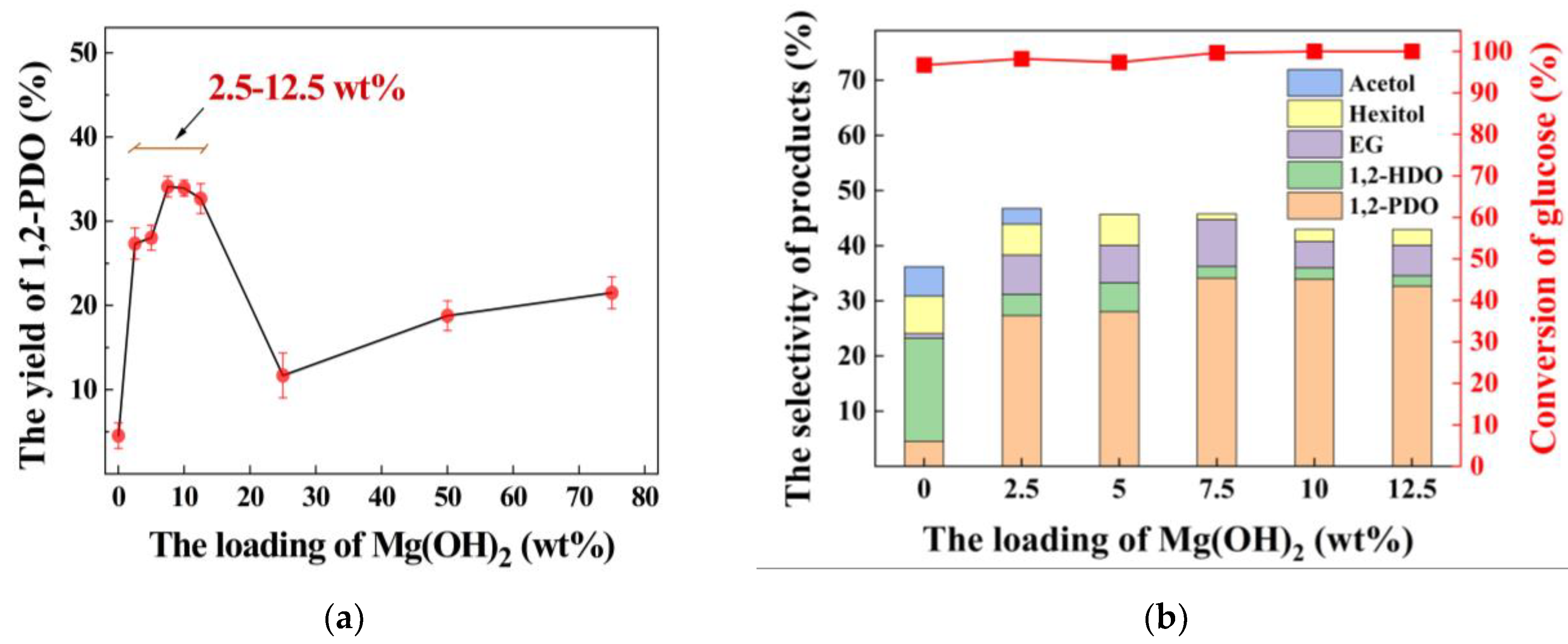
3.3. Effect of Mg(OH)2 Loading
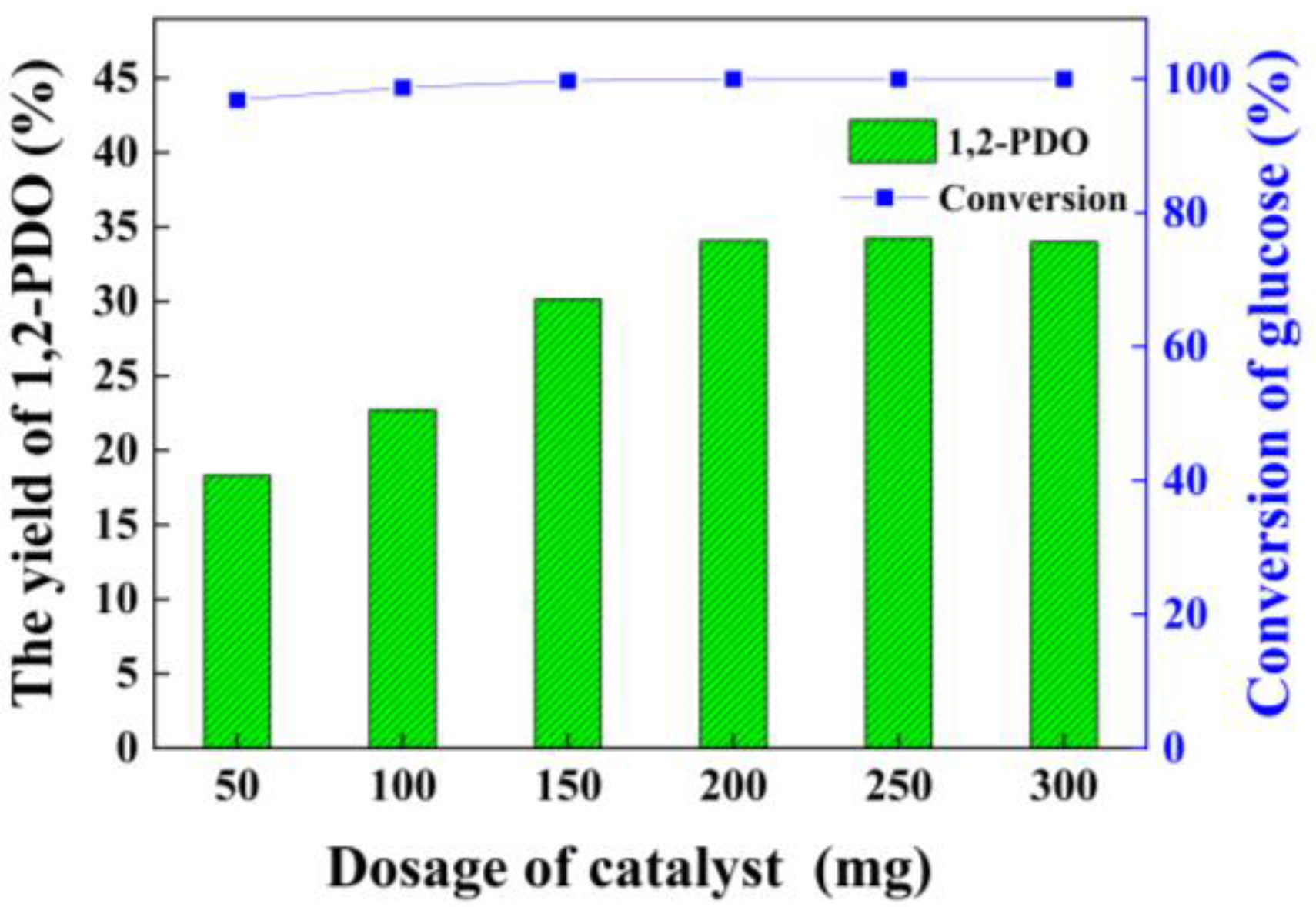
3.4. Effect of Catalyst Dosage
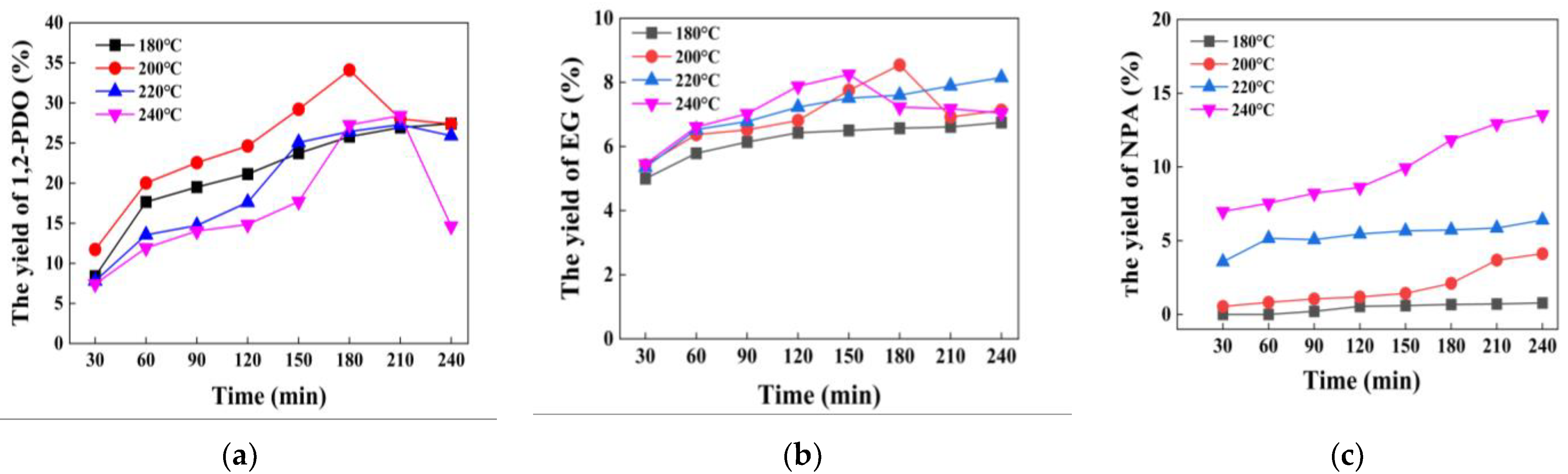
3.5. Effect of Catalytic Reaction Conditions
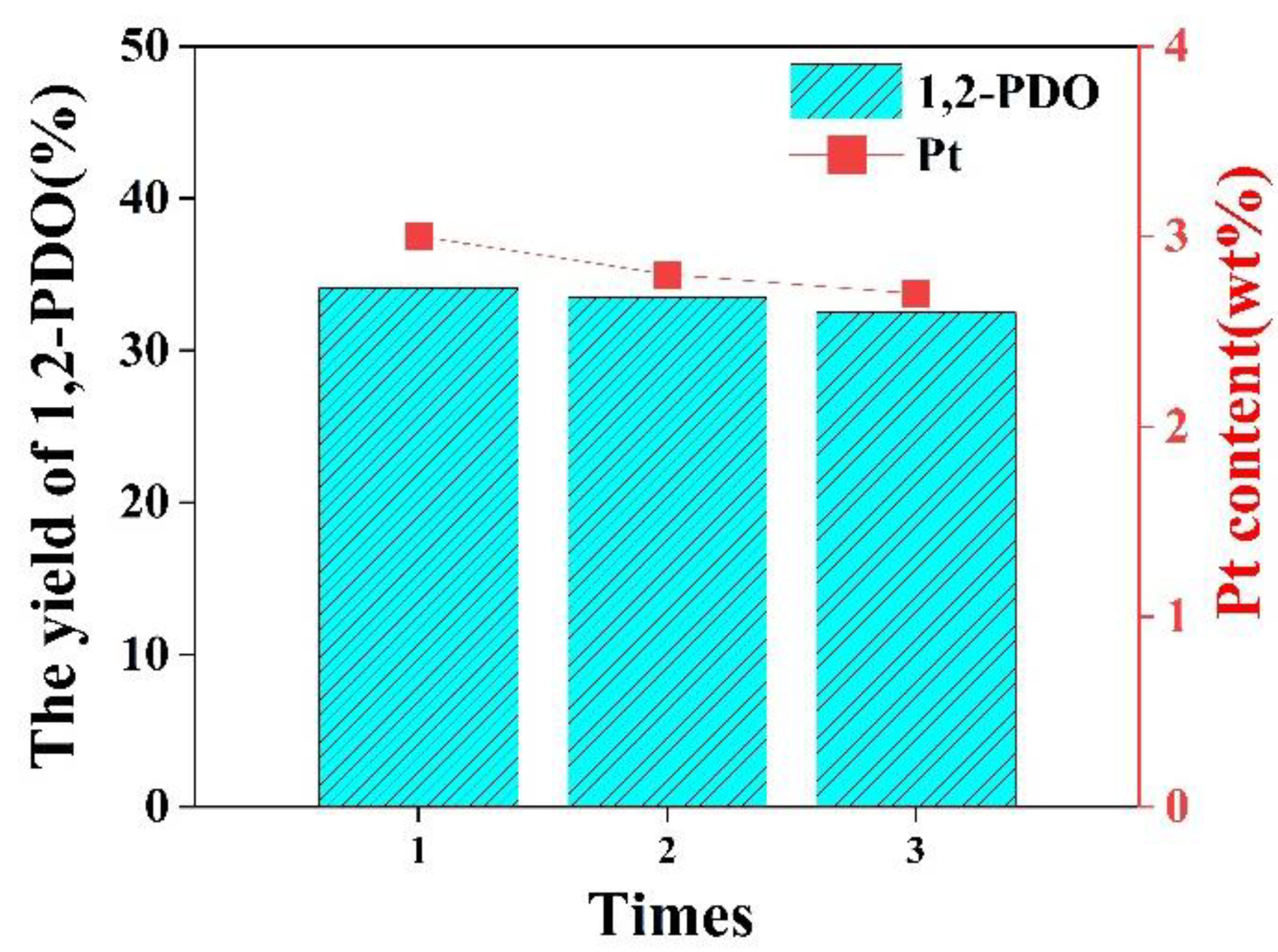
3.6. Catalyst Reusability
4. Discussion
4.1. Characterization of Catalysts
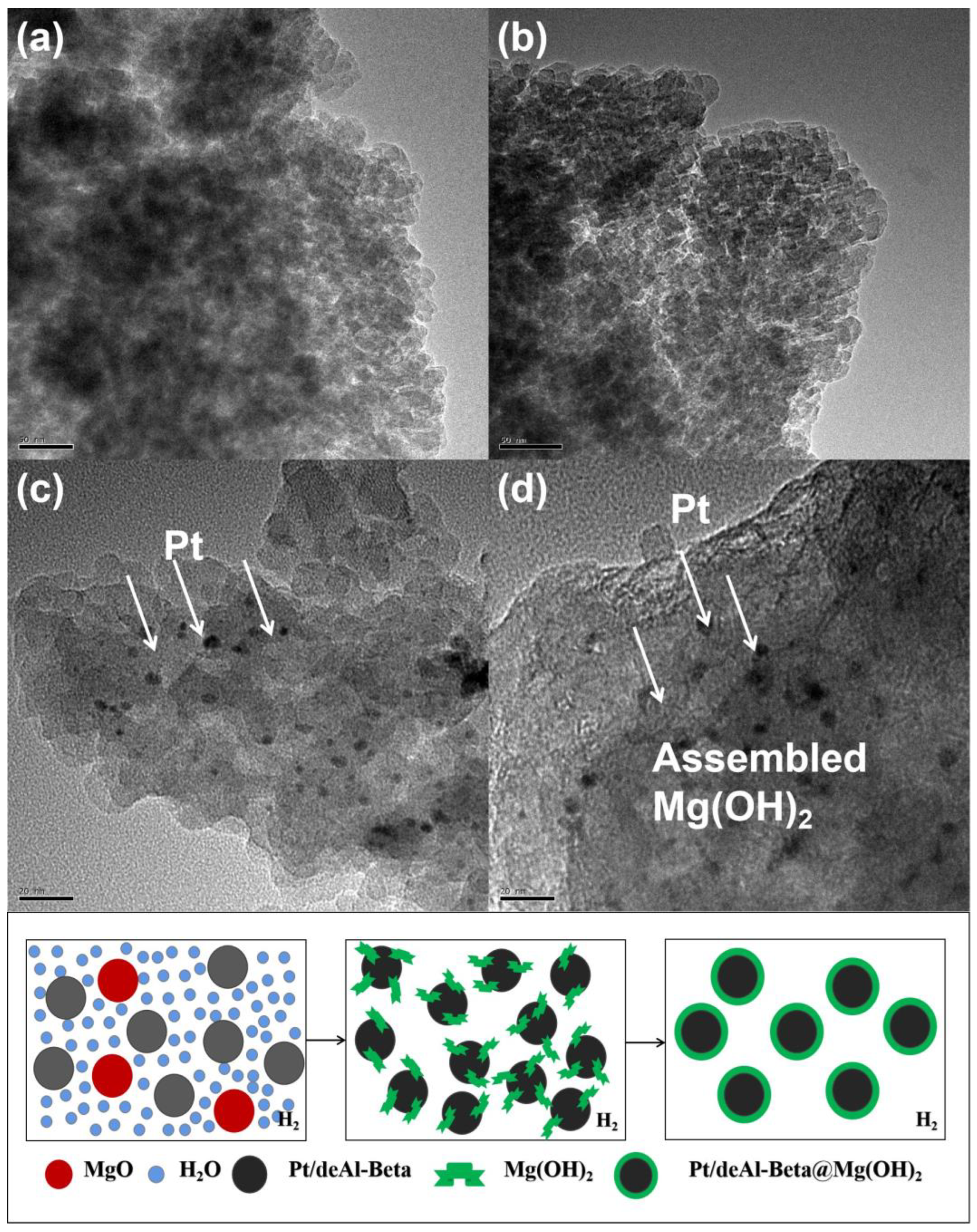
4.1.1. TEM Analysis
4.1.2. BET Analysis
4.1.3. XRD Analysis
4.1.4. Acid–Base Analysis
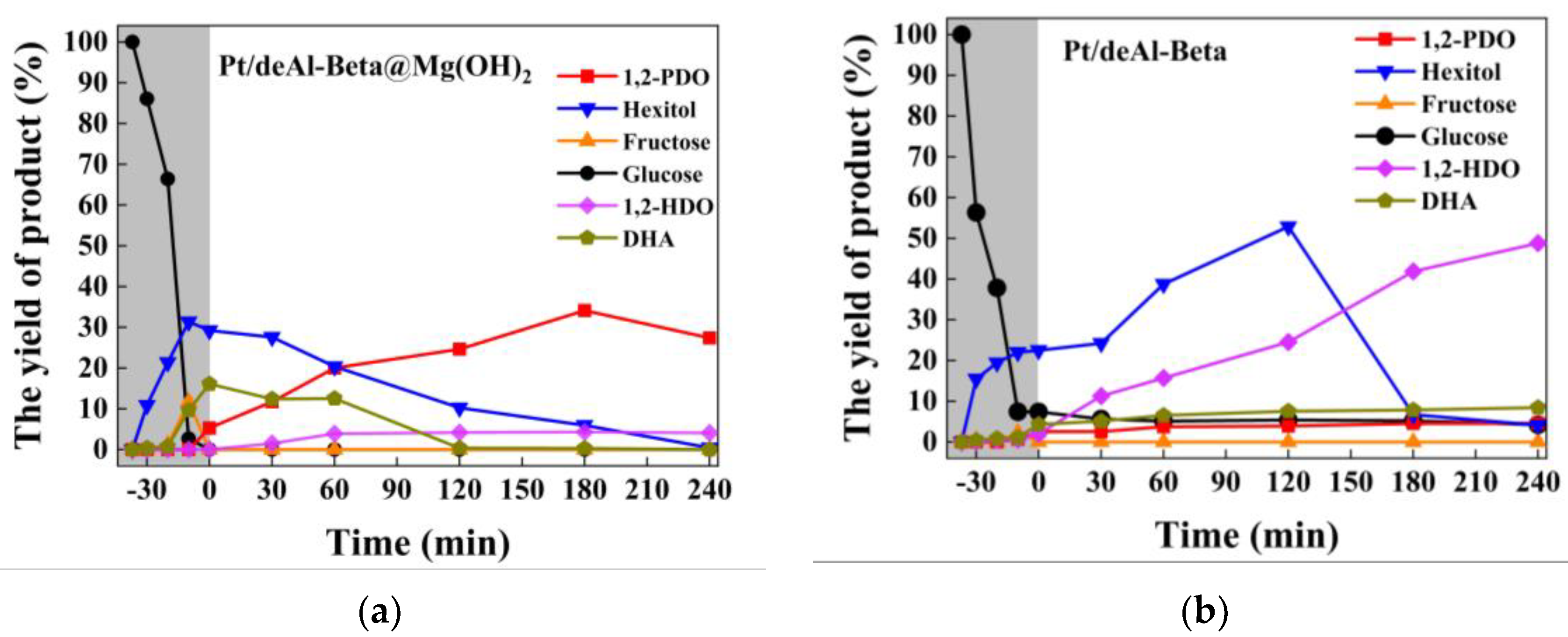
4.2. Analysis of Alkaline Catalytic Reaction Mechanism
4.3. Comparison of Two Catalytic Mechanisms
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saxena, R.; Anand, P.; Saran, S.; Isar, J.; Agarwal, L. Microbial production and applications of 1,2-propanediol. Indian J. Microbiol. 2010, 50, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Marinas, A.; Bruijnincx, P.; Ftouni, J.; Urbano, F.J.; Pinel, C. Sustainability metrics for a fossil-and renewable-based route for 1,2-propanediol production: A comparison. Catal. Today 2015, 239, 31–37. [Google Scholar] [CrossRef]
- Bower, S.; Wickramasinghe, R.; Nagle, N.J.; Schell, D.J. Modeling sucrose hydrolysis in dilute sulfuric acid solutions at pretreatment conditions for lignocellulosic biomass. Bioresour. Technol. 2008, 99, 7354–7362. [Google Scholar] [CrossRef]
- Ruppert, A.M.; Weinberg, K.; Palkovits, R. Hydrogenolysis goes bio: From carbohydrates and sugar alcohols to platform chemicals. Angew. Chem. Int. Ed. 2012, 51, 2564–2601. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Zheng, M.; Li, X.; Jiang, Y.; Zhao, Y.; Wang, A.; Wang, J.; Wang, X.; Zhang, T. Selective conversion of concentrated glucose to 1,2-propylene glycol and ethylene glycol by using RuSn/AC catalysts. Appl. Catal. B Environ. 2018, 239, 300–308. [Google Scholar] [CrossRef]
- Sun, R.; Wang, T.; Zheng, M.; Deng, W.; Pang, J.; Wang, A.; Wang, X.; Zhang, T. Versatile nickel–lanthanum (III) catalyst for direct conversion of cellulose to glycols. ACS Catal. 2015, 5, 874–883. [Google Scholar] [CrossRef]
- Treacy, M.; Newsam, J. Two new three-dimensional twelve-ring zeolite frameworks of which zeolite beta is a disordered intergrowth. Nature 1988, 332, 249–251. [Google Scholar] [CrossRef]
- Holm, M.S.; Saravanamurugan, S.; Taarning, E. Conversion of sugars to lactic acid derivatives using heterogeneous zeotype catalysts. Science 2010, 328, 602–605. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Dong, W.; Gu, M.; Chang, C.; Shen, Z.; Zhang, Y. Synergetic effects of bimetals in modified beta zeolite for lactic acid synthesis from biomass-derived carbohydrates. RSC Adv. 2018, 8, 8965–8975. [Google Scholar] [CrossRef]
- Dong, W.; Shen, Z.; Peng, B.; Gu, M.; Zhou, X.; Xiang, B.; Zhang, Y. Selective chemical conversion of sugars in aqueous solutions without alkali to lactic acid over a Zn-Sn-Beta Lewis acid-base catalyst. Sci. Rep. 2016, 6, 26713. [Google Scholar] [CrossRef]
- Guo, C.; Wang, Y.; Wang, F.; Wang, Y. Adsorption performance of amino functionalized magnetic molecular sieve adsorbent for effective removal of lead ion from aqueous solution. Nanomaterials 2021, 11, 2353. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Shen, Z.; Yang, L.; Peng, B.; Dong, W.; Zhang, W.; Zhang, Y. The effect of catalytic structure modification on hydrogenolysis of glycerol into 1, 3-propanediol over platinum nanoparticles and ordered mesoporous alumina assembled catalysts. Ind. Eng. Chem. Res. 2017, 56, 13572–13581. [Google Scholar] [CrossRef]
- Peng, B.-Y.; Su, Y.; Chen, Z.; Chen, J.; Zhou, X.; Benbow, M.E.; Criddle, C.S.; Wu, W.-M.; Zhang, Y. Biodegradation of polystyrene by dark (Tenebrio obscurus) and yellow (Tenebrio molitor) mealworms (Coleoptera: Tenebrionidae). Environ. Sci. Technol. 2019, 53, 5256–5265. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.-Y.; Chen, Z.; Chen, J.; Zhou, X.; Wu, W.-M.; Zhang, Y. Biodegradation of polylactic acid by yellow mealworms (larvae of Tenebrio molitor) via resource recovery: A sustainable approach for waste management. J. Hazard. Mater. 2021, 416, 125803. [Google Scholar] [CrossRef]
- Peng, B.-Y.; Chen, Z.; Chen, J.; Yu, H.; Zhou, X.; Criddle, C.S.; Wu, W.-M.; Zhang, Y. Biodegradation of polyvinyl chloride (PVC) in Tenebrio molitor (Coleoptera: Tenebrionidae) larvae. Environ. Int. 2020, 145, 106106. [Google Scholar] [CrossRef]
- Nemoto, K.; Hirano, Y.; Hirata, K.-i.; Takahashi, T.; Tsuneki, H.; Tominaga, K.-i.; Sato, K. Cooperative In–Sn catalyst system for efficient methyl lactate synthesis from biomass-derived sugars. Appl. Catal. B Environ. 2016, 183, 8–17. [Google Scholar] [CrossRef]
- Lee, E.H.; Park, R.S.; Kim, H.; Park, S.H.; Jung, S.C.; Jeon, J.K.; Kim, S.C.; Park, Y.W. Hydrodeoxygenation of guaiacol over Pt loaded zeolitic materials. J. Ind. Eng. Chem. 2016, 37, 18–21. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, Q.; Qin, Z.; Wu, Z.; Jiao, W.; Dong, M.; Fan, W.; Wang, J. Hierarchically structured Pt/K-Beta zeolites for the catalytic conversion of n -heptane to aromatics. Microporous Mesoporous Mater. 2021, 324, 111308. [Google Scholar] [CrossRef]
- Monteiro, C.A.A.; Costa, D.; Zotin, J.L.; Cardoso, D. Effect of metal–acid site balance on hydroconversion of decalin over Pt/Beta zeolite bifunctional catalysts. Fuel 2015, 160, 71–79. [Google Scholar] [CrossRef]
- Yao, Y.; Tian, Y.-J.; Wu, H.-H.; Lu, S.-X. Pt-promoted and Hβ zeolite-supported Ni2P catalysts for hydroisomerisation of n-heptane. Fuel Process. Technol. 2015, 133, 146–151. [Google Scholar] [CrossRef]
- Zou, S.; Zhang, M.; Mo, S.; Cheng, H.; Fu, M.; Chen, P.; Chen, L.; Shi, W.; Ye, D. Catalytic Performance of Toluene Combustion over Pt Nanoparticles Supported on Pore-Modified Macro-Meso-Microporous Zeolite Foam. Nanomaterials 2019, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Shen, Z.; Zhang, W.; Xia, M.; Jiang, J.; Dong, W.; Zhou, X.; Zhang, Y. Hydrogenolysis of Glucose into Propylene Glycol over Pt/SiO2@Mg(OH)2 Catalyst. ChemCatChem 2020, 12, 3447–3452. [Google Scholar] [CrossRef]
- Sun, J.; Liu, H. Selective hydrogenolysis of biomass-derived xylitol to ethylene glycol and propylene glycol on Ni/C and basic oxide-promoted Ni/C catalysts. Catal. Today 2014, 234, 75–82. [Google Scholar] [CrossRef]
- Li, C.; Zhao, J.; Zhang, Y. Study on Adsorption Behavior of Nickel Ions Using Silica-Based Sandwich Layered Zirconium-Titanium Phosphate Prepared by Layer-by-Layer Grafting Method. Nanomaterials 2021, 11, 2314. [Google Scholar] [CrossRef]
- Hou, X.; Qiu, Y.; Yuan, E.; Li, F.; Li, Z.; Ji, S.; Yang, Z.; Liu, G.; Zhang, X. Promotion on light olefins production through modulating the reaction pathways for n-pentane catalytic cracking over ZSM-5 based catalysts. Appl. Catal. A Gen. 2017, 543, 51–60. [Google Scholar] [CrossRef]
- Van Donk, S.; Broersma, A.; Gijzeman, O.; van Bokhoven, J.A.; Bitter, J.; De Jong, K. Combined diffusion, adsorption, and reaction studies of n-hexane hydroisomerization over Pt/H–mordenite in an oscillating microbalance. J. Catal. 2001, 204, 272–280. [Google Scholar] [CrossRef]
- Dung, N.T.; Trong, T.D.; Vu, N.T.; Binh, N.T.; Minh, T.T.L.; Luan, L.Q. Radiation Synthesis of Selenium Nanoparticles Capped with β-Glucan and Its Immunostimulant Activity in Cytoxan-Induced Immunosuppressed Mice. Nanomaterials 2021, 11, 2439. [Google Scholar] [CrossRef]
- Liu, C.; Carraher, J.M.; Swedberg, J.L.; Herndon, C.R.; Fleitman, C.N.; Tessonnier, J.-P. Selective base-catalyzed isomerization of glucose to fructose. Acs Catal. 2014, 4, 4295–4298. [Google Scholar] [CrossRef]
- Lee, H.W.; Jeon, J.-K.; Jeong, K.-E.; Kim, C.-U.; Jeong, S.-Y.; Han, J.; Park, Y.-K. Hydroisomerization of n-dodecane over Pt/Y zeolites with different acid characteristics. Chem. Eng. J. 2013, 232, 111–117. [Google Scholar] [CrossRef]
- Dong, W.; Peng, B.; Wang, K.; Miao, J.; Zhang, W.; Zhang, Y.; Shen, Z. An effective method and pathways of acrylonitrile degradation to acrylic acid through an alkaline hydrothermal system. Environ. Technol. 2017, 38, 1702–1707. [Google Scholar] [CrossRef]
- Assary, R.S.; Curtiss, L.A. Theoretical study of 1, 2-hydride shift associated with the isomerization of glyceraldehyde to dihydroxy acetone by Lewis acid active site models. J. Phys. Chem. A 2011, 115, 8754–8760. [Google Scholar] [CrossRef] [PubMed]
| Catalyst | Temperature °C | B Acid (m mol/g) | L Acid (m mol/g) | Total Acid (m mol/g) | L Acid Rate L/Total % |
|---|---|---|---|---|---|
| Pt/deAl-Beta | 150 | 0.02 | 0.43 | 0.45 | 95.56 |
| 250 | 0.01 | 0.28 | 0.28 | 100.00 | |
| 350 | 0.00 | 0.04 | 0.04 | 100.00 | |
| 450 | 0.00 | 0.02 | 0.02 | 100.00 | |
| Pt/deAl-Beta@Mg(OH)2 * | 150 | 0.02 | 0.38 | 0.38 | 95.00 |
| 250 | 0.01 | 0.21 | 0.21 | 95.45 | |
| 350 | 0.00 | 0.08 | 0.08 | 100.00 | |
| 450 | 0.00 | 0.02 | 0.02 | 100.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Jiang, J.; Gu, M.; Song, Y.; Zhao, J.; Shen, Z.; Zhou, X.; Zhang, Y. Glucose Hydrogenolysis into 1,2-Propanediol Using a Pt/deAl@Mg(OH)2 Catalyst: Expanding the Application of a Core–Shell Structured Catalyst. Nanomaterials 2022, 12, 3771. https://doi.org/10.3390/nano12213771
Wang S, Jiang J, Gu M, Song Y, Zhao J, Shen Z, Zhou X, Zhang Y. Glucose Hydrogenolysis into 1,2-Propanediol Using a Pt/deAl@Mg(OH)2 Catalyst: Expanding the Application of a Core–Shell Structured Catalyst. Nanomaterials. 2022; 12(21):3771. https://doi.org/10.3390/nano12213771
Chicago/Turabian StyleWang, Shizhuo, Jikang Jiang, Minyan Gu, Yuanbo Song, Jiang Zhao, Zheng Shen, Xuefei Zhou, and Yalei Zhang. 2022. "Glucose Hydrogenolysis into 1,2-Propanediol Using a Pt/deAl@Mg(OH)2 Catalyst: Expanding the Application of a Core–Shell Structured Catalyst" Nanomaterials 12, no. 21: 3771. https://doi.org/10.3390/nano12213771
APA StyleWang, S., Jiang, J., Gu, M., Song, Y., Zhao, J., Shen, Z., Zhou, X., & Zhang, Y. (2022). Glucose Hydrogenolysis into 1,2-Propanediol Using a Pt/deAl@Mg(OH)2 Catalyst: Expanding the Application of a Core–Shell Structured Catalyst. Nanomaterials, 12(21), 3771. https://doi.org/10.3390/nano12213771







