Facile Synthesis of Silane-Modified Mixed Metal Oxide as Catalyst in Transesterification Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Calcium Oxide (CaO)
2.3. Synthesis of Calcium-Oxide–Copper-(II)-Oxide (CaO-CuO)
2.4. Surface Modification of CaO-CuO
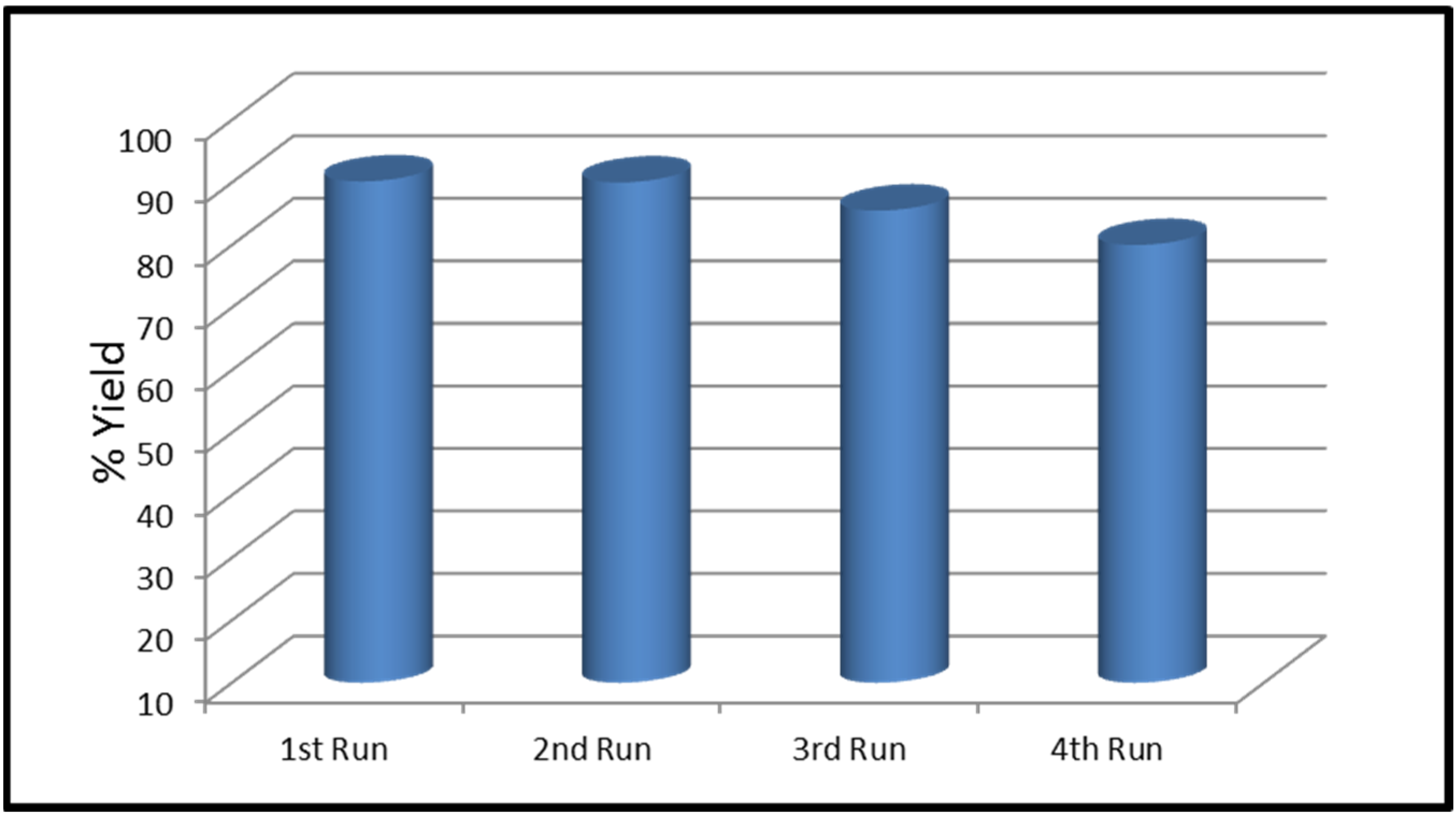
2.5. Transesterification Reaction
2.6. Characterization of Synthesized Solid Catalysts
2.7. Characterization of Transesterification Product
- ∑AFAME = Total area of FAME peaks;
- AMH = Area of MH peak;
- VMH = Volume of MH solution (mL);
- CMH = Actual concentration of MH solution (g/mL);
- m = Actual weight of the FAME sample (g).
- MFAME = Weight of FAME obtained after the reaction and separation process (g);
- Moil = Weight of the initial oil sample (g);
- Fp = FAME weight fraction obtained from FAME purity.
3. Results and Discussion
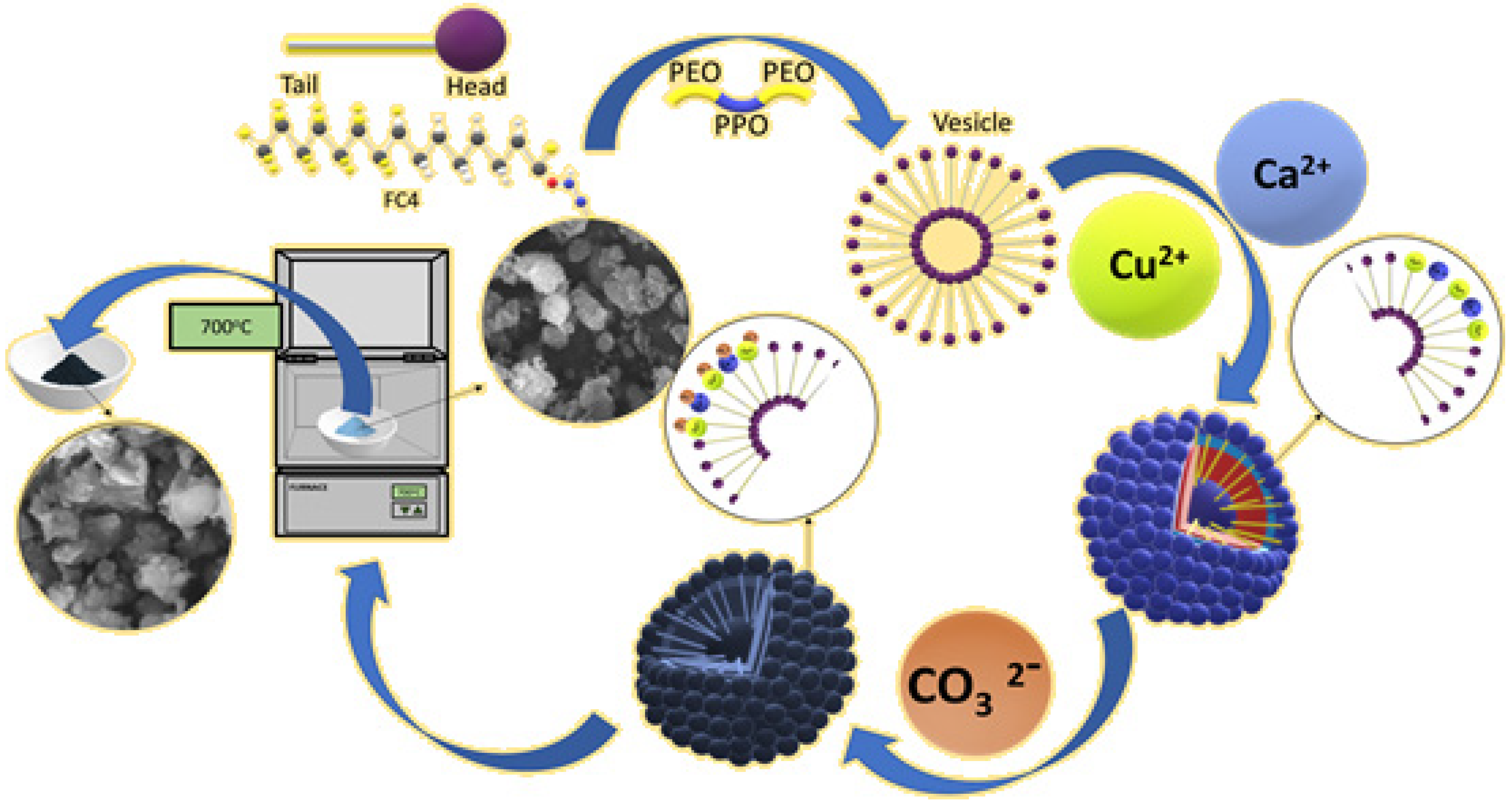
3.1. Synthesis and Modification of Modified Mixed Metal Oxide (CaO-CuO/C6)
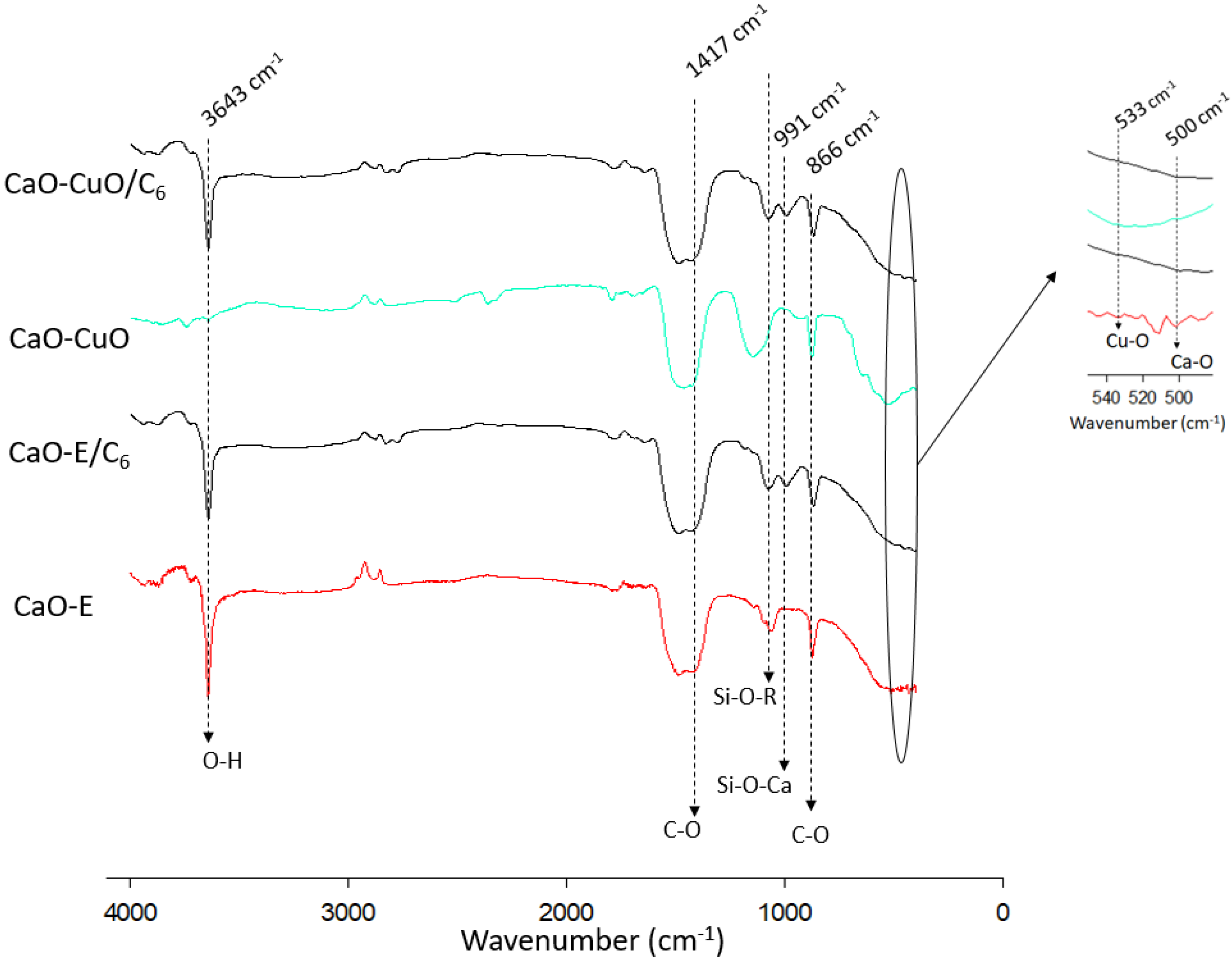
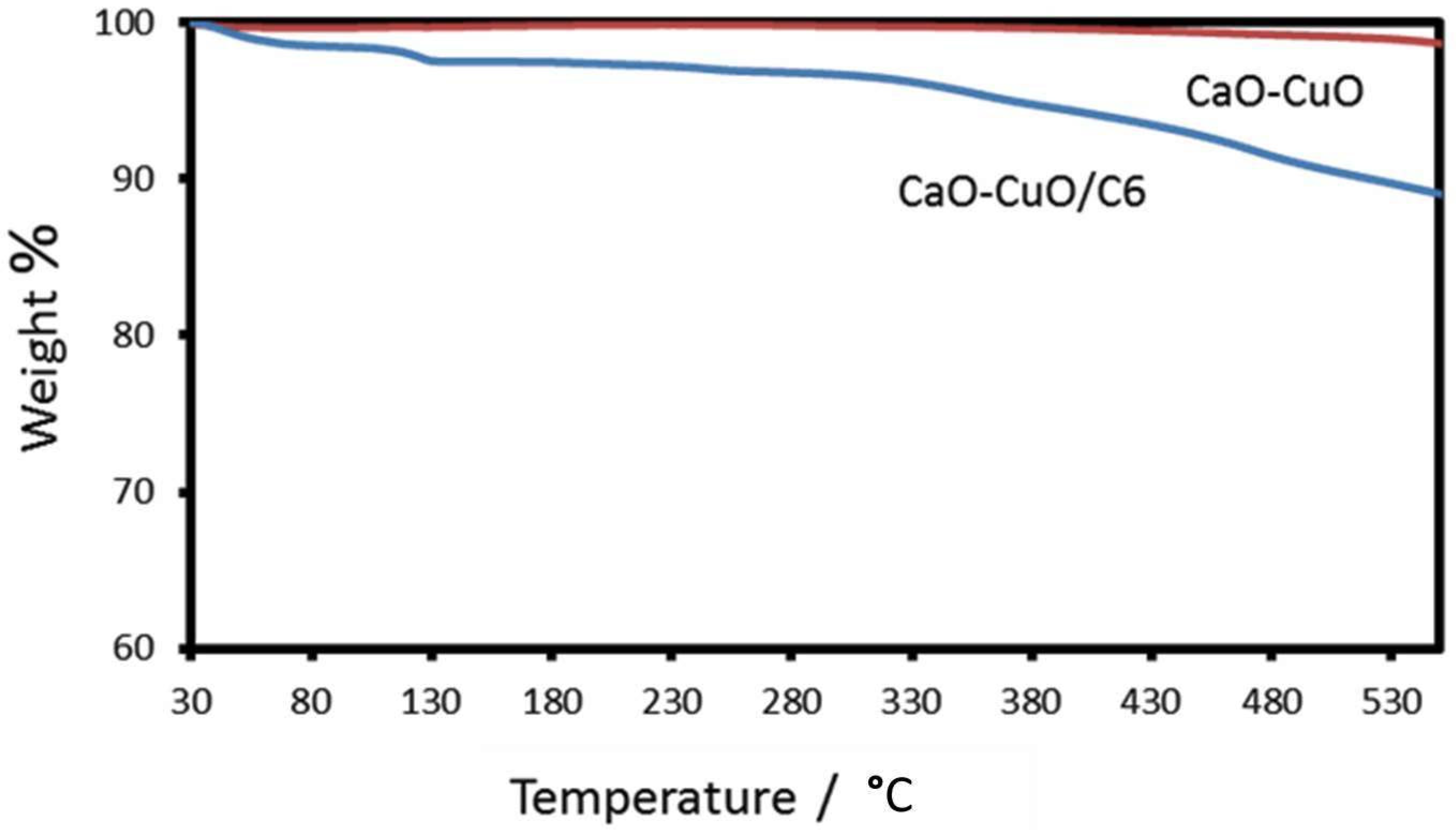
3.2. Charaterization of Silane-Modified Mixed Metal Oxide (CaO-CuO/C6)
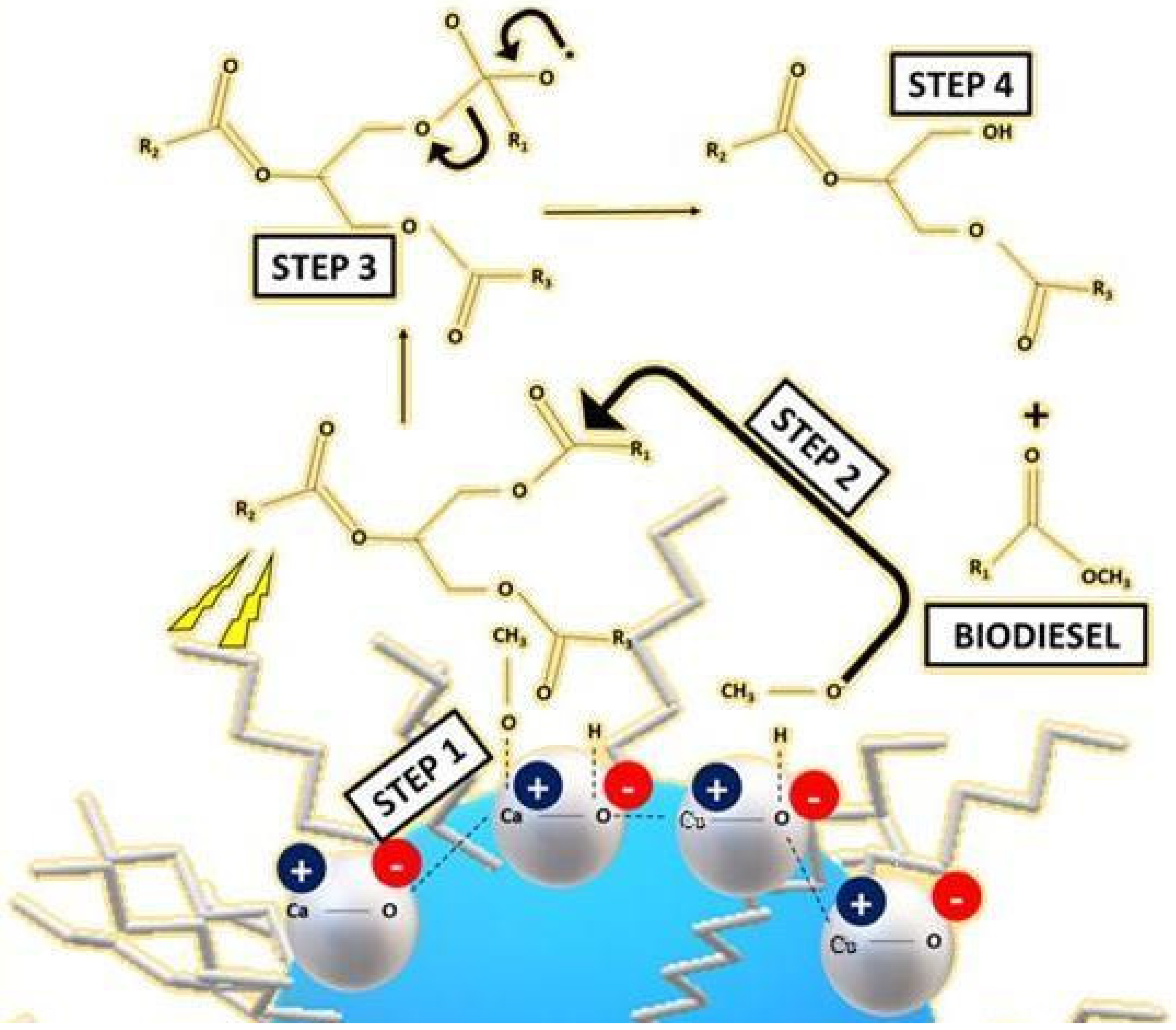
3.3. Transesterification Mechanism to Produce Biodiesel from Vegetable Oil Triglycerides by Using Silane-Modified Mixed Metal Oxide (CaO-CuO/C6)
- Step 1:
- C6 ((hexyl)silane) increases the wetness of the basic catalyst mixed metal oxide (CaO-CuO) with hydrophobic soya oil. The basic CaO-CuO catalyst induces proton removal from methanol (CH3OH) to form methoxide anions (CH3O−);
- Step 2:
- Methoxide anions (CH3O−) initiate transesterification by binding to the carbonyl carbon of triglycerides and creating an alkoxy carbonyl intermediate;
- Step 3:
- The alkoxy carbonyl intermediate transforms via bonding breakdown;
- Step 4:
- The formation of fatty acid methyl ester (FAME) (R1COOCH3) and diglycerides. The sequence is repeated to form a fatty acid from R2 and R3.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Changmai, B.; Vanlalveni, C.; Ingle, A.P.; Bhagat, R.; Rokhum, S.L. Widely used catalysts in biodiesel production: A review. RSC Adv. 2020, 10, 41625–41679. [Google Scholar] [CrossRef]
- Wongjaikham, W.; Wongsawaeng, D.; Ratnitsai, V.; Kamjam, M.; Ngaosuwan, K.; Kiatkittipong, W.; Hosemann, P.; Assabumrungrat, S. Low-cost alternative biodiesel production apparatus based on household food blender for continuous biodiesel production for small communities. Sci. Rep. 2021, 11, 13827. [Google Scholar] [CrossRef]
- Gebremariam, S.N.; Marchetti, J.M. Biodiesel production process using solid acid catalyst: Influence of market variables on the process’s economic feasibility. Biofuels Bioprod. Biorefining 2021, 15, 815–824. [Google Scholar] [CrossRef]
- Mishra, V.K.; Goswami, R. A review of production, properties and advantages of biodiesel. Biofuels 2018, 9, 273–289. [Google Scholar] [CrossRef]
- Bai, H.X.; Shen, X.Z.; Liu, X.H.; Liu, S.Y. Synthesis of porous CaO microsphere and its application in catalyzing transesterification reaction for biodiesel. Trans. Nonferrous Met. Soc. China 2009, 19, s674–s677. [Google Scholar] [CrossRef]
- Sudarsanam, P.; Zhong, R.; Van den Bosch, S.; Coman, S.M.; Parvulescu, V.I.; Sels, B.F. Functionalised heterogeneous catalysts for sustainable biomass valorisation. Chem. Soc. Rev. 2018, 47, 8349–8402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohlouli, A.; Mahdavian, L. Catalysts used in biodiesel production: A review. Biofuels 2021, 12, 885–898. [Google Scholar] [CrossRef]
- Wang, A.; Sudarsanam, P.; Xu, Y.; Zhang, H.; Li, H.; Yang, S. Functionalized magnetic nanosized materials for efficient biodiesel synthesis via acid–base/enzyme catalysis. Green Chem. 2020, 22, 2977–3012. [Google Scholar] [CrossRef]
- Mazaheri, H.; Ong, H.C.; Amini, Z.; Masjuki, H.H.; Mofijur, M.; Su, C.H.; Anjum Badruddin, I.; Khan, T.M.Y. An Overview of Biodiesel Production via Calcium Oxide Based Catalysts: Current State and Perspective. Energies 2021, 14, 3950. [Google Scholar] [CrossRef]
- Chouhan, A.P.S.; Sarma, A.K. Modern heterogeneous catalysts for biodiesel production: A comprehensive review. Renew. Sustain. Energy Rev. 2011, 15, 4378–4399. [Google Scholar] [CrossRef]
- Shylesh, S.; Schünemann, V.; Thiel, W.R. Magnetically Separable Nanocatalysts: Bridges between Homogeneous and Heterogeneous Catalysis. Angew. Chem. Int. Ed. 2010, 49, 3428–3459. [Google Scholar] [CrossRef]
- Seied Mahdi Pourmortazavi, M.R.-N.; Ahmadi, F.; Ganjali, M.R. CuCO3 and CuO nanoparticles; facile preparation and evaluation as photocatalysts. J. Mater. Sci. Mater. Electron. 2018, 29, 9442–9451. [Google Scholar] [CrossRef]
- Rahimi-Nasrabadi, M.; Naderi, H.R.; Karimi, M.S.; Ahmadi, F.; Pourmortazavi, S.M. Cobalt carbonate and cobalt oxide nanoparticles synthesis, characterization and supercapacitive evaluation. J. Mater. Sci. Mater. Electron. 2017, 28, 1877–1888. [Google Scholar] [CrossRef]
- Hanafy, N.A.N.; De Giorgi, M.L.; Nobile, C.; Rinaldi, R.; Leporatti, S. Control of colloidal CaCO3 suspension by using biodegradable polymers during fabrication. Beni-Suef Univ. J. Basic Appl. Sci. 2015, 4, 60–70. [Google Scholar] [CrossRef] [Green Version]
- Zheng, T.; Zhang, X.; Yi, H. Spherical vaterite microspheres of calcium carbonate synthesized with poly (acrylic acid) and sodium dodecyl benzene sulfonate. J. Cryst. Growth 2019, 528, 125275. [Google Scholar] [CrossRef]
- Song, J.; Wang, R.; Liu, Z.; Zhang, H. Preparation and characterization of calcium carbonate microspheres and their potential application as drug carriers. Mol. Med. Rep. 2018, 17, 8403–8408. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Ying, J.Y. Generalized Fluorocarbon-Surfactant-Mediated Synthesis of Nanoparticles with Various Mesoporous Structures. Angew. Chem. Int. Ed. 2005, 44, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, N.F.; Ramly, N.I.; Abd Mubin, M.H.; Lee, S.L. Transition metal oxide (NiO, CuO, ZnO)-doped calcium oxide catalysts derived from eggshells for the transesterification of refined waste cooking oil. RSC Adv. 2021, 11, 21781–21795. [Google Scholar] [CrossRef]
- Yang, R.; Su, M.; Li, M.; Zhang, J.; Hao, X.; Zhang, H. One-pot process combining transesterification and selective hydrogenation for biodiesel production from starting material of high degree of unsaturation. Bioresour. Technol. 2010, 101, 5903–5909. [Google Scholar] [CrossRef] [PubMed]
- Niju, S.; Raj, F.R.; Anushya, C.; Balajii, M. Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil. Green Processing Synth. 2019, 8, 756–775. [Google Scholar] [CrossRef]
- Björk, E.M.; Militello, M.P.; Tamborini, L.H.; Coneo Rodriguez, R.; Planes, G.A.; Acevedo, D.F.; Moreno, M.S.; Odén, M.; Barbero, C.A. Mesoporous silica and carbon based catalysts for esterification and biodiesel fabrication—The effect of matrix surface composition and porosity. Appl. Catal. A Gen. 2017, 533, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Hartono, S.B.; Jin, Y.G.; Li, Z.; Lu, G.Q.; Qiao, S.Z. A facile vesicle template route to multi-shelled mesoporous silica hollow nanospheres. J. Mater. Chem. 2010, 20, 4595–4601. [Google Scholar] [CrossRef]
- Liu, J.; Qiao, S.Z.; Budi Hartono, S.; Lu, G.Q. Monodisperse Yolk–Shell Nanoparticles with a Hierarchical Porous Structure for Delivery Vehicles and Nanoreactors. Angew. Chem. Int. Ed. 2010, 49, 4981–4985. [Google Scholar] [CrossRef] [PubMed]
- Helmy, R.; Fadeev, A.Y. Self-Assembled Monolayers Supported on TiO2: Comparison of C18H37SiX3 (X = H, Cl, OCH3), C18H37Si(CH3)2Cl, and C18H37PO(OH)2. Langmuir 2002, 18, 8924–8928. [Google Scholar] [CrossRef]
- Riza, A.; Yun Ii, G.; Maier, R.; Harun, S.W.; Ahmad Anas, S.B. Hygroscopic Materials and Characterization Techniques for Fiber Sensing Applications: A Review. Sens. Mater. 2020, 32, 3755. [Google Scholar] [CrossRef]
- Rodriguez-garcia, M.E. Characterization of Calcium Carbonate, Calcium Oxide, and Calcium This material may be downloaded for personal use only. Any other use requires prior permission of the American Society of Civil Engineers. Final draft. J. Mater. Civ. Eng. 2009, 21, 694–698. [Google Scholar]
- Launer, P.J. Infrared analysis of organosilicon compounds: Spectra-structure correlations. Silicone Compd. Regist. Rev. 1987, 16, 100–103. [Google Scholar]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Memic, A. Size-dependent antimicrobial properties of CuO nanoparticles against Gram-positive and -negative bacterial strains. Int. J. Nanomedicine 2012, 7, 3527–3535. [Google Scholar] [CrossRef] [Green Version]
- Ji, T.; Ma, C.; Brisbin, L.; Mu, L.; Robertson, C.G.; Dong, Y.; Zhu, J. Organosilane grafted silica: Quantitative correlation of microscopic surface characters and macroscopic surface properties. Appl. Surf. Sci. 2017, 399, 565–572. [Google Scholar] [CrossRef] [Green Version]
- Biasin, A.; Segre, C.U.; Strumendo, M. CaCO3 Crystallite Evolution during CaO Carbonation: Critical Crystallite Size and Rate Constant Measurement by In-Situ Synchrotron Radiation X-ray Powder Diffraction. Cryst. Growth Des. 2015, 15, 5188–5201. [Google Scholar] [CrossRef]
- Tang, Z.-X.; Yu, Z.; Zhang, Z.-L.; Zhang, X.-Y.; Pan, Q.-Q.; Shi, L.-E. Sonication-assisted preparation of CaO nanoparticles for antibacterial agents. Quím. Nova 2013, 36, 933–936. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.H.; Nguyen, V.T. Copper Oxide Nanomaterials Prepared by Solution Methods, Some Properties, and Potential Applications: A Brief Review. Int. Sch. Res. Not. 2014, 2014, 856592. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, T.; Kurayama, F.; Shiba, M.; Kadota, R.; Sato, M.; Suzuki, N. CaO-loaded alginate capsule modified with silane coupling agents for transesterification of rapeseed oil with methanol. Chem. Eng. J. 2016, 288, 473–481. [Google Scholar] [CrossRef]
- Asikin-Mijan, N.; Lee, H.V.; Taufiq-Yap, Y.H. Synthesis and catalytic activity of hydration–dehydration treated clamshell derived CaO for biodiesel production. Chem. Eng. Res. Des. 2015, 102, 368–377. [Google Scholar] [CrossRef]

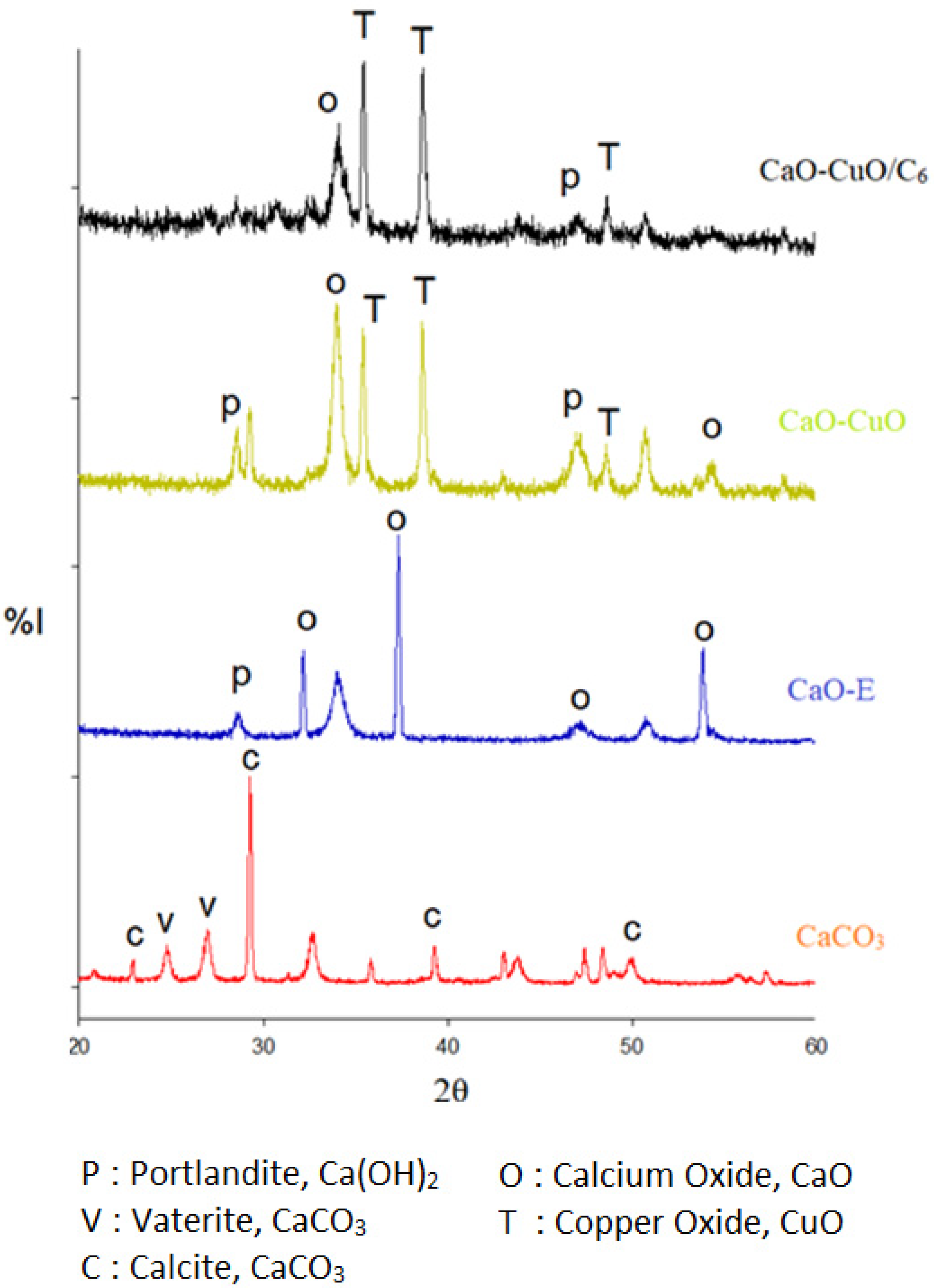
| Solid Particles | Crystallite Size (nm) | |||
|---|---|---|---|---|
| CaCO3 | CaO | Ca(OH)2 | CuO | |
| CaCO3 | 39.53 | - | - | - |
| CaO-R | - | 18.99 | 18.05 | - |
| CaO-E | - | 24.10 | 21.90 | - |
| CaO-CuO | - | 11.36 | 20.25 | 26.82 |
| CaCuO2/C6 | - | 11.73 | 27.31 | 27.75 |
| Atom | %wt | |||||
|---|---|---|---|---|---|---|
| CaCO3-E | CaO-E | CaO-E/C6 | CaCO3-CuCO3 | CaO-CuO | CaO-CuO/C6 | |
| C | 20.97% | 17.19% | 26.43% | 51.53% | 11.58% | 13.87% |
| O | 50.65% | 48.94% | 47.58% | 10.67% | 8.70% | 6.71% |
| Ca | 28.39% | 33.87% | 25.72% | 28.36% | 68.17% | 57.49% |
| Cu | - | - | - | 9.44% | 11.54% | 13.62% |
| Si | - | - | 0.28% | - | - | 8.30% |
| 100.00% | 100.00% | 100.00% | 100.00% | 100.00% | 100.00% | |
| Catalyst | Yield (%) | Purity of FAME (%) | Yield of FAME (%) |
|---|---|---|---|
| CaO-R | 60.10 | 96.80 | 58.18 |
| CaO-E2 | 70.51 | 92.54 | 65.22 |
| CaO-E2/C6 | 74.28 | 96.75 | 71.90 |
| CaO-CuO | 83.80 | 96.50 | 80.87 |
| CaO-CuO/C6 | 92.48 | 97.53 | 90.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pranyoto, N.; Dewi Susanti, Y.; Joseph Ondang, I.; Angkawijaya, A.E.; Edi Soetaredjo, F.; Santoso, S.P.; Yuliana, M.; Ismadji, S.; Budi Hartono, S. Facile Synthesis of Silane-Modified Mixed Metal Oxide as Catalyst in Transesterification Processes. Nanomaterials 2022, 12, 245. https://doi.org/10.3390/nano12020245
Pranyoto N, Dewi Susanti Y, Joseph Ondang I, Angkawijaya AE, Edi Soetaredjo F, Santoso SP, Yuliana M, Ismadji S, Budi Hartono S. Facile Synthesis of Silane-Modified Mixed Metal Oxide as Catalyst in Transesterification Processes. Nanomaterials. 2022; 12(2):245. https://doi.org/10.3390/nano12020245
Chicago/Turabian StylePranyoto, Nugroho, Yuni Dewi Susanti, Immanuel Joseph Ondang, Artik Elisa Angkawijaya, Felycia Edi Soetaredjo, Shella Permatasari Santoso, Maria Yuliana, Suryadi Ismadji, and Sandy Budi Hartono. 2022. "Facile Synthesis of Silane-Modified Mixed Metal Oxide as Catalyst in Transesterification Processes" Nanomaterials 12, no. 2: 245. https://doi.org/10.3390/nano12020245
APA StylePranyoto, N., Dewi Susanti, Y., Joseph Ondang, I., Angkawijaya, A. E., Edi Soetaredjo, F., Santoso, S. P., Yuliana, M., Ismadji, S., & Budi Hartono, S. (2022). Facile Synthesis of Silane-Modified Mixed Metal Oxide as Catalyst in Transesterification Processes. Nanomaterials, 12(2), 245. https://doi.org/10.3390/nano12020245








