3.1. Characterization of ALS Loaded Niosomes
The stratum corneum (SC) in the outermost layer of the skin restricts the skin penetration of hydrophilic and macromolecular drugs [
19]. To solve this problem, a number of strategies were used to improve transdermal drug delivery and to broaden the number of drugs delivered transdermally [
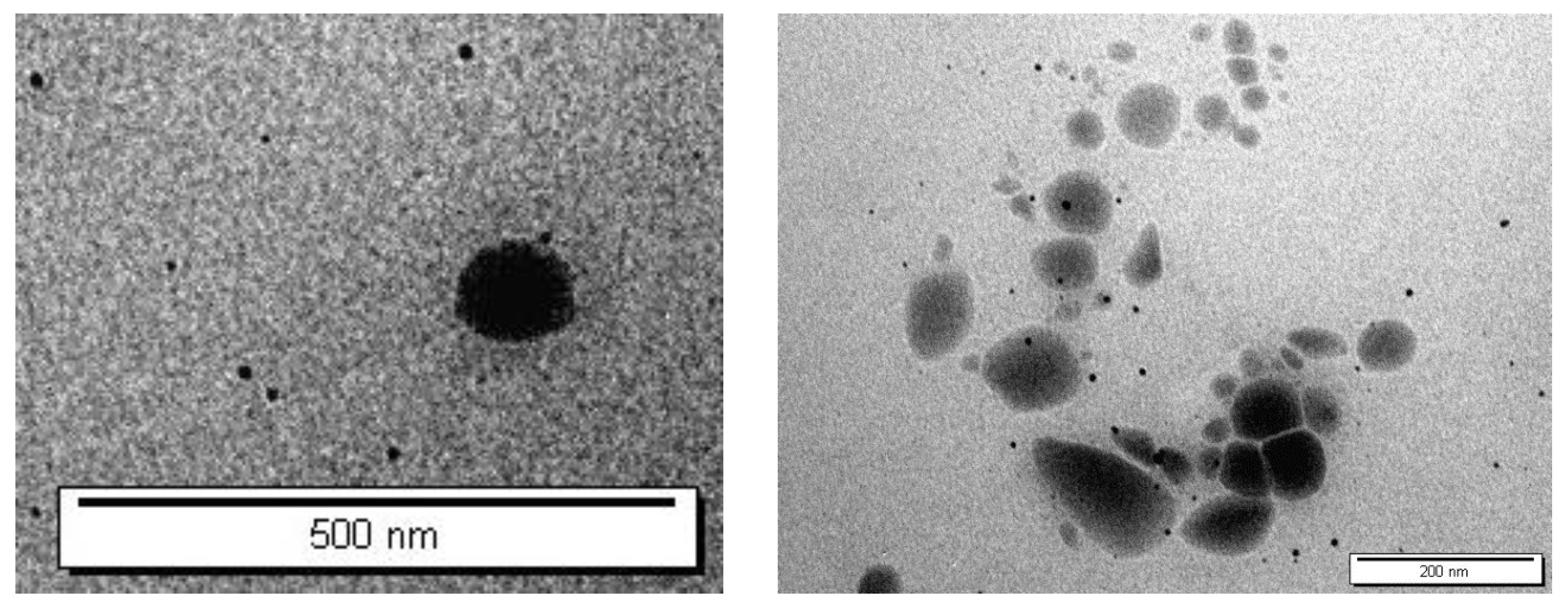
6]. Therefore, various formulations of ALS-loaded niosomes were successfully prepared using the ether injection method. The bilayer vesicles were produced using different molar ratios of nonionic surfactant (Span 60, Tween 60, and Tween 80) and Chol. As a negative charged inducing agent, DCP was also used. The TEM micrographs of the niosomes are given in
Figure 3. The TEM images confirmed the formation of niosomes. Indeed, TEM was employed to characterize niosomes in terms of shape, which illustrates that niosomes were of a spherical shape.
The PS of the niosomal formulations was in the range of 99.6 ± 0.9 to 464.3 ± 6.1 nm, as summarized in
Table 3. Particle size is an essential characteristic of drug delivery systems, affecting loading and release rates [
20]. Skin deposition was not observed in studies where the particle size of carriers was greater than 600 nm [
21]. A size of less than 300 nm may lead to an excessive amount of transdermal drug transport, whereas smaller carriers, such as those with a 300 nm particle size, enhance dermal delivery [
22]. All formulations with a PS of more than 350 nm were excluded. This is because the approximate PS of vesicles to be able to deliver their contents into the deeper skin layers is 300 nm or below [
23]. The hydrophilic–lipophilic balance (HLB) value is a critical factor in the formulation of niosomes. It has been noted that for higher niosome encapsulation efficiency, a HLB value between 4 and 8 is strongly advised [
24]. Tween 60 is a surfactant with a high hydrophilicity (HLB = 14.9), whereas Span 60 is a nonionic surfactant with a hydrophobic portion (HLB = 4.7) and limited water solubility [
25]. By combining Tween 60 with Span 60, the PS of the prepared niosome was larger than those containing Span 60 alone. Basiri, Rajabzadeh et al. (2017) observed that the lower hydrophilicity and bigger critical packing parameter (CPP) of Span 60 versus Tween 60 led into an increase in the average volume sizes [
20]. According to the results, the size of niosomes showed a regular increase with an increase in the surfactant HLB values. An increase in the HLB value of the surfactant mixture and tighter packing of molecules inside the niosome could be the cause of this. A previous study that showed a similar outcome found that the size of niosomes increased when 20% Tween 60 was added. This finding may be related to the slightly larger hydrophilic portion of the Tween 60 molecule compared to the Span 60 using cephalexin [
25]. The mean PS of the niosomes is also influenced by the membrane composition. The formula of niosomes with high ratio of Chol shows a bigger size than others. This might be interpreted in context of the fact that Chol would be more inclined to increase the number of bilayers [
26]. This outcome is consistent with a prior study which showed that an increase in Chol induced the size of vesicles loaded with ciprofloxacin to increase [
27]. The presence of Chol was found to be significantly effective in increasing the niosomal size (
p > 0.05) [
20].
The PDI is a measure of a sample’s heterogeneity [
28]. The PDI ranged from 0 to 1, where values near to zero suggesting homogeneous dispersion [
29], and less than 0.5 indicating a monodispersed sample [
21]. As shown in
Table 3, PDI ranged from 0.005 ± 0.00 to 0.334 ± 0.021, indicating that the vesicles are homogenous in size [
30]. Therefore, the low PDI values indicated that the niosomal suspension had a narrow size dispersion and was homogenous. The ZP of colloidal systems is one of the characteristics used to interpret their stability. The charged particles repel one another as the ZP increases, stabilizing the system against aggregation. Colloidal systems with a zeta potential of higher than +30 mV or lower than −30 mV are considered stable [
31,
32]. Here, the ZP values of all formulations were noted to be in the range of −27.6 ± 0.22 and −42.27 ± 2.25 mV, as shown in
Table 3, which is an indication of a stable system due to the electrostatic repulsion between nanovesicles [
33].
Vesicle size, surfactant type, and Chol concentration are the factors affecting the effectiveness of entrapment [
34]. Unencapsulated drug separated from the niosomal solution using centrifugation. After this step the encapsulated drug can be released from niosomes by lysing of vesicles. By completely disrupting the vesicle with isopropanol, the amount of drug entrapped in niosomes is evaluated [
35]. Based on
Table 3, the highest EE% of ALS were (65.94 ± 13.13%), (70.7 ± 7.35%), and (54.66 ± 1.69%) from F4, F6, and F10, respectively. The results indicated that the EE% for niosomes prepared using the mixtures of Span 60 and Tween 60 with HLB value 6.8 were superior to those prepared using solely Span 60 or others. The results are in agreement with a previous study of quercetin, which reported that using a mixture of Tween 60 and Span 60 results in the highest EE% (78.9%) of quercetin. Therefore, larger head groups and longer alkyl chains in the structure can result in greater vesicles to entrap larger amounts of quercetin [
36]. The HLB value of the surfactant was modified using a mixture of Tween 60 (HLB = 14.9) and Span 60 (HLB = 4.7) to obtain a HLB value around 6.8, in order to obtain greater entrapment efficiency [
37]. Ghafelehbashi, Akbarzadeh et al. (2019) observed that the amount of encapsulated cephalexin enhanced with an increase in the HLB value, which was in agreement with our results [
25].
Charge-inducing agents are known to stabilize niosomes by raising their zeta potential. Additionally, they increase niosome membrane permeability to water, resulting in the production of large niosomes [
38]. Here, F4, F6, and F10 were also prepared without using DCP. The EE% significantly decreased (
p > 0.05) when removing DCP from the niosomal formulations, as shown in
Table 4. Indeed, DCP, as a negatively charged molecule, is usually used in the niosomal formulation to prevent aggregation, which increases the stability of the niosomal dispersion. When a charge-inducing substance is added to the niosome membrane, water is allowed to enter the bilayer and the gap between the bilayers is increased [
39]. Therefore, the incorporation of DCP was found to enhance the EE% significantly. This is because double hydrocarbon chains in DCP contributed to a tighter packing of the bilayer membrane which increased the EE%. This result was similar to a previous study [
40], in which they revealed the influence of a stabilizer on EE%. Moreover, the phosphate groups of DCP aligned next to the polar heads of Span 60 and Tween 60 [
20].
3.2. Attenuated Total Reflectance—Fourier Transform Infrared (ATR—FTIR) Analysis
In this study, ATR–FTIR analysis was used to evaluate functional groups found in the structure of ALS and to determine molecular interactions between niosome excipients and ALS.
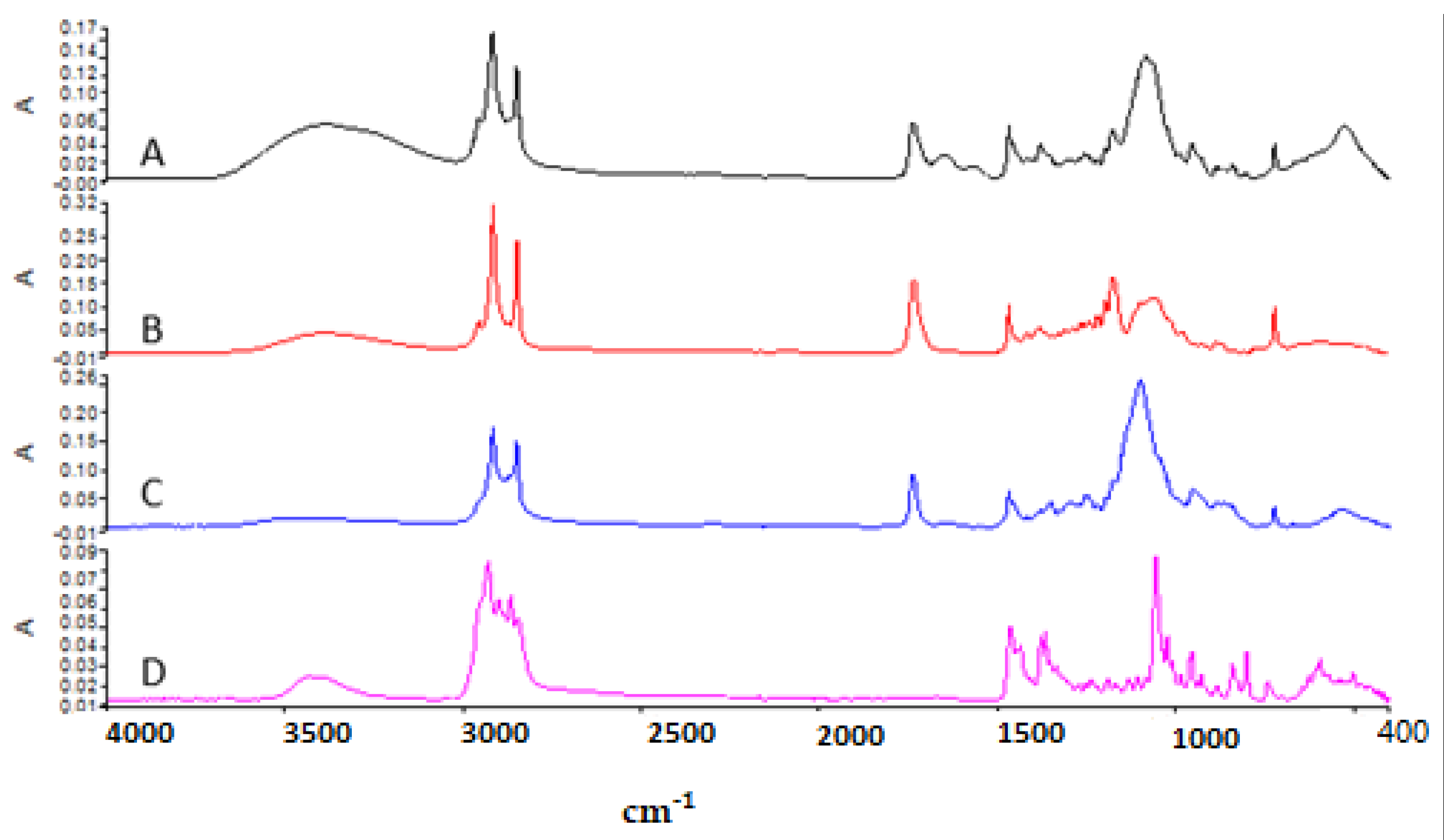
Figure 4 shows the ATR–FTIR of (A) blank niosome, (B) Span 60, (C) Tween 60, and (D) Chol. Span 60 showed the peaks around 3395 cm
−¹ due to O–H stretching, at 2956 cm
−¹ due to –CH stretching, and the strong C=O ester bond at 1736 cm
−¹, which have also been reported in previous studies [
41]. The ATR–FTIR spectrum of Tween 60 had a characteristic sharp peak around 1735 cm
−¹ which is attributed to the stretching vibration of ester carbonyl [
41]. Chol shows the wave number 3432 cm
−¹ due to O–H stretching, 2930 cm
−¹ due to C–H stretching, 1454 cm
−¹ due to C=C stretching, and 1054 cm
−¹ due to C–O bending vibrations [
41]. The strong characteristic band in the region 3393 cm
−¹ in blank niosomes is due to the existence of the O–H stretching vibration of the Chol, Span 60,and Tween 60 molecules [
25].
Figure 5 shows the ATR-FTIR of (A) Blank niosome, (B) ALS and (C) ALS-niosome. Major absorption peaks of ALS mainly appeared in wavenumber as follows; the ALS spectrum presented specific peaks on the region 1200–900 cm
−¹ that correspond to C–O and P=O stretches, respectively [
17]. The peak at 913 cm
−¹ is due to hydroxyl group bending vibration, and the absorption peaks at 1016 cm
−¹ and 1046 cm
−¹ are due to P=O stretching vibrations, which are the characteristic peaks of ALS [
42]. The characteristic peaks of the blank niosome was seen at 3393 cm
−¹. This peak likely corresponds to O–H stretching of the ingredient between (Span 60, Tween 60, and Chol). In the ALS niosome, the OH– stretching peak was shifted to 3365 cm
−¹, and this suggests the formation of H-bonds between ALS and the niosomes. Miladi et al. (2015) observed that the characteristic peak of ALS in the region 900 cm
−¹ is also seen in chitosan nanoparticles loaded with ALS and absent in blank nanoparticles. These results confirmed that ALS is entrapped within niosomes [
17].
In the spectrum of blank niosomes, the carbonyl dimer was observed to be shifted to 2918 cm
−1, while the C=O stretching peak was observed to be shifted to 1737 cm
−1. The shifts in the peaks corresponding to the carbonyl groups may be due to Span–Chol interactions, specifically hydrogen bonding, which is a characteristic of the formation of niosomes [
43].
3.3. Stability Studies
The stability of niosome suspensions has always been a critical factor in the formulation process. A stable niosomal dispersion needs to have constant drug entrapment levels and particle sizes at storage conditions. Therefore, short-term stability testing of the highest drug entrapment efficiencies, namely F4, F6, and F10, was carried out for two months. The changes in PS, PDIs, ZP, and EE% during storage at 4 °C were summarized in
Table 5.
In all formulations over the period of the two months of storage, drug leakage was not noticed, since there was no significant difference in EE% (
p > 0.05), as shown in
Table 5. After two months, the EE% of ALS was 62.69 ± 3.01%, 67.27 ± 3.17%, and 52.22 ± 2.66% for K4, K6, and K10, respectively. The PS of the niosomes did not significantly (
p > 0.05) change. Moreover, PDI values were found to be less than 0.1, which indicates the homogenous distribution and the stability of niosomes. In addition, there were no significant changes in the physicochemical parameters, such as appearance and color, and no precipitation was seen during the storage. Moreover, the ZP of all niosomes was within −35 mV, indicating a high formulation stability, and there were no significant changes (
p > 0.05). The above results revealed that the F4, F6, and F10 formulations showed good physical stability at 4 °C after two months.
3.4. In Vitro Release Study
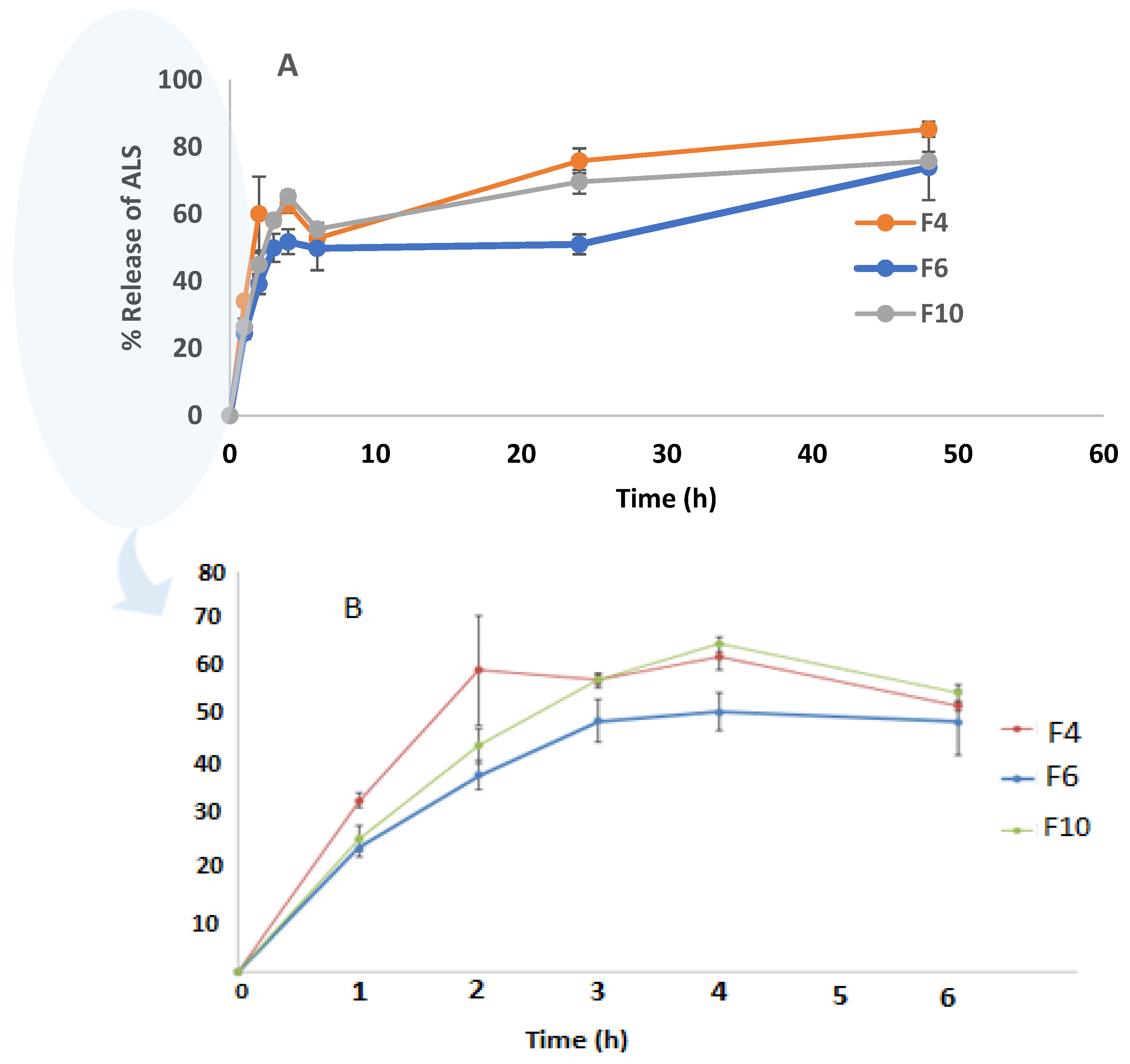
The drug release study was conducted for the highest EE% formulations (F4, F6, and F10) at up to 48 h, as shown in
Figure 6. Drug release from the niosomes in F6 and F10 was slower than F4, which contained the lowest amount of Chol. Increasing the Chol content resulted in a decrease in the release rate. Shirvany, Rezayan et al. (2021) reported that Chol increases the strength of the membrane and reduces the release of cefazolin [
44]. The release percentages of ALS from F4, F6, and F10 were 85.26 ± 2.3%, 73.91 ± 9.72%, and 76.00 ± 2.71%, respectively, after 48 h. There was no significant difference in the release of ALS between F4 and F6 (
p = 0.120). Akbari et al. (2015) reported that there were no significant differences (
p > 0.05) among the overall released amounts of ciprofloxacin (a hydrophilic drug) from the different surfactant type niosomes [
45].
A rapid initial release that lasts for about 6 h was followed by a slower but continuous release period, resulting in the biphasic release of ALS from niosomes. This hydrophilic drug’s biphasic re-release behavior appears to be a characteristic of bilayer vesicles. Similar results were reported in the previous study for ciprofloxacin (a hydrophilic drug) niosomes which have a biphasic release [
26]. The rapid initial phase may be attributed to drug desorption from niosome surface. Following the initial burst release, a continuous ALS release was seen for the next 48 h, which was caused by ALS diffusion from the lipid bilayer [
46,
47]. Based on the above release, F4 had the highest drug release out of all of the formulations. This is because the increase in the Chol amount lowered the release percentage from F6 and F10 niosomal formulations comparing with F4. Nishu, Karmoker et al. (2018) observed that Chol reduces the leakage or permeability of the encapsulating drug by decreasing the niosomal membrane fluidity of the soluble drug linagliptin, which may decrease release percentage [
48].
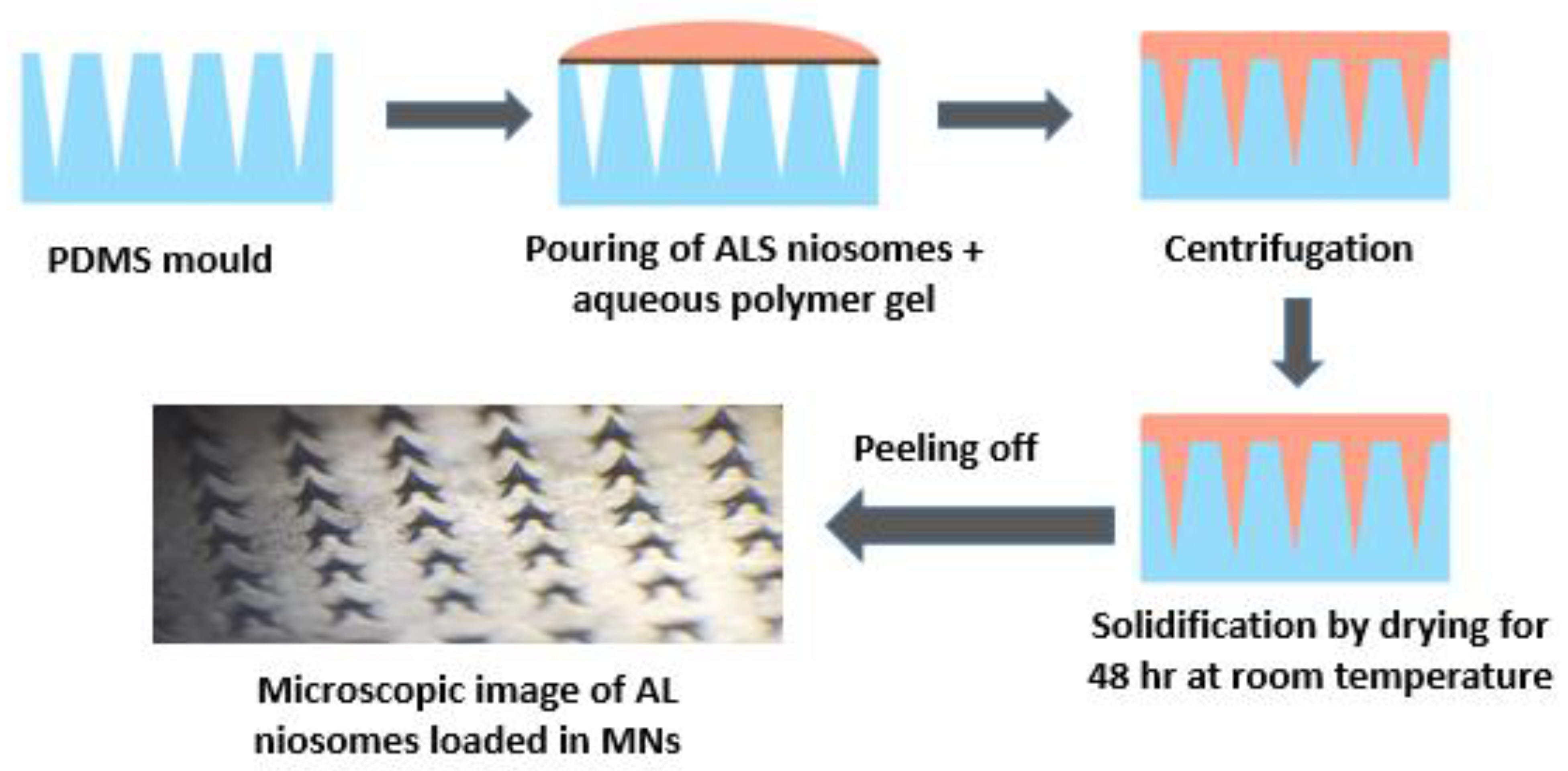
3.6. Fabrication of Polymeric Microneedle Loaded ALS Niosomes
A wider range of drugs including hydrophilic drugs can now be administered through the skin due to advances in transdermal drug delivery, particularly with MNs [
6]. Polymeric MNs have been powerful as a novel transdermal drug delivery platform for effective drug permeation, and have been widely used in the treatment of various diseases [
50]. Polymeric MNs can eliminate sharp biohazard wastes and allow loading of non-potent drugs [
51,
52]. They can also facilitate appropriate therapeutic dosing by controlling the release kinetics of a pre-loaded drug [
53].
Based on the characterization results of niosomes that have been obtained, F4 showed the highest percentage of drug entrapment and release percentage and, therefore, it was selected to be loaded into polymeric MN formulations. Those formulations showed the best physical characteristics upon removal from the mold, as they were hard but not brittle, and sharp, homogenous, and perfectly formed with an elegant appearance. Other formulations were neglected due to their poor physical characteristics upon removal from the mold, and some of them were very hard, brittle or swellable.
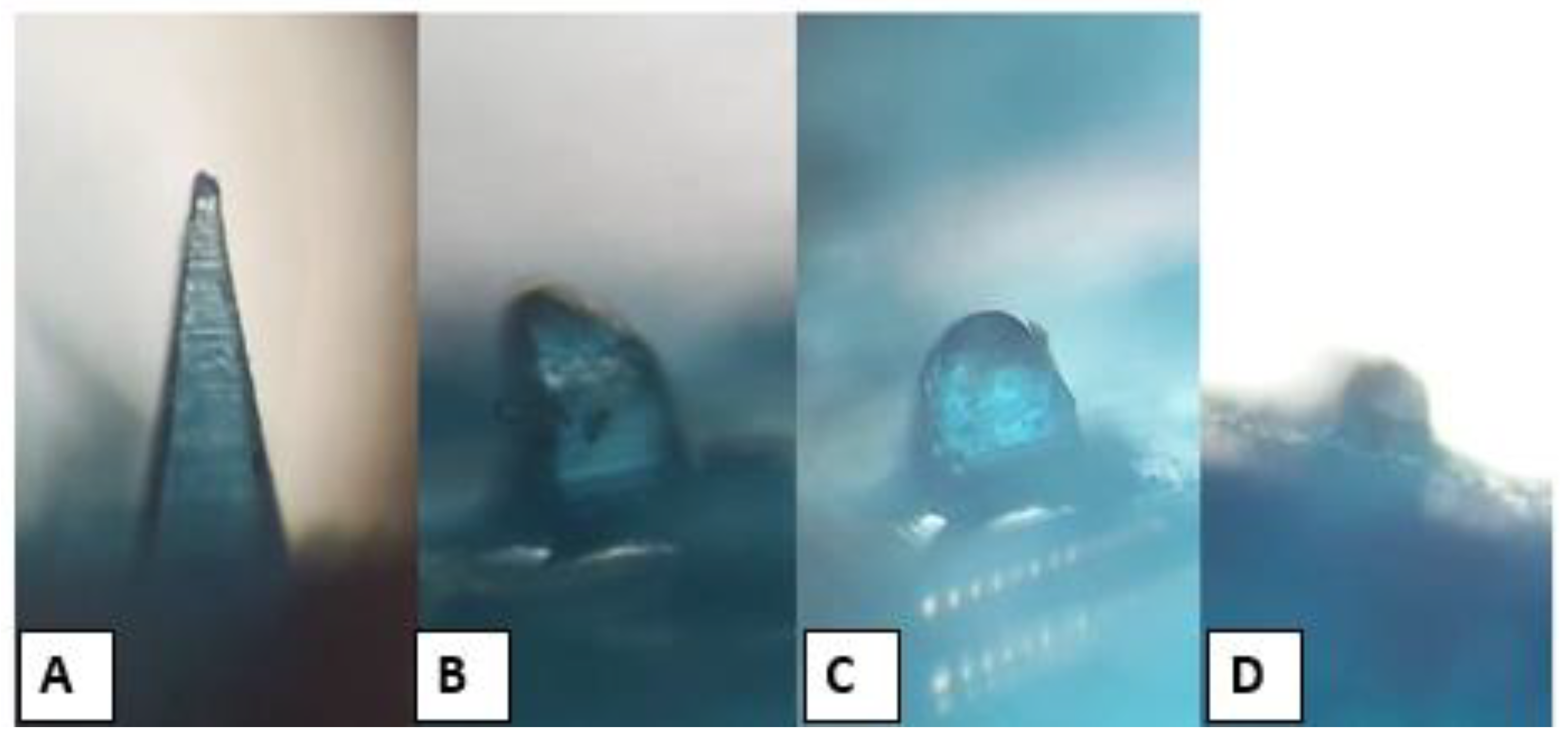
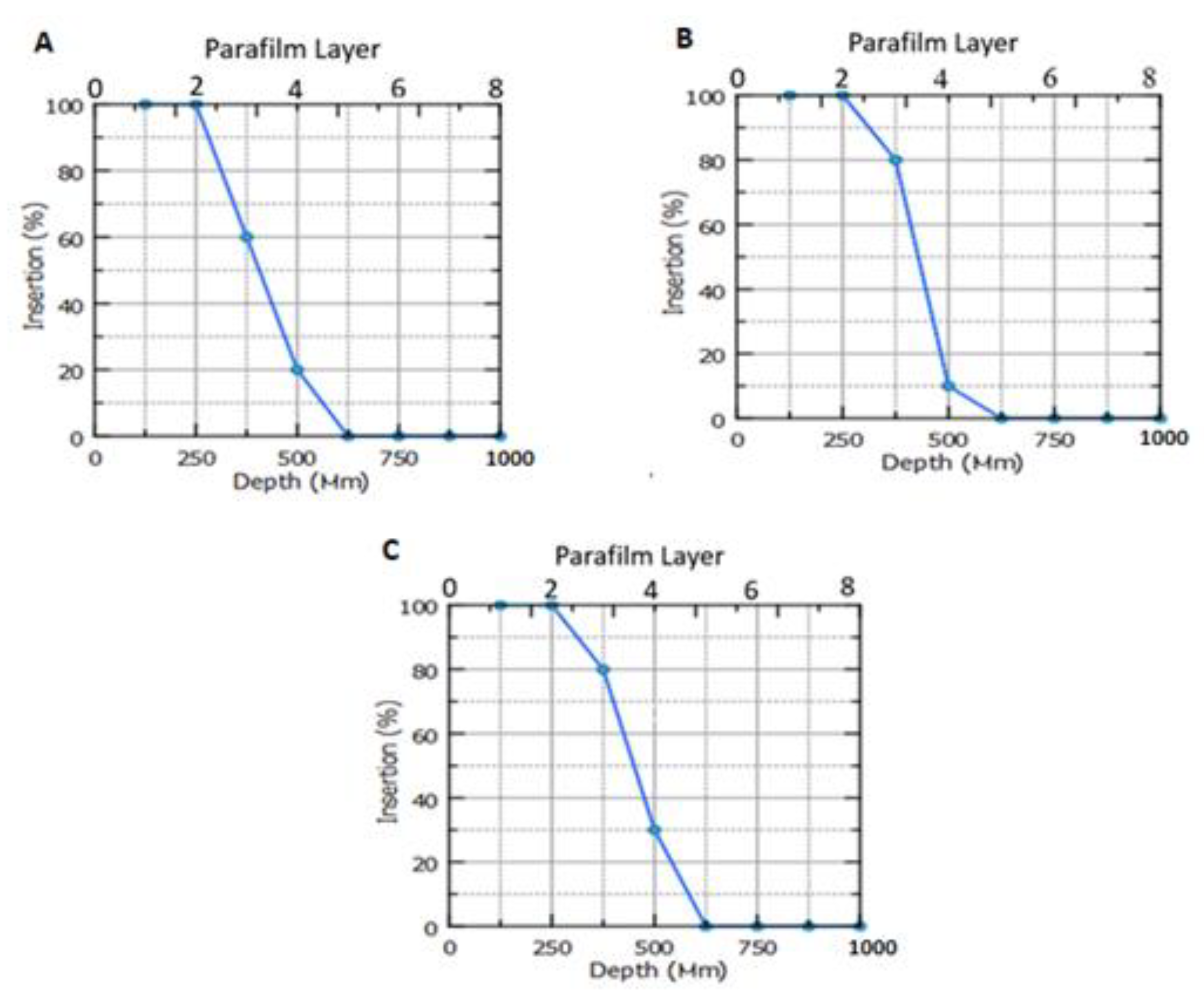
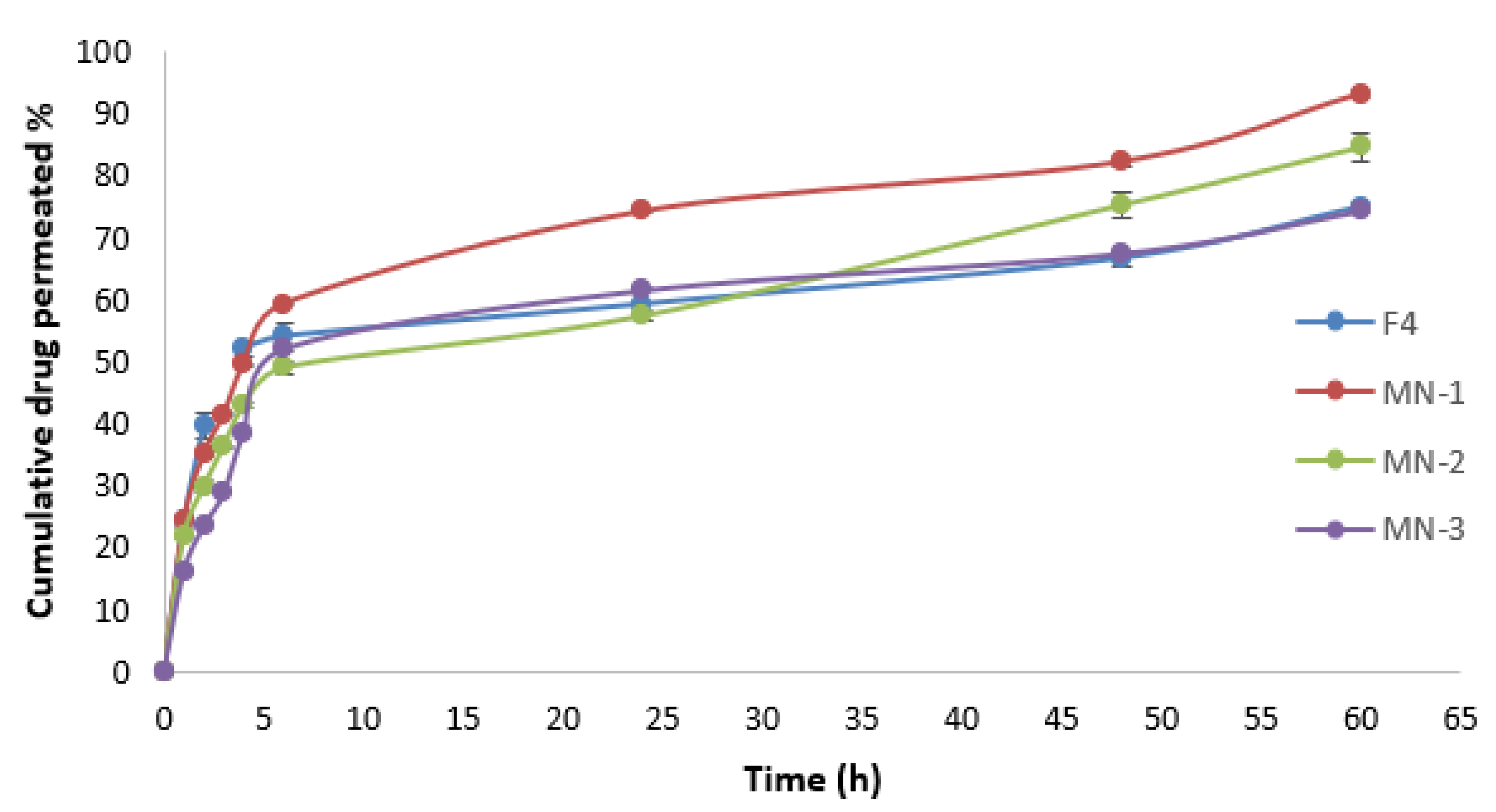
3.13. Drug Permeation Study
Figure 11 depicts the ALS permeation profile across rat skin after the application of niosome-loaded MNs and ALS niosomes as a control. It was found that the MN-1 delivered 1366.66 µg of ALS over a 60 h period, which equates to 93.44% of ALS being delivered. Meanwhile, the MN-2 delivered 1192.43 µg over a 60 h period, which equates to 81.53% of ALS being delivered, and MN-3 delivered 1108.77 µg over a 60 h period. This equates to 75.81% of ALS being delivered. The cumulative amount of ALS (Q) for different formulations was in the following order: MN-1 (containing 30% PVP) > MN-2 (containing 30% PVP:15% PVA (2:1)) > MN-3 (containing 30% PVP:15% PVA (1:1)), as seen in
Table 9. These results may be attributed to the fact that PVP improves the solubility of PVA patches within the skin. Putri, Utami et al. reported that the greater the concentration of PVA, the slower the permeation of the ceftriaxone from DMN with a mixture of 40% PVP and 15% PVA [
61].
The Jss of ALS was 62.18 ± 1.74, 53.64 ± 2.75, and 45.90 ± 4.05 µg/cm²/h for MN-1, MN-2, and MN-3, respectively. The Jss for different formulations was in the following order: MN-1 > MN-2 > MN-3, as summarized in
Table 9. There was a significant difference (
p < 0.05) in Jss between them. The findings demonstrated that an increase in PVA proportion reduces the drug release and enables sustained drug release, probably because considerably slower dissolution kinetics are involved [
62], which is in agreement with a Lee, He et al. (2015) study which reported that using a mixture of PVP and PVA for fabrication of MNs can provide a sustained release [
57].
Ex vivo skin permeation experiments revealed that the cumulative ALS permeated percentage observed using MN loaded niosomes (passive and active method) was better than the cumulative permeated percentage of ALS that permeated through F4 niosomes (passive method); the cumulative permeated percentages of ALS from F4 and MN-1 were 74.88 ± 0.79% and 93.14 ± 0.49%, respectively. The biggest difficulty with transdermal drug delivery is SC. Numerous strategies have been developed in order to penetrate the skin’s primary barrier. Due to its adaptability and capacity for sustained release, the integration of MNs with nano-systems has become more popular during the past 20 years [
63,
64]. Therefore, integrating physical and chemical technology provides a significant improvement in drug delivery. The use of MNs in combination with niosomes is the best possible approach to enhance the permeability and sustain the release of hydrophilic drugs [
65]. This is because the encapsulation ALS in niosomes could increase the concentration of ALS by helping the niosomes to decrease transepidermal water loss, which improves SC hydration and loosens the stratum corneum’s closely packed cellular structure. In addition, niosome adsorption and/or fusion on the skin’s surface can result in a high drug thermodynamic activity gradient at the interface, which is the driving factor for drug permeation to be attached to the SC [
66]. Additionally, MN-created micropores offer extra routes for niosome delivery to the dermis layer of the skin after the disruption of the SC barrier, making more of them available for systemic absorption through dermal microcirculation. Several previous studies were found in the literature describing MN-assisted permeation of nanoparticles [
67,
68]. Without utilizing any specific procedures, a new niosomally encapsulated DMN was produced in simple conditions at ambient temperature. The dual-delivery strategy utilizing niosomes, and MNs can enhance TDD while fostering the prolonged release of therapeutic substances [
6]. The synergistic improvement in skin permeation caused by the combination of MNs and niosomes was explored for the first time in this study.