Chemokine-Decorated Nanoparticles Target Specific Subpopulations of Primary Blood Mononuclear Leukocytes
Abstract
1. Introduction
2. Materials and Methods
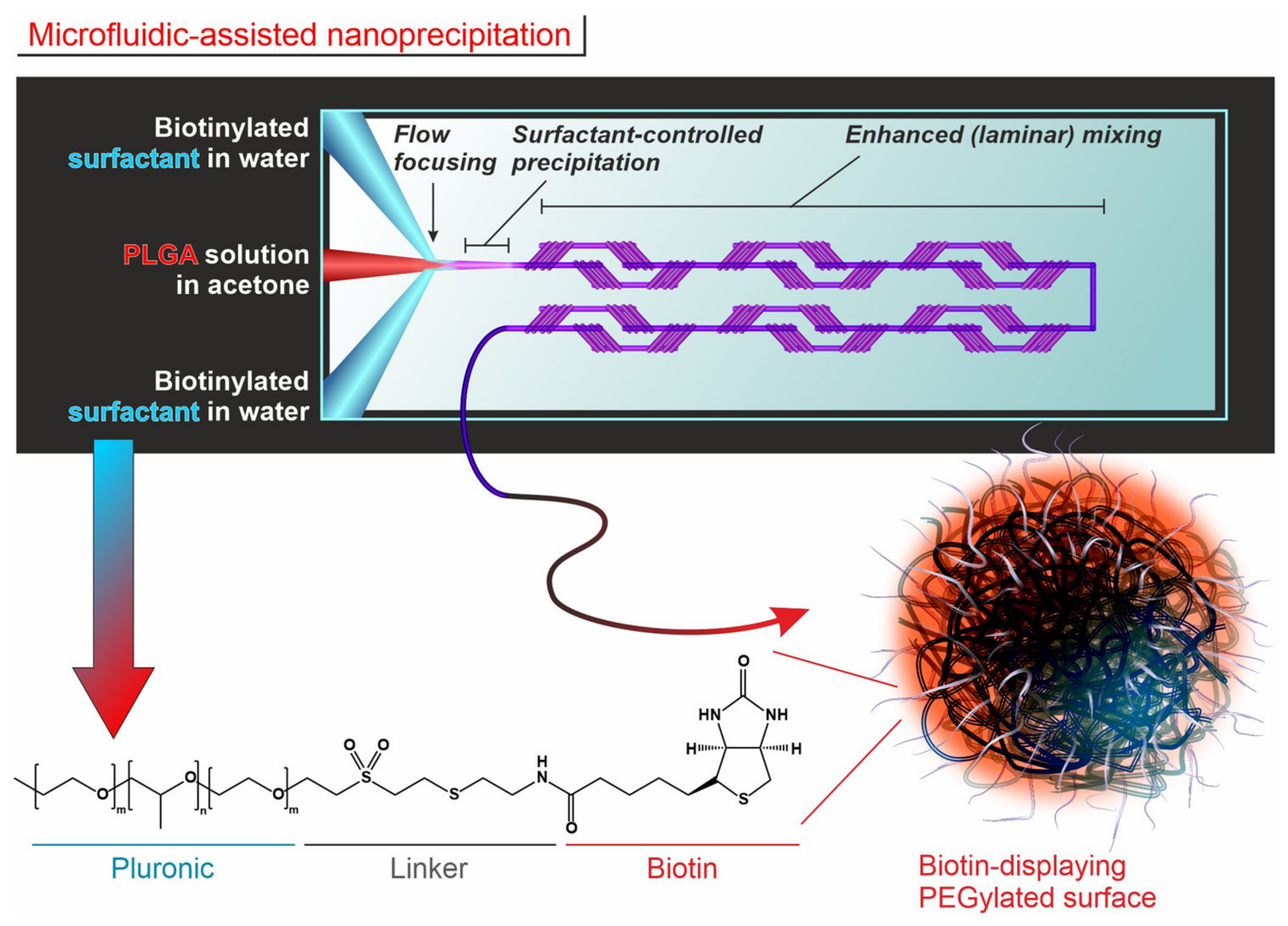
2.1. Nanoparticle Synthesis and Functionalization
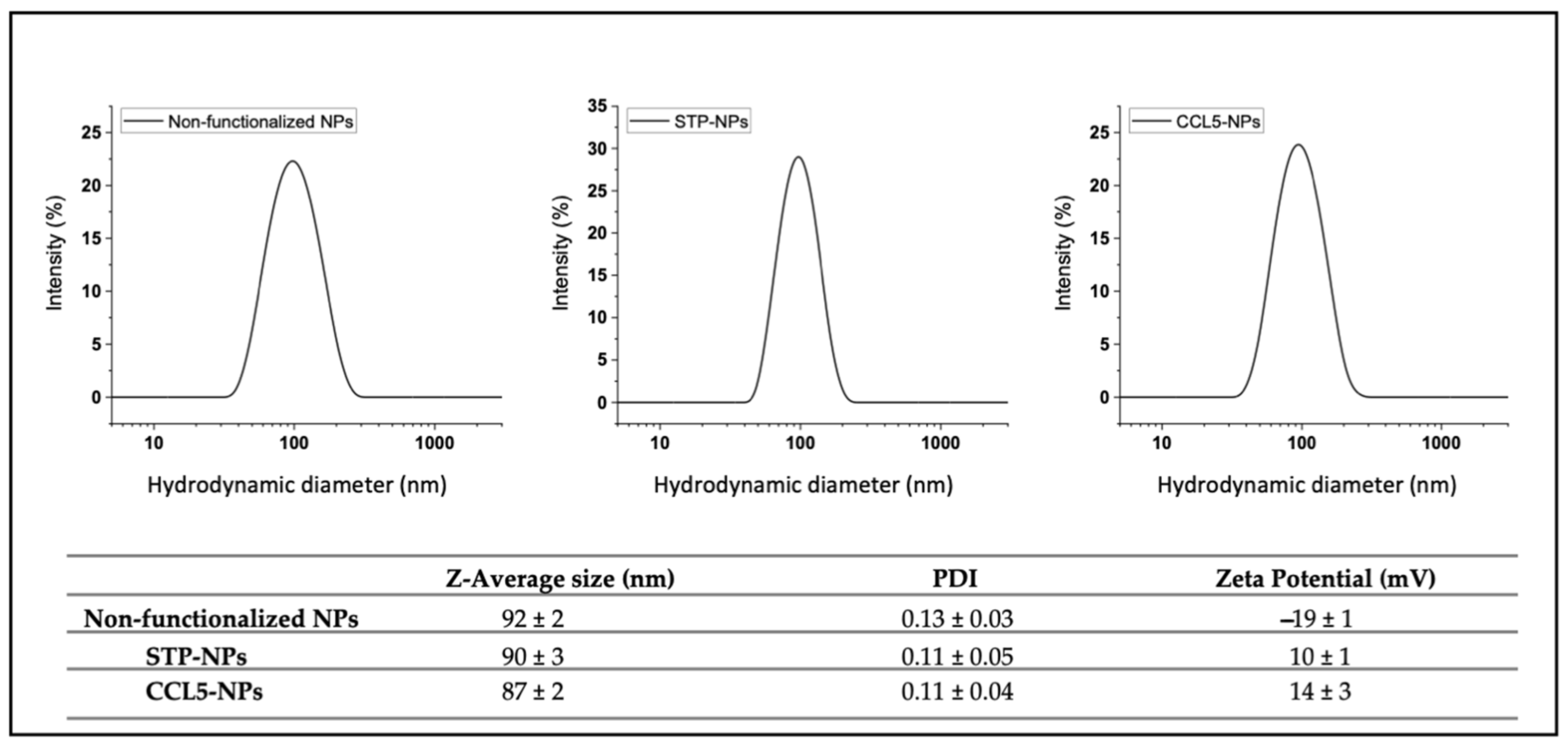
2.2. Nanoparticle Characterization
2.3. Cell Cultures
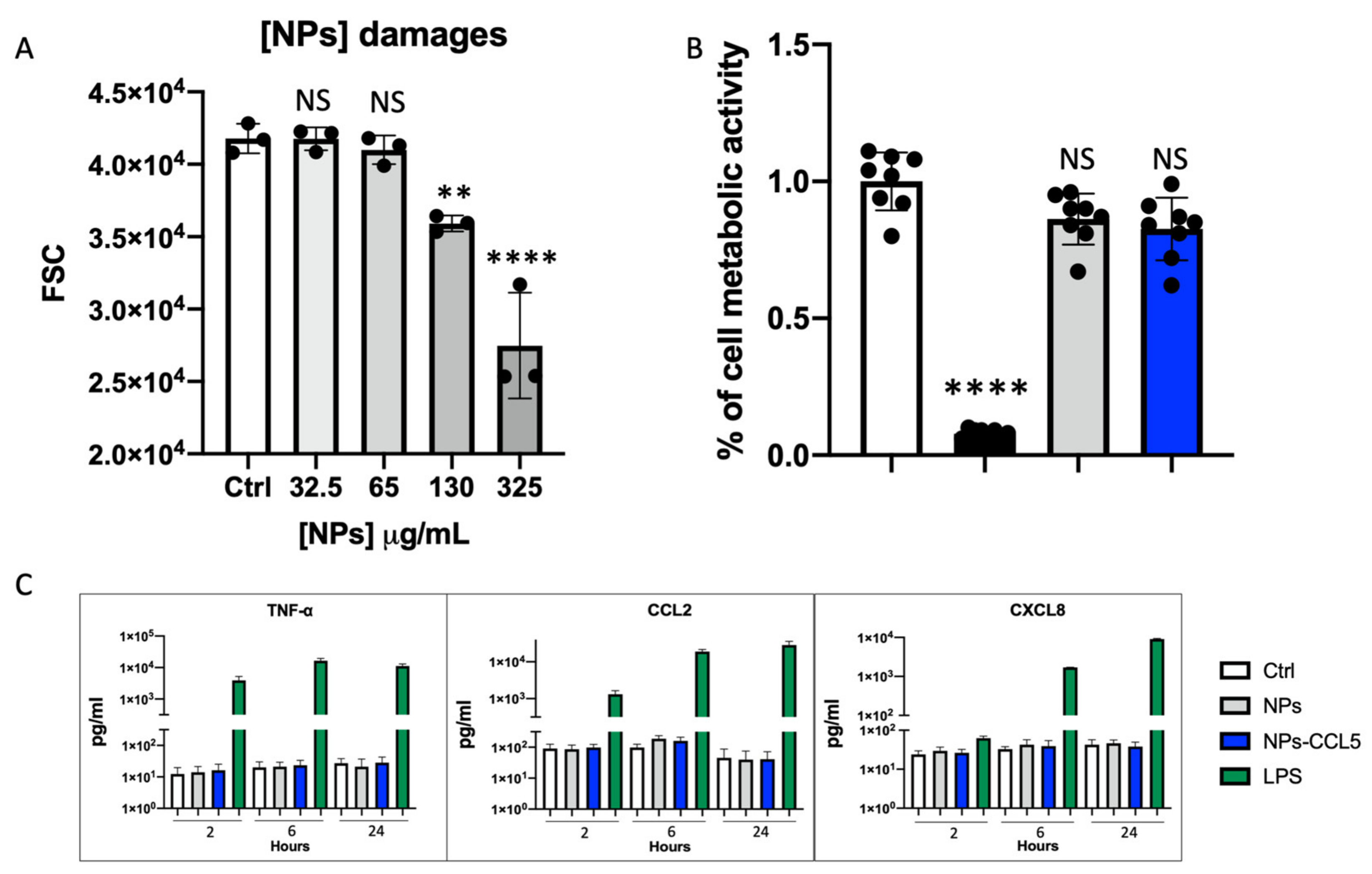
2.4. Cytotoxicity Assays
2.5. Cytokine Release
2.6. NPs Internalization Assays
2.7. Statistical Analysis
3. Results and Discussion
3.1. Nanoparticle Synthesis and Functionalization
3.2. Nanoparticle Biocompatibility
3.3. CCL5 Nanoparticle Internalization through CCR5
3.4. CCL5 Nanoparticle Specific/Selective? Internalization in CCR5high Primary Human Monocytes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rios De La Rosa, J.M.; Spadea, A.; Donno, R.; Lallana, E.; Lu, Y.; Puri, S.; Caswell, P.; Lawrence, M.J.; Ashford, M.; Tirelli, N. Microfluidic-assisted preparation of RGD-decorated nanoparticles: Exploring integrin-facilitated uptake in cancer cell lines. Sci. Rep. 2020, 10, 14505. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Kozma, G.T.; Shimizu, T.; Ishida, T.; Szebeni, J. Anti-PEG antibodies: Properties, formation, testing and role in adverse immune reactions to PEGylated nano-biopharmaceuticals. Adv. Drug Deliv. Rev. 2020, 154–155, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.; Abu Lila, A.S.; Shimizu, T.; Alaaeldin, E.; Hussein, A.; Sarhan, H.A.; Szebeni, J.; Ishida, T. PEGylated liposomes: Immunological responses. Sci. Technol. Adv. Mater. 2019, 20, 710–724. [Google Scholar] [CrossRef] [PubMed]
- Sanità, G.; Carrese, B.; Lamberti, A. Nanoparticle Surface Functionalization: How to Improve Biocompatibility and Cellular Internalization. Front. Mol. Biosci. 2020, 7, 587012. [Google Scholar] [CrossRef] [PubMed]
- Cagliani, R.; Gatto, F.; Bardi, G. Protein adsorption: A feasible method for nanoparticle functionalization? Materials 2019, 12, 1991. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. AMD3100/CXCR4 inhibitor. Front. Immunol. 2015, 6, 276. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Zhang, T.-Y.; Koyanagi, Y.; Tanaka, Y.; Kim, J.; Suzuki, Y.; Minoguchi, S.; Tamamura, H.; Waki, M.; Matsumoto, A.; et al. Inhibitory Mechanism of the CXCR4 Antagonist T22 against Human Immunodeficiency Virus Type 1 Infection. J. Virol. 1999, 73, 7489–7496. [Google Scholar] [CrossRef] [PubMed]
- Bardi, G. Chemokines and nanomaterials: Interaction for useful immune-applications. Explor. Immunol. 2022, 2, 637–647. [Google Scholar] [CrossRef]
- De la Torre, C.; Casanova, I.; Acosta, G.; Coll, C.; Moreno, M.J.; Albericio, F.; Aznar, E.; Mangues, R.; Royo, M.; Sancenón, F.; et al. Gated Mesoporous Silica Nanoparticles Using a Double-Role Circular Peptide for the Controlled and Target-Preferential Release of Doxorubicin in CXCR4-Expresing Lymphoma Cells. Adv. Funct. Mater. 2015, 25, 687–695. [Google Scholar] [CrossRef]
- Liu, J.Y.; Chiang, T.; Liu, C.H.; Chern, G.G.; Lin, T.T.; Gao, D.Y.; Chen, Y. Delivery of siRNA Using CXCR4-targeted nanoparticles modulates tumor microenvironment and achieves a potent antitumor response in liver cancer. Mol. Ther. 2015, 23, 1772–1782. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Nakashima, H.; Ueda, T.; Naba, H.; Ikoma, R.; Otaka, A.; Terakawa, Y.; Tamamura, H.; Ibuka, T.; Murakami, T.; et al. A novel anti-HIV synthetic peptide, T-22 ([Tyr5,12,Lys7]-polyphemusin II). Biochem. Biophys. Res. Commun. 1992, 189, 845–850. [Google Scholar] [CrossRef]
- Rioja-Blanco, E.; Gallardo, A.; Arroyo-Solera, I.; Álamo, P.; Casanova, I.; Unzueta, U.; Serna, N.; Sánchez-García, L.; Quer, M.; Villaverde, A.; et al. A Novel CXCR4-Targeted Diphtheria Toxin Nanoparticle Inhibits Invasion and Metastatic Dissemination in a Head and Neck Squamous Cell Carcinoma Mouse Model. Pharmaceutics 2022, 14, 887. [Google Scholar] [CrossRef] [PubMed]
- Lesniak, W.G.; Azad, B.B.; Chatterjee, S.; Lisok, A.; Pomper, M.G. An Evaluation of CXCR4 Targeting with PAMAM Dendrimer Conjugates for Oncologic Applications. Pharmaceutics 2022, 14, 655. [Google Scholar] [CrossRef] [PubMed]
- Cagliani, R.; Gatto, F.; Cibecchini, G.; Marotta, R.; Catalano, F.; Sanchez-Moreno, P.; Pompa, P.P.; Bardi, G. CXCL5 Modified Nanoparticle Surface Improves CXCR2+ Cell Selective Internalization. Cells 2019, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Pisani, A.; Donno, R.; Gennari, A.; Cibecchini, G.; Catalano, F.; Marotta, R.; Pompa, P.P.; Tirelli, N.; Bardi, G. CXCL12-PLGA/Pluronic Nanoparticle Internalization Abrogates CXCR4-Mediated Cell Migration. Nanomaterials 2020, 10, 2304. [Google Scholar] [CrossRef] [PubMed]
- Donno, R.; Gennari, A.; Lallana, E.; De La Rosa, J.M.R.; D’Arcy, R.; Treacher, K.; Hill, K.; Ashford, M.; Tirelli, N. Nanomanufacturing through microfluidic-assisted nanoprecipitation: Advanced analytics and structure-activity relationships. Int. J. Pharm. 2017, 534, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Pisani, A.; Bardi, G. Immunology of biodegradable nanoparticles: A brief overview on a wide growing field. Explor. Immunol. 2021, 1, 48–60. [Google Scholar] [CrossRef]
- Hughes, C.E.; Nibbs, R.J.B. A guide to chemokines and their receptors. Febs J. 2018, 285, 2944. [Google Scholar] [CrossRef] [PubMed]
- Hernaez, B.; Alcamí, A. Virus-encoded cytokine and chemokine decoy receptors. Curr. Opin. Immunol. 2020, 66, 50–56. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisani, A.; Donno, R.; Valenti, G.; Pompa, P.P.; Tirelli, N.; Bardi, G. Chemokine-Decorated Nanoparticles Target Specific Subpopulations of Primary Blood Mononuclear Leukocytes. Nanomaterials 2022, 12, 3560. https://doi.org/10.3390/nano12203560
Pisani A, Donno R, Valenti G, Pompa PP, Tirelli N, Bardi G. Chemokine-Decorated Nanoparticles Target Specific Subpopulations of Primary Blood Mononuclear Leukocytes. Nanomaterials. 2022; 12(20):3560. https://doi.org/10.3390/nano12203560
Chicago/Turabian StylePisani, Anissa, Roberto Donno, Giulio Valenti, Pier Paolo Pompa, Nicola Tirelli, and Giuseppe Bardi. 2022. "Chemokine-Decorated Nanoparticles Target Specific Subpopulations of Primary Blood Mononuclear Leukocytes" Nanomaterials 12, no. 20: 3560. https://doi.org/10.3390/nano12203560
APA StylePisani, A., Donno, R., Valenti, G., Pompa, P. P., Tirelli, N., & Bardi, G. (2022). Chemokine-Decorated Nanoparticles Target Specific Subpopulations of Primary Blood Mononuclear Leukocytes. Nanomaterials, 12(20), 3560. https://doi.org/10.3390/nano12203560







