The Golden Fig: A Plasmonic Effect Study of Organic-Based Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
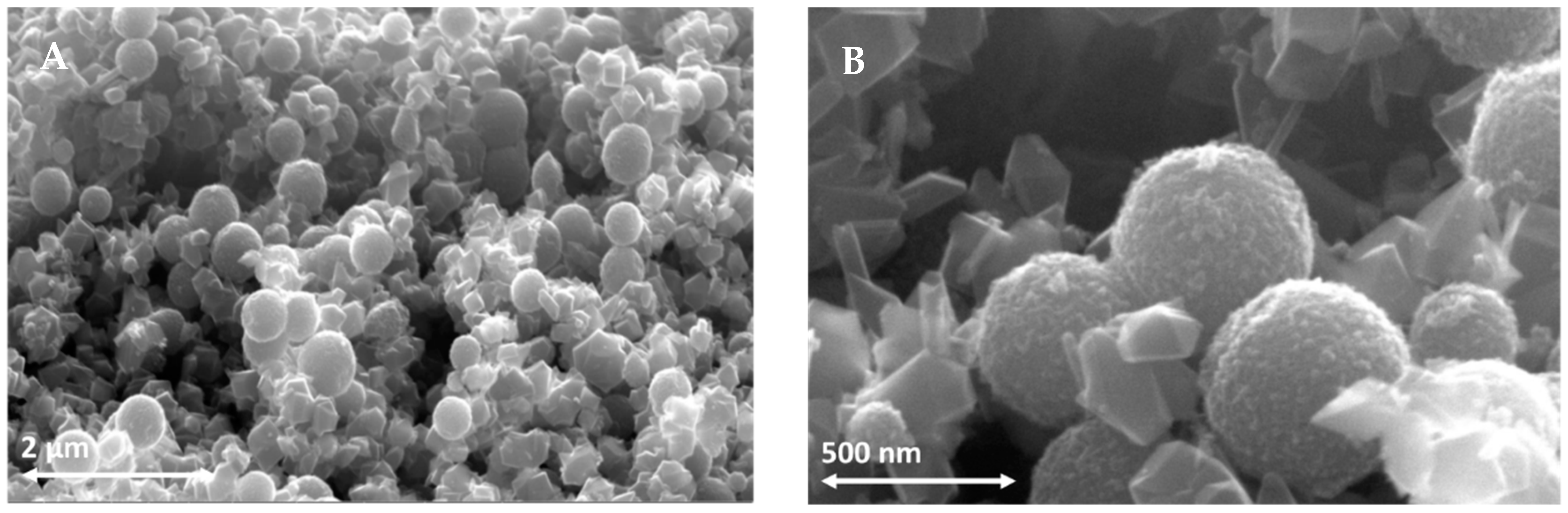
2.1. Preparation of TiO2 Sub-Micrometric Powder and Paste for Scattering
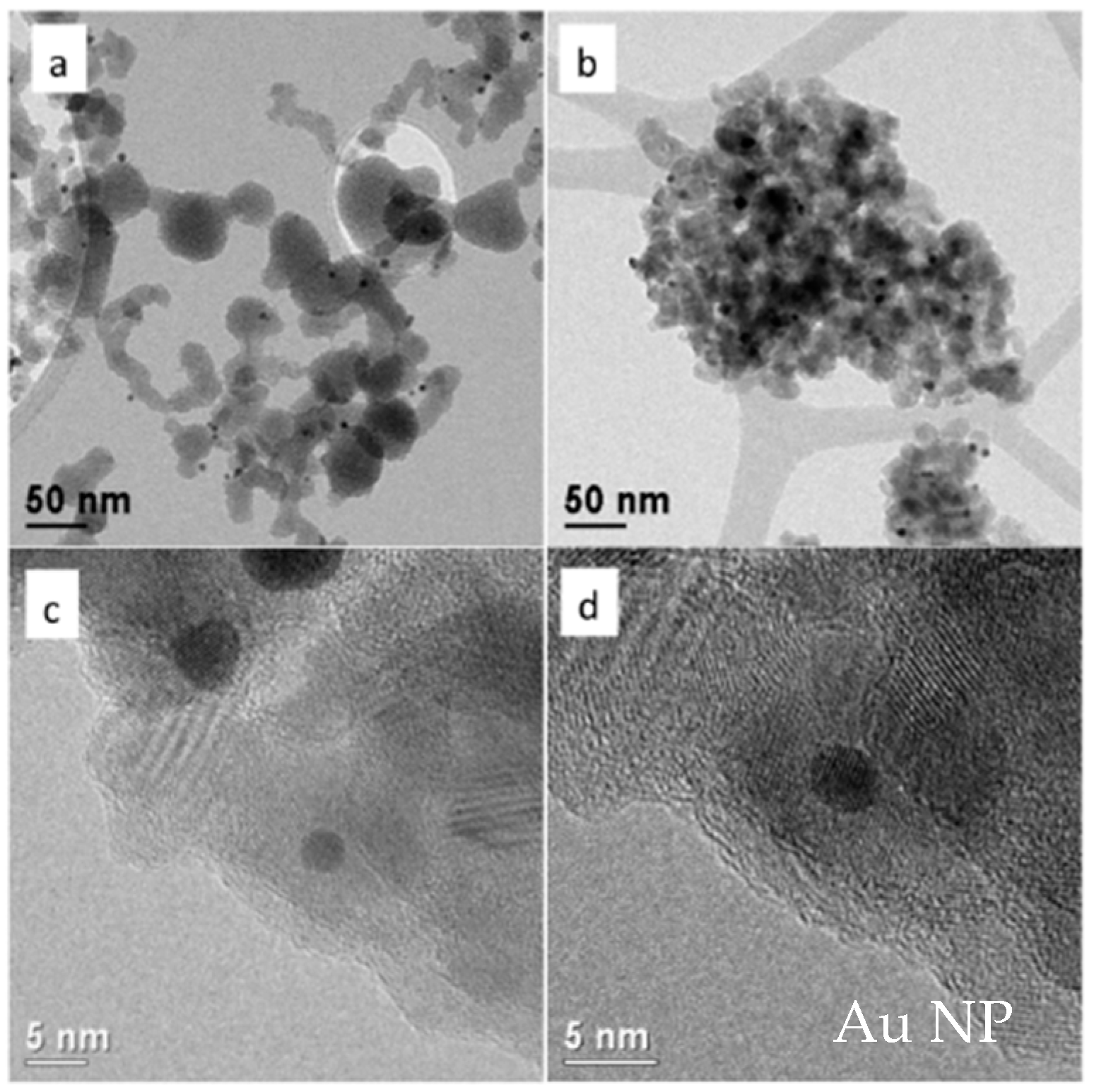
2.2. Preparation of Au@TiO2 Nanoparticles
- (a)
- The Au NPs are synthesized by adding 1 mL of 11.2 mM NaBH4 dropwise under vigorous stirring to an aqueous solution of 20 mL of 1 mM HAuCl4 and 1.6 mL of 38.8 mM TC, the latter working as a capping agent, which restricts crystal growth to the desired size. By the addition of NaBH4, the colorless solution immediately turns ruby red, indicating the formation of the Au NPs. The sol is stable for about one month, after which some black precipitate starts to form.
- (b)
- The TC capping agent is substituted by sodium 3-mercaptopropionate (NaMP), by adding it dropwise, under stirring and at room temperature, to the solution containing the Au NPs. Dropping must be carried out very slowly (1 drop every 20 s), otherwise the solution turns a violet color, indicating that the particles have increased in size.
- (c)
- To embed the Au NPs into TiO2 nanoparticles, 80 μL of titanium tetrabutooxide (TTB) is dissolved in 100 mL of ethanol. Then, 50 mL of the solution containing the NaMP-capped Au NPs is added dropwise and, finally, it is left in reflux for 45 min at 80 °C. The dispersion becomes turbid and assumes a violet color. The solution is finally centrifuged 3 times at 9000 rpm for 30 min and the particles are thermally treated in an oven at 500 °C for 30 min.
2.3. Preparation of the TiO2 Paste
2.4. Preparation of Electrodes
- J8* (LiI 0.1 M, I2 0.05 M, MPII 0.6 M, TBP 0.5 M in AN:VN 70:30) for N719 [40];
2.5. Realization of the DSSC Module
2.6. Preparation of Dye Sensitizer
2.7. Measurement
3. Results
3.1. The Scattering Layer
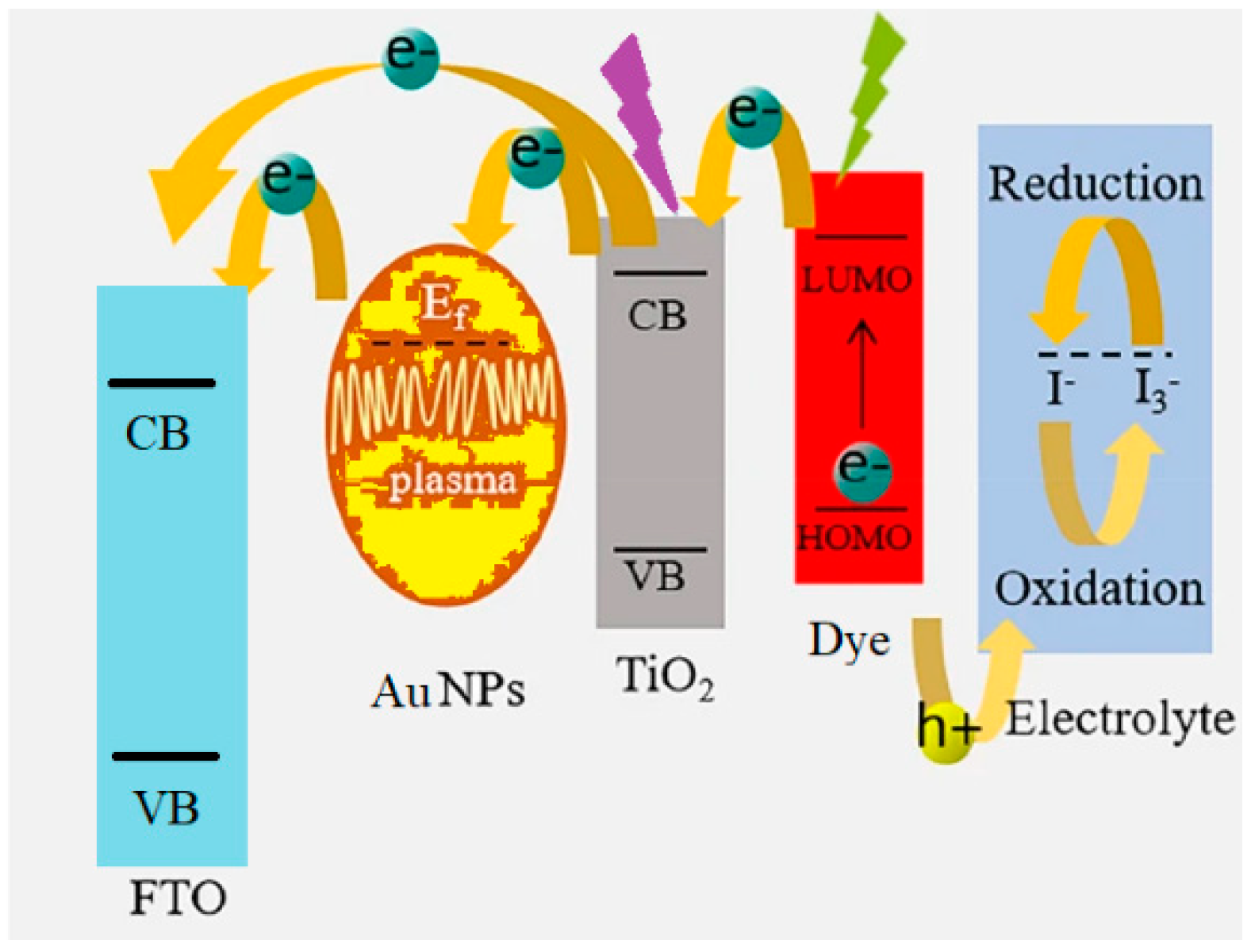
3.2. Au@TiO2
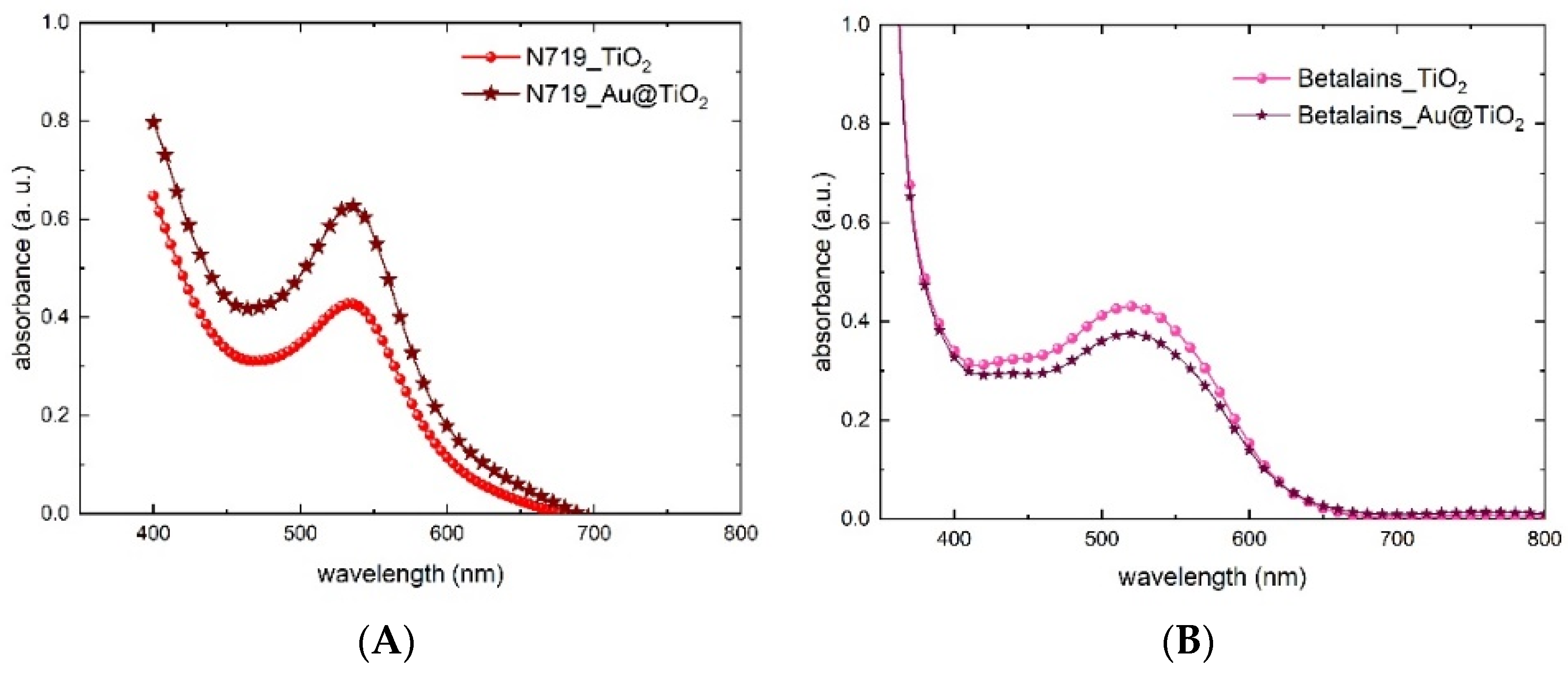
3.3. The Working Electrode
3.4. Photovoltaic Performances
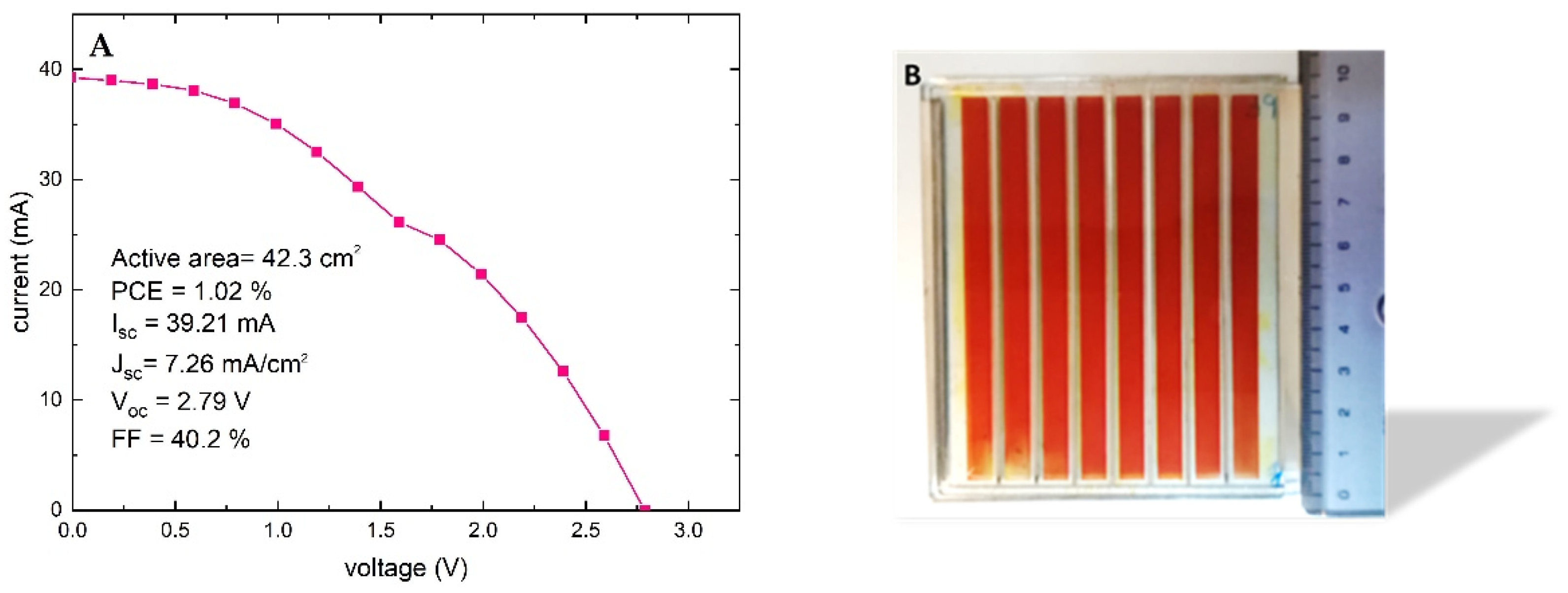
3.5. Natural-Based Dye-Sensitized Solar Module
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy trans-formation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Parida, B.; Iniyan, S.; Goic, R. A review of solar photovoltaic technologies. Renew. Sustain. Energy Rev. 2011, 15, 1625–1636. [Google Scholar] [CrossRef]
- Bellani, S.; Bartolotta, A.; Agresti, A.; Calogero, G.; Grancini, G.; Di Carlo, A.; Kymakis, E.; Bonaccorso, F. Solution-processed two-dimensional materials for next-generation photovoltaics. Chem. Soc. Rev. 2021, 50, 11870–11965. [Google Scholar] [CrossRef] [PubMed]
- NERL. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 31 October 2021).
- Available online: https://scholar.google.com/scholar?q=DSSC&hl=it&as_sdt=0%2C5&as_rr=1&as_vis=1&as_ylo=2020&as_yhi=2021 (accessed on 31 October 2021).
- Shakeel Ahmada, M.; Pandeya, A.K.; Abd Rahima, N. Advancements in the development of TiO2 photoanodes and its fabrication methods for dye sensitized solar cell (DSSC) applications. A review. Renew. Sustain. Energy Rev. 2017, 77, 89–108. [Google Scholar] [CrossRef]
- Gong, J.; Sumathy, K.; Qiao, Q.; Zhou, Z. Review on dye-sensitized solar cells (DSSCs): Advanced techniques and research trends. Renew. Sustain. Energy Rev. 2017, 68, 234–246. [Google Scholar] [CrossRef]
- Devadiga, D.; Selvakumar, M.; Shetty, P.; Santosh, M.S. Dye-Sensitized Solar Cell for Indoor Applications: A Mini-Review. J. Electron. Mater. 2021, 50, 3187–3206. [Google Scholar] [CrossRef]
- Freitag, M.; Teuscher, J.; Saygili, Y.; Zhang, X.; Giordano, F.; Liska, P.; Hua, J.; Zakeeruddin, S.M.; Moser, J.-E.; Grätzel, M.; et al. Dye-sensitized solar cells for efficient power generation under ambient lighting. Nat. Photon 2017, 11, 372–378. [Google Scholar] [CrossRef]
- O’Reagan, B.; Grätzel, M. A low-cost, high-efficiency Solar-Cell based on Dye-Sensitized Colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Sharifi, N.; Tajabadi, F.; Taghavinia, N. Recent Developments in Dye-Sensitized Solar Cells. ChemPhysChem 2014, 15, 3902–3927. [Google Scholar] [CrossRef] [PubMed]
- Nazeeruddin, M.K.; De Angelis, F.; Fantacci, S.; Selloni, A.; Viscardi, G.; Liska, P.; Ito, S.; Takeru, B.; Grätzel, M. Combined ex-perimental and DFT-TDDFT computational study of photoelectrochemical cell ruthenium sensitizers. J. Am. Chem. Soc. 2005, 127, 16835–16847. [Google Scholar] [CrossRef]
- Liu, X.; Iocozzia, J.; Wang, Y.; Cui, X.; Chen, Y.; Zhao, S.; Li, Z.; Lin, Z. Noble metal–metal oxide nanohybrids with tailored nanostructures for efficient solar energy conversion, photocatalysis and environmental remediation. Energy Environ. Sci. 2017, 10, 402–434. [Google Scholar] [CrossRef]
- Atwater, H.A.; Polman, A. Plasmonics for improved photovoltaic devices. Nat. Mater. 2010, 9, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Clavero, C. Plasmon-induced hot-electron generation at nanoparticle/metal-oxide interfaces for photovoltaic and photocatalytic devices. Nat. Photonics 2014, 8, 95–103. [Google Scholar] [CrossRef]
- Liu, Z.-X.; You, C.; Wang, B.; Dong, H.; Xiong, H.; Wu, Y. Nanoparticle-mediated chiral light chaos based on non-Hermitian mode coupling. Nanoscale 2020, 12, 2118–2125. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Si, L.-G.; Guo, J.F.; Lü, X.-Y.; Yang, X. Classical theory of cylindrical nonlinear optics: Second-harmonic generation. Phys. Rev. A 2011, 83, 063845. [Google Scholar] [CrossRef]
- Wen, C.; Ishikawa, K.; Kishima, M.; Yamada, K. Effects of silver particles on the photovoltaic properties of dyesensitized TiO2 thin films. Sol. Energy Mater. Sol. Cells 2000, 61, 339–351. [Google Scholar] [CrossRef]
- Hou, W.; Pavaskar, P.; Liu, Z.; Theiss, J.; Aykol, M.; Cronin, S.B. Plasmon resonant enhancement of dye sensitized solar cells. Energy Environ. Sci. 2011, 4, 4650–4655. [Google Scholar] [CrossRef]
- Naphade, R.A.; Tathavadekar, M.; Jog, J.P.; Agarkar, S.; Ogale, S. Plasmonic light harvesting of dye sensitized solar cells by Au-nanoparticle loaded TiO2 nanofibers. J. Mater. Chem. A 2014, 2, 975–984. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, F.; Liu, Y.; Cui, K.; Feng, X.; Zhang, W.; Huang, Y. Broadband light absorption enhancement in dye-sensitized solar cells with Au-Ag alloy popcorn nanoparticles. Sci. Rep. 2013, 3, 2112. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.; Koo, B.; Kim, J.-Y.; Kim, T.; Son, H.J.; Kim, B.; Kim, J.Y.; Lee, D.-K.; Kim, H.; Cho, J.; et al. Enhanced Photovoltaic Properties and Long-Term Stability in Plasmonic Dye-Sensitized Solar Cells via Noncorrosive Redox Mediator. ACS Appl. Mater. Interfaces 2014, 6, 19191–19200. [Google Scholar] [CrossRef]
- Erwin, W.R.; Zarick, H.F.; Talbert, E.M.; Bardhan, R. Light trapping in mesoporous solar cells with plasmonic nanostructures. Energy Environ. Sci. 2016, 9, 1577–1601. [Google Scholar] [CrossRef] [Green Version]
- Jang, Y.H.; Jang, Y.J.; Kim, S.; Quan, L.N.; Chung, K.; Kim, D.H. Plasmonic Solar Cells: From Rational Design to Mechanism Overview. Chem. Rev. 2016, 116, 14982–15034. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Liu, S.; Xu, Y.-J. Recent progress on metal core@semiconductor shell nanocomposites as a promising type of photocatalyst. Nanoscale 2012, 4, 2227–2238. [Google Scholar] [CrossRef]
- Li, G.; Tang, Z. Nanoscale, Noble metal nanoparticle@metal oxide core/yolk–shell nanostructures as catalysts: Recent progress and perspective. Nanoscale 2014, 6, 3995–4011. [Google Scholar] [CrossRef]
- Rai, P.; Majhi, S.M.; Yu, Y.-T.; Lee, J.-H. Noble metal@metal oxide semiconductor core@shell nano-architectures as a new platform for gas sensor applications. RSC Adv. 2015, 5, 76229–76248. [Google Scholar] [CrossRef]
- Jiang, R.; Li, B.; Fang, C.; Wang, J. Unraveling the Evolution and Nature of the Plasmons in (Au Core)–(Ag Shell) Nanorods. Adv. Mater. 2014, 26, 5274–5309. [Google Scholar] [CrossRef]
- Rai, P.; Yoon, J.W.; Jeong, H.M.; Hwang, S.J.; Kwak, C.H.; Lee, J.H. Design of highly sensitive and selective Au@NiO yolk–shell nanoreactors for gas sensor applications. Nanoscale 2014, 6, 8292–8299. [Google Scholar] [CrossRef]
- Choi, H.; Chen, W.T.; Kamat, P.V. Know Thy Nano Neighbor. Plasmonic versus Electron Charging Effects of Metal Nano-particles in Dye-Sensitized Solar Cells. ACS Nano 2012, 6, 4418–4427. [Google Scholar] [CrossRef]
- Hore, S.; Vetter, C.; Kern, R.; Smit, H.; Hinsch, A. Influence of scattering layers on efficiency of dye-sensitized solar cells. Sol. Energy Mater. Sol. Cells 2006, 90, 1176–1188. [Google Scholar] [CrossRef]
- Lee, J.-K.; Jeong, B.-H.; Jang, S.-I.; Kim, Y.-G.; Jang, Y.-W.; Lee, S.-B.; Kim, M.-R. Preparations of TiO2 pastes and its application to light-scattering layer for dye-sensitized solar cells. J. Ind. Eng. Chem. 2009, 15, 724–729. [Google Scholar] [CrossRef]
- Calogero, G.; Bartolotta, A.; Di Marco, G.; Di Carlo, A.; Bonaccorso, F. Vegetable-based dye-sensitized solar cells. Chem. Soc. Rev. 2015, 44, 3244–3294. [Google Scholar] [CrossRef] [PubMed]
- Bartolotta, A.; Calogero, G. Dye-sensitized solar cells: From synthetic dyes to natural pigments. Solar Cells Light Manag. 2019, 107–161. [Google Scholar] [CrossRef]
- Chen, D.; Cao, L.; Huang, F.; Imperia, P.; Cheng, Y.-B.; Caruso, R.A. Synthesis of Monodisperse Mesoporous Titania Beads with Controllable Diameter, High Surface Areas, and Variable Pore Diameters (14−23 nm). J. Am. Chem. Soc. 2010, 132, 4438–4444. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hua, C.Z. Size Tuning, Functionalization, and Reactivation of Au in TiO2 Nanoreactors. Angew. Chem. Intern. Ed. 2005, 44, 4342–4345. [Google Scholar] [CrossRef] [PubMed]
- Samal, A.K.; Sreeprasad, T.S.; Pradeep, T. Investigation of the role of NaBH4 in the chemical synthesis of gold nanorods. J. Nanopart. Res. 2009, 12, 1777–1786. [Google Scholar] [CrossRef]
- Barbé, C.J.; Arendse, F.; Comte, P.; Jirousek, M.; Lenzmann, F.; Shklover, V.; Grätzel, M. Nanocrystalline Titanium Oxide Electrodes for Photovoltaic Applications. J. Am. Ceram. Soc. 2005, 80, 3157–3171. [Google Scholar] [CrossRef]
- Shklover, V.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Barbe, C.; Kay, A.; Haibach, T.; Steurer, W.; Hermann, R.; Nissen, H.-U.; Grätzel, M. Structure of nanocrystalline TiO2 powders and precursor to their highly efficient photosensitizer. Chem. Mater. 1997, 9, 430–439. [Google Scholar] [CrossRef]
- Calogero, G.; Yum, J.-H.; Sinopoli, A.; Di Marco, G.; Grätzel, M.; Nazeeruddin, M.K. Anthocyanins and betalains as light-harvesting pigments for dye-sensitized solar cells. Sol. Energy 2012, 86, 1563–1575. [Google Scholar] [CrossRef]
- Calogero, G.; Barichello, J.; Citro, I.; Mariani, P.; Vesce, L.; Bartolotta, A.; Di Carlo, A.; Di Marco, G. Photoelectrochemical and spectrophotometric studies on dye-sensitized solar cells (DSCs) and stable modules (DSCMs) based on natural apocarotenoids pigments. Dye. Pigment. 2018, 155, 75–83. [Google Scholar] [CrossRef]
- Mariani, P.; Vesce, L.; Di Carlo, A. The role of printing techniques for large-area dye sensitized solar cells. Semicond. Sci. Technol. 2015, 30, 104003. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of Size and Concentration of Gold Nanoparticles from UV−Vis Spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.D.; Suteewong, T.; Kumar, R.S.S.; D’Innocenzo, V.; Petrozza, A.; Lee, M.M.; Wiesner, U.; Snaith, H.J. Plasmonic Dye-Sensitized Solar Cells Using Core−Shell Metal−Insulator Nanoparticles. Nano Lett. 2011, 11, 438–445. [Google Scholar] [CrossRef]
- Shah, A.A.; Umar, A.A.; Salleh, M.M. Efficient quantum capacitance enhancement in DSSC by gold nanoparticles plasmonic effect. Electrochim. Acta 2016, 195, 134–142. [Google Scholar] [CrossRef]
- Calogero, G.; Di Marco, G.; Cazzanti, S.; Caramori, S.; Argazzi, R.; Di Carlo, A.; Bignozzi, C.A. Efficient Dye-Sensitized Solar Cells Using Red Turnip and Purple Wild Sicilian Prickly Pear Fruits. Int. J. Mol. Sci. 2010, 11, 254–267. [Google Scholar] [CrossRef]
- Liu, C.; Liang, M.; Khaw, C. Effect of gold nanoparticles on the performances of TiO2 dye sensitised solar cell. Ceram. Int. 2018, 44, 5926–5931. [Google Scholar] [CrossRef]
- Calogero, G.; Di Marco, G.; Caramori, S.; Cazzanti, S.; Argazzi, R.; Bignozzi, C.A. Natural dye senstizers for photoelectrochemical cells. Energy Environ. Sci. 2009, 2, 1162–1172. [Google Scholar] [CrossRef]
- Vesce, L.; Guidobaldi, A.; Mariani, P.; DI Carlo, A.; Parisi, M.L.; Maranghi, S.; Basosi, R. Scaling-up of Dye Sensitized Solar Modules. In World Scientific Reference of Hybrid Materials; World Scientific: Singapore, 2019; pp. 423–485. [Google Scholar]
- Giordano, F.; Guidobaldi, A.; Petrolati, E.; Vesce, L.; Riccitelli, R.; Reale, A.; Brown, T.M.; Di Carlo, A. Realization of high performance large area Z-series-interconnected opaque dye solar cell modules. Prog. Photovolt. Res. Appl. 2012, 21, 1653–1658. [Google Scholar] [CrossRef]
- Barichello, J.; Vesce, L.; Mariani, P.; Leonardi, E.; Braglia, R.; Di Carlo, A.; Canini, A.; Reale, A. Stable Semi-Transparent Dye-Sensitized Solar Modules and Panels for Greenhouse Application. Energies 2021, 14, 6393. [Google Scholar] [CrossRef]



| TiO2 + scatt. D | TiO2 + scatt. J | ||
|---|---|---|---|
| Jsc (mA/cm²) | 20.0 ± 0.7 | 21.5 ± 0.3 | +7% |
| Voc (V) | 0.69 ± 0.01 | 0.69 ± 0.01 | - |
| FF (%) | 64 ± 1 | 65 ± 1 | +1% |
| η (%) | 9.1 ± 0.4 | 9.8 ± 0.2 | +8% |
| TiO2@N719 | Au@TiO2@N719 | ||
|---|---|---|---|
| Jsc (mA/cm²) | 21.5 ± 0.3 | 22.1 ± 0.4 | +3% |
| Voc (V) | 0.69 ± 0.01 | 0.72 ± 0.02 | +4% |
| FF (%) | 65 ± 1 | 65 ± 1 | / |
| η (%) | 9.8 ± 0.2 | 10.3 ± 0.3 | +5% |
| Sample | Anode | Irradiation (mW/cm2) | Jsc (mA/cm2) | Voc (V) | FF (%) | PCE (%) |
|---|---|---|---|---|---|---|
| Betalains | TiO2@Au | 100 | 11.3 | 0.34 | 52 | 2.0 |
| Betalains | TiO2 | 100 | 6.5 | 0.30 | 57 | 1.1 |
| Betalains | TiO2@Au | 10 (LED) | 8.6 | 0.33 | 54 | 15.3 |
| Betalains | TiO2 | 10 (LED) | 4.4 | 0.31 | 57 | 7.9 |
| N719 | TiO2@Au | 100 | 23.3 | 0.71 | 65 | 10.8 |
| N719 | TiO2 | 100 | 22.0 | 0.69 | 65 | 9.9 |
| N719 | TiO2@Au | 10 (LED) | 12.8 | 0.71 | 70 | 63.6 |
| N719 | TiO2 | 10 (LED) | 12.0 | 0.65 | 72 | 56.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barichello, J.; Mariani, P.; Matteocci, F.; Vesce, L.; Reale, A.; Di Carlo, A.; Lanza, M.; Di Marco, G.; Polizzi, S.; Calogero, G. The Golden Fig: A Plasmonic Effect Study of Organic-Based Solar Cells. Nanomaterials 2022, 12, 267. https://doi.org/10.3390/nano12020267
Barichello J, Mariani P, Matteocci F, Vesce L, Reale A, Di Carlo A, Lanza M, Di Marco G, Polizzi S, Calogero G. The Golden Fig: A Plasmonic Effect Study of Organic-Based Solar Cells. Nanomaterials. 2022; 12(2):267. https://doi.org/10.3390/nano12020267
Chicago/Turabian StyleBarichello, Jessica, Paolo Mariani, Fabio Matteocci, Luigi Vesce, Andrea Reale, Aldo Di Carlo, Maurizio Lanza, Gaetano Di Marco, Stefano Polizzi, and Giuseppe Calogero. 2022. "The Golden Fig: A Plasmonic Effect Study of Organic-Based Solar Cells" Nanomaterials 12, no. 2: 267. https://doi.org/10.3390/nano12020267
APA StyleBarichello, J., Mariani, P., Matteocci, F., Vesce, L., Reale, A., Di Carlo, A., Lanza, M., Di Marco, G., Polizzi, S., & Calogero, G. (2022). The Golden Fig: A Plasmonic Effect Study of Organic-Based Solar Cells. Nanomaterials, 12(2), 267. https://doi.org/10.3390/nano12020267













