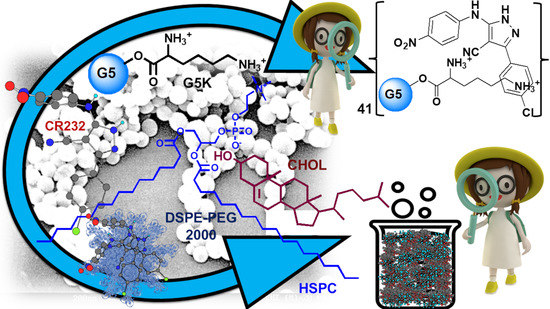
Successful Dendrimer and Liposome-Based Strategies to Solubilize an Antiproliferative Pyrazole Otherwise Not Clinically Applicable
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Substances and Instruments
2.2. Experimental Procedures Concerning Dendrimer NPs
2.2.1. Synthesis of the Fifth-Generation αN,εN-(tert-Butoxycarbonyl)lysine Dendrimer (G5BK)
2.2.2. Removal of tert-Butoxycarbonyl-Protecting Groups to Achieve G5K Hydrochloride Salt
2.2.3. Determination of the Molecular Weight (MW) of Dendrimer G5K by Volumetric Titration
2.2.4. Cytotoxicity Studies on G5K
2.2.5. CR232-Loaded Dendrimer NPs (CR232-G5K NPs): Experimental Procedure
2.2.6. Spectroscopic Characterization of CR232-G5K NPs
2.2.7. UV-Vis Analyses
2.2.8. CR232 Calibration Curve
2.2.9. Estimation of CR232 Contained in CR232-G5K NPs
2.2.10. Molecular Weight of CR232-G5K NPs
2.2.11. Morphology and Average Size of G5K and CR232-G5K NPs
2.2.12. Potentiometric Titrations of CR232-G5K NPs
2.2.13. In Vitro CR232 Release Profile from CR232-G5K NPs
2.3. Experimental Procedures Concerning Liposomes
2.3.1. CR232-Loaded Unilamellar Liposomes (CR232-SUVs): Experimental Procedure
2.3.2. Spectroscopic Characterization of SUVs and of CR232-SUVs
2.3.3. Estimation of CR232 Contained in CR232-SUVs
2.3.4. In Vitro CR232 Release Profile from CR232-SUVs
2.4. Analytical Experiments Concerning Both Dendrimer NPs and Liposomes
2.4.1. Principal Component Analysis (PCA) of ATR-FTIR Spectral Data
2.4.2. Water Solubility Studies
2.4.3. Dynamic Light Scattering (DLS) Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Dendrimer NPs
3.1.1. Synthesis of Boc-Protected Dendrimer G5BK Containing Lysine Residues
3.1.2. Removal of tert-Butoxycarbonyl-Protecting Groups to Achieve G5K Hydrochloride Salt
3.1.3. Determination of the MW of Dendrimer G5K by Volumetric Titration
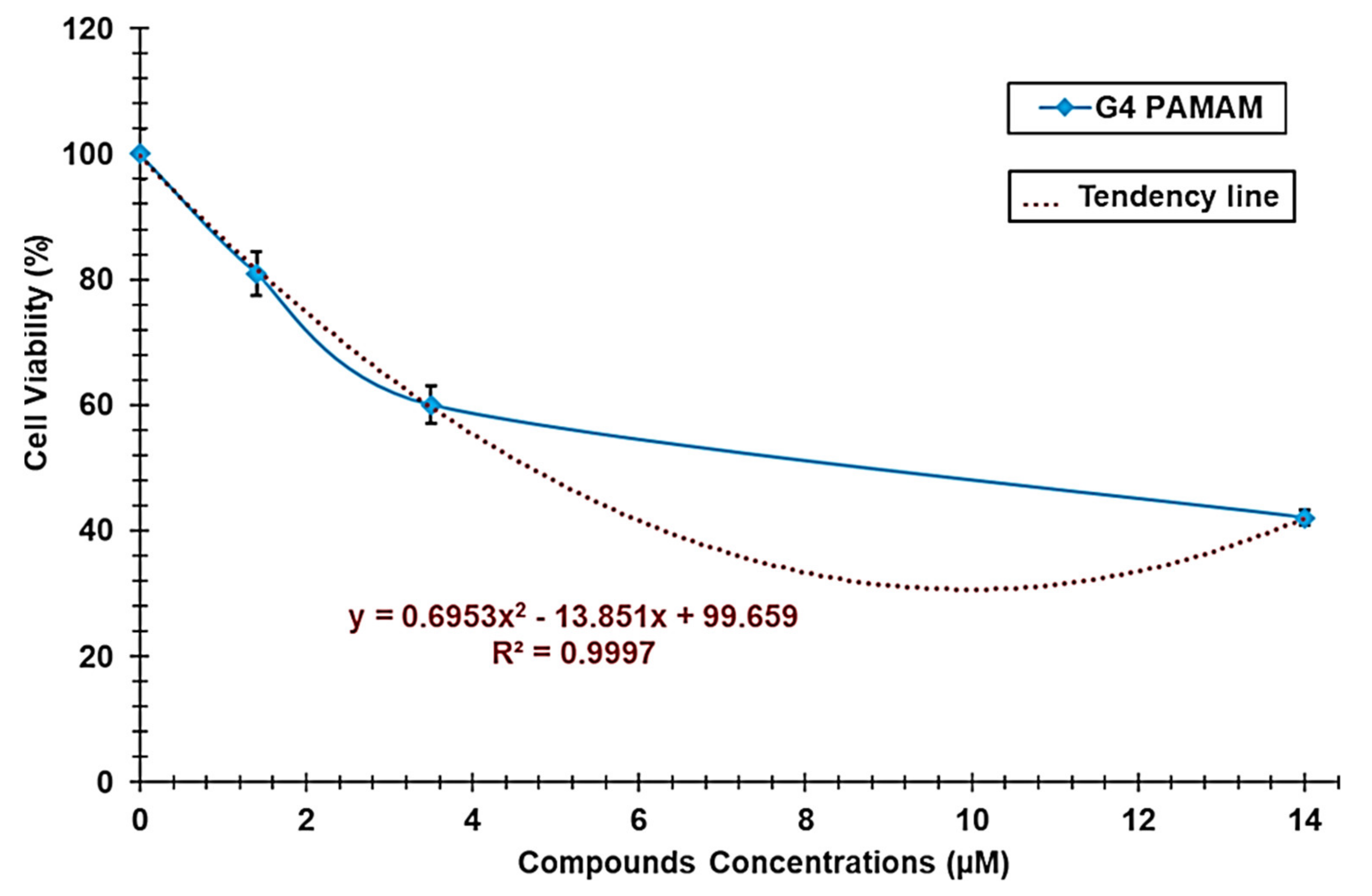
3.1.4. Cytotoxicity Studies
3.1.5. Preparation of CR232-G5K NPs
3.1.6. NMR Analyses
3.1.7. UV-Vis Spectra of G5K, CR232, and CR232-G5K
3.1.8. CR232 Calibration Curve
3.1.9. Determination of CR232 Contained in the CR232-G5K NPs and DL% and EE%
3.1.10. Determination of CR232-G5K MW
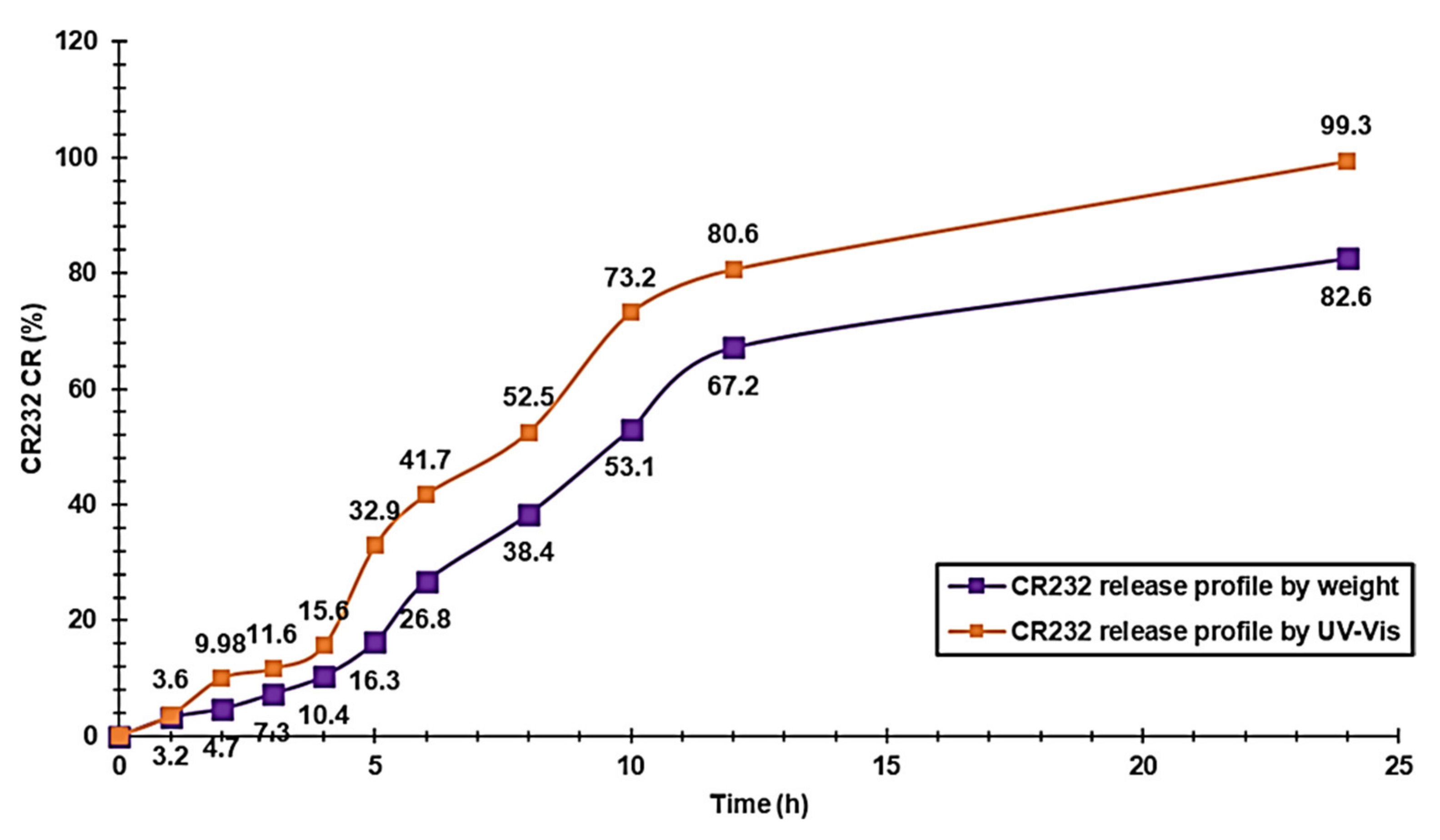
3.1.11. CR232-G5K NP Release Profile
3.1.12. Morphology of Particles of G5K and CR232-G5K NPs by SEM
3.1.13. Potentiometric Titrations
3.2. Liposomes
3.2.1. Preparation of CR232-SUVs
3.2.2. Freeze-Drying of Liposome Suspensions (SUVs and CR232-SUVs)
3.2.3. Determination of the CR232 Concentration in the Prepared CR232-SUVs and the EE% and DL%
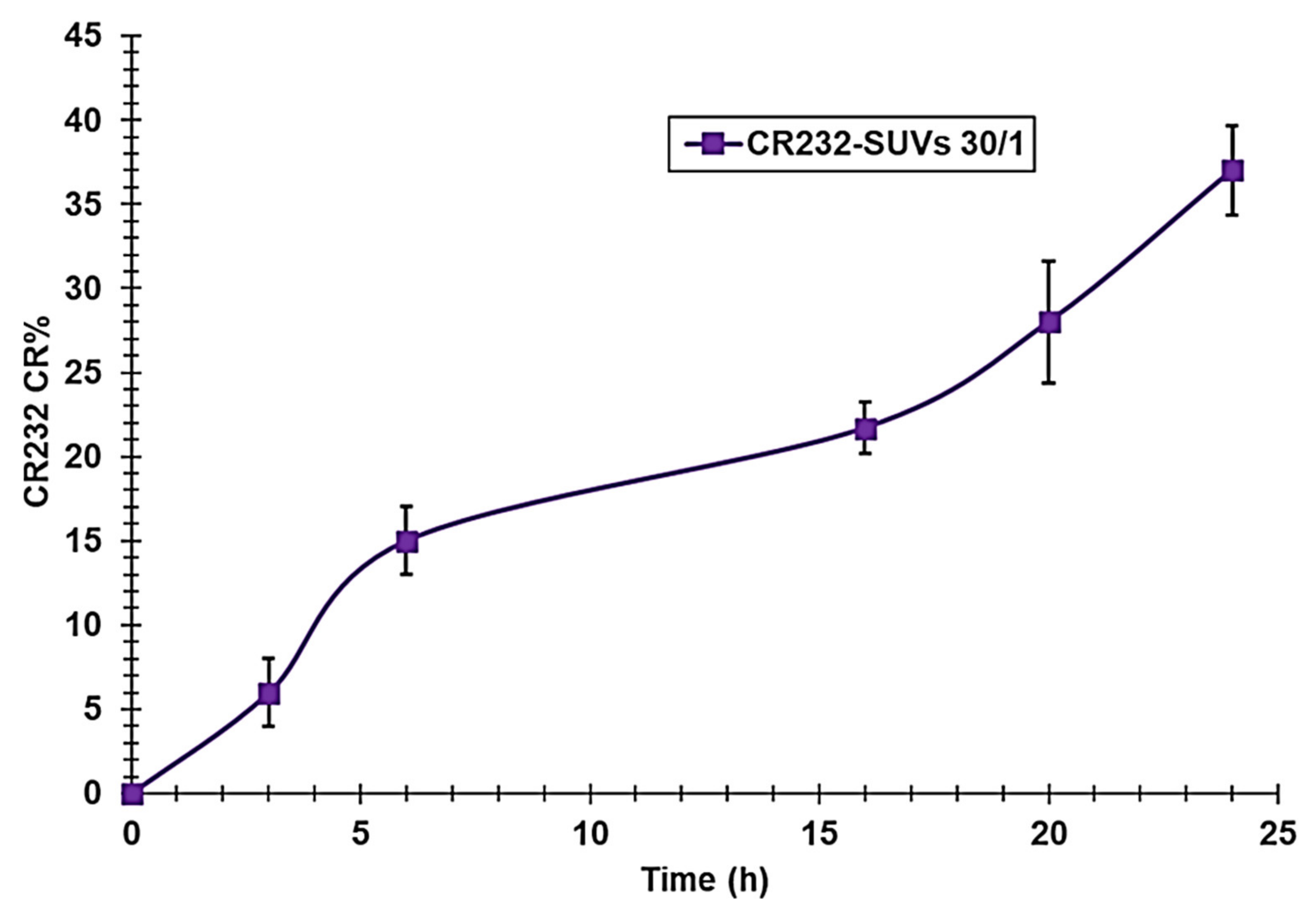
3.2.4. CR232-SUV Release Profile
3.3. Dendrimer NPs and Liposomes
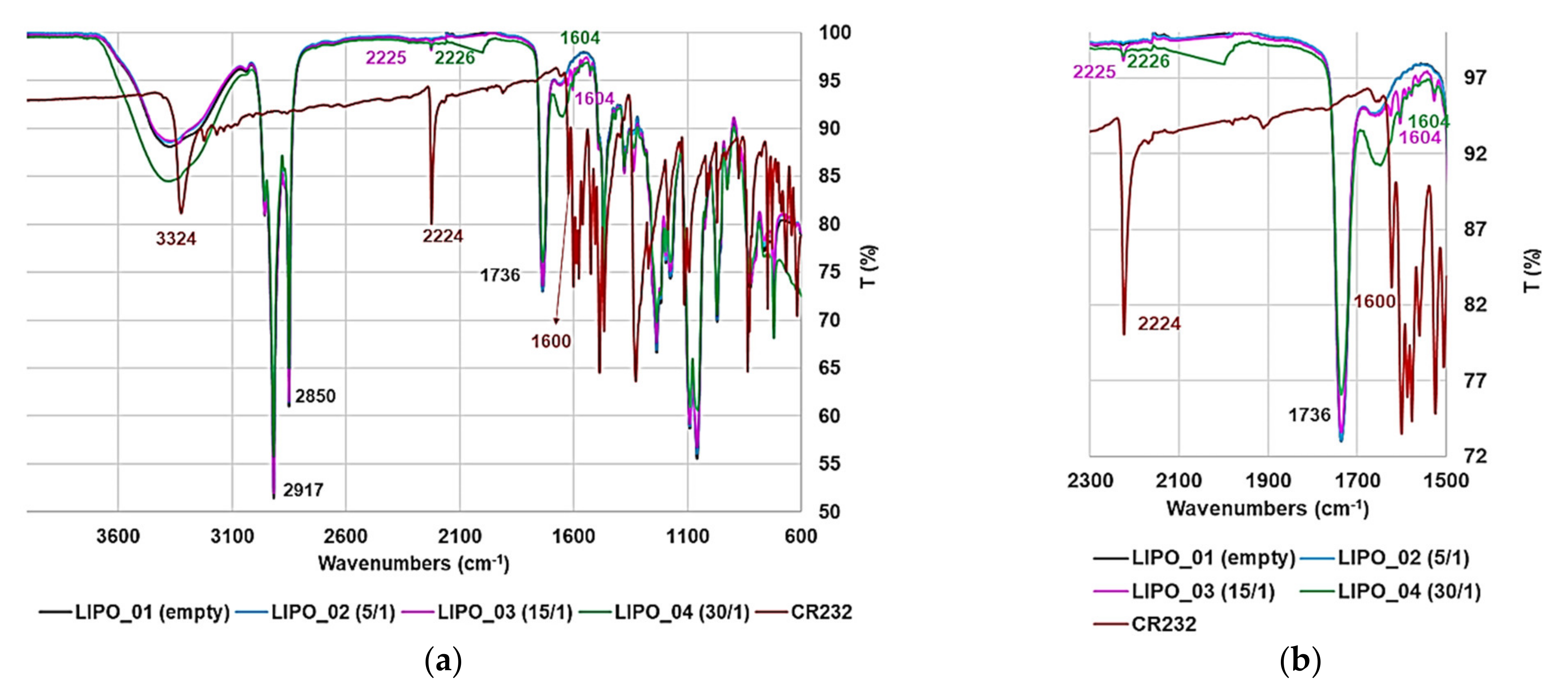
3.3.1. ATR-FTIR Spectroscopy
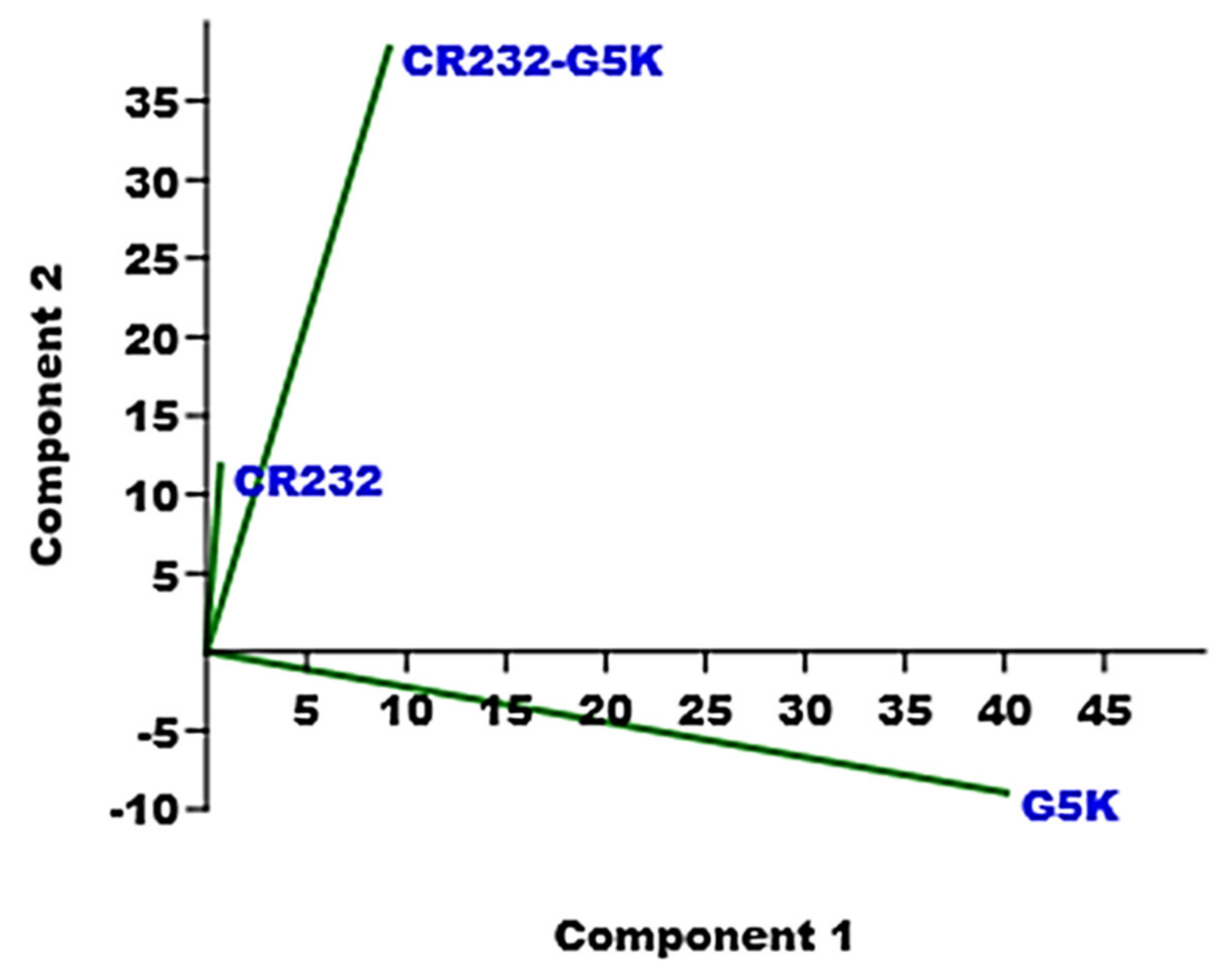
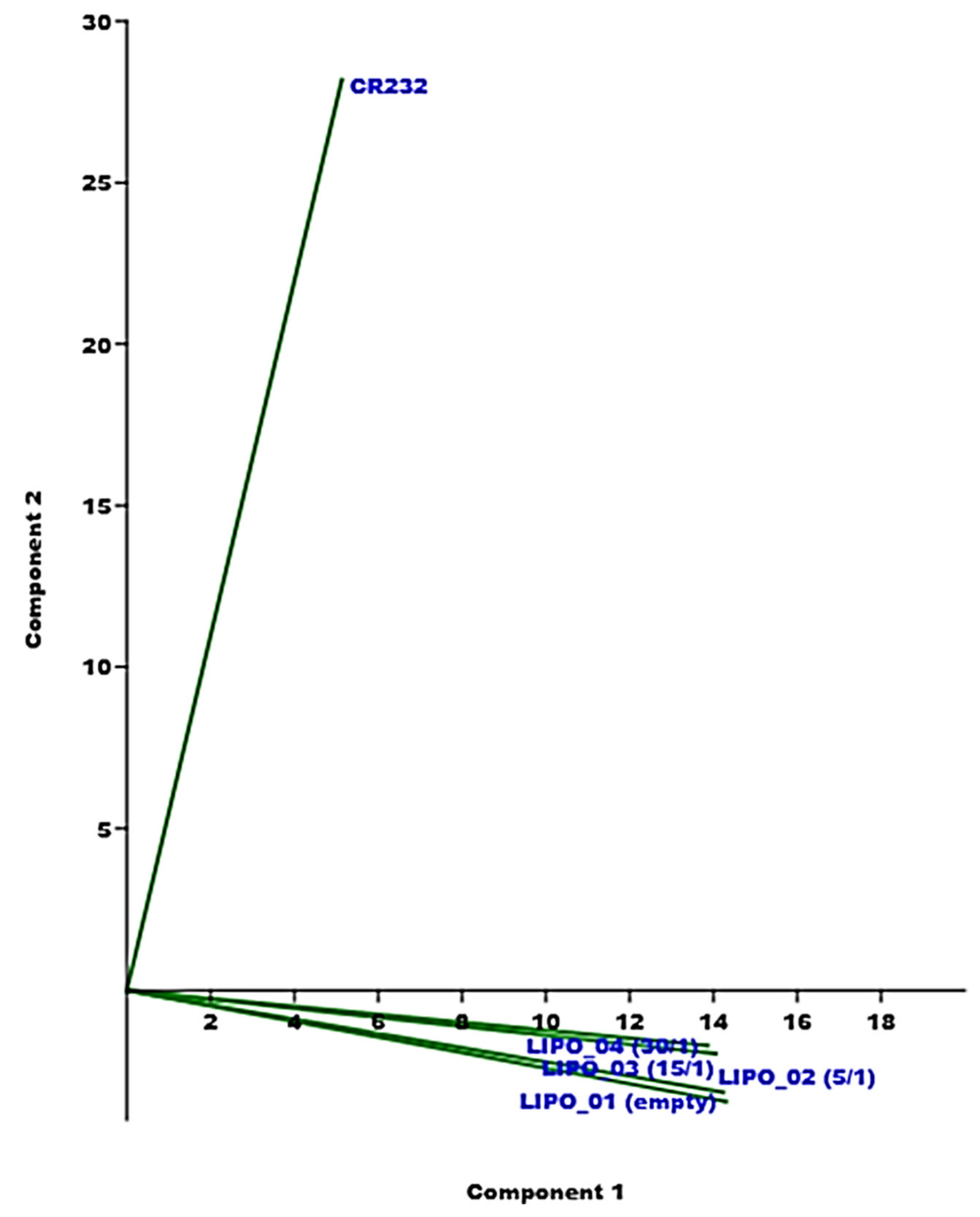
3.3.2. PCA of the ATR-FTIR Spectral Data
3.3.3. Water Solubility Determinations
3.3.4. Dynamic Light-Scattering Analysis (DLS)
3.4. Summary of the Main Physicochemical Properties of CR232-G5K NPs and CR232-SUVs for Easy Comparison
4. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jamwal, A.; Javed, A.; Bhardwaj, V. A review on Pyrazole derivatives of pharmacological potential. J. Pharm. BioSci. 2013, 3, 114–123. [Google Scholar]
- Karrouchi, K.; Radi, S.; Ramli, Y.; Taoufik, J.; Mabkhot, Y.N.; Al-aizari, F.A.; Ansar, M. Synthesis and Pharmacological Activities of Pyrazole Derivatives: A Review. Molecules 2018, 23, 134. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Verma, S.K.; Rakesh, K.P.; Girish, Y.R.; Ashrafizadeh, M.; Kumar, K.S.S.; Rangappa, K.S. Pyrazole-based analogs as potential antibacterial agents against methicillin-resistance staphylococcus aureus (MRSA) and its SAR elucidation. Eur. J. Med. Chem. 2021, 212, 113134. [Google Scholar] [CrossRef]
- Dewar, M.J.S. Attempts to find new antimalarials. Part XXI. J. Chem. Soc. Resumed 1944, 615–619. [Google Scholar] [CrossRef]
- Bondavalli, F.; Bruno, O.; Ranise, A.; Schenone, P.; Addonizio, P.; de Novellis, V.; Loffreda, A.; Marmo, E. 3,5-Diphenyl-1H-pyrazole derivatives. Farm. Sci. Ed. 1988, 43, 725–743. [Google Scholar]
- Akbas, E.; Berber, I.; Sener, A.; Hasanov, B. Synthesis and antibacterial activity of 4-benzoyl-1-methyl-5- phenyl-1H-pyrazole-3-carboxylic acid and derivatives. Farmaco 2005, 60, 23–26. [Google Scholar] [CrossRef]
- Wei, F.; Zhao, B.-X.; Huang, B.; Zhang, L.; Sun, C.-H.; Dong, W.-L.; Shin, D.-S.; Miao, J.-Y. Design, synthesis, and preliminary biological evaluation of novel ethyl 1-(2′-hydroxy-3′-aroxypropyl)-3-aryl-1H-pyrazole-5-carboxylate. Bioorg. Med. Chem. Lett. 2006, 16, 6342–6634. [Google Scholar] [CrossRef]
- Kumar, V.; Kaur, K.; Gupta, G.K.; Sharma, A.K. Pyrazole containing natural products: Synthetic preview and biological significance. Eur. J. Med. Chem. 2013, 69, 735–753. [Google Scholar] [CrossRef]
- Prokopp, C.R.; Rubin, M.A.; Sauzem, P.D.; de Souza, A.H.; Berlese, D.B.; Lourega, R.V.; Muniz, M.N.; Bonacorso, H.G.; Zanatta, N.; Martins, M.A.P.; et al. A pyrazolyl-thiazole derivative causes antinociception in mice. Braz. J. Med. Biol. Res. 2006, 39, 795–799. [Google Scholar] [CrossRef]
- Balbi, A.; Anzaldi, A.; Mazzei, M.; Miele, M.; Bertolotto, M.; Ottonello, L.; Dallegri, F. Synthesis and biological evaluation of novel heterocyclic ionone-like derivatives as anti-inflammatory agents. Bioorg. Med. Chem. 2006, 14, 5152–5160. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.V.; Bell, R.; Majest, S.; Henry, R.; Kolasa, T. Synthesis of 4,5-Diaryl-1H-pyrazole-3-ol Derivatives as Potential COX-2 Inhibitors. J. Org. Chem. 2004, 69, 7058–7065. [Google Scholar] [CrossRef] [PubMed]
- Süleyman, H.; Büyükokuroğlu, M.E. The effects of newly synthesized pyrazole derivatives on formaldehyde-, carrageenan-, and dextran-induced acute paw edema in rats. Biol. Pharm. Bull. 2001, 24, 1133–1136. [Google Scholar] [CrossRef][Green Version]
- Shchegolkov, E.; Khudina, O.; Anikinam, L.; Burgart, Y.; Saloutin, V. Synthesis, analgesic and antipyretic activity of 2-(antipyrin-4-yl)hydrazones of 1,2,3-triketones and their derivatives. Pharm. Chem. J. 2006, 40, 373–376. [Google Scholar]
- Bailey, D.M.; Hansen, P.E.; Hlavac, A.G.; Baizman, E.R.; Pearl, J.; Defelice, A.F.; Feigenson, M.E. 3,4-Di-phenyl-1H-pyrazole-1-propanamine Antidepressants. J. Med. Chem. 1985, 28, 256–260. [Google Scholar] [CrossRef]
- Dardari, Z.; Lemrani, M.; Sebban, A.; Bahloul, A.; Hassar, M.; Kitane, S.; Berrada, M.; Boudouma, M. Antileishmanial and Antibacterial Activity of a New Pyrazole Derivative Designated 4-[2-(1-(Ethylamino)-2-methyl-propyl)phenyl]-3-(4-methyphenyl)-1-phenylpyrazole. Arch. Pharm. 2006, 339, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, R.N.; Havaldar, F.H.; Fernandes, P.S. Syntheses and biological activity of heterocycles derived from 3-methoxy-i-phenyl-ih-pyrazole-5-carboxylate. J. Indian Chem. Soc. 1991, 68, 245–246. [Google Scholar]
- Wardakhan, W.W.; Louca, N.A. Synthesis of Novel Pyrazole, Coumarin and Pyridazine Derivatives Evaluated As Potential Antimicrobial And Antifungal Agents. J. Chil. Chem. Soc. 2007, 52, 1145–1149. [Google Scholar] [CrossRef]
- Elguero, J. Comprehensive Heterocyclic Chemistry II; Katritzky, A.R., Rees, C.V., Scriven, E.F.V., Eds.; Elsevier Ltd.: Oxford, UK, 1996; Volume 2, Chapter 3.01; pp. 1–75. [Google Scholar]
- Sutharchanadevi, M.; Murugan, R. Comprehensive Heterocyclic Chemistry II; Katritzky, A.R., Rees, C.V., Scriven, E.F.V., Eds.; Elsevier Ltd.: Oxford, UK, 1996; Volume 3, Chapter 3.03; pp. 221–260. [Google Scholar]
- Dawood, D.H.; Nossier, E.S.; Ali, M.M.; Mahmoud, A.E. Synthesis and molecular docking study of new pyrazole derivatives as potent anti-breast cancer agents targeting VEGFR-2 kinase. Bioorg. Chem. 2020, 101, 103916. [Google Scholar] [CrossRef]
- Ravula, P.; Vamaraju, H.B.; Paturi, M.; Chandra, J.N.G.N.S. Design, synthesis, in silico and antiproliferative evaluation of novel pyrazole derivatives as VEGFR-2 inhibitors. Arch. Pharm. Chem. Life Sci. 2018, 351, e1700234. [Google Scholar] [CrossRef]
- Lusardi, M.; Rotolo, C.; Ponassi, M.; Iervasi, E.; Rosano, C.; Spallarossa, A. One-pot synthesis and antiproliferative activity of highly functionalized pyrazole derivatives. ChemMedChem 2022, 17, e202100670. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). No Time to Wait: Securing the Future from Drug-Resistant Infections. Report to the Secretary-General of the United Nations. Interagency Coordination Group on Antimicrobial Resistance. WHO: Geneva, Switzerland, 2019. Available online: https://www.who.int/antimicrobial-resistance/interagency-coordination-group/IACG_final_report_EN.pdf?ua=1 (accessed on 29 October 2021).
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Songmee, B.; Jaehoon, L.; Jaehwa, L.; Eunah, K.; Sunhwa, L.; Jaeyon, Y.; Yeonho, K. Antimicrobial Resistance in Haemophilus influenzae Respiratory Tract Isolates in Korea: Results of a Nationwide Acute Respiratory Infections Surveillance. Antimicrob. Agents Chemother. 2010, 54, 65–71. [Google Scholar] [CrossRef]
- Nahak, P.; Karmakar, G.; Chettri, P.; Roy, B.; Guha, P.; Besra, S.E.; Soren, A.; Bykov, A.G.; Akentiev, A.V.; Noskov, B.A.; et al. Influence of lipid core material on physicochemical characteristics of an ursolic acid-loaded nanostructured lipid carrier: An attempt to enhance anticancer activity. Langmuir 2016, 32, 9816–9825. [Google Scholar] [CrossRef]
- Hu, X.L.; Liu, G.H.; Li, Y.; Wang, X.R.; Liu, S.Y. Cell-penetrating hyperbranched polyprodrug amphiphiles for synergistic reductive milieu-triggered drug release and enhanced magnetic resonance signals. J. Am. Chem. Soc. 2015, 137, 362–368. [Google Scholar] [CrossRef]
- Li, X.; Qian, Y.; Liu, T.; Hu, X.; Zhang, G.; You, Y.; Liu, S. Amphiphilic multiarm star block copolymer-based multifunctional unimolecular micelles for cancer targeted drug delivery and MR imaging. Biomaterials 2011, 32, 6595–6605. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Catena, S.; Ponassi, M.; Rosano, C.; Zoppi, V.; Spallarossa, A. Hydrophilic and amphiphilic water-soluble dendrimer prodrugs suitable for parenteral administration of a non-soluble non-nucleoside HIV-1 reverse transcriptase inhibitor thiocarbamate derivative. Eur. J. Pharm. Sci. 2018, 124, 153–164. [Google Scholar] [CrossRef]
- Alfei, S.; Turrini, F.; Catena, S.; Zunin, P.; Parodi, B.; Zuccari, G.; Pittaluga, A.M.; Boggia, R. Preparation of Ellagic Acid Micro and Nano Formulations with Amazingly Increased Water Solubility by Its Entrapment in Pectin or Non-PAMAM Dendrimers Suitable for Clinical Applications. New J. Chem. 2019, 43, 2438–2448. [Google Scholar] [CrossRef]
- Alfei, S.; Taptue, G.B.; Catena, S.; Bisio, A. Synthesis of Water-Soluble, Polyester-Based Dendrimer Prodrugs for Exploiting Therapeutic Properties of Two Triterpenoid Acids. Chin. J. Polym. Sci. 2018, 36, 999–1010. [Google Scholar] [CrossRef]
- Alfei, S.; Zuccari, G.; Schito, A.M. Considerable Improvement of Ursolic Acid Water Solubility by its Encapsulation in Dendrimer Nanoparticles: Design, Synthesis and Physicochemical Characterization. Nanomaterials 2021, 11, 2196. [Google Scholar] [CrossRef]
- Alfei, S.; Brullo, C.; Caviglia, D.; Zuccari, G. Preparation and Physicochemical Characterization of Water-Soluble Pyrazole-Based Nanoparticles by Dendrimer Encapsulation of an Insoluble Bioactive Pyrazole Derivative. Nanomaterials 2021, 11, 2662. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Marengo, B.; Domenicotti, C. Polyester-Based Dendrimer Nanoparticles Combined with Etoposide Have an Improved Cytotoxic and Pro-Oxidant Effect on Human Neuroblastoma Cells. Antioxidants 2020, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Nada, A.; Al-Moghazy, M.; Soliman, A.A.F.; Rashwan, G.M.T.; Eldawy, T.H.A.; Hassan, A.A.E.; Sayed, G.H. Pyrazole-based compounds in chitosan liposomal emulsion for antimicrobial cotton fabrics. Int. J. Biol. Macromol. 2018, 107, 585–594. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, L.; Gao, M.; Que, X.; Zhou, C.; Zhu, D.; Cai, Y. Nanoformulation of a Novel Pyrano[2,3-c] Pyrazole Heterocyclic Compound AMDPC Exhibits Anti-Cancer Activity via Blocking the Cell Cycle through a P53-Independent Pathway. Molecules 2019, 24, 624. [Google Scholar] [CrossRef] [PubMed]
- Mulet, X.; Boyd, B.J.; Drummond, C.J. Advances in drug delivery and medical imaging using colloidal lyotropic liquid crystalline dispersions. J. Colloid Interface Sci. 2013, 393, 1–20. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Lee M., K. Liposomes for Enhanced Bioavailability of Water-Insoluble Drugs: In Vivo Evidence and Recent Approaches. Pharmaceutics 2020, 12, 264. [Google Scholar] [CrossRef]
- Milla, P.; Dosio, F.; Cattel, L. PEGylation of proteins and liposomes: A powerful and flexible strategy to improve the drug delivery. Curr. Drug Metab. 2012, 13, 105–119. [Google Scholar] [CrossRef]
- Qi, R.; Zhang, H.; Xu, L.; Shen, W.; Chen, C.; Wang, C.; Cao, Y.; Wang, Y.; van Dongen, M.A.; He, B.; et al. G5 PAMAM dendrimer versus liposome: A comparison study on the in vitro transepithelial transport and in vivo oral absorption of simvastatin. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1141–1151. [Google Scholar] [CrossRef]
- Lee, W.-H.; Loo, C.-Y.; Traini, D.; Young, P.M. Nano-and micro-based inhaled drug delivery systems for targeting alveolar macrophages. Expert Opin. Drug Deliv. 2015, 12, 1009–1026. [Google Scholar] [CrossRef]
- Ihre, H.; Hult, A.; Fréchet, J.M.J.; Gitsov, I. Double-stage convergent approach for the synthesis of functionalized dendritic aliphatic polyesters based on 2,2-bis(hydroxymethyl)propionic acid. Macromolecules 1998, 31, 4061–4068. [Google Scholar] [CrossRef]
- Alfei, S.; Castellaro, S.; Taptue, G.B. Synthesis and NMR characterization of dendrimers based on 2,2-bis-(hydroxymethyl)-propanoic acid (bis-HMPA) containing peripheral amino acid residues for gene transfection. Org. Commun. 2017, 10, 144–177. [Google Scholar] [CrossRef]
- Alfei, S.; Castellaro, S. Synthesis and characterization of polyester-based dendrimers containing peripheral arginine or mixed amino acids as potential vectors for gene and drug delivery. Macromol. Res. 2017, 25, 1172–1186. [Google Scholar] [CrossRef]
- Alfei, S.; Catena, S.; Turrini, F. Biodegradable and biocompatible spherical dendrimer nanoparticles with a gallic acid shell and a double-acting strong antioxidant activity as potential device to fight diseases from “oxidative stress”. Drug Deliv. Transl. Res. 2019, 10, 259–270. [Google Scholar] [CrossRef]
- Alfei, S.; Signorello, M.G.; Schito, A.M.; Catena, S.; Turrini, F. Reshaped as polyester-based nanoparticles, gallic acid inhibits platelet aggregation, reactive oxygen species production and multi-resistant Gram-positive bacteria with an efficiency never obtained. Nanoscale Adv. 2019, 1, 4148–4157. [Google Scholar] [CrossRef]
- Alfei, S.; Catena, S. Synthesis and characterization of versatile amphiphilic dendrimers peripherally decorated with positive charged amino acids. Polym. Int. 2018, 67, 1572–1584. [Google Scholar] [CrossRef]
- Alfei, S.; Catena, S. Synthesis and characterization of fourth generation polyester-based dendrimers with cationic amino acids-modified crown as promising water soluble biomedical devices. Polym. Adv. Technol. 2018, 29, 2735–2749. [Google Scholar] [CrossRef]
- Zuccari, G.; Alfei, S.; Zorzoli, A.; Marimpietri, D.; Turrini, F.; Baldassari, S.; Marchitto, L.; Caviglioli, G. Resveratrol-loaded D-tocopheryl polyethylene glycol 1000 succinate micelles as nutritional supplement for children with chronic liver disease. Pharmaceutics 2021, 13, 1128. [Google Scholar] [CrossRef] [PubMed]
- Benns, J.M.; Choi, J.S.; Mahato, R.I.; Park, J.S.; Kim, S.W. pH-sensitive cationic polymer gene delivery vehicle: N-Ac-poly(L-histidine)-graft-poly(L-lysine) comb shaped polymer. Bioconj. Chem. 2000, 11, 637–645. [Google Scholar] [CrossRef]
- Parise, A.; Milelli, A.; Tumiatti, V.; Minarini, A.; Neviani, P.; Zuccari, G. Preparation, Characterization, and in Vitro Evaluation of Sterically Stabilized Liposome Containing a Naphthalenediimide Derivative as Anticancer Agent. Drug Deliv. 2015, 22, 590–597. [Google Scholar] [CrossRef][Green Version]
- Zuccari, G.; Baldassari, S.; Alfei, S.; Marengo, B.; Valenti, G.E.; Domenicotti, C.; Ailuno, G.; Villa, C.; Marchitto, L.; Caviglioli, G. D-α-Tocopherol-Based Micelles for Successful Encapsulation of Retinoic Acid. Pharmaceuticals 2021, 14, 212. [Google Scholar] [CrossRef]
- Vogel, A.I. Elementary Practical Organic Chemistry. Part III. Quantitative Organic Analysis, 1st ed.; Longman Ed.: London, UK, 1958; Chapter 20; p. 7. [Google Scholar]
- Buzea, C.; Pacheco, I. 28—Toxicity of Nanoparticles. In Nanotechnology in Eco-Efficient Construction, 2nd ed.; Pacheco-Torgal, F., Diamanti, M.V., Nazari, A., Granqvist, C.G., Pruna, A., Amirkhanian, S., Eds.; Woodhead Publishing Series in Civil and Structural Engineering; Woodhead Publishing: Sawston, UK, 2019; pp. 705–754. ISBN 978-0-08-102641-0. [Google Scholar]
- Chauhan, A.S. Dendrimers for Drug Delivery. Molecules 2018, 23, 938. [Google Scholar] [CrossRef]
- Thiele, W.; Kyjacova, L.; Köhler, A.; Sleeman, J.P. A cautionary note: Toxicity of polyethylene glycol 200 injected intraperitoneally into mice. Lab. Anim. 2020, 54, 391–396. [Google Scholar] [CrossRef]
- Palanisamy, S. Synthesis and Evaluation of Polyamidoamine (PAMAM) dendrimer as a carrier of cefixime drug. World J. Pharm. Pharm. Sci. 2016, 5, 858–867. [Google Scholar]
- Jain, K.; Kesharwani, P.; Gupta, U.; Jain, N.K. Dendrimer toxicity: Let’s meet the challenge. Int. J. Pharm. 2010, 394, 122–142. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Marengo, B.; Valenti, G.E.; Domenicotti, C. Synthesis of Polystyrene-Based Cationic Nanomaterials with Pro-Oxidant Cytotoxic Activity on Etoposide-Resistant Neuroblastoma Cells. Nanomaterials 2021, 11, 977. [Google Scholar] [CrossRef]
- Alfei, S.; Schito, A.M. From Nanobiotechnology, Positively Charged Biomimetic Dendrimers as Novel Antibacterial Agents: A Review. Nanomaterials 2020, 10, 2022. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Schito, A.M. Positively Charged Polymers as Promising Devices against Multidrug Resistant Gram-Negative Bacteria: A Review. Polymers 2020, 12, 1195. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Narmani, A.; Jafari, S.M. Dendrimers as efficient nanocarriers for the protection and delivery of bioactive phytochemicals. Adv. Colloid Interface Sci. 2020, 278, 102125. [Google Scholar] [CrossRef]
- Mircioiu, C.; Voicu, V.; Anuta, V.; Tudose, A.; Celia, C.; Paolino, D.; Fresta, M.; Sandulovici, R.; Mircioiu, I. Mathematical Modeling of Release Kinetics from Supramolecular Drug Delivery Systems. Pharmaceutics 2019, 11, 140. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, M.L. (Ed.) Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Sawston, UK, 2015; pp. 63–86. ISBN 978-0-08-100092-2. [Google Scholar]
- Alfei, S.; Marengo, B.; Zuccari, G.; Turrini, F.; Domenicotti, C. Dendrimer Nanodevices and Gallic Acid as Novel Strategies to Fight Chemoresistance in Neuroblastoma Cells. Nanomaterials 2020, 10, 1243. [Google Scholar] [CrossRef]
- Ankit, J.; Sanjay, K.J. In vitro release kinetics model fitting of liposomes: An insight. Chem. Phys. Lipids 2016, 201, 28–40. [Google Scholar]
- Jafari-Aghdam, N.; Adibkia, K.; Payab, S.; Barzegar-Jalali, M.; Parvizpur, A.; Mohammadi, G.; Sabzevari, A. Methylprednisolone acetate–Eudragit® RS100 electrospuns: Preparation and physicochemical characterization. Artif. Cells Nanomed. Biotechnol. 2016, 44, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, V.; Kosmidis, K.; Vlachou, M.; Macheras, P. On the use of the Weibull function for the discernment of drug release mechanisms. Int. J. Pharm. 2006, 309, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Kaminskas, L.M.; Boyd, B.J.; Porter, C.J.H. Dendrimer pharmacokinetics: The effect of size, structure and surface characteristics on ADME properties. Nanomedicine 2011, 6, 1063–1084. [Google Scholar] [CrossRef]
- Rizvi, A.A.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef]
- Von Seel, F. Grundlagen der Analytischen Chemie, 5th ed.; Geier, G., Ed.; Verlag Chemie: Weinheim, Germany, 1970; Volume 82, p. 962. [Google Scholar]
- Aravindan, L.; Bicknell, K.A.; Brooks, G.; Khutoryanskiya, V.V.; Williams, A.C. Effect of acyl chain length on transfection efficiency and toxicity of polyethylenimine. Int. J. Pharm. 2009, 378, 201–210. [Google Scholar] [CrossRef]
- Abedi-Gaballu, F.; Dehghan, G.; Ghaffari, M.; Yekta, R.; Abbaspour-Ravasjani, S.; Baradaran, B.; Dolatabadi, J.; Hamblin, M.R. PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl. Mater. Today 2018, 12, 177–190. [Google Scholar] [CrossRef]
- Deshpande, P.P.; Biswas, S.; Torchilin, V.P. Current trends in the use of liposomes for tumor targeting. Nanomedicine 2013, 8, 1509–1528. [Google Scholar] [CrossRef]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef]
- Van Winden, E.C.A. Freeze-Drying of Liposomes: Theory and Practice. Methods Enzymol. 2003, 367, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Van Winden, E.C.A.; Zhang, W.; Crommelin, D.J.A. Effect of freezing rate on the stability of liposomes during freeze-drying and rehydration. Pharm. Res. 1997, 14, 1151–1160. [Google Scholar] [CrossRef]
- Crowe, O.J.H.; Leslie, S.B.; Crowe, L.M. Is vitrification sufficient to preserve liposomes during freeze-drying? Cryobiology 1994, 31, 355–366. [Google Scholar] [CrossRef]
- Wolfe, J.; Bryant, G. Freezing, drying, and/or vitrification of membrane- solute-water systems. Cryobiology 1999, 39, 103–129. [Google Scholar] [CrossRef]
- Koster, K.L.; Lei, Y.P.; Anderson, M.; Martin, S.; Bryant, G. Effects of Vitrified and Nonvitrified Sugars on Phosphatidylcholine Fluid-to-Gel Phase Transitions. Biophys. J. 2000, 78, 1932–1946. [Google Scholar] [CrossRef]
- Hawach Scientific. Why Is Trehalose Such a Good Cryoprotectant? Available online: https://www.hawachdryer.com/why-is-trehalose-such-a-good-cryoprotectant/ (accessed on 14 December 2021).
- Henriksen, I.; Sande, S.A.; Smistad, G.; Agren, T.; Karlsen, J. In vitro evaluation of drug release kinetics from liposomes by fractional dialysis. Int. J. Pharm. 1995, 119, 231–238. [Google Scholar] [CrossRef]
- Paveli, Z.; Basnet, K.N.; Schubert, R. Liposomal gels for vaginal drug delivery. Int. J. Pharm. 2001, 219, 139–149. [Google Scholar] [CrossRef]
- Alfei, S.; Oliveri, P.; Malegori, C. Assessment of the Efficiency of a Nanospherical Gallic Acid Dendrimer for Long-Term Preservation of Essential Oils: An Integrated Chemometric-Assisted FTIR Study. Chem. Sel. 2019, 4, 8891–8901. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Domenicotti, C. Development of a Fast, Low-Cost, Conservative and Ecological Method for Quantifying Gallic Acid in Polymeric Formulations by FTIR Spectroscopy in Solution. Chem. Sel. 2020, 5, 4381–4388. [Google Scholar] [CrossRef]
- Plöger, G.F.; Hofsäss, M.A.; Dressman, J.B. Solubility Determination of Active Pharmaceutical Ingredients Which Have Been Recently Added to the List of Essential Medicines in the Context of the Biopharmaceutics Classification System–Biowaiver. J. Pharm. Sci. 2018, 107, 1478–1488. [Google Scholar] [CrossRef]
- Rommasi, F.; Esfandiari, N. Liposomal Nanomedicine: Applications for Drug Delivery in Cancer Therapy. Nanoscale Res. Lett. 2021, 16, 95. [Google Scholar] [CrossRef]
- Buzea, C.; Pacheco, I.I.; Robbieet, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Askari, G.; Yarmand, M.S.; Salami, M.; EmamDjomeh, Z. Development, modification and characterization of ursolic acid-loaded gelatin nanoparticles through electrospraying technique. Food Bioprod. Process. 2020, 124, 329–341. [Google Scholar] [CrossRef]
- Lasoń, E.; Sikora, E.; Ogonowski, J.; Tabaszewska, M.; Skoczylas, L. Release study of selected terpenes from nanostructured lipid carriers. Colloids Surf. A Physicochem. Eng. Asp. 2016, 510, 87–92. [Google Scholar] [CrossRef]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef]
- Akinc, A.; Battaglia, G. Exploiting endocytosis for nanomedicines. Cold Spring Harb. Perspect. Biol. 2013, 5, a016980. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Mccrate, J.M.; Lee, J.C.-M.; Li, H. The Role of Surface Charge on the Uptake and Biocompatibility of Hydroxyapatite Nanoparticles with Osteoblast Cells. Nanotechnology 2011, 22, 105708. [Google Scholar] [CrossRef]
- Jambhrunkar, S.; Yu, M.; Yang, J.; Zhang, J.; Shrotri, A.; Endo-Munoz, L.; Moreau, J.; Lu, G.; Yu, C. Stepwise Pore Size Reduction of Ordered Nanoporous Silica Materials at Angstrom Precision. J. Am. Chem. Soc. 2013, 135, 8444–8447. [Google Scholar] [CrossRef]
- Petri-Fink, A.; Chastellain, M.; Juillerat-Jeanneret, L.; Ferrari, A.; Hofmann, H. Development of Functionalized Superparamagnetic Iron Oxide Nanoparticles for Interaction with Human Cancer Cells. Biomaterials 2005, 26, 2685–2694. [Google Scholar] [CrossRef]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of Particle Size and Surface Charge on Cellular Uptake and Biodistribution of Polymeric Nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef] [PubMed]
- Gregoriadis, G. Liposome Technology: Liposome Preparation and Related Techniques, 3rd ed.; Gregoriadis, G., Ed.; Informa Helthcare USA, Inc.: New York, NY, USA, 2006; Volume I, p. 324. [Google Scholar]
- Malvern Panalytical. Liposomes and The Use of Zeta Potential Measurements to Study Sterically Stabilized Liposomes. AZoNano. Available online: https://www.azonano.com/article.aspx?ArticleID=1214 (accessed on 27 October 2021).
- Lunov, O.; Syrovets, T.; Loos, C.; Beil, J.; Delacher, M.; Tron, K.; Nienhaus, G.U.; Musyanovych, A.; Mailänder, V.; Landfester, K.; et al. Differential uptake of functionalized polystyrene nanoparticles by human macrophages and a monocytic cell line. ACS Nano 2011, 5, 1657–1669. [Google Scholar] [CrossRef]




| Dendrimer | MW 1 | MW 2 | Error % |
|---|---|---|---|
| G5K | 30,224 | 28,966 | 4.2 |
| Dendrimer | Equations | R2 | LD50 (µM) | |
|---|---|---|---|---|
| G5K | y = 0.0056x2 − 1.2139x + 104.96 | (6) | 0.9820 | 64.4 |
| G4-PAMAM-NH2 | y = 0.6953x2 − 13.851x + 99.659 | (7) | 0.9997 | 4.7 |
| CR232-G5K NPs | ||||||
|---|---|---|---|---|---|---|
| [A] (mAU) | CCR232 (µg/mL) | CR232 in CR232-G5K | DL (%) EE (%) | Molecular Formula | MW | Error (%) |
| 2.35350 | 0.03903 | 1.9121 ± 0.0389 mg * 39.01 ± 0.80 mg § 41.2 ± 0.7 # | 31.7 ± 0.6 98.3 ± 2.0 | C1705H2512N398O460Cl233 | 44,219.5 ± 237.8 1 44,153.1 2 | 0.15 3 |
| 1.18140 | 0.01948 | |||||
| 0.57283 | 0.00937 | |||||
| 0.54493 | 0.00890 | |||||
| 0.33907 | 0.00548 | |||||
| Entry | β (pH Value) | β Mean * (mL/pH) |
|---|---|---|
| G5K | 0.047 | 0.1100 |
| CR232-G5K | 0.3076 | 0.1871 |
| PEI-b | 0.0760 | 0.5170 |
| PAMAM G4 1 | 0.0014 | 0.01717 |
| PAMAM G4R 2 | 0.0015 | 0.01818 |
| PAMAM G4HHR 3 | 0.0038 | 0.04127 |
| CR232-SUV | CCR232 (mg/mL) in CR232-SUVs 1 | EE (%) | CCR232 (mM) in CR232-SUVs 2 | DL (%) |
|---|---|---|---|---|
| 5/1 3 | 0.07 ± 0.03 | 17.03 ± 5.52 24.85 ± 4.25 90.18 ± 3.30 | 0.206 ± 0.038 | 3.95 ± 0.03 4.02 ± 0.05 4.34 ± 0.15 |
| 15/1 3 | 0.10 ± 0.03 | 0.294 ± 0.014 | ||
| 30/1 3 | 0.08 ± 0.03 | 0.235 ± 0.018 |
| Experimental Data | Solubility Data (mg/mL) | Water-Solubility Improvement | CR232 Water Solubility (mg/mL) | |
|---|---|---|---|---|
| Substance | Mg | |||
| CR232-G5K NPs | 5.2 ± 0.05 1 | 5.2 ± 0.05 | 2311.1 | 0.00225 ± 0.0001 |
| CR232 contained in solubilized CR232-G5K | 1.65 ± 0.02 2 | 1.65 ± 0.02 | 733.3 | |
| CR232 in CR232-SUVs 5/1 | N.C. | 0.07 ± 0.03 | 31.1 | |
| CR232 in CR232-SUVs 15/1 | N.C. | 0.10 ± 0.03 | 44.4 | |
| CR232 in CR232-SUVs 30/1 | N.C. | 0.08 ± 0.03 | 35.6 | |
| CR232-SUVs 30/1 | 1.3 ± 0.0 3 | 3.97 ± 0.47 | 1764.4 | |
| Measure | G5K NPs | CR232-G5K NPs | CR232-SUVs |
|---|---|---|---|
| Z-Ave 1 (nm) | 175.7 ± 1.8 | 529.7 ± 33.5 | 173.4 ± 0.8 |
| PDI | 0.129 ± 0.035 | 0.472 ± 0.054 | 0.118 ± 0.030 |
| ζ-p (mV) | +48.0 ± 6.4 | +37.2 ± 7.0 | +17.8 ± 4.5 |
| Analysis | CR232-G5K NPs | CR232-SUVs | |
|---|---|---|---|
| FTIR [cm−1] | 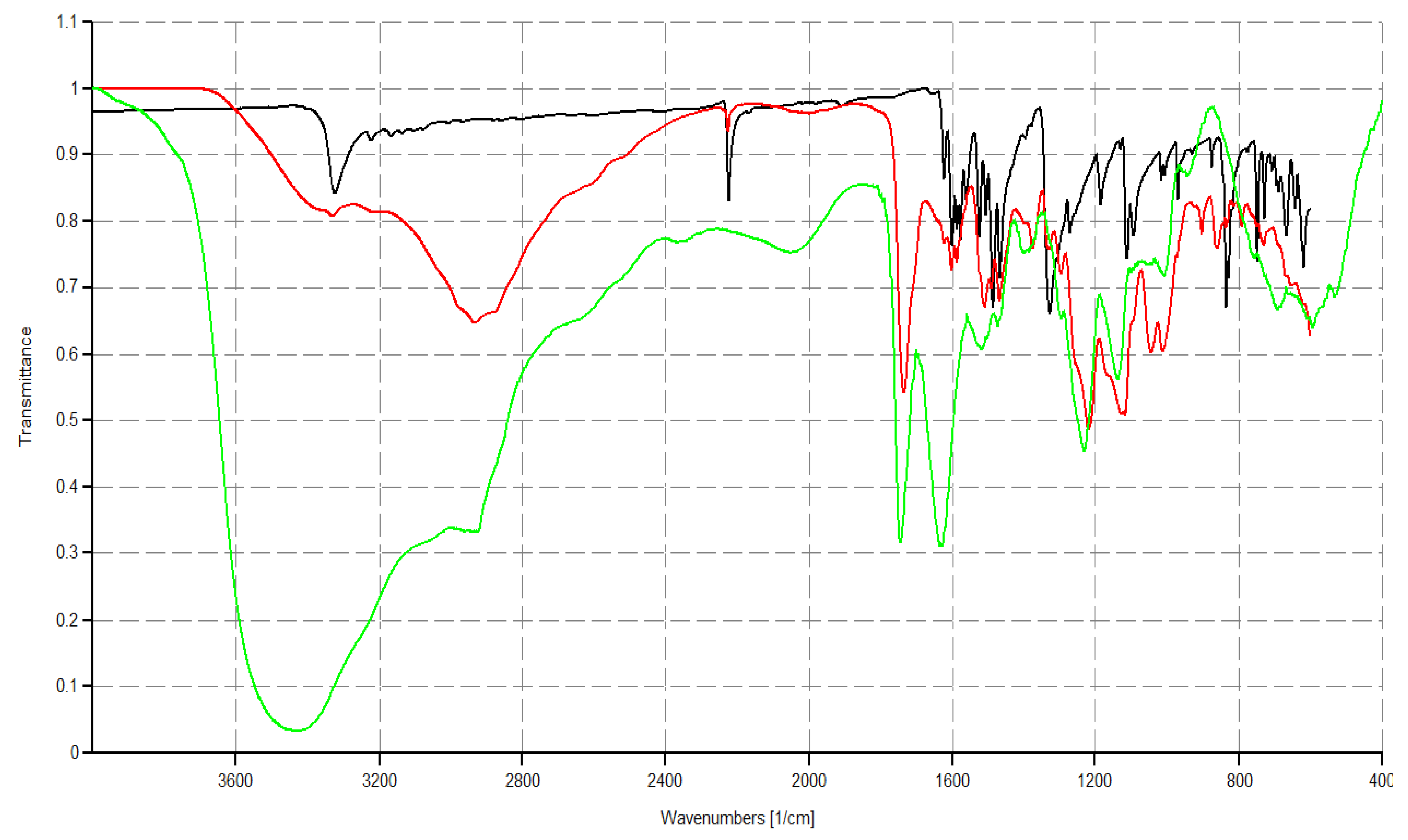 G5K (green line), CR232 (black line), CR232-G5K NPs (red line) | 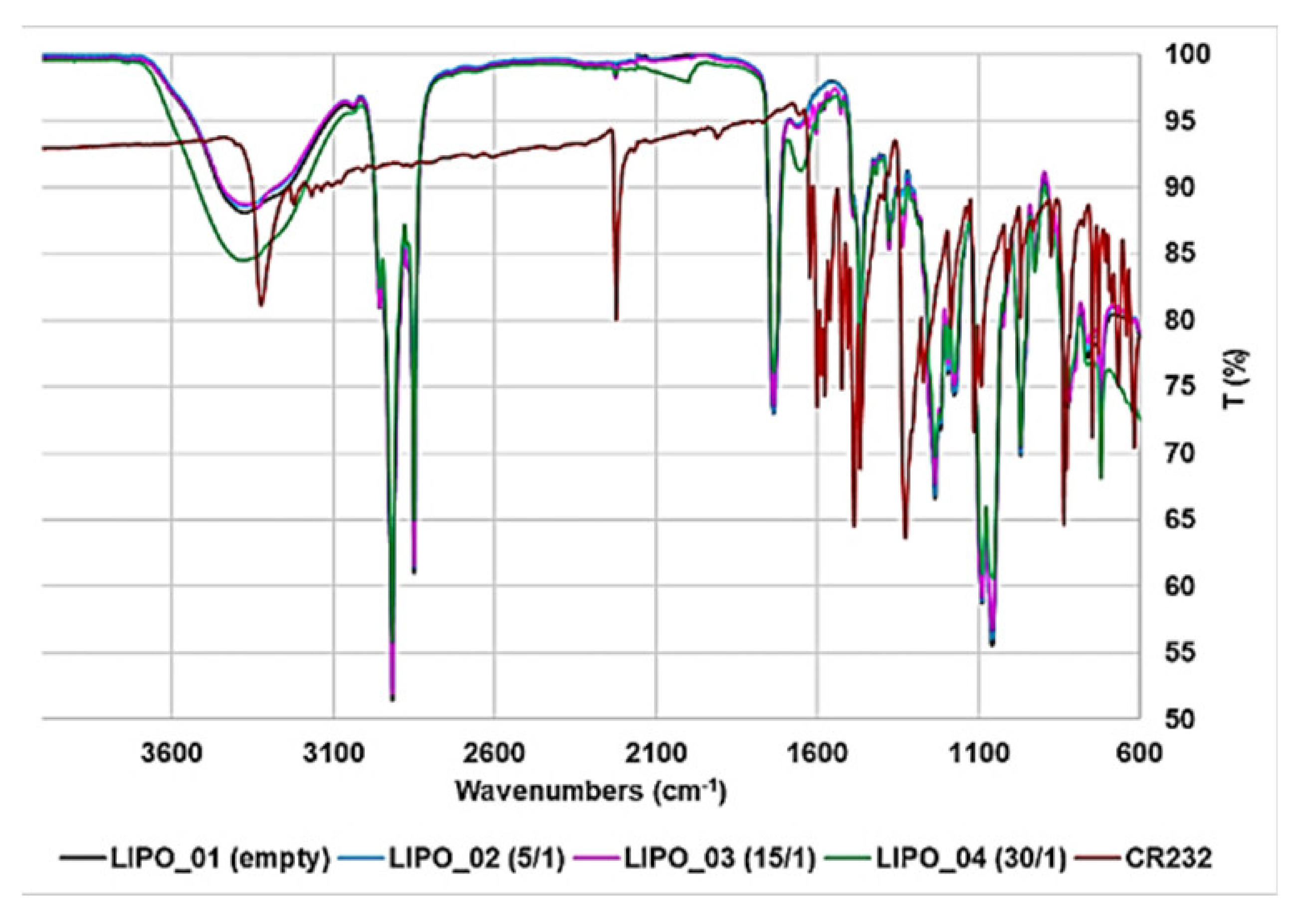 | |
| 1H NMR (400 MHz, DMSO-d6) [ppm] |  | N.A. | |
| 13C NMR (100 MHz, DMSO-d6) [ppm] | 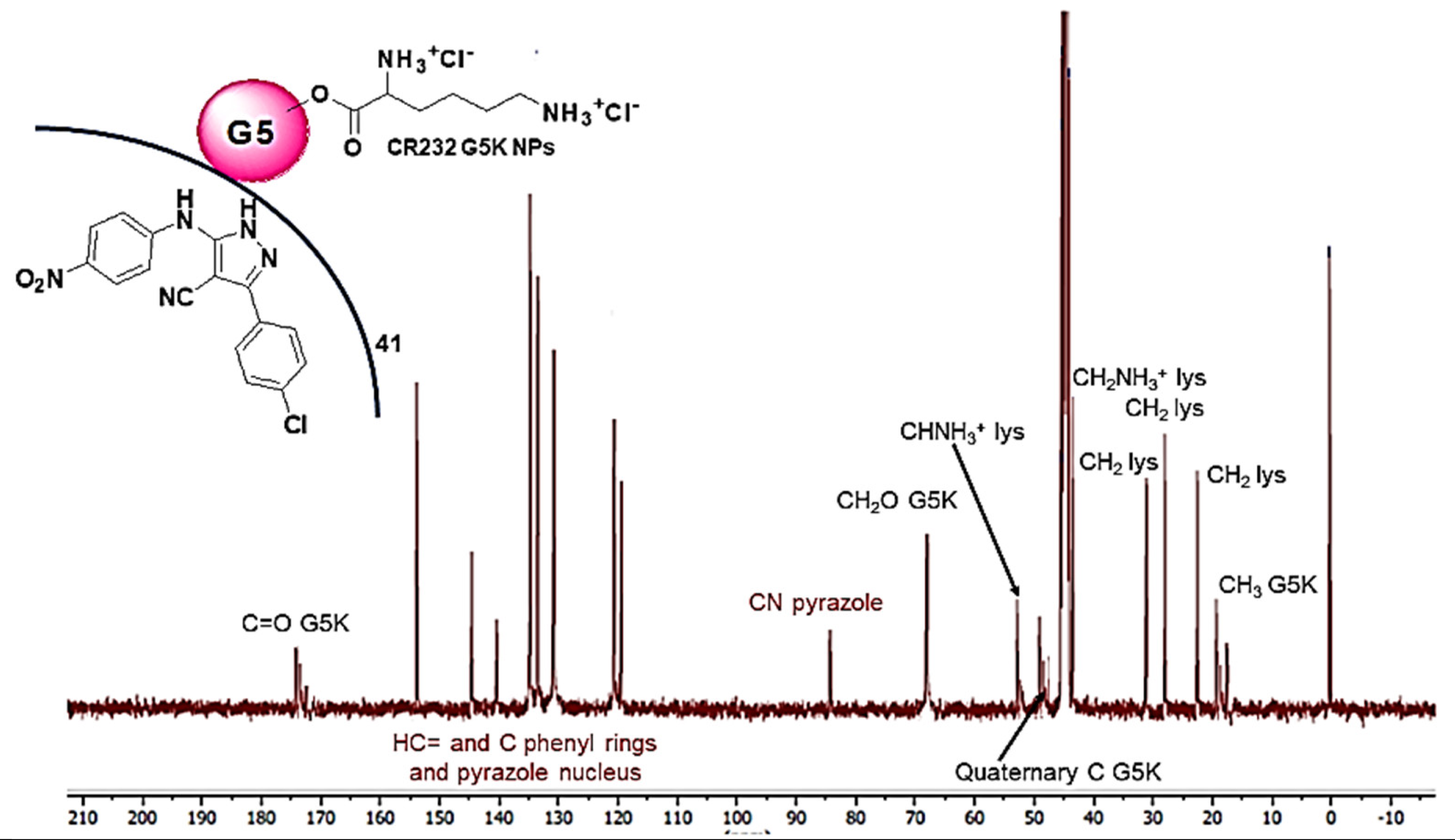 | N.A. | |
| UV-Vis | Ultraviolet Spectrum | ʎabs = 328 nm | N.A. |
| UV-Vis | DL (%) | 31.7 ± 0.6 | 3.95 ± 0.03 |
| 4.02 ± 0.05 | |||
| 4.34 ± 0.15 | |||
| EE (%) | 98.3 ± 2.0 | 17.0 ± 5.0 | |
| 24.0 ± 4.0 | |||
| 90.0 ± 3.0 | |||
| 1H NMR | MW | 44,153.1 | N.AP. |
| DL% (UV-Vis) | 44,219.5 ± 237.8 | ||
| Scanning Electron Microscopy (SEM) | Morphology | Spherical | N.A. |
| Average Size | ≃ 500 nm | ||
| DLS 1 Analysis | Z-Ave 2 (nm) PDI 3 | 529.7 ± 33.5 5 0.427 ± 0.054 5 | 173.4 ± 0.8 0.118 ± 0.03 |
| ζ-p 4 (mV) | +37.2 ± 7.0 5 | +17.8 ± 4.5 | |
| Solubilization Essay | Water Solubility (mg/mL) | 5.2 ± 0.05 6,§,8 | 3.97 ± 0.47 6,§,10 |
| 0.07 ± 0.03 7,§,11 | |||
| 1.65 ± 0.02 7,§,9 | 0.10 ± 0.03 7,§,12 | ||
| 0.08 ± 0.03 7,§,13 | |||
| Dialysis Method (UV-Vis) | Cumulative Release (%, 24 h) | 99.3 | 37% |
| Mathematical Model | Weibull (β > 1) | Zero order | |
| Mechanism | Complex Mechanisms | Slow and constant drug release concentration-independent | |
| Cytotoxicity G5K (HeLa Cells) | LD50 | 64.4 µM * | N.D. |
| Potentiometric Titration # | Buffer Capacity (β) | 0.3076 | N.D. |
| Average Buffer Capacity (β mean) | 0.1871 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfei, S.; Spallarossa, A.; Lusardi, M.; Zuccari, G. Successful Dendrimer and Liposome-Based Strategies to Solubilize an Antiproliferative Pyrazole Otherwise Not Clinically Applicable. Nanomaterials 2022, 12, 233. https://doi.org/10.3390/nano12020233
Alfei S, Spallarossa A, Lusardi M, Zuccari G. Successful Dendrimer and Liposome-Based Strategies to Solubilize an Antiproliferative Pyrazole Otherwise Not Clinically Applicable. Nanomaterials. 2022; 12(2):233. https://doi.org/10.3390/nano12020233
Chicago/Turabian StyleAlfei, Silvana, Andrea Spallarossa, Matteo Lusardi, and Guendalina Zuccari. 2022. "Successful Dendrimer and Liposome-Based Strategies to Solubilize an Antiproliferative Pyrazole Otherwise Not Clinically Applicable" Nanomaterials 12, no. 2: 233. https://doi.org/10.3390/nano12020233
APA StyleAlfei, S., Spallarossa, A., Lusardi, M., & Zuccari, G. (2022). Successful Dendrimer and Liposome-Based Strategies to Solubilize an Antiproliferative Pyrazole Otherwise Not Clinically Applicable. Nanomaterials, 12(2), 233. https://doi.org/10.3390/nano12020233