TiO2 Gas Sensors Combining Experimental and DFT Calculations: A Review
Abstract
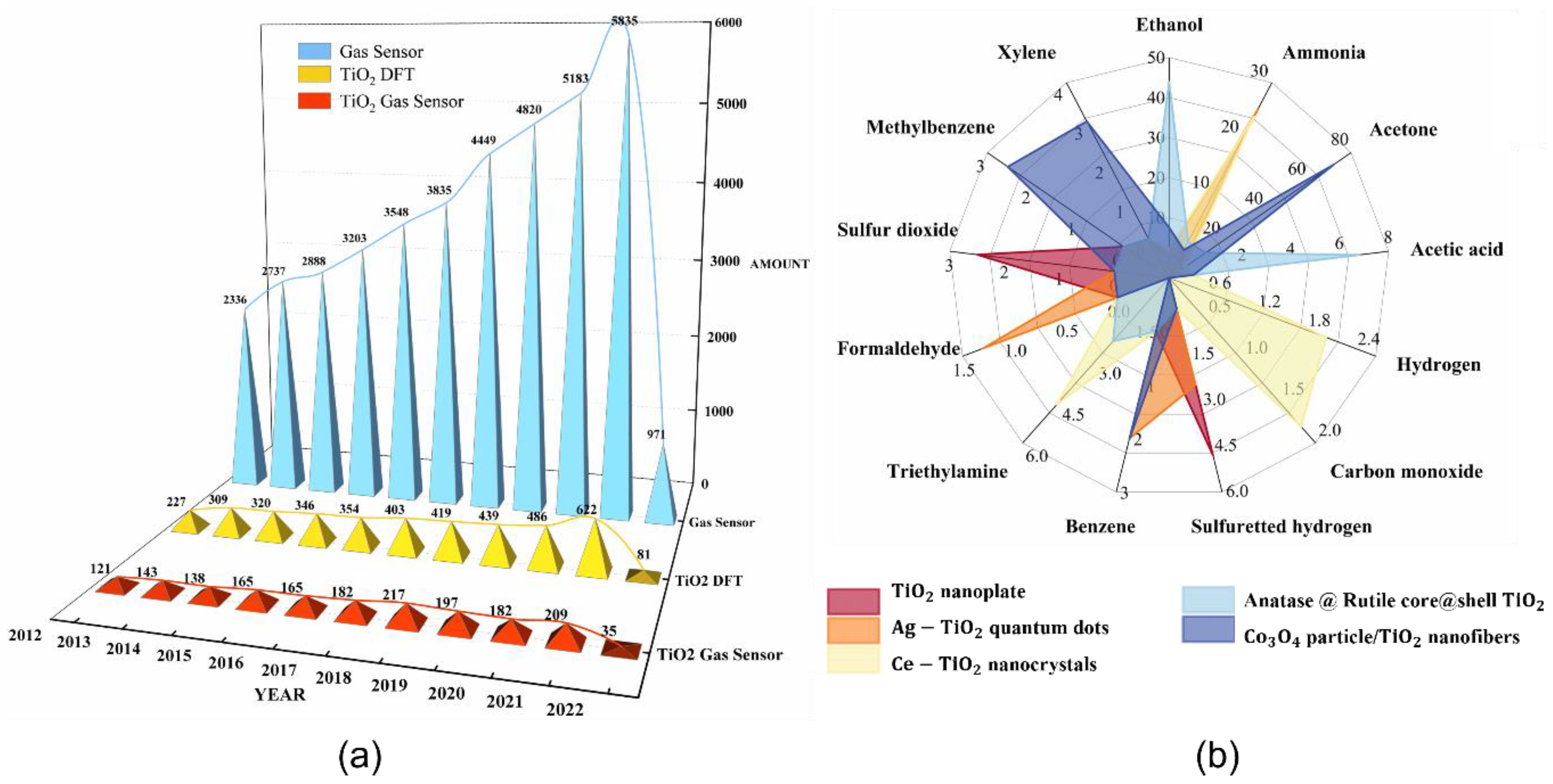
1. Introduction
2. TiO2 Nanostructure-Based Gas Sensors
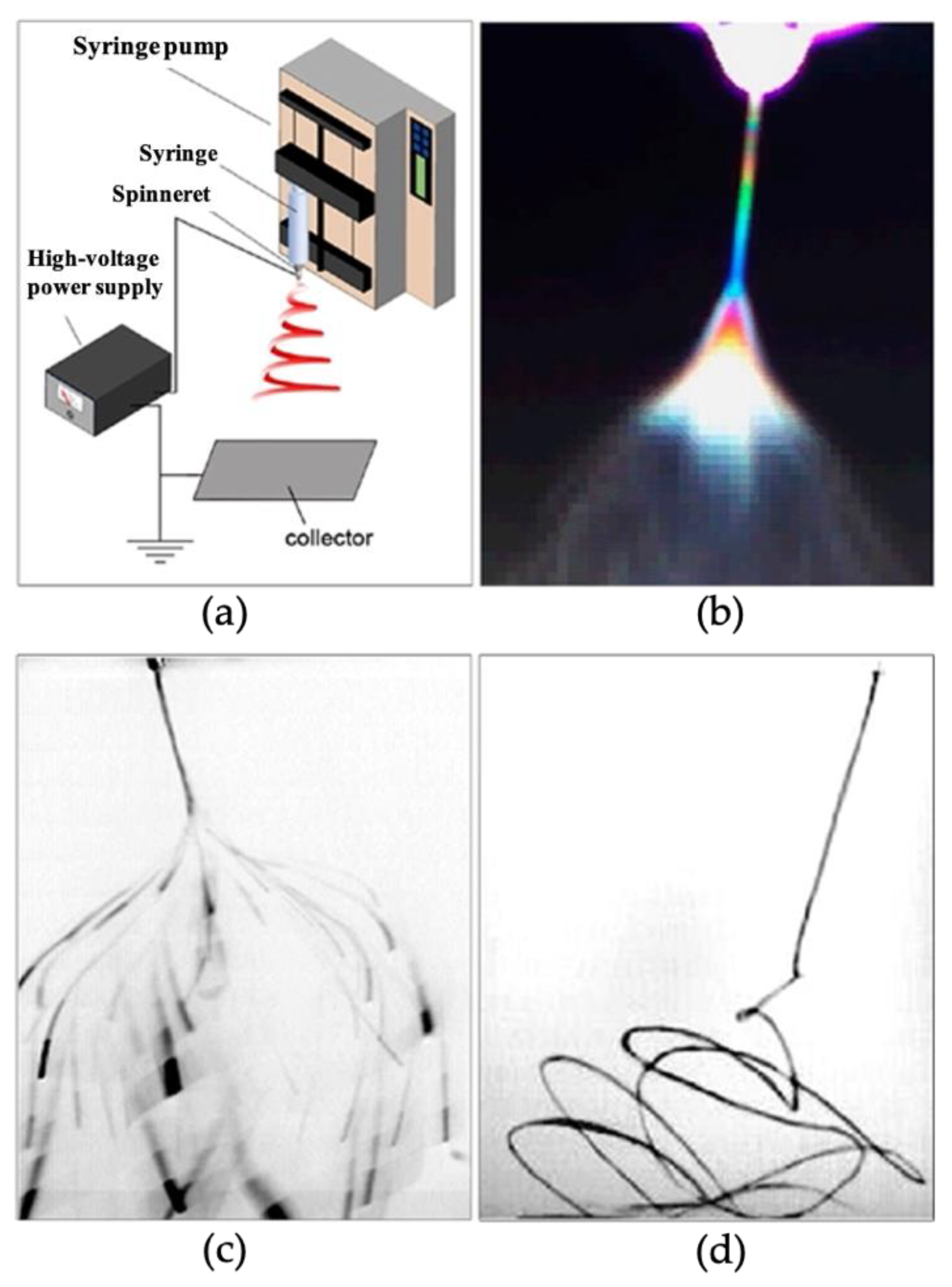
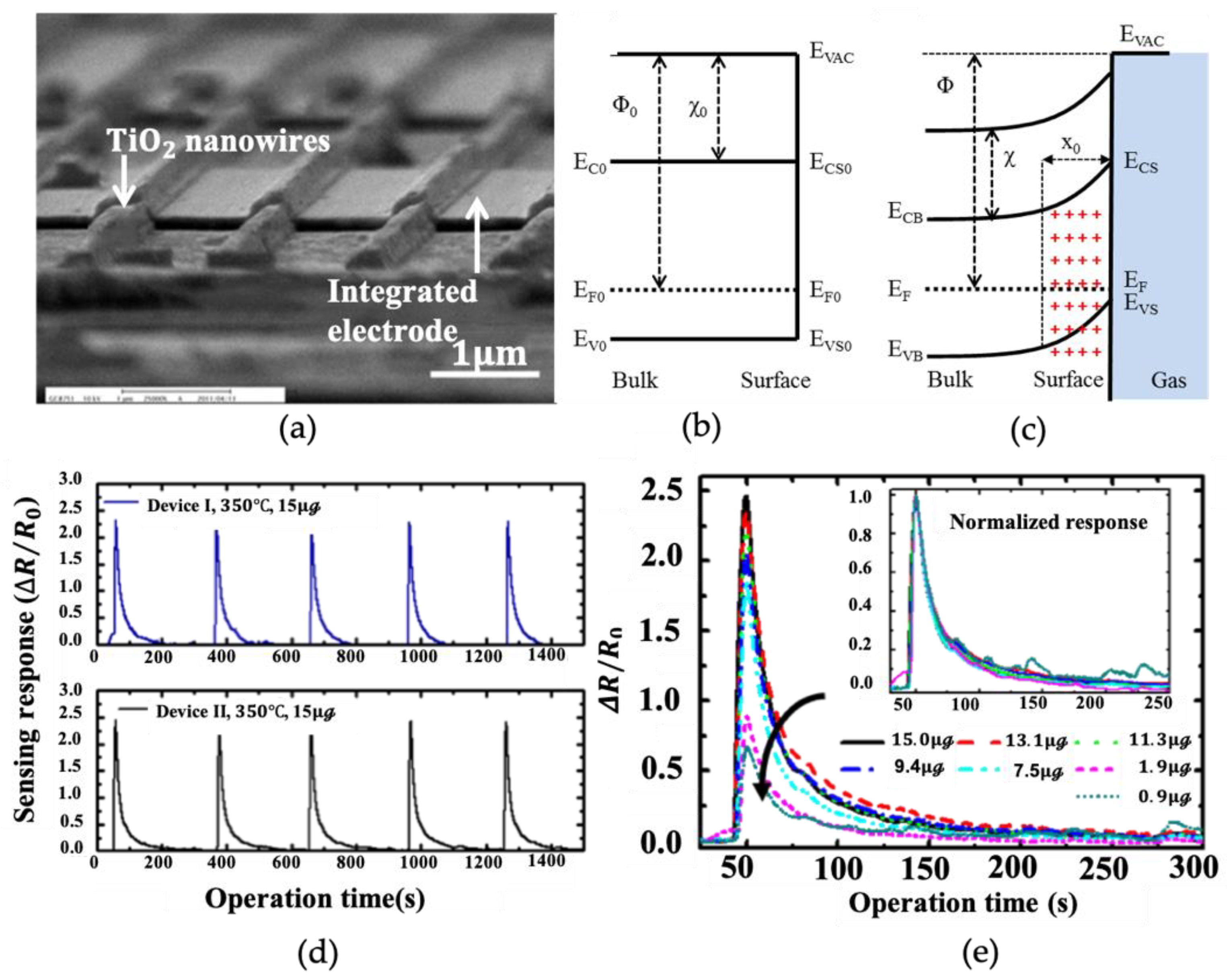
2.1. Pure Gas Sensors
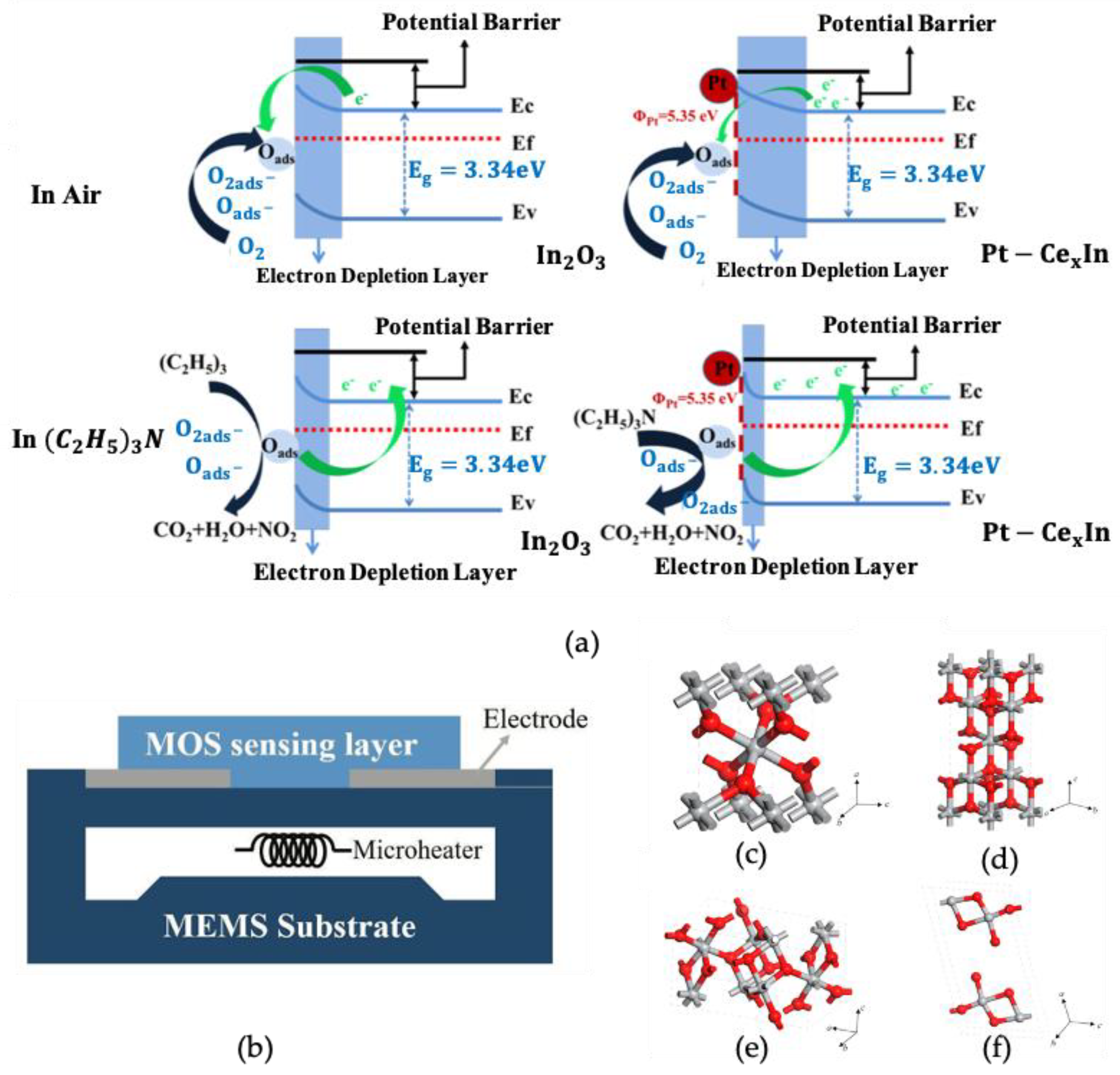
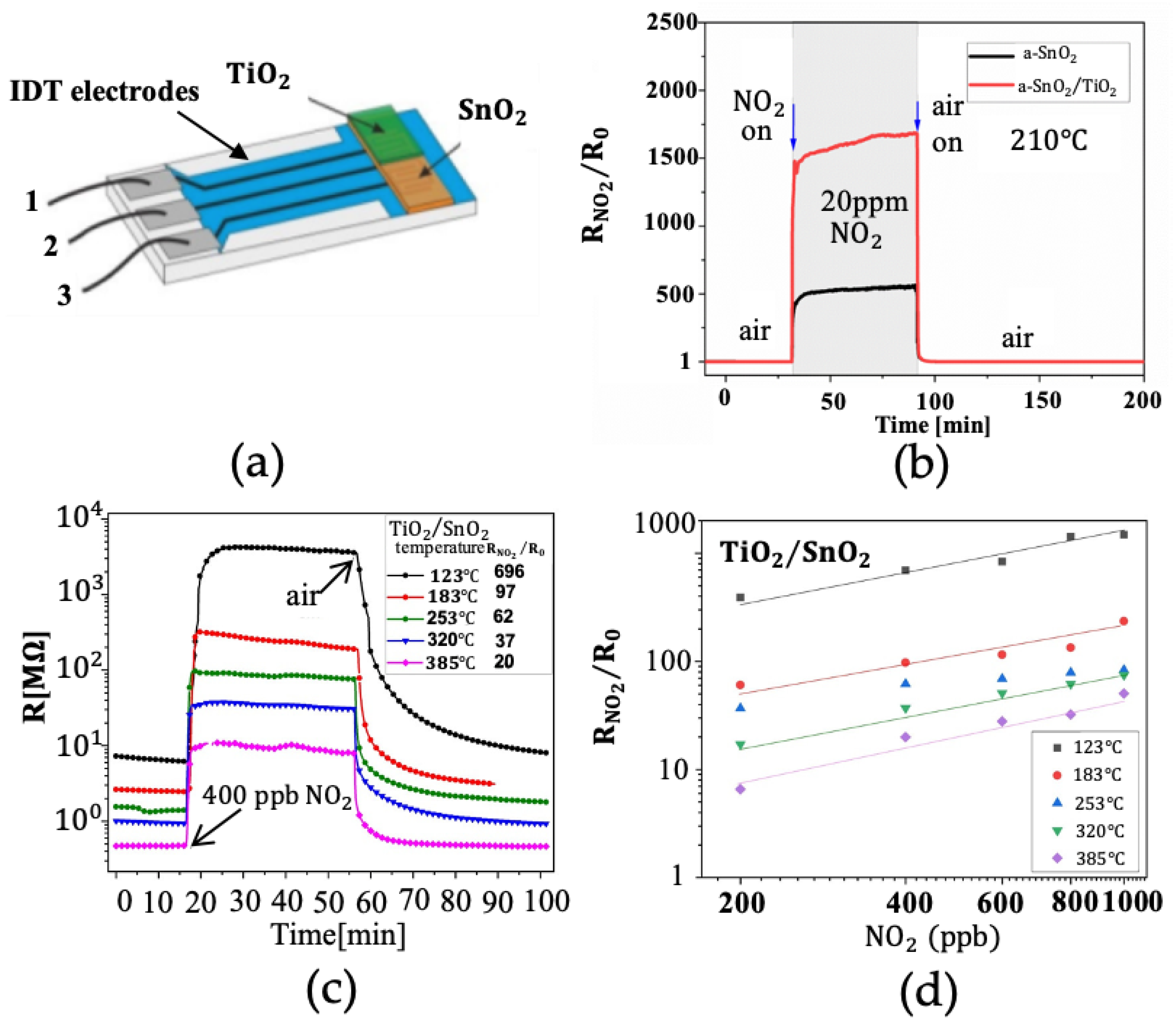
2.2. Onefold Element-Doped Gas Sensors
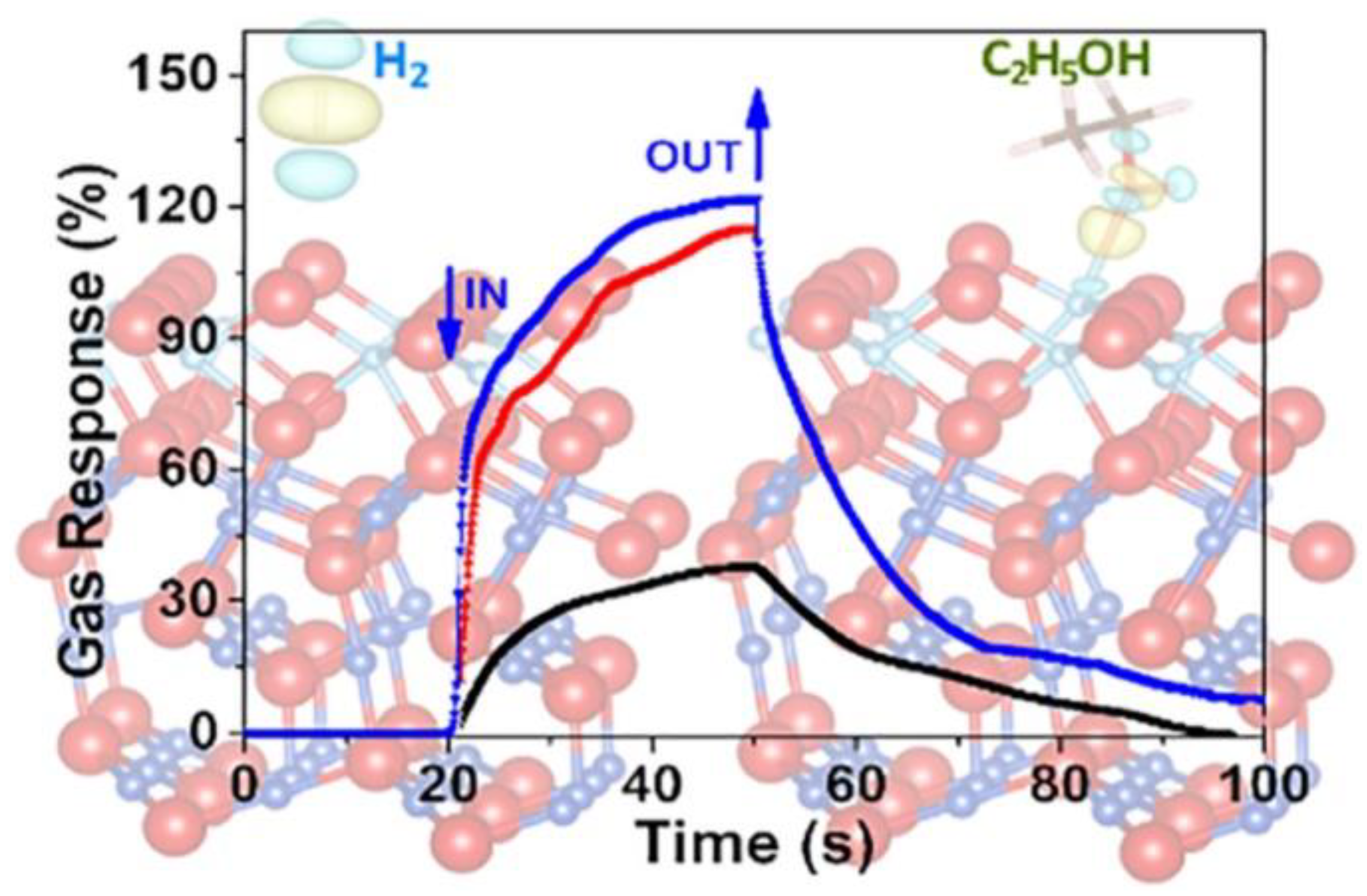
2.3. Other Substances Doped with
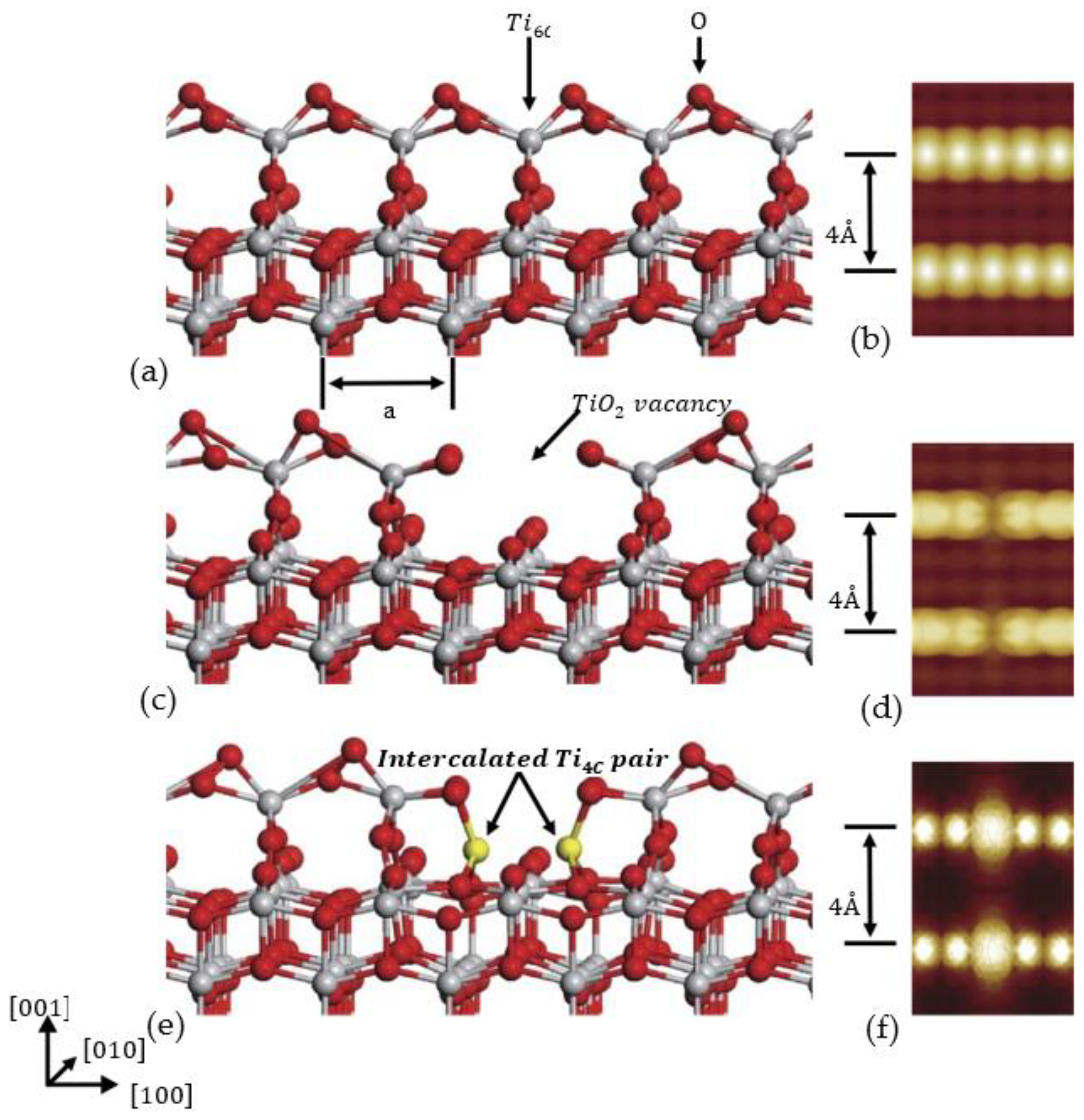
3. DFT Calculation
3.1. The DFT Calculation Combined with Experiment
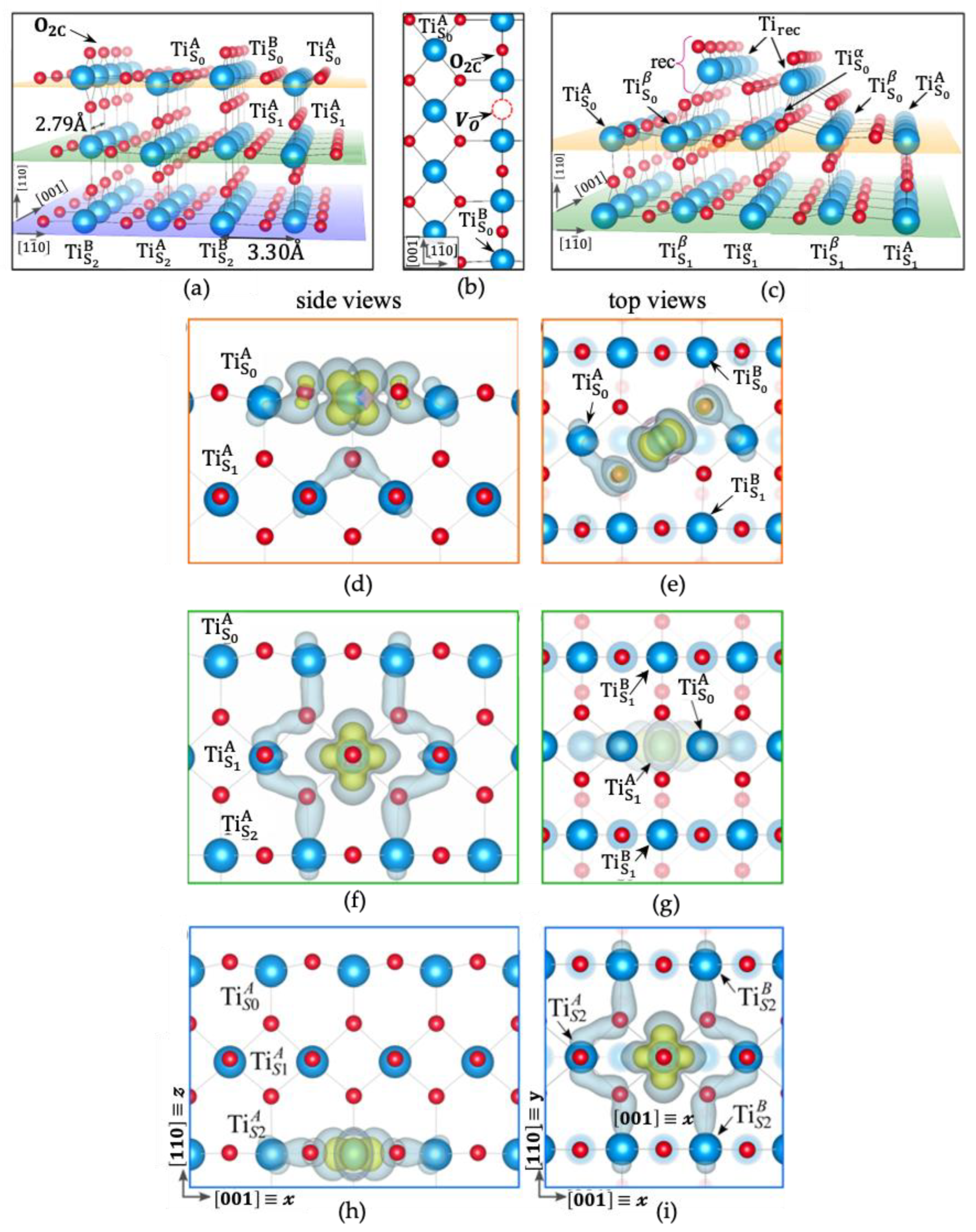
3.2. More In-Depth Theoretical Calculations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, L.; Zeng, W. Room-temperature gas sensing of ZnO-based gas sensor: A review. Sens. Actuators A Phys. 2017, 267, 242–261. [Google Scholar] [CrossRef]
- Wang, Q.; Shen, L.; Xue, T.; Cheng, G.; Huang, C.Z.; Fan, H.J.; Feng, Y.P. Single-Crystalline TiO2(B) Nanobelts with Unusual Large Exposed {100} Facets and Enhanced Li-Storage Capacity. Adv. Funct. Mater. 2020, 31, 11. [Google Scholar] [CrossRef]
- Yang, W.; Wang, Z.; Tan, W.; Peng, R.; Wu, X.; Lu, Y. A first-principles computational comparison of defect-free and disordered, fluorinated anatase TiO2 (001) interfaces with water. Sci. China Mater. 2019, 63, 364–374. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, T. An overview: Facet-dependent metal oxide semiconductor gas sensors. Sens. Actuators B Chem. 2018, 277, 604–633. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.; Ning, H. A Survey on Gas Sensing Technology. Sensors 2012, 12, 9635–9665. [Google Scholar] [CrossRef]
- Li, Z.; Yao, Z.; Haidry, A.A.; Plecenik, T.; Xie, L.; Sun, L.; Fatima, Q. Resistive-type hydrogen gas sensor based on TiO2: A review. Int. J. Hydrog. Energy 2018, 43, 21114–21132. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Nigam, S.; Sarkarb, P.; Majumder, C. DFT study of H2O adsorption on TiO2 (110) and SnO2 (110) surfaces. AIP Conf. Proc. 2013, 1512, 3. [Google Scholar] [CrossRef]
- Markovits, A.; Minot, C. Comparative creation of surface Schottky defects on SnO2(110) and TiO2(110). J. Phys. Conf. Ser. 2008, 117, 012021. [Google Scholar] [CrossRef]
- Hong, N.H.; Sakai, J.; Poirot, N.; Brizé, V. Room-temperature ferromagnetism observed in undoped semiconducting and insulating oxide thin films. Phys. Rev. B 2006, 73, 132404. [Google Scholar] [CrossRef]
- Kosaka, W.; Liu, Z.; Zhang, J.; Sato, Y.; Hori, A.; Matsuda, R.; Kitagawa, S.; Miyasaka, H. Gas-responsive porous magnet distinguishes the electron spin of molecular oxygen. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Uzer, E.; Kumar, P.; Kisslinger, R.; Kar, P.; Thakur, U.K.; Shankar, K.; Nilges, T. Vapor growth of binary and ternary phosphorusbased semiconductors into TiO2 nanotube arrays and application in visible light driven water splitting. Nanoscale Adv. 2019, 1, 2881–2890. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lin, Z.-X.; Wang, W.-H.; Kuo, C.L.; Hwang, K.C.; Hong, C.-C. Self-regenerating Photocatalytic Sensor Based on Dielectrophoretically-Assembled TiO2 Nanowires for Chemical Vapor Sensing. Sens. Actuators B Chem. 2014, 194, 1–9. [Google Scholar] [CrossRef]
- Li, Z.; Haidry, A.A.; Gao, B.; Wang, T.; Yao, Z. The effect of Co-doping on the humidity sensing properties of ordered mesoporous TiO2. Appl. Surf. Sci. 2017, 412, 638–647. [Google Scholar] [CrossRef]
- Oldroyd, R.D.; Sankar, G.; Thomas, J.M.; Ozkaya, D. Enhancing the Performance of a Supported Titanium Epoxidation Catalyst by Modifying the Active Center. J. Phys. Chem. B 1998, 102, 1849–1855. [Google Scholar] [CrossRef]
- Formenti, M.; Juillet, F.; Meriaudeau, P.; Teichner, S.; Vergnon, P. Preparation in a Hydrogen—Oxygen Flame of Ultrafine Metal Oxide Particles. J. Colloid Interface Sci. 1972, 39, 79–89. [Google Scholar] [CrossRef]
- Wen, X.; Zhao, S.; Asuha, S. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. J. Nanomater. 2019, 2019, 12. [Google Scholar] [CrossRef]
- Kwon, H.; Lee, Y.; Hwang, S.; Kim, J.K. Highly-sensitive H2 sensor operating at room temperature using Pt/TiO2 nanoscale Schottky contacts. Sens. Actuators B Chem. 2017, 241, 985–992. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Teleki, A.; Pratsinis, S.E.; Kalyanasundaram, K.; Gouma, P.I. Sensing of organic vapors by flame-made TiO2 nanoparticles. Sens. Actuators B Chem. 2006, 119, 683–690. [Google Scholar] [CrossRef]
- Sun, H.; Tao, L.-Q.; Li, T.; Gao, X.; Sang, T.; Li, Y.; Wei, Y.; Wang, G.; Peng, Z.; Gui, Y.; et al. TiO2–Doped GeSe Monolayer: A highly selective gas sensor for SF6 decomposed species detection based on DFT method. Appl. Surf. Sci. 2022, 572, 6. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Arafat, M.M.; Dinan, B.; Akbar, S.A.; Haseeb, A.S.M.A. Gas Sensors Based on One Dimensional Nanostructured Metal-Oxides: A Review. Sensors 2012, 12, 7207–7258. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Brock, C.; Matt, A.; Bevan, K.H. Implications of the DFT+U method on polaron properties in energy materials. Phys. Rev. B 2017, 96, 13. [Google Scholar] [CrossRef]
- Guo, W.; Feng, Q.; Tao, Y.; Zheng, L.; Han, Z.; Ma, J. Systematic investigation on the gas-sensing performance of TiO2 nanoplate sensors for enhanced detection on toxic gases. Mater. Res. Bull. 2016, 73, 302–307. [Google Scholar] [CrossRef]
- Liu, H.; Shen, W.; Chen, X. A room temperature operated ammonia gas sensor based on Ag-decorated TiO2 quantum dot clusters. RSC Adv. 2019, 9, 24519–24526. [Google Scholar] [CrossRef]
- Wu, K.; Debliquy, M.; Zhang, C. Room temperature gas sensors based on Ce doped TiO2 nanocrystals for highly sensitive NH3 detection. Chem. Eng. J. 2022, 444, 136449. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, C.; Lu, H.; Leng, D.; Li, G.; Liu, Y.; Liang, Q.; Gao, J.; Wang, C.; Zhu, B. Construction of anatase@rutile core@shell TiO2 nanosheets with controllable shell layer thicknesses for enhanced ethanol sensing. Sens. Actuators B Chem. 2020, 325, 12. [Google Scholar] [CrossRef]
- Cai, Z.; Park, S. Highly selective acetone sensor based on Co3O4-decorated porous TiO2 nanofibers. J. Alloys Compd. 2022, 919, 165875. [Google Scholar] [CrossRef]
- Amir, H.; Ponpandian, N.; Viswanathan, C. An electrochemical dopamine sensor based on RF magnetron sputtered TiO2/SS thin film electrode. Mater. Lett. 2021, 300, 4. [Google Scholar] [CrossRef]
- Atashbar, M.Z.; Sunb, H.T.; Gong, B.; Wlodarskia, W.; Lamb, R. XPS study of Nb-doped oxygen sensing TiO2 thin films prepared by sol-gel method. Thin Solid Film. 1998, 326, 238–244. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Zheng, W.; Wang, W.; Huang, H.; Wang, C.; MacDiarmid, A.G.; Wei, Y. Highly Sensitive and Stable Humidity Nanosensors Based on LiCl Doped TiO2 Electrospun Nanofibers. J. Am. Chem. Soc. 2008, 130, 5036–5307. [Google Scholar] [CrossRef] [PubMed]
- Derek, R.M.; Sheikh, A.A.; Patricia, A.M. Nanoscale Metal Oxide-Based Heterojunctions for Gas Sensing: A Review. Sens. Actuators B Chem. 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Karunagaran, B.; Uthirakumar, P.; Chung, S.J.; Velumani, S.; Suh, E.-K. TiO2 thin film gas sensor for monitoring ammonia. Mater. Charact. 2007, 58, 680–684. [Google Scholar] [CrossRef]
- Tai, H.; Jiang, Y.; Xie, G.; Yu, J.; Chen, X. Fabrication and gas sensitivity of polyaniline–Titanium dioxide nanocomposite thin film. Sens. Actuators B 2007, 125, 644–650. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, X.; Cai, L.; Cao, Y.; Gan, L.; Liu, S.; Wang, Z.; Zhang, H.; Li, L. TiO2-decorated graphenes as efficient photoswitches with high oxygen sensitivity. Chem. Sci. 2011, 2, 1860–1864. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, L.; Tie, J.; Dong, X. Gas Sensitivity and Sensing Mechanism Studies on Au-Doped TiO2 Nanotube Arrays for Detecting SF6 Decomposed Components. Sensors 2014, 14, 19517–19532. [Google Scholar] [CrossRef]
- Nikfarjam, A.; Salehifar, N. Improvement in gas-sensing properties of TiO2 nanofiber sensor by UV irradiation. Sens. Actuators B Chem. 2015, 211, 146–156. [Google Scholar] [CrossRef]
- Wang, B.; Deng, L.; Sun, L.; Lei, Y.; Wu, N.; Wang, Y. Growth of TiO2 nanostructures exposed {001} and {110} facets on SiC ultrafine fibers for enhanced gas sensing performance. Sens. Actuators B Chem. 2018, 276, 57–64. [Google Scholar] [CrossRef]
- Wang, L.; Chai, R.; Lou, Z.; Shen, G. Highly sensitive hybrid nanofiber-based room-temperature CO sensors: Experiments and density functional theory simulations. Nano Res. 2018, 11, 1029–1037. [Google Scholar] [CrossRef]
- Tong, X.; Shen, W.; Chen, X. Enhanced H2S sensing performance of cobalt doped free-standing TiO2 nanotube array film and theoretical simulation based on density functional theory. Appl. Surf. Sci. 2019, 469, 414–422. [Google Scholar] [CrossRef]
- Nowak, P.; Maziarz, W.; Rydosz, A.; Kowalski, K.; Ziabka, M.; Zakrzewska, K. SnO2/TiO2 Thin Film n-n Heterostructures of Improved Sensitivity to NO2. Sensors 2020, 20, 6830. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Song, H.; Yu, W.; Ma, Q.; Dong, X.; Wang, J.; Liu, G. Electrospun TiO2//SnO2 Janus nanofibers and its application in ethanol sensing. Mater. Lett. 2020, 262, 4. [Google Scholar] [CrossRef]
- Thangamani, G.J.; Pasha, S.K.K. Titanium dioxide (TiO2) nanoparticles reinforced polyvinyl formal (PVF) nanocomposites as chemiresistive gas sensor for sulfur dioxide (SO2) monitoring. Chemosphere 2021, 275, 129960. [Google Scholar] [CrossRef] [PubMed]
- Kumar, U.; Yang, Y.-H.; Deng, Z.-Y.; Lee, M.-W.; Huang, W.-M.; Wu, C.-H. In situ growth of ternary metal sulfide based quantum dots to detect dual gas at extremely low levels with theoretical investigations. Sens. Actuators B Chem. 2022, 353, 11. [Google Scholar] [CrossRef]
- Zhou, S.; Lu, Q.; Chen, M.; Li, B.; Wei, H.; Zi, B.; Zeng, J.; Zhang, Y.; Zhang, J.; Zhu, Z.; et al. Platinum-Supported Cerium-Doped Indium Oxide for Highly Sensitive Triethylamine Gas Sensing with Good Antihumidity. ACS Appl. Mater. Interfaces 2020, 12, 42962–42970. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Chowdhury, N.K.; Roy, S.C.; Bhowmik, B. Review of Thin Film Transistor Gas Sensors: Comparison with Resistive and Capacitive Sensors. J. Electron. Mater. 2022, 51, 1974–2003. [Google Scholar] [CrossRef]
- Oh, J.; Kim, S.H.; Lee, M.-J.; Hwang, H.; Ku, W.; Lim, J.; Hwang, I.-S.; Lee, J.-H.; Hwang, J.-H. Machine learning-based discrimination of indoor pollutants using an oxide gas sensor array: High endurance against ambient humidity and temperature. Sens. Actuators B Chem. 2022, 364, 131894. [Google Scholar] [CrossRef]
- Nazemi, H.; Joseph, A.; Park, J.; Emadi, A. Advanced Micro- and Nano-Gas Sensor Technology: A Review. Sensors 2019, 19, 1285. [Google Scholar] [CrossRef]
- Bindra, P.; Hazra, A. Selective detection of organic vapors using TiO2 nanotubes based single sensor at room temperature. Sens. Actuators B Chem. 2019, 290, 7. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, T.; Zhou, Y.; Meng, C.; Zhu, W.; Liu, L. TiO2-Based Nanoheterostructures for Promoting Gas Sensitivity Performance: Designs, Developments, and Prospects. Sensors 2017, 17, 1971. [Google Scholar] [CrossRef]
- Sharma, B.; Sharma, A.; Myung, J.-H. Highly selective detection of acetone by TiO2-SnO2 heterostructures for environmental biomarkers of diabetes. Sens. Actuators B Chem. 2021, 349, 13. [Google Scholar] [CrossRef]
- Kaur, N.; Singh, M.; Moumen, A.; Duina, G.; Comini, E. 1D Titanium Dioxide: Achievements in Chemical Sensing. Materials 2020, 13, 2974. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Xue, Q.; Qi, X.; Cheng, C.; Yang, M.; Yang, T.; Chen, F.; Qiu, F.; Quan, X. DFT and experimental study on visible-light driven photocatalysis of rare-earth-doped TiO2. Vacuum 2022, 200, 110972. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; John, T.; Yates, J. Photocatalysis on TiOn Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Paola, A.D.; Bellardita, M.; Palmisano, L. Brookite, the Least Known TiO2 Photocatalyst. Catalysts 2013, 3, 36–73. [Google Scholar] [CrossRef]
- Tian, W.; Ho, Y.-H.; Chen, C.-H.; Kuo, C.-Y. Sensing Performance of Precisely Ordered TiO2 Nanowire Gas. Sensors 2013, 13, 865–874. [Google Scholar] [CrossRef]
- Galstyan, V.; Comini, E.; Faglia, G.; Sberveglieri, G. TiO2 Nanotubes: Recent Advances in Synthesis and Gas. Sensors 2013, 13, 14813–14838. [Google Scholar] [CrossRef]
- Güntner, A.T.; Pineau, N.J.; Pratsinis, S.E. Flame-made chemoresistive gas sensors and devices. Prog. Energy Combust. Sci. 2022, 90, 100992. [Google Scholar] [CrossRef]
- Xue, J.; Xie, J.; Liu, W.; Xia, Y. Electrospun Nanofibers: New Concepts, Materials, and Applications. Acc. Chem. Res. 2017, 50, 1976–1987. [Google Scholar] [CrossRef]
- Si, Y.; Yu, J.; Tang, X.; Ge, J.; Ding, B. Ultralight nanofibre-assembled cellular aerogels with superelasticity and multifunctionality. Nat. Commun. 2014, 5, 9. [Google Scholar] [CrossRef]
- Haidry, A.A.; Schlosser, P.; Durina, P.; Mikula, M.; Tomasek, M.; Plecenik, T.; Roch, T.; Pidik, A.; Stefecka, M.; Noskovic, J.; et al. Hydrogen gas sensors based on nanocrystalline TiO2 thin films. Cent. Eur. J. Phys. 2011, 9, 1351–1356. [Google Scholar] [CrossRef]
- Tolmachoff, E.; Memarzadeh, S.; Wang, H. Nanoporous Titania Gas Sensing Films Prepared in a Premixed Stagnation Flame. J. Phys. Chem. C 2011, 115, 21620–21628. [Google Scholar] [CrossRef]
- Park, J.-A.; Moon, J.; Lee, S.-J.; Kim, S.H.; Zyung, T.; Chu, H.Y. Structural, electrical and gas sensing properties of eletrospun TiO2 nanofibers. Thin Solid Film. 2010, 518, 6642–6645. [Google Scholar] [CrossRef]
- Khan, M.A.H.; Rao, M.V. Gallium Nitride (GaN) Nanostructures and Their Gas Sensing Properties: A Review. Sensors 2020, 20, 3889. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wen, Y.; Hu, X.; Ji, X.; Yan, M.; Mai, L.; Hu, P.; Shan, B.; Huang, Y. Na+ intercalation pseudocapacitance in graphene-coupled titanium oxide enabling ultra-fast sodium storage and long-term cycling. Nat. Commun. 2015, 6, 5. [Google Scholar] [CrossRef]
- Teleki, A.; Bjelobrk, N.; Pratsinis, S.E. Flame-made Nb- and Cu-doped TiO2 sensors for CO and ethanol. Sens. Actuators B Chem. 2008, 130, 449–457. [Google Scholar] [CrossRef]
- Lahmar, A.; Pfeiffer, N.; Habouti, S.; Es-Souni, M. Microstructure and property control in TiO2–Pt nanocomposite thin films. Ceram. Int. 2015, 41, 443–449. [Google Scholar] [CrossRef]
- Chomkitichai, W.; Ninsonthi, H.; Liewhiran, C.; Wisitsoraat, A.; Sriwichai, S.; Phanichphant4, S. Flame-Made Pt-Loaded TiO2 Thin Films and Their Application as H2 Gas Sensors. J. Nanomater. 2013, 8, 6. [Google Scholar] [CrossRef]
- Aldon, L.; Kubiak, P.; Picard, A.; Jumas, J.-C.; Olivier-Fourcade, J. Size Particle Effects on Lithium Insertion into Sn-doped TiO2 Anatase. Chem. Mater. 2006, 18, 1401–1406. [Google Scholar] [CrossRef]
- Phanichphant, S.; Liewhiran, C.; Wetchakun, K.; Wisitsoraat, A.; Tuantranont, A. Flame-Made Nb-Doped TiO2 Ethanol and Acetone Sensors. Sensors 2011, 11, 472–484. [Google Scholar] [CrossRef]
- Xiong, C.; Kenneth, J.; Balkus, J. Mesoporous Molecular Sieve Derived TiO2 Nanofibers Doped with SnO2. J. Phys. Chem. C 2007, 111, 10359–10367. [Google Scholar] [CrossRef]
- Bakr, Z.H.; Wali, Q.; Ismail, J.; Elumalai, N.K.; Uddin, A.; Jose, R. Synergistic combination of electronic and electrical properties of SnO2 and TiO2 in a single SnO2-TiO2 composite nanofiber for dye-sensitized solar cells. Electrochim. Acta 2018, 263, 524–532. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, D.D.; Guo, P.; Leckie, J.O. An Efficient Bicomponent TiO2/SnO2 Nanofiber Photocatalyst Fabricated by Electrospinning with a Side-by-Side Dual Spinneret Method. Nano Lett. 2007, 7, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhai, L.; Liu, H.; Li, B.; Li, M.; Wang, B. A novel H2O2 photoelectrochemical sensor based on ternary RGO/Ag-TiO2 nanotube arrays nanocomposite. Electrochim. Acta 2021, 374, 137851. [Google Scholar] [CrossRef]
- Mohd Chachuli, S.A.; Hamidon, M.N.; Mamat, M.S.; Ertugrul, M.; Abdullah, N.H. A Hydrogen Gas Sensor Based on TiO(2) Nanoparticles on Alumina Substrate. Sensors 2018, 18, 2483. [Google Scholar] [CrossRef]
- Chen, B.; Li, P.; Wang, B.; Wang, Y. Flame-annealed porous TiO2/CeO2 nanosheets for enhenced CO gas sensors. Appl. Surf. Sci. 2022, 593, 153418. [Google Scholar] [CrossRef]
- Conti, P.P.; Andre, R.S.; Mercante, L.A.; Fugikawa-Santos, L.; Correa, D.S. Discriminative detection of volatile organic compounds using an electronic nose based on TiO2 hybrid nanostructures. Sens. Actuators B Chem. 2021, 344, 130124. [Google Scholar] [CrossRef]
- Xia, S.-Y.; Tao, L.-Q.; Jiang, T.; Sun, H.; Li, J. Rh-doped h-BN monolayer as a high sensitivity SF6 decomposed gases sensor: A DFT study. Appl. Surf. Sci. 2021, 536, 12. [Google Scholar] [CrossRef]
- Nikolaev, A.V.; Chtchelkatchev, N.M.; Bibikov, A.V.; Salamatin, D.A.; Tsvyashchenko, A.V. Ab initio based description of the unusual increase of the electric field gradient with temperature at Ti sites in rutile TiO2. Phys. Rev. B 2020, 102, 8. [Google Scholar] [CrossRef]
- Freysoldt, C.; Grabowski, B.; Hickel, T.; Neugebauer, J. First-principles calculations for point defects in solids. Rev. Mod. Phys. 2014, 86, 53. [Google Scholar] [CrossRef]
- Muscat, J.; Harrison, N.M.; Thornton, G. First-principles study of potassium adsorption on TiO2 surfaces. Phys. Rev. B 1999, 59, 7. [Google Scholar] [CrossRef]
- Kirchner-Hall, N.E.; Zhao, W.; Xiong, Y.; Timrov, I.; Dabo, I. Extensive Benchmarking of DFT+U Calculations for Predicting Band Gaps. Appl. Sci. 2021, 11, 2395. [Google Scholar] [CrossRef]
- Myers, W.D.; Swiatecki, W.J. Table of Nuclear Masses According to the 1994 Thomas-Fermi Model; U.S. Department of Energy: Washington, DC, USA, 1994; p. Medium: ED.; 141p.
- Hohenberg, P.; KonN, W. Inhomgeneous Electron Gas. Phys. Rev. B 1964, 136, 8. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. B 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Andriotis, A.N. LDA exchange-energy functional. Phys. Rev. B 1998, 58, 15300–15303. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Wang, Y. Generalized gradient approximation for the exchange-correlation hole of a many-electron system. Phys. Rev. B 1996, 54, 16533–16539. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Zhao, W.; Lin, H.; Li, Y.; Zhang, Y.; Huang, X.; Chen, W. Growth mechanism of palladium clusters on rutile TiO2(110) surface. J. Nat. Gas Chem. 2012, 21, 544–555. [Google Scholar] [CrossRef]
- Zhang, M.; Meng, F.; Zhang, S.; Zeng, Y.; Zhong, Q. Sulfur-doping promoting peroxone reaction over TiO2 for highly effective NO oxidation at low temperature: Experimental and DFT studies. Chem. Eng. J. 2022, 429, 11. [Google Scholar] [CrossRef]
- Gui, Y.H.; Hua, S.C.; Zhang, Q.S.; Jin, Z.; Gang, L.; Campbell, S.S.; Ming, C.H.; Qing, L.G. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef]
- An, J.-W.; Wang, G.-C. Titania crystal-plane-determined activity of copper cluster in water-gas shift reaction. Appl. Surf. Sci. 2022, 591, 153145. [Google Scholar] [CrossRef]
- Nolan, M.; Iwaszuk, A.; Tada, H. Molecular Metal Oxide Cluster-Surface Modified Titanium(IV) Dioxide Photocatalysts. Aust. J. Chem. 2012, 65, 624–632. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Q.; Fang, P.; Pan, C.; Xiao, W. First principles study of the adsorption of a NO molecule on N-doped anatase nanoparticles. Appl. Surf. Sci. 2012, 258, 8312–8318. [Google Scholar] [CrossRef]
- L’opez-Caballero, P.; Opez, J.M.R.-L.; Giovanetti, L.J.; Buceta, D.; Miret-Artes, S.; Lopez-Quintela, M.A.; Requejo, F.G.; de Lara-Castells, M.P. Exploring the properties of Ag5–TiO2 interfaces: Stable surface polaron formation, UV-Vis optical response, and CO2 photoactivation. J. Mater. Chem. A 2020, 8, 6842–6853. [Google Scholar] [CrossRef]
- Tian, X.; Yao, L.; Cui, X.; Zhao, R.; Chen, T.; Xiao, X.; Wang, Y. A two-dimensional Ti3C2TX Xene@TiO2/MoS2 heterostructure with excellent selectivity for the room temperature detection of ammonia. J. Mater. Chem. A 2022, 10, 5505–5519. [Google Scholar] [CrossRef]
- Lupan, O.; Santos-Carballal, D.; Ababii, N.; Magariu, N.; Hansen, S.; Vahl, A.; Zimoch, L.; Hoppe, M.; Pauporte, T.; Galstyan, V.; et al. TiO2/Cu2O/CuO Multi-Nanolayers as Sensors for H2 and Volatile Organic Compounds: An Experimental and Theoretical Investigation. ACS Appl. Mater. Interfaces 2021, 13, 32363–32380. [Google Scholar] [CrossRef] [PubMed]
- Muscat, J.; Swamy, V.; Harrison, N.M. First-principles calculations of the phase stability of TiO2. Phys. Rev. B 2002, 65, 15. [Google Scholar] [CrossRef]
- Krylow, S.; Garcia, M.E. Ab initio study of temperature- and laser-induced phase transitions in TiO2. Phys. Rev. B 2019, 100, 6. [Google Scholar] [CrossRef]
- Zeng, W.; Liu, T.; Wang, Z.; Tsukimoto, S.; Saito, M.; Ikuhara, Y. Oxygen Adsorption on Anatase TiO2 (101) and (001) Surfaces from First Principles. Mater. Trans. 2010, 51, 171–175. [Google Scholar] [CrossRef]
- Quirk, J.A.; Lazarov, V.K.; McKenna, K.P. Electronic Properties of{112} and {110} Twin Boundaries in Anatase TiO2. Adv. Theory Simul 2019, 2, 7. [Google Scholar] [CrossRef]
- Reticcioli, M.; Setvin, M.; Schmid, M.; Diebold, U.; Franchini, C. Formation and dynamics of small polarons on the rutile TiO2(110) surface. Phys. Rev. B 2018, 98, 14. [Google Scholar] [CrossRef]
- Su, W.; Zhao, R.; Zheng, S. Electronic Structures of Cu/S Co-doped/Anatase TiO2 by First-principles. Mater. -Rio De Jan. 2016, 21, 599–605. [Google Scholar]
- Wu, Y.; Tian, Y.; Zheng, S. First Principles Study on the Electronic Structure and Optical Property of Nd-C Codoped Anatase TiO2. Mater. -Rio De Jan. 2016, 21, 301–306. [Google Scholar]
- Zhang, X.; Zhang, J.; Cui, H. Adsorption mechanism of SF6 decomposition components onto N, F-codoped TiO2: A DFT study. J. Fluor. Chem. 2018, 23, 18–23. [Google Scholar] [CrossRef]
- Yamamoto, T.; Ohno, T. Screened hybrid density functional study on Nb- and Ta-doped TiO2. Phys. Rev. B 2012, 85, 4. [Google Scholar] [CrossRef]
- Kang, Y.; Peelaers, H.; Van de Walle, C.G. First-principles study of electron-phonon interactions and transport in anatase TiO2. Phys. Rev. B 2019, 100, 7. [Google Scholar] [CrossRef]
- Orhan, O.K.; O’Regan, D.D. First-principles Hubbard U and Hund’s J corrected approximate density functional theory predicts an accurate fundamental gap in rutile and anatase TiO2. Phys. Rev. B 2020, 101, 14. [Google Scholar] [CrossRef]
- Huang, L.; Dong, H.; Huo, N.; Zheng, Z.; Deng, H.-X.; Zhang, G.; Cheng, Y.; Li, J. Deep insights into interface engineering by buffer layer for efficient perovskite solar cells: A firstprinciples study. Sci. China Mater. 2020, 63, 1588–1596. [Google Scholar] [CrossRef]
- Nagatsuka, N.; Kato, K.; Wilde, M.; Fukutani, K. Absence of midgap states due to excess electrons donated by adsorbed hydrogen on the anatase TiO2 (101) surface. Phys. Rev. B 2022, 105, 045424. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, H.; Tan, S.; Feng, H.; Cheng, Z.; Zhao, J.; Zhao, A.; Wang, B.; Luo, Y.; Yang, J.; et al. Role of point defects on the reactivity of reconstructed anatase titanium dioxide (001) surface. Nat. Commun. 2013, 4, 8. [Google Scholar] [CrossRef]
- Lindan, P.J.; Harrison, N.M.; Gillan, M.J. Mixed Dissociative and Molecular Adsorption of Water on the Rutile (110) Surface. Phys. Rev. Lett. 1998, 80, 4. [Google Scholar] [CrossRef]
- Zhang, Q.; Gui, Y.; Qiao, H.; Chen, X.; Cao, L. Theoretical study of SF6 decomposition products adsorption on metal oxide cluster-modified single-layer graphene. J. Ind. Eng. Chem. 2022, 105, 278–290. [Google Scholar] [CrossRef]
- Fellah, M.F. The reduced graphene oxide/WO3: Sensing properties for NO2 gas detection at room temperature. Diam. Relat. Mater. 2021, 119, 9. [Google Scholar] [CrossRef]

| Materials/ Structure | Synthesis Methods | Detecting Gases/ Concentrations | Response () | Temperature () | Response/ Recovery Time | Ref./ Year |
|---|---|---|---|---|---|---|
| /nanoparticle | Flame spray pyrolysis | Isoprene/acetone (1–7.5 ppm)/ethanol/CO | NA | Acetone: 2–3 s/144 s (1 ppm), 302 s (7.5 ppm) | [19]/ 2006 | |
| /thin film | DC magnetron sputtering | /500 ppm | NA | 90 s/110 s | [33]/ 2007 | |
| polyaniline–titanium dioxide (PANI/)/thin film | In situ chemical oxidation polymerization | /47 ppm | 5 s/69 s | [34]/ 2007 | ||
| 2.33 | ||||||
| /nanofiber | Electrostatic spinning | NA | NA | 3 s/7 s | [31]/ 2008 | |
| 1000 | ||||||
| -graphene/thin film | Chemical vapor deposition | Room temperature | 130 s/260 s | [35]/ 2011 | ||
| 3.65 (5% ) | ||||||
| /nanotubes | Deposition sedimentation | decomposition gas ()/ | 110 ℃ | NA | [36]/ 2014 | |
| /Au-/nanofiber/nanofiber@ nanofilm | Electrostatic spinning | : (without UV)/ (with UV) | (without UV) | (with UV) | [37]/ 2015 | ||
| 10.1 (without UV)/96 (with UV) | ||||||
| /core–shell hierarchical | Hydrothermal method | Acetone/100 ppm | 1 s/NA | [38]/ 2018 | ||
| 19.2 | ||||||
| ultrafine nanoparticles in | Electrostatic spinning | CO/1 ppm | Room temperature | 10.0 s/12.5 s | [39]/ 2018 | |
| 1.02 | ||||||
| /QDs | Convenient hydrolysis method | Room temperature | 150 s/600 s | [25]/ 2019 | ||
| 25.1 | ||||||
| /nanotube | One-step anodization and immersion method | 14 s/4 s | [40]/ 2019 | |||
| 199.16 | ||||||
| Anatase@ rutile /core@ shell | Two-step hydrothermal method | NA | [27]/ 2020 | |||
| 8.2 | ||||||
| / nanoheterostructures | Magnetron sputtering and Langmuir–Blodgett technique | 62 s/42 s | [41]/ 2020 | |||
| 696 | ||||||
| Janus nanofiber | Electrostatic spinning | 8 s/13 s | [42]/ 2020 | |||
| 7< S < 8 | ||||||
| PVF//nanocomposite films | Solution casting | (not compounded)/ (after compounding) | 66 s/107 s | [43]/ 2021 | ||
| (not compounded)/ (after compounding) | ||||||
| /film | Successive Ionic Layer Adsorption and Reaction (SILAR) | Room temperature | 198 s/36 s 16.03 s/27 s | [44]/ 2022 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Z.; Zhang, Y.; Kang, W.; Deng, N.; Pan, Y.; Sun, W.; Ni, J.; Kang, X. TiO2 Gas Sensors Combining Experimental and DFT Calculations: A Review. Nanomaterials 2022, 12, 3611. https://doi.org/10.3390/nano12203611
Yan Z, Zhang Y, Kang W, Deng N, Pan Y, Sun W, Ni J, Kang X. TiO2 Gas Sensors Combining Experimental and DFT Calculations: A Review. Nanomaterials. 2022; 12(20):3611. https://doi.org/10.3390/nano12203611
Chicago/Turabian StyleYan, Zirui, Yaofang Zhang, Weimin Kang, Nanping Deng, Yingwen Pan, Wei Sun, Jian Ni, and Xiaoying Kang. 2022. "TiO2 Gas Sensors Combining Experimental and DFT Calculations: A Review" Nanomaterials 12, no. 20: 3611. https://doi.org/10.3390/nano12203611
APA StyleYan, Z., Zhang, Y., Kang, W., Deng, N., Pan, Y., Sun, W., Ni, J., & Kang, X. (2022). TiO2 Gas Sensors Combining Experimental and DFT Calculations: A Review. Nanomaterials, 12(20), 3611. https://doi.org/10.3390/nano12203611







