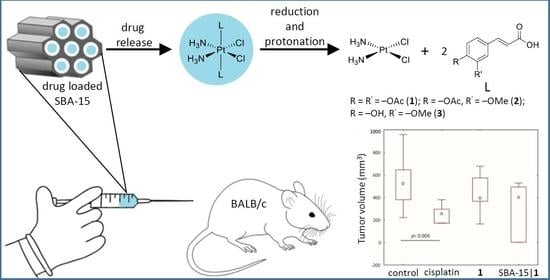
Mesoporous Silica Nanoparticles Enhance the Anticancer Efficacy of Platinum(IV)-Phenolate Conjugates in Breast Cancer Cell Lines
Abstract
1. Introduction
2. Materials and Methods
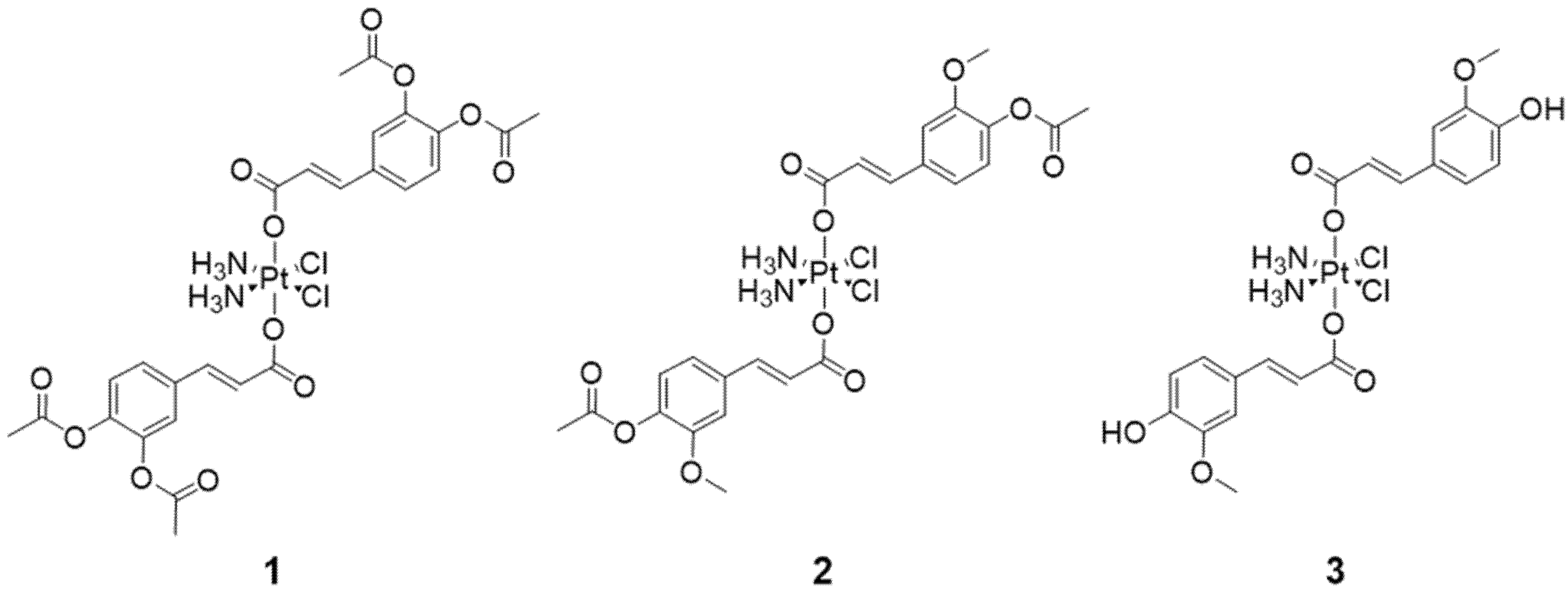
2.1. Synthesis of Platinum(IV) Conjugates 1 and 2
2.2. Deprotection of 2 to Obtain Platinum(IV) Conjugate 3
2.3. Solubility and Stability of Conjugates 1–3
2.4. Preparation of SBA-15
2.5. Preparation of SBA-15 Material Loaded with Platinum(IV) Conjugates, SBA-15|1–SBA-15|3
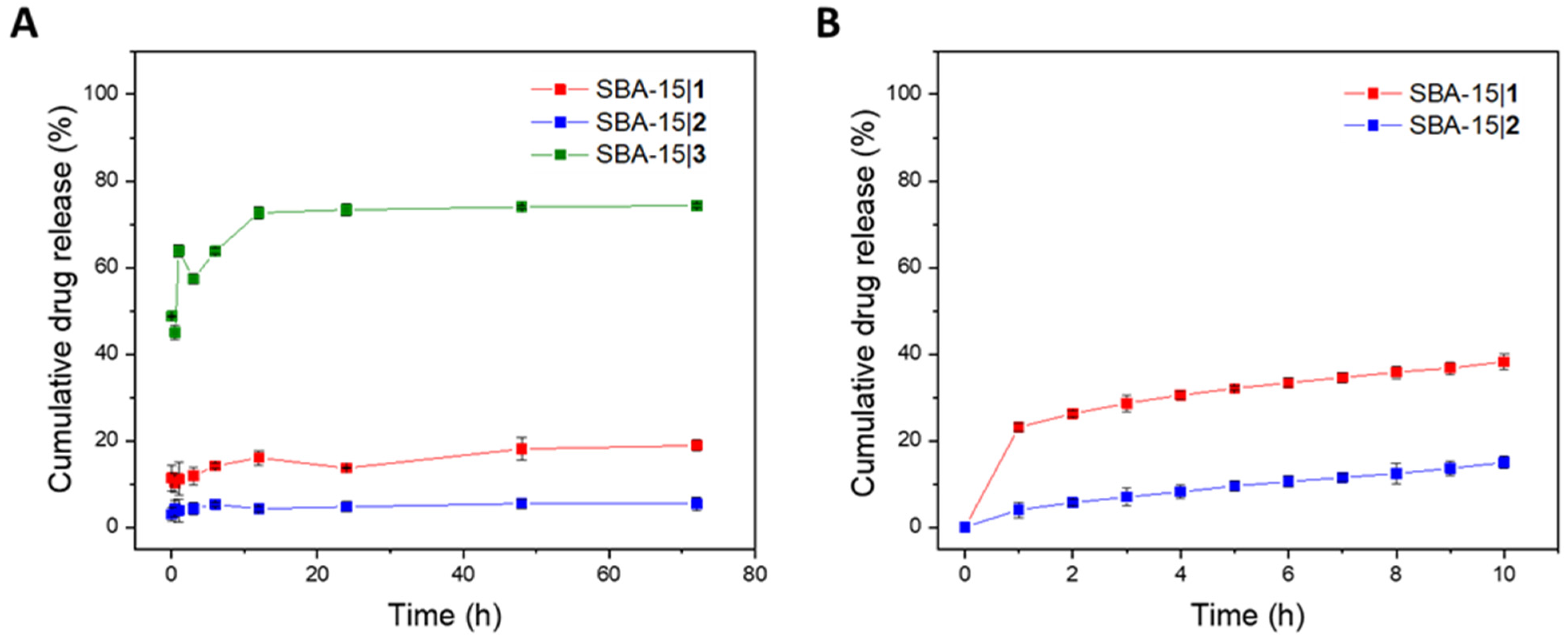
2.6. Drug Release Studies
2.7. In Vitro Studies
2.8. Cell Culture
2.9. Cell Viability Studies
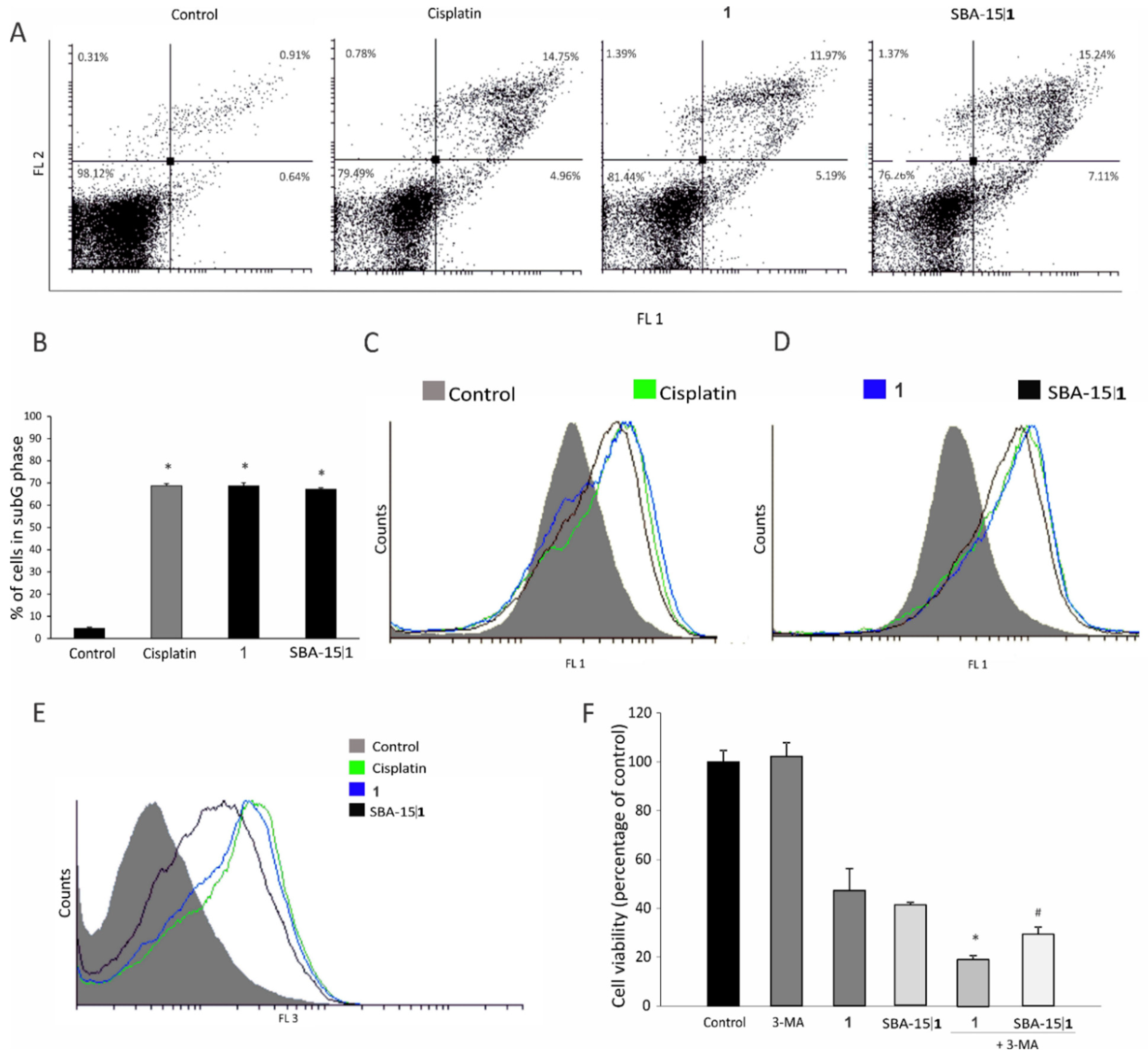
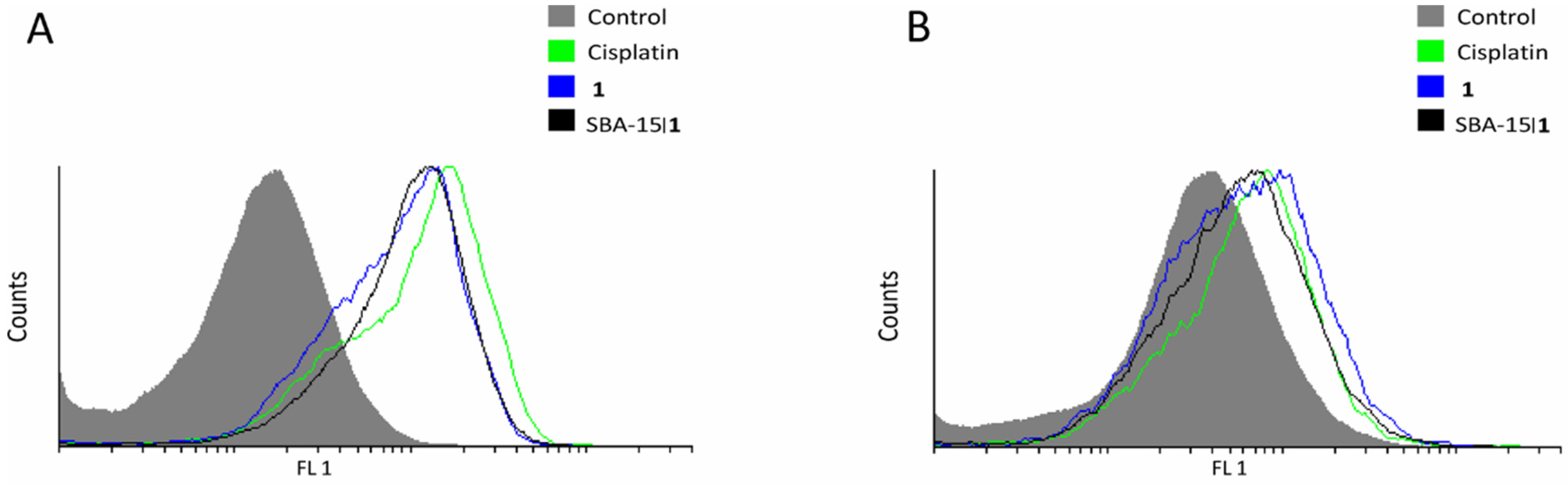
2.10. Flow Cytometry
2.11. In Vivo Studies
2.12. Induction of Tumors and In Vivo Treatment
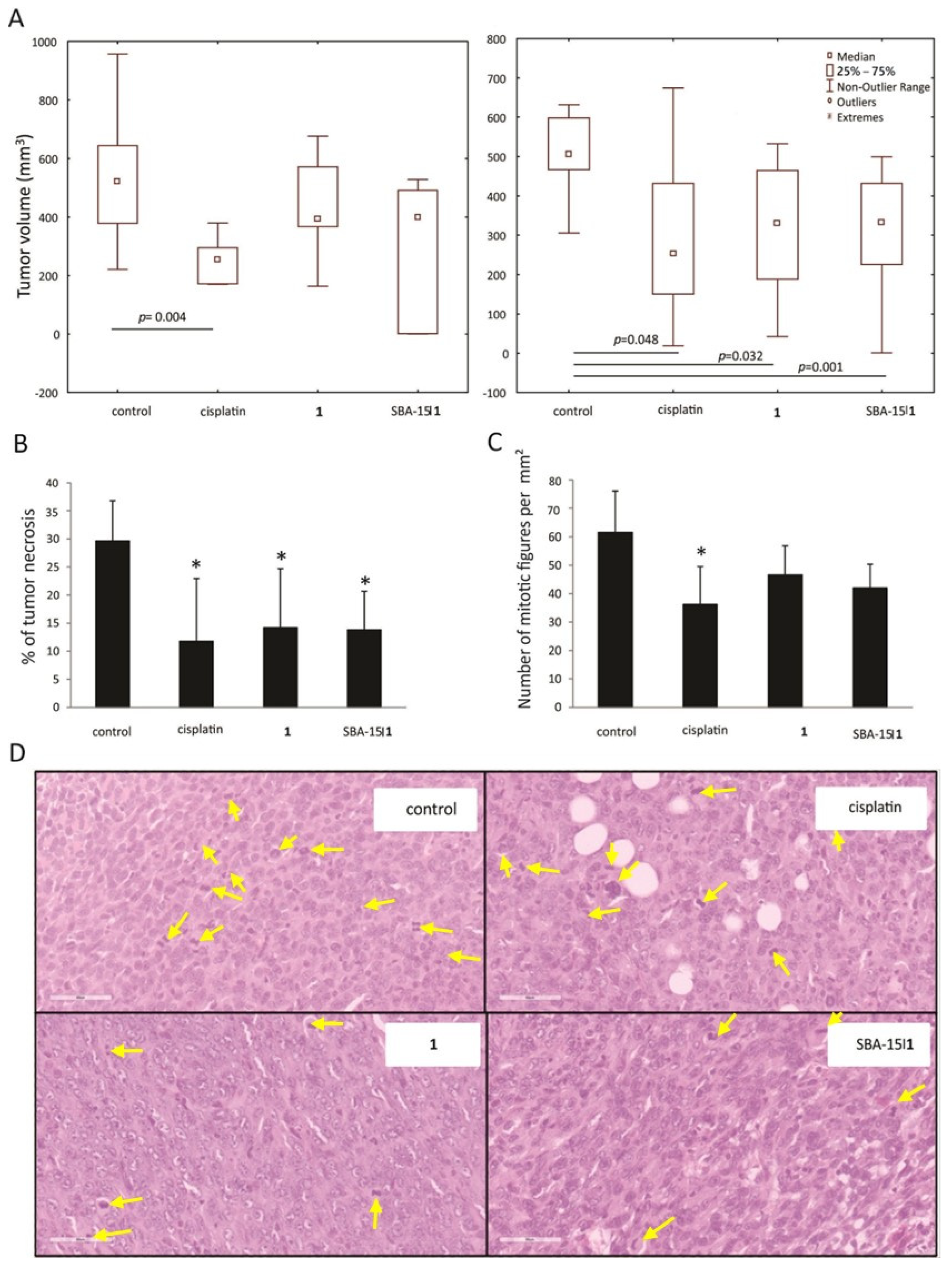
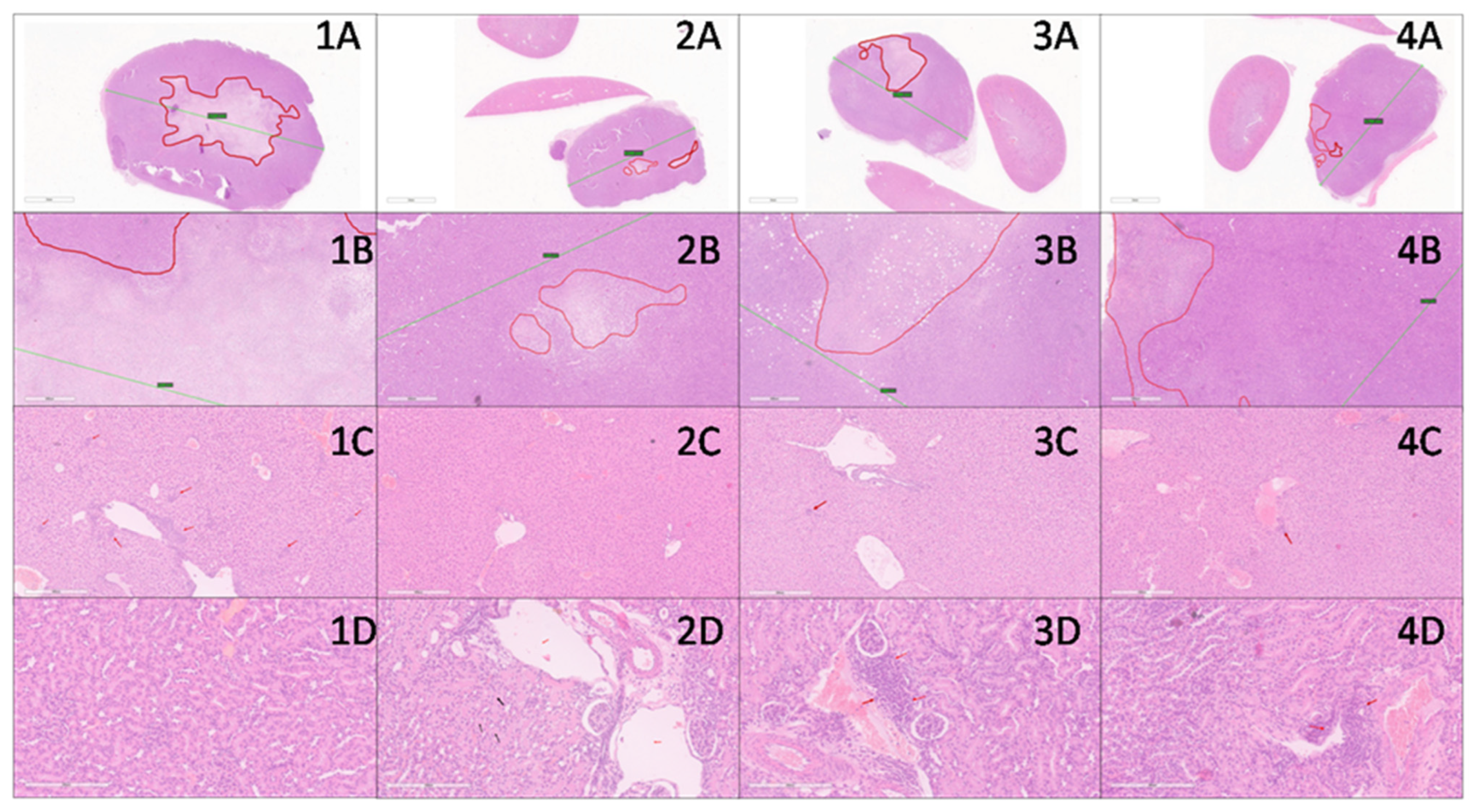
2.13. Histopathological Examination
2.14. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of Platinum(IV) Conjugates 1–3
3.2. Synthesis and Characterization of the Mesoporous Silica Materials
3.3. Release of Platinum(IV) Conjugates from MSNs
3.4. Antiproliferative Activity
3.5. Molecular Mechanism of Action
3.6. In Vivo Decrease of Breast Cancer Growth
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahidi, F.; Naczk, M. Food Phenolics: Sources, Chemistry, Effects, Applications; Technomic Pub. Co.: Lancaster, PA, USA, 1995; ISBN 1-56676-279-0. [Google Scholar]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Gupta, S.; Chand Yadav, T.; Pruthi, V.; Kumar Varadwaj, P.; Goel, N. Extrapolation of Phenolic Compounds as Multi-Target Agents against Cancer and Inflammation. J. Biomol. Struct. Dyn. 2019, 37, 2355–2369. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Kawabata, K.; Yoshimi, N.; Tanaka, T.; Murakami, T.; Okada, T.; Murai, H. Chemopreventive Effects of Ferulic Acid on Oral and Rice Germ on Large Bowel Carcinogenesis. Anticancer Res. 1999, 19, 3775–3778. [Google Scholar] [PubMed]
- Middleton, E.J.; Kandaswami, C.; Theoharides, T.C. The Effects of Plant Flavonoids on Mammalian Cells: Implications for Inflammation, Heart Disease, and Cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Rocha, L.D.; Monteiro, M.; Teodoro, A.J. Anticancer Properties of Hydroxycinnamic Acids -A Review. Cancer Clin. Oncol. 2012, 1, 109. [Google Scholar] [CrossRef]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The Potential of Plant Phenolics in Prevention and Therapy of Skin Disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef]
- Sytar, O.; Hemmerich, I.; Zivcak, M.; Rauh, C.; Brestic, M. Comparative Analysis of Bioactive Phenolic Compounds Composition from 26 Medicinal Plants. Saudi J. Biol. Sci. 2018, 25, 631–641. [Google Scholar] [CrossRef]
- Son, S.; Lewis, B.A. Free Radical Scavenging and Antioxidative Activity of Caffeic Acid Amide and Ester Analogues: Structure−Activity Relationship. J. Agric. Food Chem. 2002, 50, 468–472. [Google Scholar] [CrossRef]
- Kumar, N.; Pruthi, V. Structural Elucidation and Molecular Docking of Ferulic Acid from Parthenium Hysterophorus Possessing COX-2 Inhibition Activity. 3 Biotech 2015, 5, 541–551. [Google Scholar] [CrossRef]
- Jayaprakasam, B.; Vanisree, M.; Zhang, Y.; Dewitt, D.L.; Nair, M.G. Impact of Alkyl Esters of Caffeic and Ferulic Acids on Tumor Cell Proliferation, Cyclooxygenase Enzyme, and Lipid Peroxidation. J. Agric. Food Chem. 2006, 54, 5375–5381. [Google Scholar] [CrossRef]
- Michaluart, P.; Masferrer, J.L.; Carothers, A.M.; Subbaramaiah, K.; Zweifel, B.S.; Koboldt, C.; Mestre, J.R.; Grunberger, D.; Sacks, P.G.; Tanabe, T.; et al. Inhibitory Effects of Caffeic Acid Phenethyl Ester on the Activity and Expression of Cyclooxygenase-2 in Human Oral Epithelial Cells and in a Rat Model of Inflammation. Cancer Res. 1999, 59, 2347–2352. [Google Scholar] [PubMed]
- Kang, N.J.; Lee, K.W.; Shin, B.J.; Jung, S.K.; Hwang, M.K.; Bode, A.M.; Heo, Y.-S.; Lee, H.J.; Dong, Z. Caffeic Acid, a Phenolic Phytochemical in Coffee, Directly Inhibits Fyn Kinase Activity and UVB-Induced COX-2 Expression. Carcinogenesis 2009, 30, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Müller-Decker, K.; Fürstenberger, G. The Cyclooxygenase-2-Mediated Prostaglandin Signaling Is Causally Related to Epithelial Carcinogenesis. Mol. Carcinog. 2007, 46, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Chiang, E.-P.I.; Tsai, S.-Y.; Kuo, Y.-H.; Pai, M.-H.; Chiu, H.-L.; Rodriguez, R.L.; Tang, F.-Y. Caffeic Acid Derivatives Inhibit the Growth of Colon Cancer: Involvement of the PI3-K/Akt and AMPK Signaling Pathways. PLoS ONE 2014, 9, e99631. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, S.K. Growth Inhibition by Caffeic Acid, One of the Phenolic Constituents of Honey, in HCT 15 Colon Cancer Cells. Sci. World J. 2012, 2012, 372345. [Google Scholar] [CrossRef]
- Murad, L.D.; Soares ND, C.P.; Brand, C.; Monteiro, M.C.; Teodoro, A.J. Effects of Caffeic and 5-Caffeoylquinic Acids on Cell Viability and Cellular Uptake in Human Colon Adenocarcinoma Cells. Nutr. Cancer 2015, 67, 532–542. [Google Scholar] [CrossRef]
- Tang, H.; Yao, X.; Yao, C.; Zhao, X.; Zuo, H.; Li, Z. Anti-Colon Cancer Effect of Caffeic Acid p-Nitro-Phenethyl Ester in Vitro and in Vivo and Detection of Its Metabolites. Sci. Rep. 2017, 7, 7599. [Google Scholar] [CrossRef]
- Espíndola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Silva Rosario, A.C.R.; da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef]
- Rajendra Prasad, N.; Karthikeyan, A.; Karthikeyan, S.; Reddy, B.V. Inhibitory Effect of Caffeic Acid on Cancer Cell Proliferation by Oxidative Mechanism in Human HT-1080 Fibrosarcoma Cell Line. Mol. Cell. Biochem. 2011, 349, 11–19. [Google Scholar] [CrossRef]
- Kanimozhi, G.; Prasad, N.R. Chapter 73-Anticancer Effect of Caffeic Acid on Human Cervical Cancer Cells. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 655–661. ISBN 978-0-12-409517-5. [Google Scholar]
- Kabała-Dzik, A.; Rzepecka-Stojko, A.; Kubina, R.; Wojtyczka, R.D.; Buszman, E.; Stojko, J. Caffeic Acid Versus Caffeic Acid Phenethyl Ester in the Treatment of Breast Cancer MCF-7 Cells: Migration Rate Inhibition. Integr. Cancer Ther. 2018, 17, 1247–1259. [Google Scholar] [CrossRef]
- Rosendahl, A.H.; Perks, C.M.; Zeng, L.; Markkula, A.; Simonsson, M.; Rose, C.; Ingvar, C.; Holly, J.M.P.; Jernström, H. Caffeine and Caffeic Acid Inhibit Growth and Modify Estrogen Receptor and Insulin-like Growth Factor I Receptor Levels in Human Breast Cancer. Clin. Cancer Res. 2015, 21, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Hudson, E.A.; Dinh, P.A.; Kokubun, T.; Simmonds, M.S.; Gescher, A. Characterization of Potentially Chemopreventive Phenols in Extracts of Brown Rice That Inhibit the Growth of Human Breast and Colon Cancer Cells. Cancer Epidemiol. Biomarkers Prev. 2000, 9, 1163–1170. [Google Scholar] [PubMed]
- Min, J.; Shen, H.; Xi, W.; Wang, Q.; Yin, L.; Zhang, Y.; Yu, Y.; Yang, Q.; Wang, Z.-N. Synergistic Anticancer Activity of Combined Use of Caffeic Acid with Paclitaxel Enhances Apoptosis of Non-Small-Cell Lung Cancer H1299 Cells in Vivo and in Vitro. Cell. Physiol. Biochem. 2018, 48, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Tyszka-Czochara, M.; Konieczny, P.; Majka, M. Caffeic Acid Expands Anti-Tumor Effect of Metformin in Human Metastatic Cervical Carcinoma HTB-34 Cells: Implications of AMPK Activation and Impairment of Fatty Acids De Novo Biosynthesis. Int. J. Mol. Sci. 2017, 18, 462. [Google Scholar] [CrossRef]
- Matsunaga, T.; Tsuchimura, S.; Azuma, N.; Endo, S.; Ichihara, K.; Ikari, A. Caffeic Acid Phenethyl Ester Potentiates Gastric Cancer Cell Sensitivity to Doxorubicin and Cisplatin by Decreasing Proteasome Function. Anticancer Drugs 2019, 30, 251–259. [Google Scholar] [CrossRef]
- Koraneekit, A.; Limpaiboon, T.; Sangka, A.; Boonsiri, P.; Daduang, S.; Daduang, J. Synergistic Effects of Cisplatin-Caffeic Acid Induces Apoptosis in Human Cervical Cancer Cells via the Mitochondrial Pathways. Oncol. Lett. 2018, 15, 7397–7402. [Google Scholar] [CrossRef]
- Sirota, R.; Gibson, D.; Kohen, R. The Timing of Caffeic Acid Treatment with Cisplatin Determines Sensitization or Resistance of Ovarian Carcinoma Cell Lines. Redox Biol. 2017, 11, 170–175. [Google Scholar] [CrossRef]
- Kelland, L. The Resurgence of Platinum-Based Cancer Chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef]
- Neumann, W.; Crews, B.C.; Marnett, L.J.; Hey-Hawkins, E. Conjugates of Cisplatin and Cyclooxygenase Inhibitors as Potent Antitumor Agents Overcoming Cisplatin Resistance. ChemMedChem 2014, 9, 1150–1153. [Google Scholar] [CrossRef]
- Neumann, W.; Crews, B.C.; Sárosi, M.B.; Daniel, C.M.; Ghebreselasie, K.; Scholz, M.S.; Marnett, L.J.; Hey-Hawkins, E. Conjugation of Cisplatin Analogues and Cyclooxygenase Inhibitors to Overcome Cisplatin Resistance. ChemMedChem 2015, 10, 183–192. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Hagman, E.; Elimam, A.; Kupferschmidt, N.; Ekbom, K.; Rössner, S.; Iqbal, M.N.; Johnston, E.; Lindgren, M.; Bengtsson, T.; Danielsson, P. Oral Intake of Mesoporous Silica Is Safe and Well Tolerated in Male Humans. PLoS ONE 2020, 15, e0240030. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, S.K.; Luo, Y.; O’Donnell, M.A.; Assouline, J. Nanotechnology and Cancer: Improving Real-Time Monitoring and Staging of Bladder Cancer with Multimodal Mesoporous Silica Nanoparticles. Cancer Nanotechnol. 2016, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Li, L.; Chen, D. Mesoporous Silica Nanoparticles: Synthesis, Biocompatibility and Drug Delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef] [PubMed]
- Bharti, C.; Nagaich, U.; Pal, A.K.; Gulati, N. Mesoporous Silica Nanoparticles in Target Drug Delivery System: A Review. Int. J. Pharm. Investig. 2015, 5, 124. [Google Scholar] [CrossRef] [PubMed]
- Manzano, M.; Vallet-Regí, M. Mesoporous Silica Nanoparticles for Drug Delivery. Adv. Funct. Mater. 2020, 30, 1902634. [Google Scholar] [CrossRef]
- Sábio, R.M.; Meneguin, A.B.; Ribeiro, T.C.; Silva, R.R.; Chorilli, M. New Insights towards Mesoporous Silica Nanoparticles as a Technological Platform for Chemotherapeutic Drugs Delivery. Int. J. Pharm. 2019, 564, 379–409. [Google Scholar] [CrossRef]
- Castillo, R.R.; Lozano, D.; Vallet-Regí, M. Mesoporous Silica Nanoparticles as Carriers for Therapeutic Biomolecules. Pharmaceutics 2020, 12, 432. [Google Scholar] [CrossRef]
- Taleghani, A.S.; Nakhjiri, A.T.; Khakzad, M.J.; Rezayat, S.M.; Ebrahimnejad, P.; Heydarinasab, A.; Akbarzadeh, A.; Marjani, A. Mesoporous Silica Nanoparticles as a Versatile Nanocarrier for Cancer Treatment: A Review. J. Mol. Liq. 2021, 328, 115417. [Google Scholar] [CrossRef]
- Yu, A.; Dai, X.; Wang, Z.; Chen, H.; Guo, B.; Huang, L. Recent Advances of Mesoporous Silica as a Platform for Cancer Immunotherapy. Biosensors 2022, 12, 109. [Google Scholar] [CrossRef]
- Yuan, Z.; Pan, Y.; Cheng, R.; Sheng, L.; Wu, W.; Pan, G.; Feng, Q.; Cui, W. Doxorubicin-Loaded Mesoporous Silica Nanoparticle Composite Nanofibers for Long-Term Adjustments of Tumor Apoptosis. Nanotechnology 2016, 27, 245101. [Google Scholar] [CrossRef] [PubMed]
- Edeler, D.; Arlt, S.; Petković, V.; Ludwig, G.; Drača, D.; Maksimović-Ivanić, D.; Mijatović, S.; Kaluđerović, G.N. Delivery of [Ru(η6-p-Cymene)Cl2{Ph2P(CH2)3SPh-κP}] Using Unfunctionalized and Mercapto Functionalized SBA-15 Mesoporous Silica: Preparation, Characterization and in Vitro Study. J. Inorg. Biochem. 2018, 180, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Toms, B.; Goodisman, J.; Asefa, T. Mesoporous Silica Microparticles Enhance the Cytotoxicity of Anticancer Platinum Drugs. ACS Nano 2010, 4, 789–794. [Google Scholar] [CrossRef]
- Lin, C.H.; Cheng, S.H.; Liao, W.N.; Wei, P.R.; Sung, P.J.; Weng, C.F.; Lee, C.H. Mesoporous Silica Nanoparticles for the Improved Anticancer Efficacy of Cis-Platin. Int. J. Pharm. 2012, 429, 138–147. [Google Scholar] [CrossRef]
- Edeler, D.; Kaluđerović, M.R.; Dojčinović, B.; Schmidt, H.; Kaluđerović, G.N. SBA-15 Mesoporous Silica Particles Loaded with Cisplatin Induce Senescence in B16F10 Cells. RSC Adv. 2016, 6, 111031–111040. [Google Scholar] [CrossRef]
- Predarska, I.; Saoud, M.; Morgan, I.; Eichhorn, T.; Kaluđerović, G.N.; Hey-Hawkins, E. Cisplatin−cyclooxygenase Inhibitor Conjugates, Free and Immobilised in Mesoporous Silica SBA-15, Prove Highly Potent against Triple-Negative MDA-MB-468 Breast Cancer Cell Line. Dalton Trans. 2022, 51, 857–869. [Google Scholar] [CrossRef]
- Tan, M.-X.; Wang, Z.-F.; Qin, Q.-P.; Zou, B.-Q.; Liang, H. Complexes of Oxoplatin with Rhein and Ferulic Acid Ligands as Platinum(IV) Prodrugs with High Anti-Tumor Activity. Dalton Trans. 2020, 49, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.D.; Dillon, C.T.; Zhang, M.; Beale, P.; Cai, Z.; Lai, B.; Stampfl, A.P.; Hambley, T.W. The Cellular Distribution and Oxidation State of Platinum(II) and Platinum(IV) Antitumour Complexes in Cancer Cells. J. Biol. Inorg. Chem. 2003, 8, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Touaibia, M.; Guay, M. Natural Product Total Synthesis in the Organic Laboratory: Total Synthesis of Caffeic Acid Phenethyl Ester (CAPE), A Potent 5-Lipoxygenase Inhibitor from Honeybee Hives. J. Chem. Educ. 2011, 88, 473–475. [Google Scholar] [CrossRef]
- García-Peñas, A.; Gómez-Ruiz, S.; Pérez-Quintanilla, D.; Paschke, R.; Sierra, I.; Prashar, S.; del Hierro, I.; Kaluđerović, G.N. Study of the Cytotoxicity and Particle Action in Human Cancer Cells of Titanocene-Functionalized Materials with Potential Application against Tumors. J. Inorg. Biochem. 2012, 106, 100–110. [Google Scholar] [CrossRef]
- Kaluđerović, G.N.; Pérez-Quintanilla, D.; Sierra, I.; Prashar, S.; del Hierro, I.; Žižak, Ž.; Juranić, Z.D.; Fajardo, M.; Gómez-Ruiz, S. Study of the Influence of the Metal Complex on the Cytotoxic Activity of Titanocene-Functionalized Mesoporous Materials. J. Mater. Chem. 2010, 20, 806–814. [Google Scholar] [CrossRef]
- Smolko, L.; Smolková, R.; Samoľová, E.; Morgan, I.; Saoud, M.; Kaluđerović, G.N. Two Isostructural Co(II) Flufenamato and Niflumato Complexes with Bathocuproine: Analogues with a Different Cytotoxic Activity. J. Inorg. Biochem. 2020, 210, 111160. [Google Scholar] [CrossRef]
- Subik, K.; Lee, J.-F.; Baxter, L.; Strzepek, T.; Costello, D.; Crowley, P.; Xing, L.; Hung, M.-C.; Bonfiglio, T.; Hicks, D.G.; et al. The Expression Patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67 and AR by Immunohistochemical Analysis in Breast Cancer Cell Lines. Breast Cancer 2010, 4, 35–41. [Google Scholar] [CrossRef]
- Khan, M.F.; Nasr, F.A.; Noman, O.M.; Alyhya, N.A.; Ali, I.; Saoud, M.; Rennert, R.; Dube, M.; Hussain, W.; Green, I.R.; et al. Cichorins D–F: Three New Compounds from Cichorium Intybus and Their Biological Effects. Molecules 2020, 25, 4160. [Google Scholar] [CrossRef]
- Drača, D.; Edeler, D.; Saoud, M.; Dojčinović, B.; Dunđerović, D.; Đmura, G.; Maksimović-Ivanić, D.; Mijatović, S.; Kaluđerović, G.N. Antitumor Potential of Cisplatin Loaded into SBA-15 Mesoporous Silica Nanoparticles against B16F1 Melanoma Cells: In Vitro and in Vivo Studies. J. Inorg. Biochem. 2021, 217, 111383. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-Antioxidant Activity Relationships of Flavonoids and Phenolic Acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Spiegel, M.; Kapusta, K.; Kołodziejczyk, W.; Saloni, J.; Żbikowska, B.; Hill, G.A.; Sroka, Z. Antioxidant Activity of Selected Phenolic Acids–Ferric Reducing Antioxidant Power Assay and QSAR Analysis of the Structural Features. Molecules 2020, 25, 3088. [Google Scholar] [CrossRef]
- Scampicchio, M.; Wang, J.; Blasco, A.J.; Sanchez Arribas, A.; Mannino, S.; Escarpa, A. Nanoparticle-Based Assays of Antioxidant Activity. Anal. Chem. 2006, 78, 2060–2063. [Google Scholar] [CrossRef]
- Amini, S.M.; Akbari, A. Metal Nanoparticles Synthesis through Natural Phenolic Acids. IET Nanobiotechnol. 2019, 13, 771–777. [Google Scholar] [CrossRef]
- LeBlanc, L.M.; Paré, A.F.; Jean-François, J.; Hébert, M.J.G.; Surette, M.E.; Touaibia, M. Synthesis and Antiradical/Antioxidant Activities of Caffeic Acid Phenethyl Ester and Its Related Propionic, Acetic, and Benzoic Acid Analoguesc. Molecules 2012, 17, 14637–14650. [Google Scholar] [CrossRef]
- Wang, S.-M.; Zhang, Y.-B.; Liu, H.-M.; Yu, G.-B.; Wang, K.-R. Mild and Selective Deprotection Method of Acetylated Steroids and Diterpenes by Dibutyltin Oxide. Steroids 2007, 72, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, C.; Mahender, G.; Ravindranath, N.; Das, B. A Mild, Highly Selective and Remarkably Easy Procedure for Deprotection of Aromatic Acetates Using Ammonium Acetate as a Neutral Catalyst in Aqueous Medium. Tetrahedron 2003, 59, 1049–1054. [Google Scholar] [CrossRef]
- Dunne, A.; Palomo, J.M. Efficient and Green Approach for the Complete Deprotection of O-Acetylated Biomolecules. RSC Adv. 2016, 6, 88974–88978. [Google Scholar] [CrossRef]
- Sing, K.S. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Kankala, R.K.; Han, Y.-H.; Na, J.; Lee, C.-H.; Sun, Z.; Wang, S.-B.; Kimura, T.; Ok, Y.S.; Yamauchi, Y.; Chen, A.-Z.; et al. Nanoarchitectured Structure and Surface Biofunctionality of Mesoporous Silica Nanoparticles. Adv. Mat. 2020, 32, 1907035. [Google Scholar] [CrossRef]
- Dolete, G.; Purcăreanu, B.; Mihaiescu, D.E.; Ficai, D.; Oprea, O.-C.; Bîrcă, A.C.; Chircov, C.; Vasile, B.Ș.; Vasilievici, G.; Ficai, A.; et al. A Comparative Loading and Release Study of Vancomycin from a Green Mesoporous Silica. Molecules 2022, 27, 5589. [Google Scholar] [CrossRef]
- Costa, P.; Sousa Lobo, J.M. Modeling and Comparison of Dissolution Profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of Solute Release from Porous Hydrophilic Polymers. Inter. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Langer, R.S.; Peppas, N.A. Present and Future Applications of Biomaterials in Controlled Drug Delivery Systems. Biomaterials 1981, 2, 201–214. [Google Scholar] [CrossRef]
- Vistica, D.T.; Skehan, P.; Scudiero, D.; Monks, A.; Pittman, A.; Boyd, M.R. Tetrazolium-Based Assays for Cellular Viability: A Critical Examination of Selected Parameters Affecting Formazan Production. Cancer Res. 1991, 51, 2515–2520. [Google Scholar]
- Shoemaker, M.; Cohen, I.; Campbell, M. Reduction of MTT by Aqueous Herbal Extracts in the Absence of Cells. J. Ethnopharmacol. 2004, 93, 381–384. [Google Scholar] [CrossRef]
- Wang, P.; Henning, S.M.; Heber, D. Limitations of MTT and MTS-Based Assays for Measurement of Antiproliferative Activity of Green Tea Polyphenols. PLoS ONE 2010, 5, e10202. [Google Scholar] [CrossRef] [PubMed]
- Bruggisser, R.; von Daeniken, K.; Jundt, G.; Schaffner, W.; Tullberg-Reinert, H. Interference of Plant Extracts, Phytoestrogens and Antioxidants with the MTT Tetrazolium Assay. Planta Med. 2002, 68, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Chang, M. Tamoxifen Resistance in Breast Cancer. Biomol. Ther. 2012, 20, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Rasool, M.; Chaoudhry, H.; N Pushparaj, P.; Jha, P.; Hafiz, A.; Mahfooz, M.; Abdus Sami, G.; Azhar Kamal, M.; Bashir, S.; et al. Molecular Mechanisms and Mode of Tamoxifen Resistance in Breast Cancer. Bioinformation 2016, 12, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Dominguez, R.; Perez-Medina, M.; Lopez-Gonzalez, J.S.; Galicia-Velasco, M.; Aguilar-Cazares, D. The Double-Edge Sword of Autophagy in Cancer: From Tumor Suppression to Pro-Tumor Activity. Front. Oncol. 2020, 10, 578418. [Google Scholar] [CrossRef]
- Jung, S.; Jeong, H.; Yu, S.-W. Autophagy as a Decisive Process for Cell Death. Exp. Mol. Med. 2020, 52, 921–930. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in Cancer Therapy: The Bright Side of the Moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Mijatović, S.; Savić-Radojević, A.; Plješa-Ercegovac, M.; Simić, T.; Nicoletti, F.; Maksimović-Ivanić, D. The Double-Faced Role of Nitric Oxide and Reactive Oxygen Species in Solid Tumors. Antioxidants 2020, 9, 374. [Google Scholar] [CrossRef]
- Maksimović-Ivanić, D.; Mijatović, S.; Mirkov, I.; Stošić-Grujičić, S.; Miljković, D.; Sabo, T.J.; Trajković, V.; Kaluđerović, G.N. Melanoma Tumor Inhibition by Tetrachlorido(O,O′-Dibutyl-Ethylenediamine-N,N′-Di-3-Propionate)Platinum(IV) Complex: In Vitro and in Vivo Investigations. Metallomics 2012, 4, 1155–1159. [Google Scholar] [CrossRef]
- Bredholt, G.; Mannelqvist, M.; Stefansson, I.M.; Birkeland, E.; Hellem Bø, T.; Øyan, A.M.; Trovik, J.; Kalland, K.-H.; Jonassen, I.; Salvesen, H.B.; et al. Tumor Necrosis Is an Important Hallmark of Aggressive Endometrial Cancer and Associates with Hypoxia, Angiogenesis and Inflammation Responses. Oncotarget 2015, 6, 39676. [Google Scholar] [CrossRef] [PubMed]
- duPre’, S.A.; Hunter, K.W. Murine Mammary Carcinoma 4T1 Induces a Leukemoid Reaction with Splenomegaly: Association with Tumor-Derived Growth Factors. Exp. Mol. Pathol. 2007, 82, 12–24. [Google Scholar] [CrossRef] [PubMed]
- DuPre´, S.A.; Redelman, D.; Hunter Jr, K.W. The Mouse Mammary Carcinoma 4T1: Characterization of the Cellular Landscape of Primary Tumours and Metastatic Tumour Foci. Int. J. Exp.Pathol. 2007, 88, 351–360. [Google Scholar] [CrossRef] [PubMed]
- McSweeney, K.R.; Gadanec, L.K.; Qaradakhi, T.; Ali, B.A.; Zulli, A.; Apostolopoulos, V. Mechanisms of Cisplatin-Induced Acute Kidney Injury: Pathological Mechanisms, Pharmacological Interventions, and Genetic Mitigations. Cancers 2021, 13, 1572. [Google Scholar] [CrossRef] [PubMed]
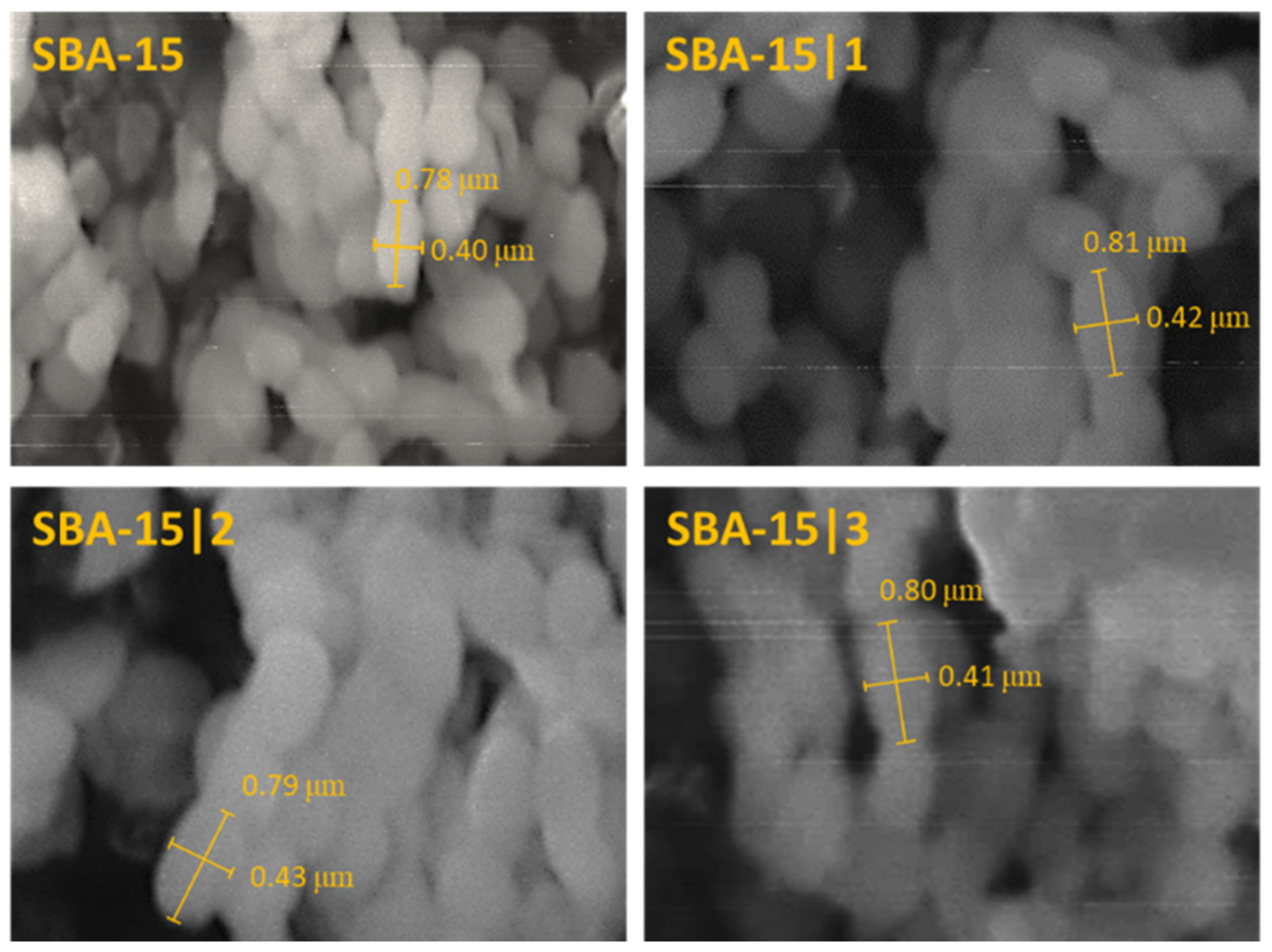
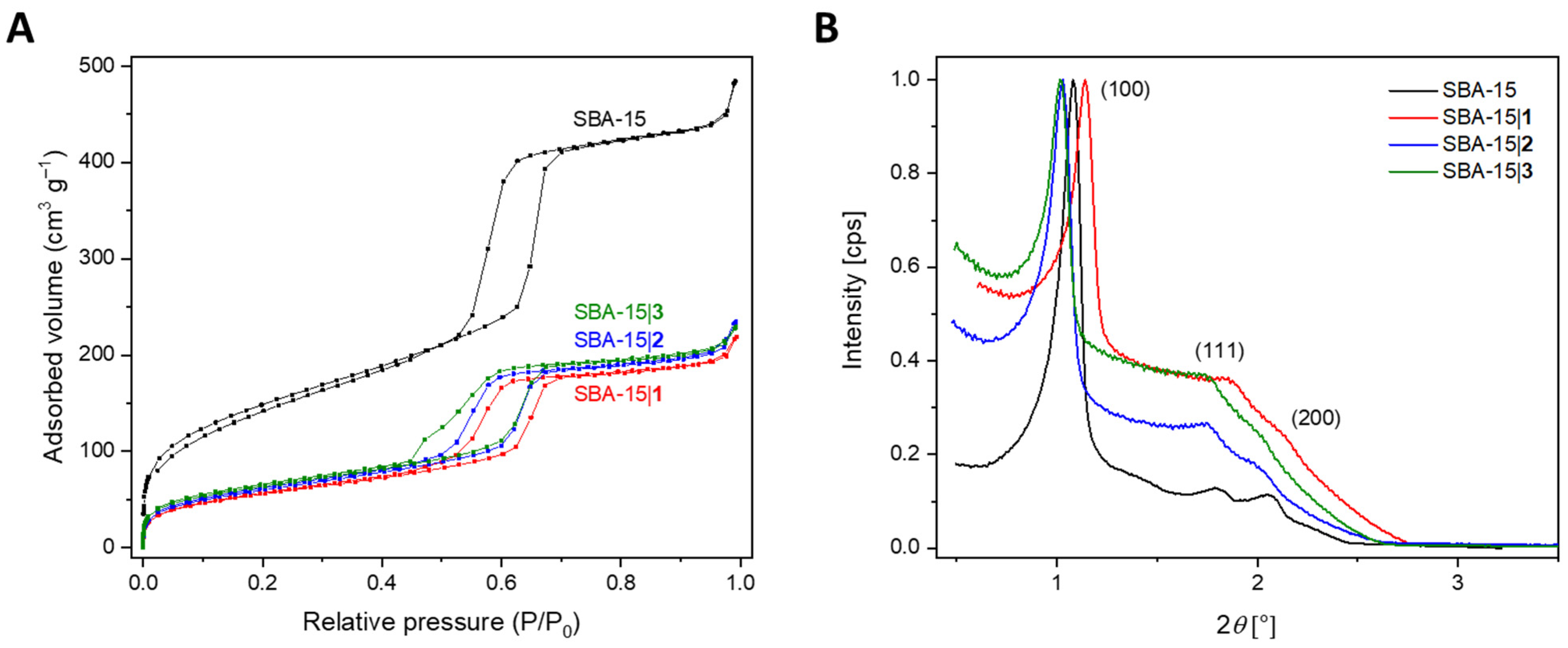
| MSNs | SBET | Wall Thickness | Pore Diameter | Pore Volume | Lattice Parameter | XRD | |
|---|---|---|---|---|---|---|---|
| [m2 g−1] | [nm] | [nm] | [cm3 g−1] | a [nm] | 2θ [°] | hkl | |
| SBA-15 [48] | 517 | 3.66 | 4.74 | 0.72 | 8.4 | 1.0802 | 100 |
| 1.8032 | 111 | ||||||
| 2.0645 | 200 | ||||||
| SBA-15|1 | 245 | 3.34 | 4.75 | 0.34 | 8.1 | 1.1404 | 100 |
| 1.8543 | 111 | ||||||
| 2.1378 | 200 | ||||||
| SBA-15|2 | 260 | 4.26 | 4.46 | 0.37 | 8.7 | 1.0311 | 100 |
| 1.7450 | 111 | ||||||
| 2.0075 | 200 | ||||||
| SBA-15|3 | 292 | 5.03 | 3.68 | 0.37 | 8.7 | 1.0149 | 100 |
| 1.7603 | 111 | ||||||
| 2.0228 | 200 | ||||||
| Compound/Material | CV | MTT | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MDA-MB-468 | HCC1937 | MCF-7 | BT-474 | 4T1 | MDA-MB-468 | HCC1937 | MCF-7 | BT-474 | 4T1 | |
| IC50 [µM] | IC50 [µM] | |||||||||
| Acetyl-protected caffeic acid | >100 | >100 | ||||||||
| Acetyl-protected ferulic acid | >100 | >100 | ||||||||
| Ferulic acid | >100 | >100 | ||||||||
| Cisplatin | 3.3 ± 0.2 | 7.6 ± 0.9 | 33.6 ± 4.8 | 54.9 ± 6.0 | 3.2 ± 0.3 | 0.6 ± 0.1 | 4.3 ± 0.7 | 32.0 ± 4.3 | 70.3 ± 8.5 | 1.3 ± 0.1 |
| Cisplatin-acetyl protected caffeate conjugate (1) | 2.6 ± 0.7 | 8.9 ± 0.3 | 16.0 ± 2.3 | 5.4 ± 0.3 | 4.4 ± 0.7 | 2.7 ± 0.8 | 9.9 ± 0.5 | > 50 | 4.5 ± 0.4 | 3.3 ± 0.4 |
| Cisplatin-acetyl protected ferulate conjugate (2) | 3.5 ± 0.5 | 10.4 ± 1.1 | 8.4 ± 2.2 | 5.9 ± 0.4 | 3.8 ± 0.8 | 5.5 ± 4.3 | 27.6 ± 2.6 | 9.7 ± 1.2 | ||
| Cisplatin-ferulate conjugate (3) | 7.1 ± 0.3 | 11.1 ± 0.8 | 19.9 ± 1.3 | >50 | 8.3 ± 0.7 | 15.1 ± 2.1 | >50 | >50 | ||
| SBA-15|1 (a) | 0.3 ± 0.03 | 1.7 ± 0.1 | 1.6 ± 0.1 | 0.0001 ± 0.00001 | 1.0 ± 0.2 | 0.1 ± 0.001 | 0.3 ± 0.02 | 0.3 ± 0.1 | 0.1 ± 0.001 | 0.8 ± 0.04 |
| SBA-15|2 (a) | 0.1 ± 0.01 | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.0017 ± 0.0001 | 0.02 ± 0.002 | 0.2 ± 0.02 | / | 0.1 ± 0.02 | ||
| SBA-15|3 (a) | 1.6 ± 0.1 | 2.6 ± 0.5 | 4.4 ± 0.5 | 0.7 ± 0.1 | 0.4 ± 0.01 | 1.0 ± 0.2 | 3.7 ± 0.2 | 1.3 ± 0.2 | ||
| MC50 [µg mL−1] | MC50 [µg mL−1] | |||||||||
| SBA-15 | >100 | >100 | ||||||||
| SBA-15|1 | 3.0 ± 0.5 | 19.9 ± 1.0 | 18.5 ± 1.0 | 0.0017 ± 0.0002 | 11.9 ± 1.9 | 0.6 ± 0.02 | 3.3 ± 0.3 | 7.3 ± 0.8 | 0.7 ± 0.02 | 9.7 ± 0.5 |
| SBA-15|2 | 3.6 ± 0.4 | 32.9 ± 2.1 | 35.0 ± 3.0 | 0.6 ± 0.006 | 0.6 ± 0.1 | 6.5 ± 0.7 | >50 | 4.3 ± 0.7 | ||
| SBA-15|3 | 3.9 ± 0.2 | 6.4 ± 1.2 | 10.7 ± 1.3 | 1.7 ± 0.3 | 1.1 ± 0.03 | 2.3 ± 0.4 | 5.0 ± 0.5 | 3.3 ± 0.6 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Predarska, I.; Saoud, M.; Drača, D.; Morgan, I.; Komazec, T.; Eichhorn, T.; Mihajlović, E.; Dunđerović, D.; Mijatović, S.; Maksimović-Ivanić, D.; et al. Mesoporous Silica Nanoparticles Enhance the Anticancer Efficacy of Platinum(IV)-Phenolate Conjugates in Breast Cancer Cell Lines. Nanomaterials 2022, 12, 3767. https://doi.org/10.3390/nano12213767
Predarska I, Saoud M, Drača D, Morgan I, Komazec T, Eichhorn T, Mihajlović E, Dunđerović D, Mijatović S, Maksimović-Ivanić D, et al. Mesoporous Silica Nanoparticles Enhance the Anticancer Efficacy of Platinum(IV)-Phenolate Conjugates in Breast Cancer Cell Lines. Nanomaterials. 2022; 12(21):3767. https://doi.org/10.3390/nano12213767
Chicago/Turabian StylePredarska, Ivana, Mohamad Saoud, Dijana Drača, Ibrahim Morgan, Teodora Komazec, Thomas Eichhorn, Ekatarina Mihajlović, Duško Dunđerović, Sanja Mijatović, Danijela Maksimović-Ivanić, and et al. 2022. "Mesoporous Silica Nanoparticles Enhance the Anticancer Efficacy of Platinum(IV)-Phenolate Conjugates in Breast Cancer Cell Lines" Nanomaterials 12, no. 21: 3767. https://doi.org/10.3390/nano12213767
APA StylePredarska, I., Saoud, M., Drača, D., Morgan, I., Komazec, T., Eichhorn, T., Mihajlović, E., Dunđerović, D., Mijatović, S., Maksimović-Ivanić, D., Hey-Hawkins, E., & Kaluđerović, G. N. (2022). Mesoporous Silica Nanoparticles Enhance the Anticancer Efficacy of Platinum(IV)-Phenolate Conjugates in Breast Cancer Cell Lines. Nanomaterials, 12(21), 3767. https://doi.org/10.3390/nano12213767