Sorption of Nanomaterials to Sandstone Rock
Abstract
:1. Introduction
2. Overall Aim
- Assessing rock sample characterisation by means of routine core analysis, particle size distribution, Brunauer–Emett–Teller (BET) and Zeta potential experiments;
- Evaluating the fluid–fluid (brine–nanomaterial) interaction using a compatibility test;
- Evaluating nanomaterial and rock interaction using static batch sorption experiments and ultraviolet–visible (UV–Vis) spectroscopy;
- Define the effect of the nanomaterials on the reservoir rock through single-phase core floods and size and concentration analysis of the effluents.
3. Materials and Methods
3.1. Fluids and Rock Material
3.1.1. Core Plugs
3.1.2. Synthetic Brines
3.1.3. Alkali Solution
3.1.4. Nanofluids
3.1.5. Tracer
3.2. Batch Sorption Experiments
3.2.1. UV–Vis Spectrophotometry
3.2.2. Limitations of Measurement—Exclusion Criterion
3.3. Single-Phase Core Flooding
3.3.1. Permeability Measurement and Nanofluids Injection
3.3.2. Effluent Analysis Purposes
3.4. Effluents Analysis Methods
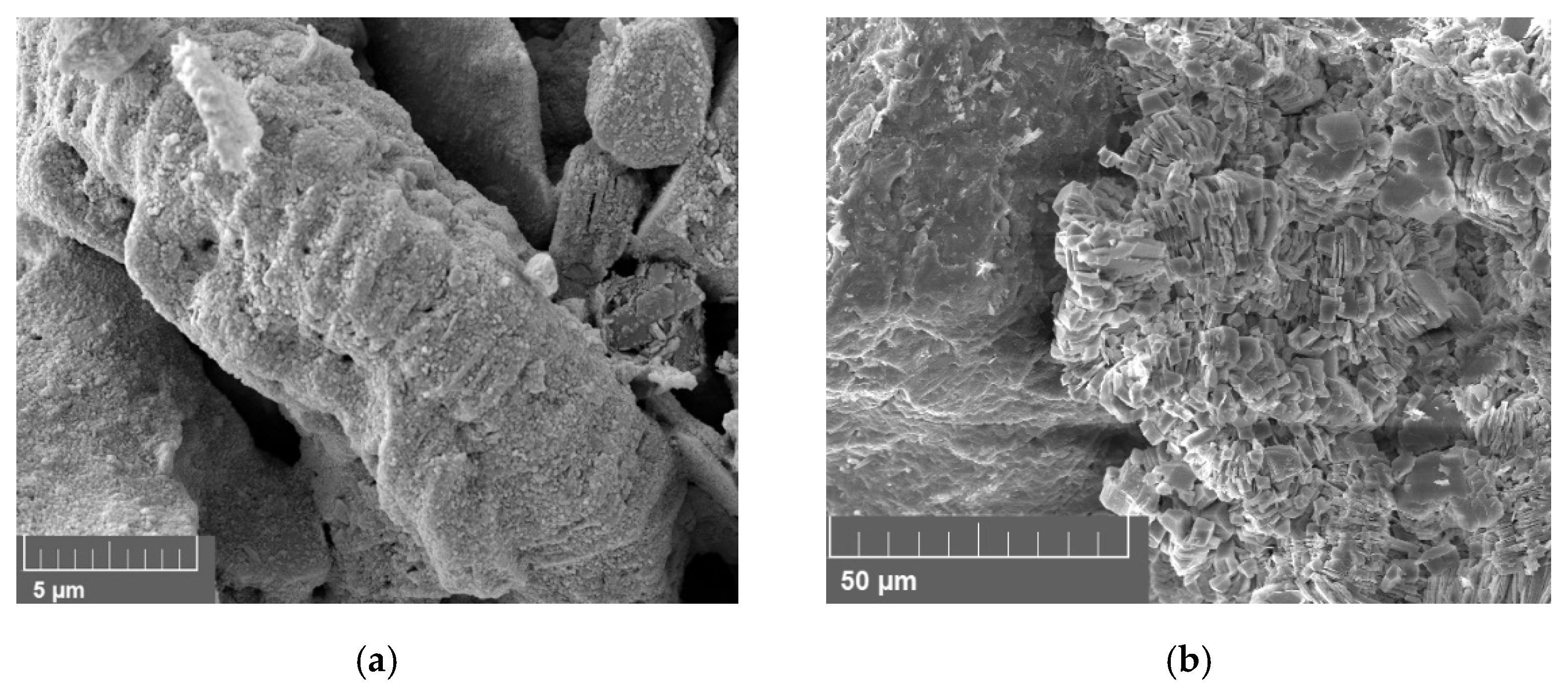
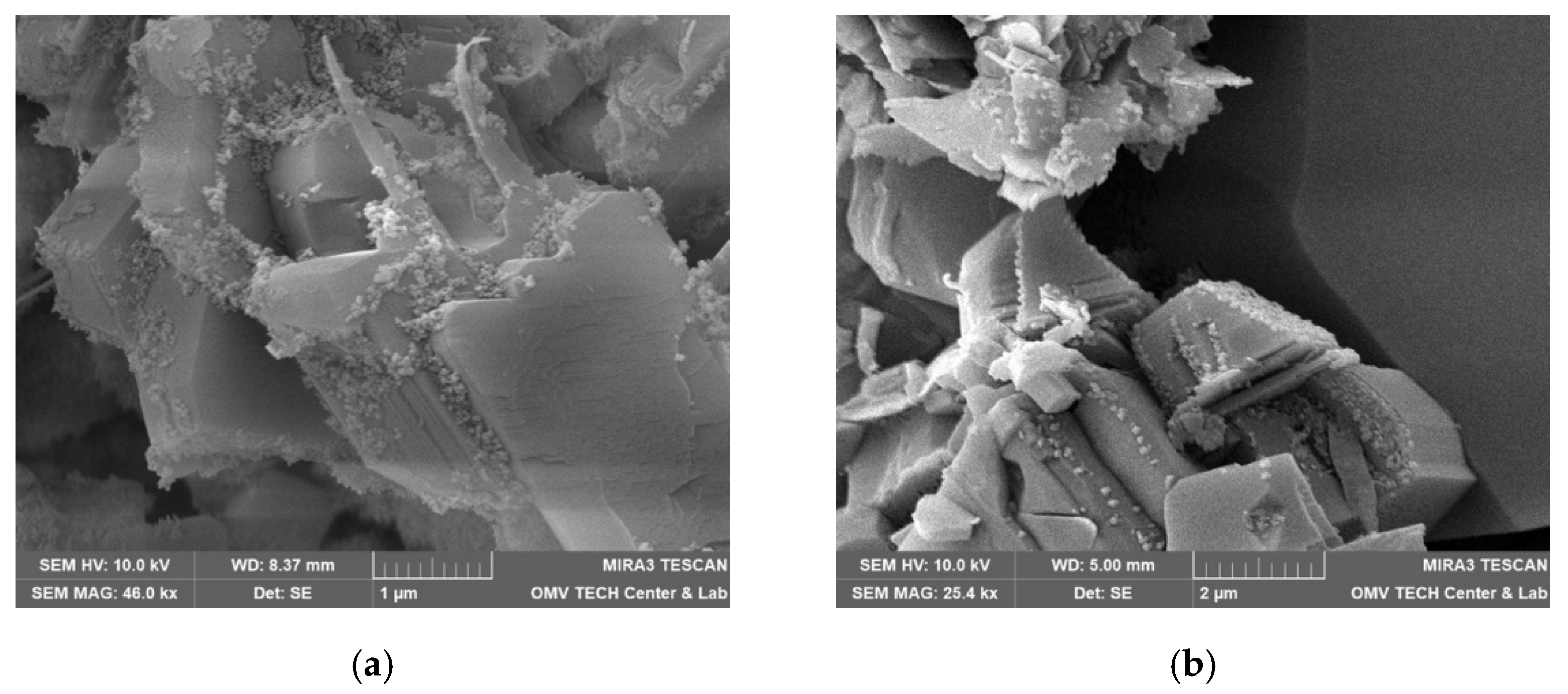
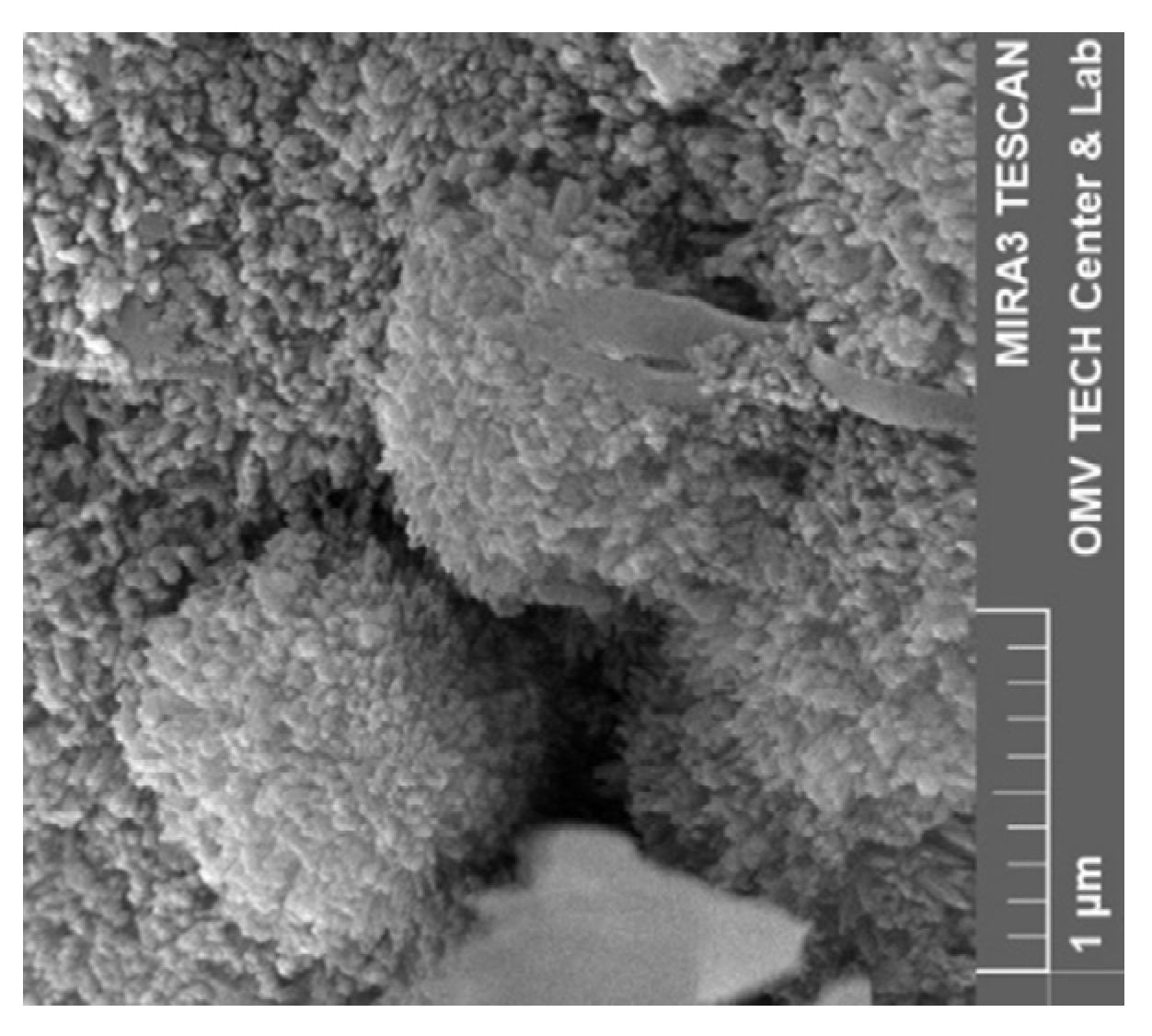
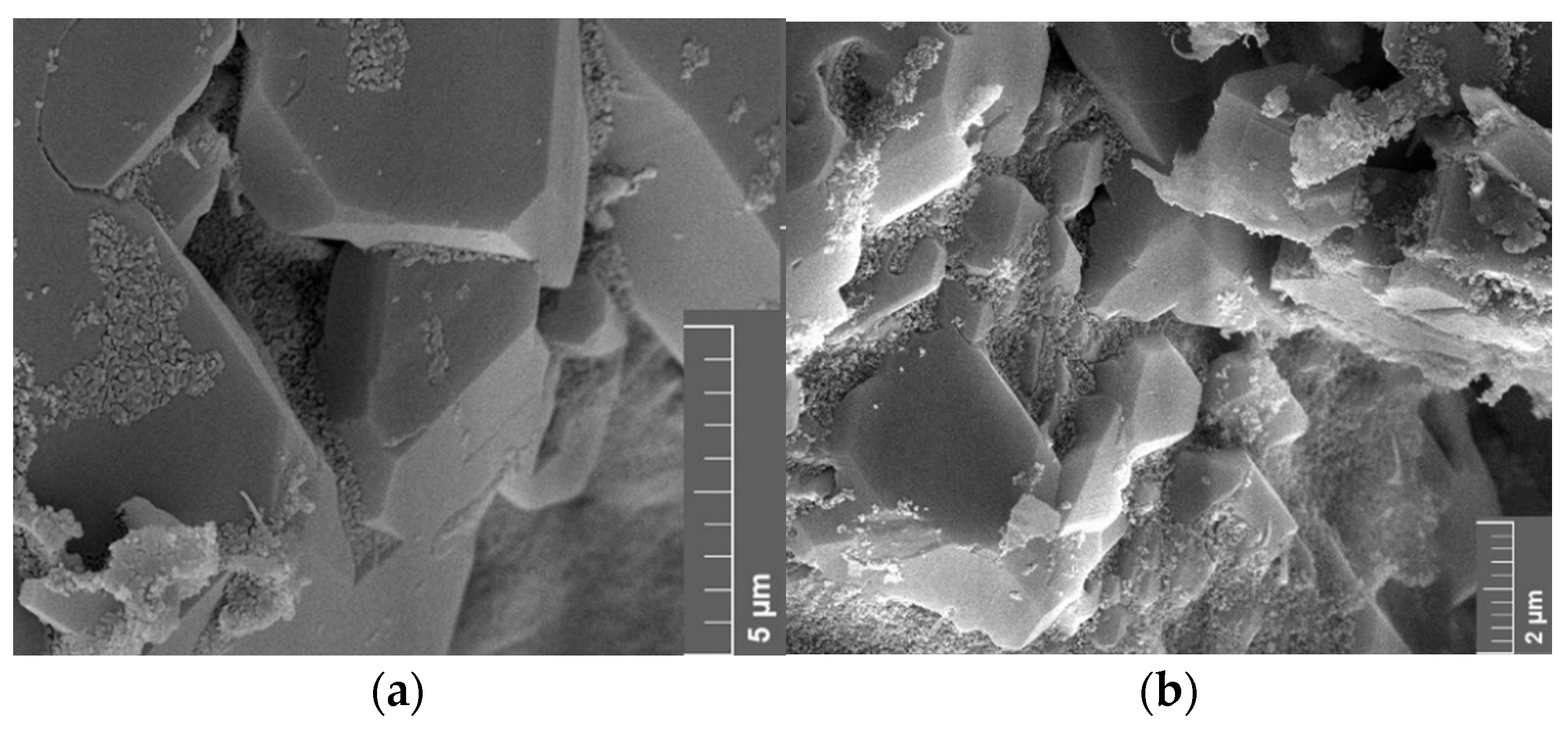
3.5. Scanning Electron Microscope (SEM)
4. Results and Discussion
4.1. Batch Sorption
4.1.1. Nanofluid A
4.1.2. Nanofluid B
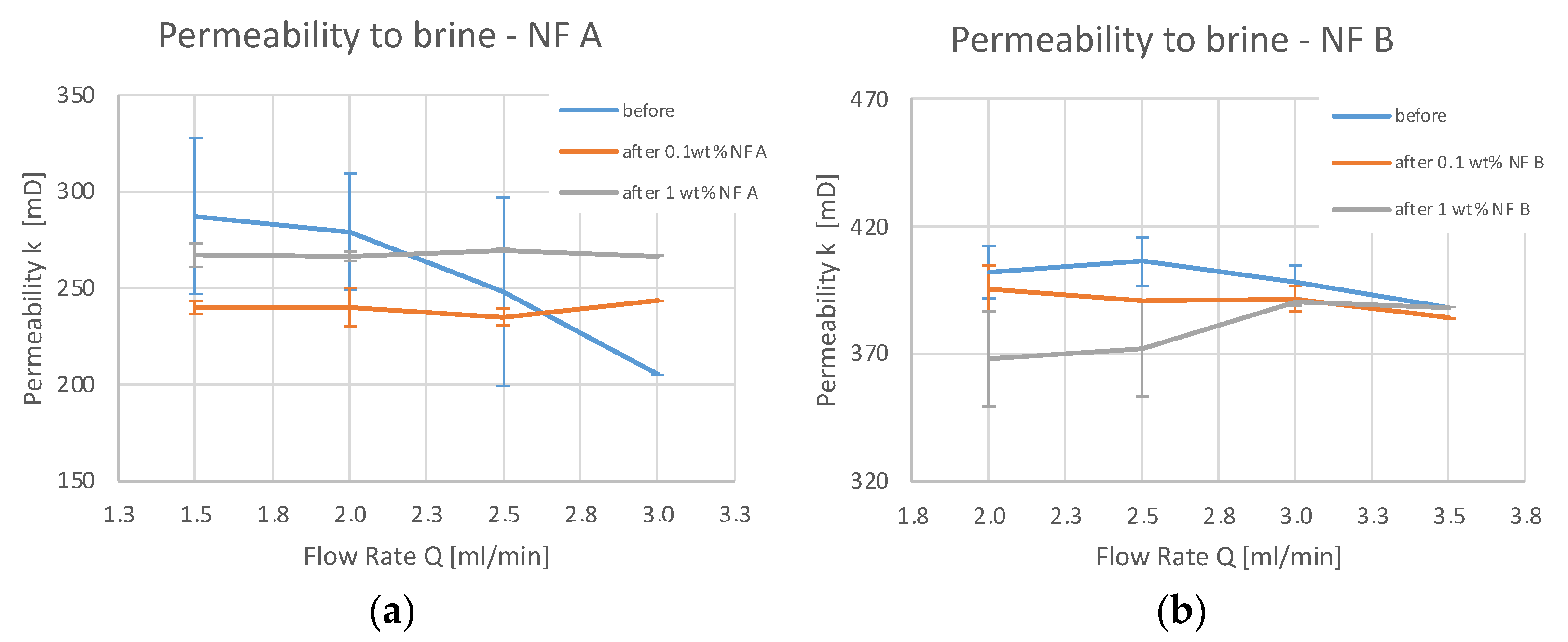
4.2. Core Flooding—Permeability Measurements
4.3. Core Flooding—Eflluents Analysis
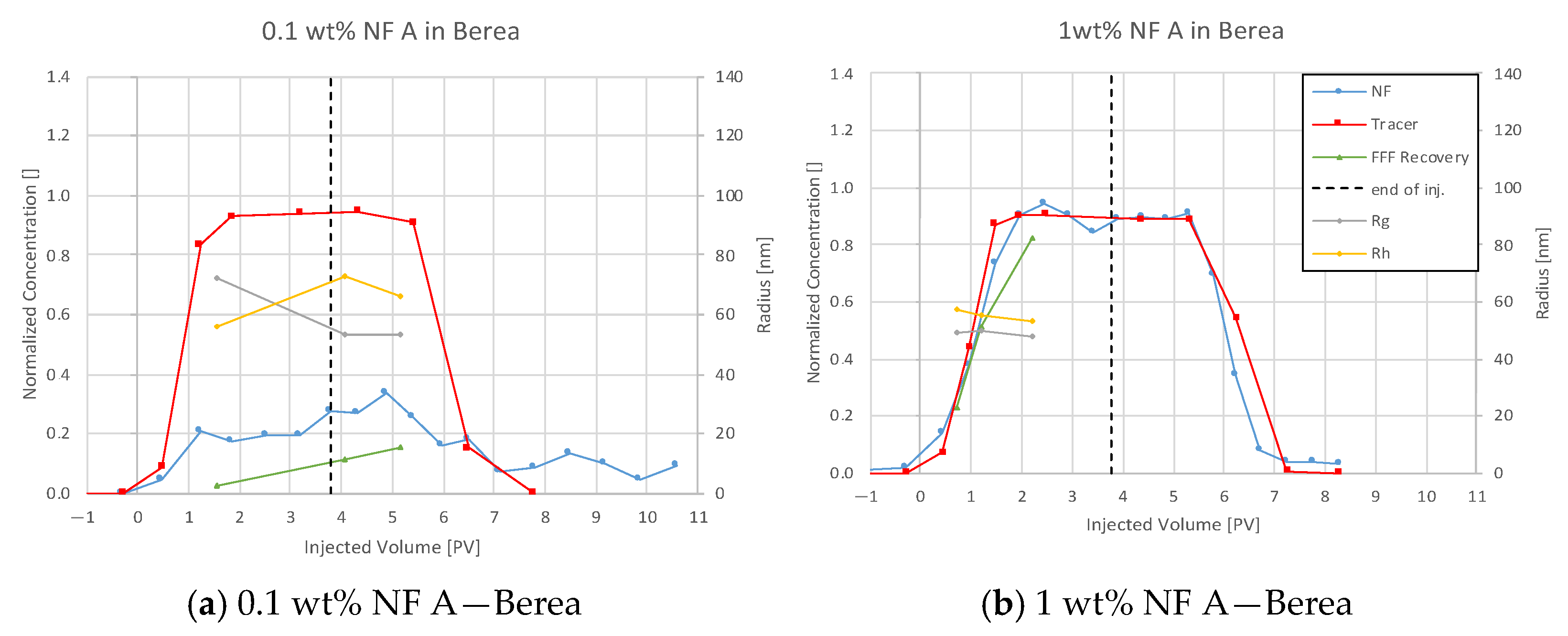
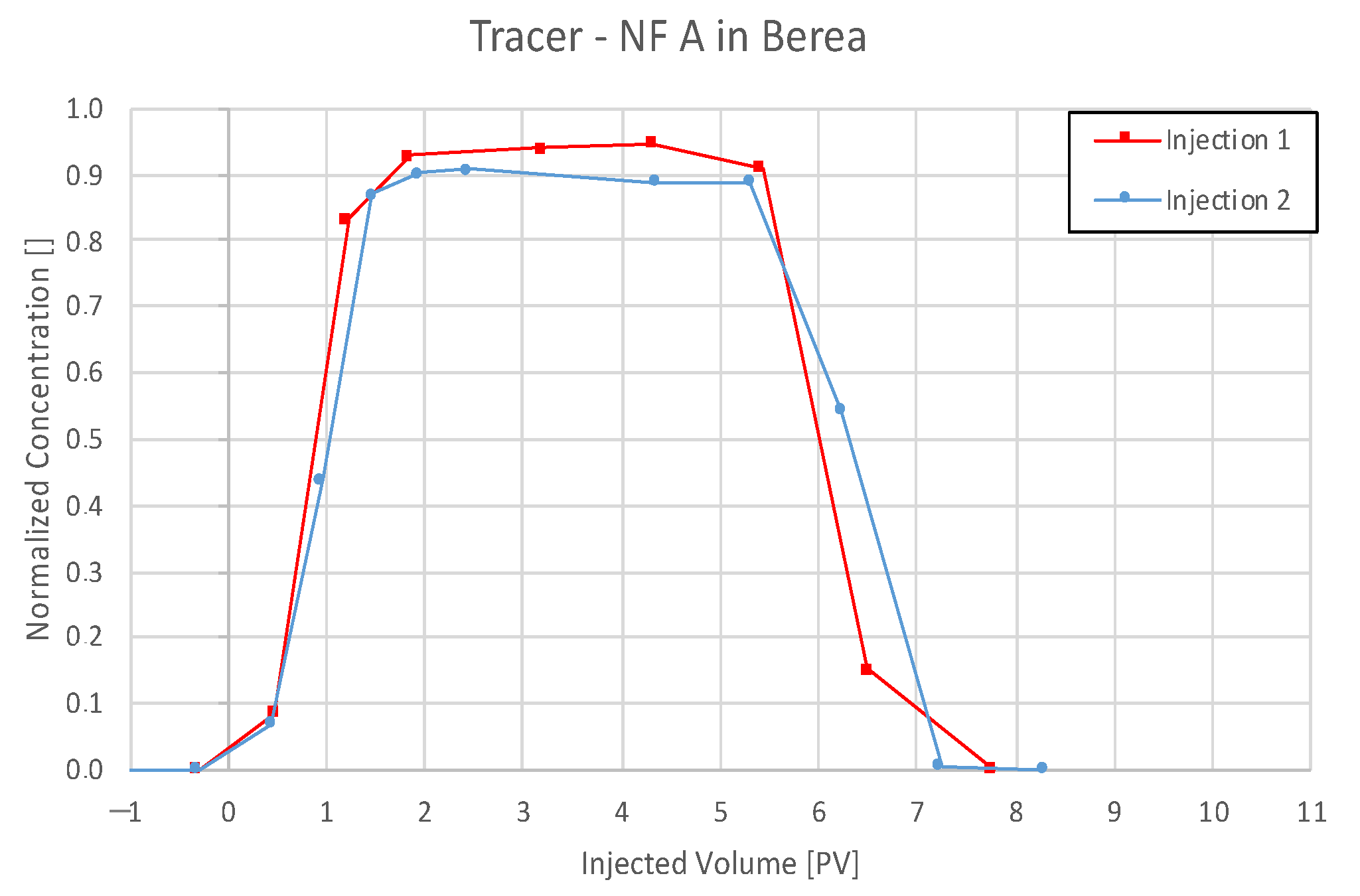
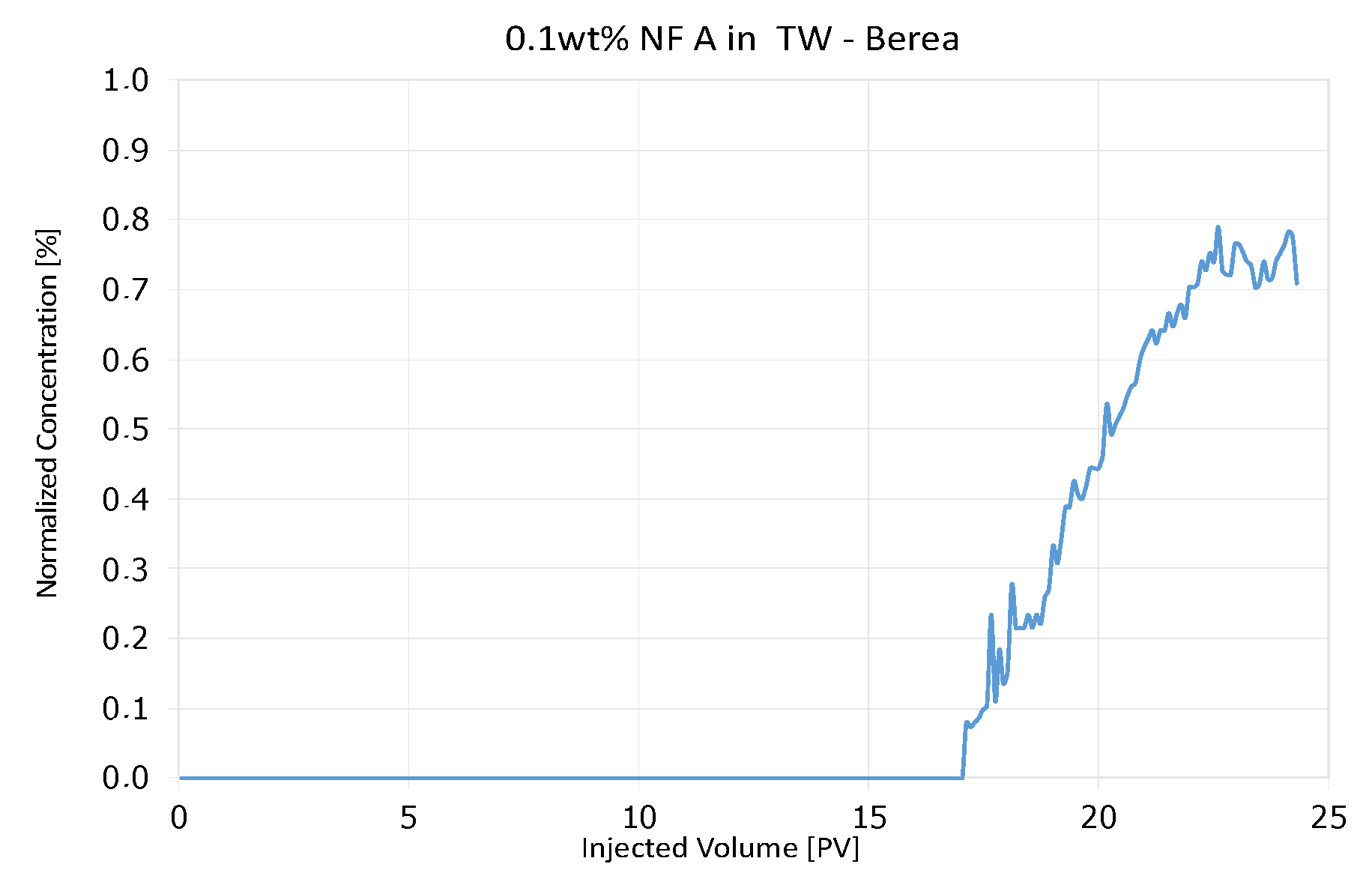
4.3.1. Nanofluid A in Berea
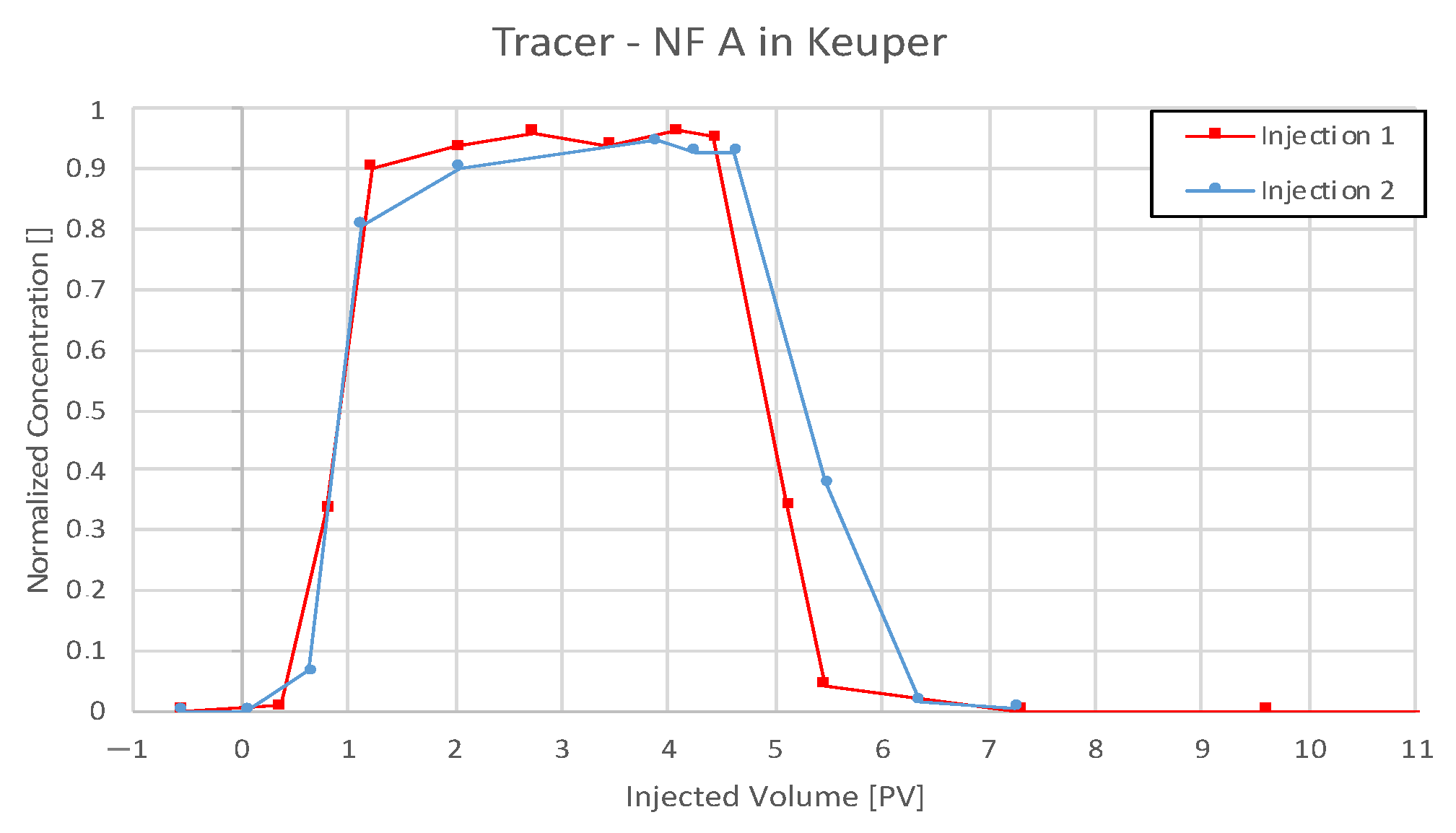
4.3.2. Nanofluid A in Keuper
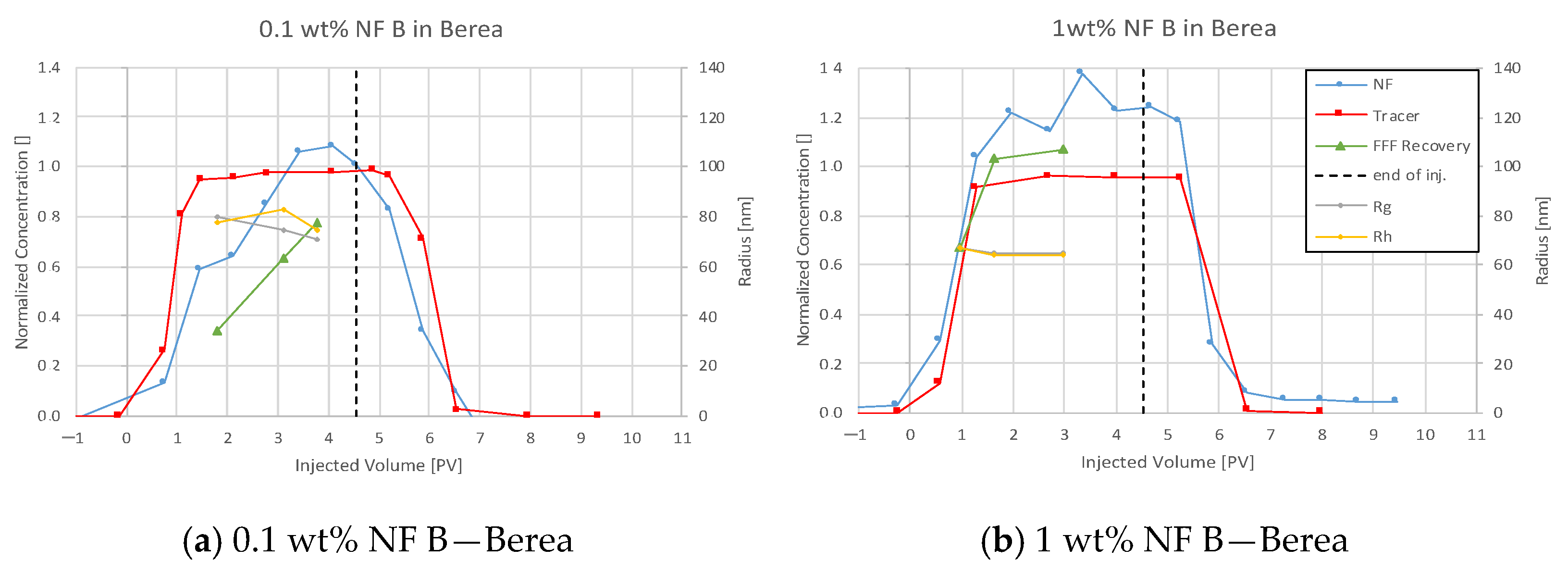
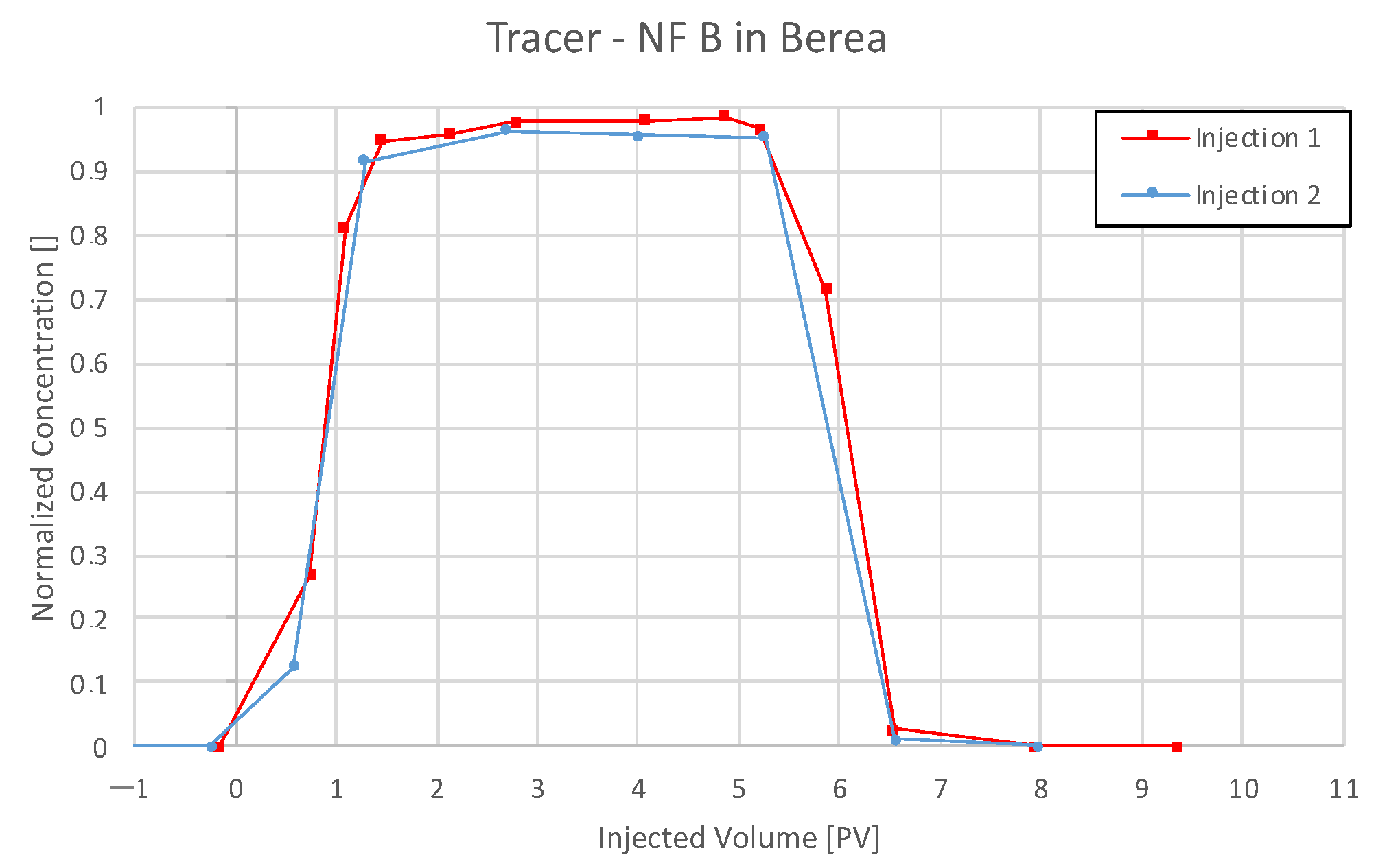
4.3.3. Nanofluid B in Berea
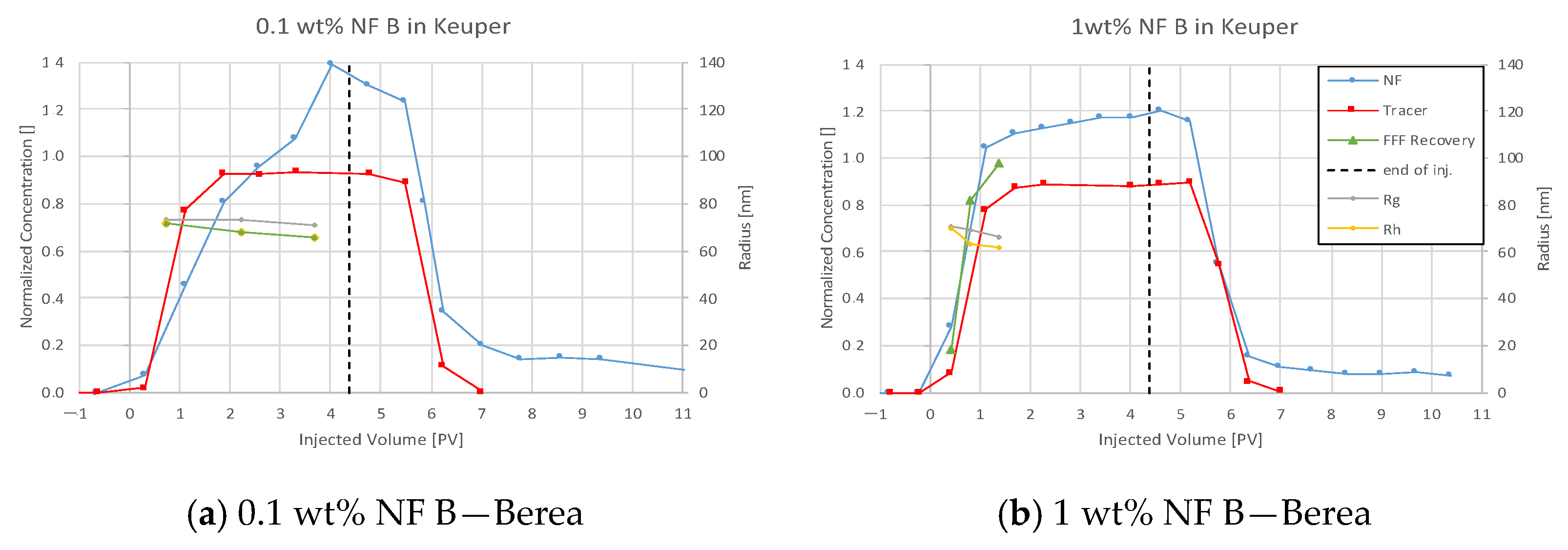
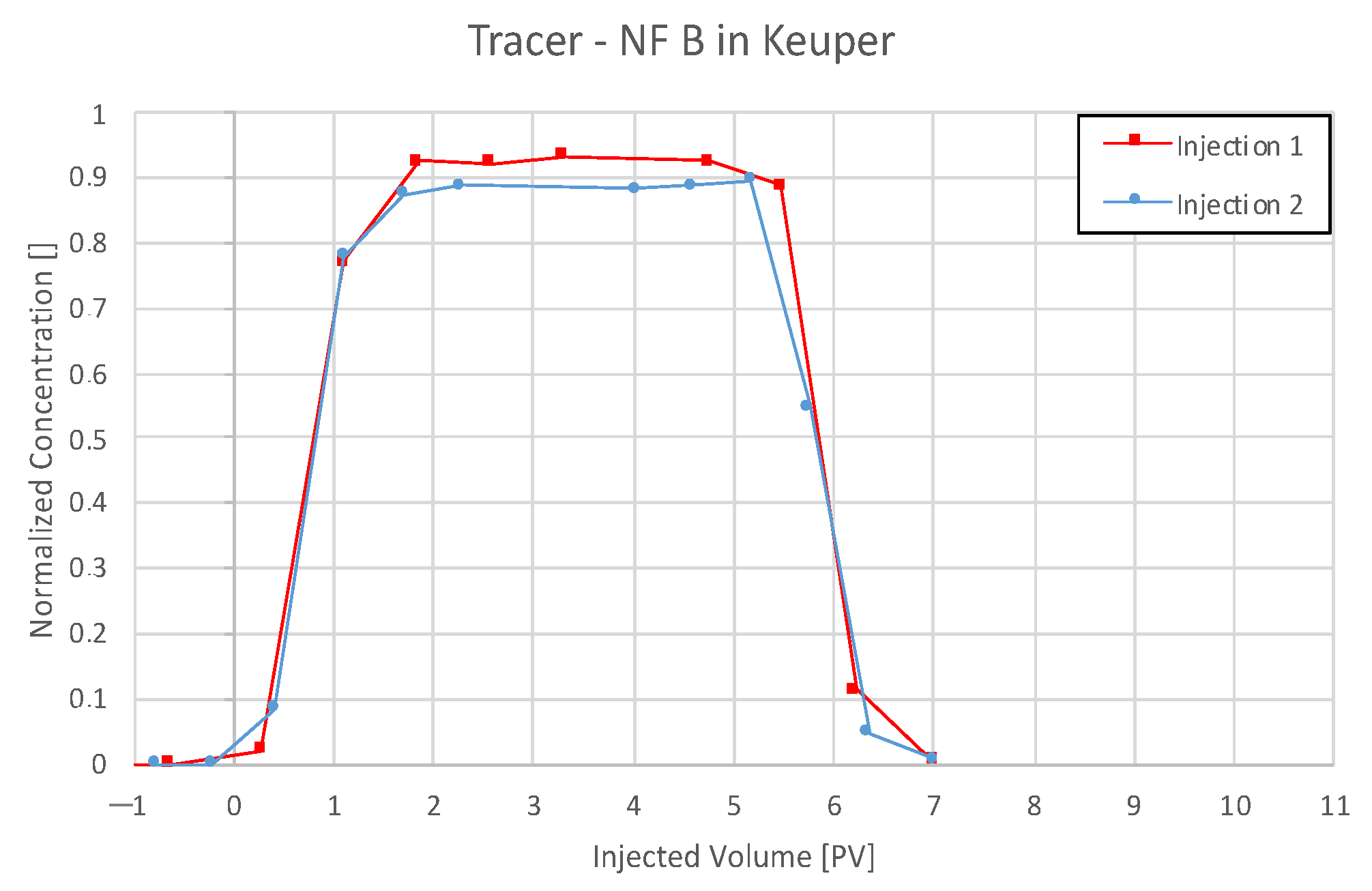
4.3.4. Nanofluid B in Keuper
4.4. Flow Field Flow Fractionation (FFF) and Particle Size Measurements
4.5. Scanning Electron Microscopy (SEM)
4.5.1. Effect of Minerology
4.5.2. Effect of Brine
4.5.3. Vacuum Saturation vs. Core Flood
5. Discussion
5.1. Discussion of Batch Sorption Results
5.2. Discussion of Core Flooding—Permeability Measurements
5.3. Discussion of Core Flooding—Effluent Analysis Nanofluid A—Berea and Keuper
5.4. Discussion of Core Flooding—Effluent Analysis Nanofluid B—Berea and Keuper
5.5. Discussion of Flow Field Flow Fractionation (FFF) and Particle Size Measurements
5.6. Discussion of Scanning Electron Microscopy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saleh, S.; Neubauer, E.; Borovina, A.; Hincapie, R.E.; Clemens, T.; Ness, D. Wettability Changes Due to Nanomaterials and Alkali—A Proposed Formulation for EOR. Nanomaterials 2021, 11, 2351. [Google Scholar] [CrossRef] [PubMed]
- Gbadamosi, A.O.; Junin, R.; Manan, M.A.; Agi, A.; Yusuff, A.S. An overview of chemical enhanced oil recovery: Recent advances and prospects. Int. Nano Lett. 2019, 9, 171–202. [Google Scholar] [CrossRef] [Green Version]
- Ko, S.; Huh, C. Use of nanoparticles for oil production applications. J. Pet. Sci. Eng. 2019, 172, 97–114. [Google Scholar] [CrossRef]
- McDonald, M.J. A Novel Potassium Silicate for Use in Drilling Fluids Targeting Unconventional Hydrocarbons. In Proceedings of the SPE Canadian Unconventional Resources Conference, Calgary, Alberta, 30 October–1 November 2012. [Google Scholar]
- Sharma, M.M.; Zhang, R.; Chenevert, M.E.; Ji, L.; Guo, Q.; Friedheim, J. A New Family of Nanoparticle Based Drilling Fluids. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 8–10 October 2012. [Google Scholar]
- Contreras, O.; Hareland, G.; Husein, M.; Nygaard, R.; Alsaba, M. Application of In-House Prepared Nanoparticles as Filtration Control Additive to Reduce Formation Damage. In Proceedings of the SPE International Symposium and Exhibition on Formation Damage Control, Lafayette, LA, USA, 26–28 February 2014. [Google Scholar]
- Other Water-Based Uses; Elsevier BV: Amsterdam, The Netherlands, 2015; pp. 179–207.
- Omurlu, C.; Pham, H.; Nguyen, Q.P. Interaction of surface-modified silica nanoparticles with clay minerals. Appl. Nanosci. 2016, 6, 1167–1173. [Google Scholar] [CrossRef] [Green Version]
- Pham, H.; Nguyen, Q.P. Effect of silica nanoparticles on clay swelling and aqueous stability of nanoparticle dispersions. J. Nanopart. Res. 2014, 16, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Alomair, O.A.; Matar, K.M.; Alsaeed, Y.H. Nanofluids Application for Heavy Oil Recovery. In Proceedings of the SPE Asia Pacific Oil & Gas Conference and Exhibition, Adelaide, Australia, 14–16 October 2014. [Google Scholar] [CrossRef]
- Sandeep, R.; Jain, S.; Agrawal, A. Application of Nanoparticles-Based Technologies in the Oil and Gas Industry. In Nanotech-nology for Energy and Environmental Engineering. Green Energy and Technology; Ledwani, L., Sangwai, J., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Ledwani, L.; Sangwai, J.S. Nanotechnology for Energy and Environmental Engineering. In Nano-Technology for Energy and Environmental Engineering; Springer Nature: Cham, Switzerland, 2020; pp. 257–327. [Google Scholar]
- Ehtesabi, H.; Ahadian, M.M.; Taghikhani, V. Enhanced Heavy Oil Recovery Using TiO2 Nanoparticles: Investigation of Deposition during Transport in Core Plug. Energy Fuels 2014, 29, 1–8. [Google Scholar] [CrossRef]
- Lashari, N.; Ganat, T. Emerging applications of nanomaterials in chemical enhanced oil recovery: Progress and perspective. Chin. J. Chem. Eng. 2020, 28, 1995–2009. [Google Scholar] [CrossRef]
- Bera, A.; Belhaj, H. Application of nanotechnology by means of nanoparticles and nanodispersions in oil recovery—A comprehensive review. J. Nat. Gas Sci. Eng. 2016, 34, 1284–1309. [Google Scholar] [CrossRef]
- Kamal, M.S.; Adewunmi, A.A.; Sultan, A.S.; Al-Hamad, M.F.; Mehmood, U. Recent Advances in Nanoparticles Enhanced Oil Recovery: Rheology, Interfacial Tension, Oil Recovery, and Wettability Alteration. J. Nanomater. 2017, 2017, 1–15. [Google Scholar] [CrossRef]
- Neubauer, E.; Hincapie, R.E.; Clemens, T.; Cornelius, M. Selection of Nanomaterials as Emulsion Stabilizers in Alkali-Polymer EOR of High-TAN Number Oil. In Proceedings of the SPE Improved Oil Recovery Conference, Virtual, 31 August–4 September 2020. [Google Scholar]
- Neubauer, E.; Hincapie, R.E.; Borovina, A.; Biernat, M.; Clemens, T.; Ahmad, Y.K. Influence of Nanofluids on Wettability Changes and Interfacial Tension Reduction. In Proceedings of the SPE Europec, Virtual, 1–3 December 2020. [Google Scholar]
- Joonaki, E.; Ghanaatian, S. The Application of Nanofluids for Enhanced Oil Recovery: Effects on Interfacial Tension and Coreflooding Process. Pet. Sci. Technol. 2014, 32, 2599–2607. [Google Scholar] [CrossRef]
- Cheraghian, G.; Rostami, S.; Afrand, M. Nanotechnology in Enhanced Oil Recovery. Process 2020, 8, 1073. [Google Scholar] [CrossRef]
- Petosa, A.R.; Jaisi, D.P.; Quevedo, I.R.; Elimelech, M.; Tufenkji, N. Aggregation and Deposition of Engineered Nanomaterials in Aquatic Environments: Role of Physicochemical Interactions. Environ. Sci. Technol. 2010, 44, 6532–6549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Murphy, M.J.; Yu, H.; Bagaria, H.G.; Yoon, K.Y.; Neilson, B.M.; Bielawski, C.W.; Johnston, K.P.; Huh, C.; Bryant, S.L. Investigation of Nanoparticle Adsorption During Transport in Porous Media. SPE J. 2015, 20, 667–677. [Google Scholar] [CrossRef]
- Li, S.; Torsaeter, O.; Lau, H.C.; Hadia, N.J.; Stubbs, L.P. The Impact of Nanoparticle Adsorption on Transport and Wettability Alteration in Water-Wet Berea Sandstone: An Experimental Study. Front. Phys. 2019, 7. [Google Scholar] [CrossRef]
- Huh, C.; Daigle, H.; Prigiobbe, V.; Prodanović, M. Practical Nanotechnology for Petroleum Engineers; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Carvalho, P.M.; Felício, M.R.; Santos, N.C.; Gonçalves, S.; Domingues, M.M. Application of Light Scattering Techniques to Nanoparticle Characterization and Development. Front. Chem. 2018, 6, 237. [Google Scholar] [CrossRef] [PubMed]
- Abhishek, R.; Hamouda, A.A. Effect of Various Silica Nanofluids: Reduction of Fines Migrations and Surface Modification of Berea Sandstone. Appl. Sci. 2017, 7, 1216. [Google Scholar] [CrossRef] [Green Version]
- Lauth, G.J.; Kowalczyk, J. Einführung in Die Physik Und Chemie Der Grenzflächen Und Kolloide; Springer Spektrum: Berlin, Germany, 2016. [Google Scholar]
- Arekhov, V.; Hincapie, R.E.; Clemens, T.; Tahir, M. Variations in Wettability and Interfacial Tension during Alkali–Polymer Application for High and Low TAN Oils. Polymers 2020, 12, 2241. [Google Scholar] [CrossRef]
- Abhishek, R. Interaction of Silica Nanoparticles with Chalk and Sandstone Minerals: Adsorption, Fluid/Rock Interactions in the Absence and Presence of Hydrocarbons; University of Stavanger: Stavanger, Norway, 2020. [Google Scholar]
- Tanikawa, W.; Shimamoto, T. Comparison of Klinkenberg-corrected gas permeability and water permeability in sedimentary rocks. Int. J. Rock Mech. Min. Sci. 2009, 46, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Miller, J. How To Measure Zeta Potential More Confidently—Slides and Notes. 2019. Enlighten Scientific LLC. Available online: https://www.researchgate.net/publication/331996588_How_To_Measure_Zeta_Potential_More_Confidently_-_Slides_and_notes (accessed on 1 August 2021).
- Metin, C.O.; Lake, L.W.; Miranda, C.R.; Nguyen, Q.P. Stability of aqueous silica nanoparticle dispersions. J. Nanoparticle Res. 2011, 13, 839–850. [Google Scholar] [CrossRef]
- Pham, H.; Nguyen, Q.P. Adsorption of Functionalized Silica Nanoparticles on Swelling Clay Minerals. TechConnect Briefs 2014, 3, 497–500. [Google Scholar]
- Qiu, C.; Eng, P.J.; Hennig, C.; Schmidt, M. Competitive Adsorption of ZrO2 Nanoparticle and Alkali Cations (Li+–Cs+) on Muscovite (001). Langmuir 2018, 34, 12270–12278. [Google Scholar] [CrossRef]
- Pol, E.V.D.; van Rijn, C.; Southwick, J. Alkali and Surfactant Consumption in Sandstone Outcrop Rocks. In Proceedings of the IOR 2015 18th European Symposium on Improved Oil Recovery, Dresden, Germany, 14–16 April 2015. cp-445-00067. [Google Scholar]
- Bila, A.; Torsæter, O. Experimental Investigation of Polymer-Coated Silica Nanoparticles for EOR under Harsh Reservoir Conditions of High Temperature and Salinity. Nanomaterials 2021, 11, 765. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.C.; Jang, J.; Jung, J.; Waite, W.F.; Collett, T.S.; Kumar, P. 2D micromodel study of clogging behavior of fine-grained particles associated with gas hydrate production in NGHP-02 gas hydrate reservoir sediments. Mar. Pet. Geol. 2019, 108, 714–730. [Google Scholar] [CrossRef]
- Khilar, K.; Fogler, H. The existence of a critical salt concentration for particle release. J. Colloid Interface Sci. 1984, 101, 214–224. [Google Scholar] [CrossRef] [Green Version]
- Nowack, B.; Bucheli, T.D. Occurrence, behavior and effects of nanoparticles in the environment. Environ. Pollut. 2007, 150, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; An, C.; Mo, D.; Liu, N.; Lee, R.L. Study of Adsorption and Transportation Behavior of Nanoparticles in Three Different Porous Media. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 14–18 April 2012. [Google Scholar]
- Abhishek, R.; Hamouda, A.A.; Ayoub, A. Effect of Silica Nanoparticles on Fluid/Rock Interactions during Low Salinity Water Flooding of Chalk Reservoirs. Appl. Sci. 2018, 8, 1093. [Google Scholar] [CrossRef] [Green Version]

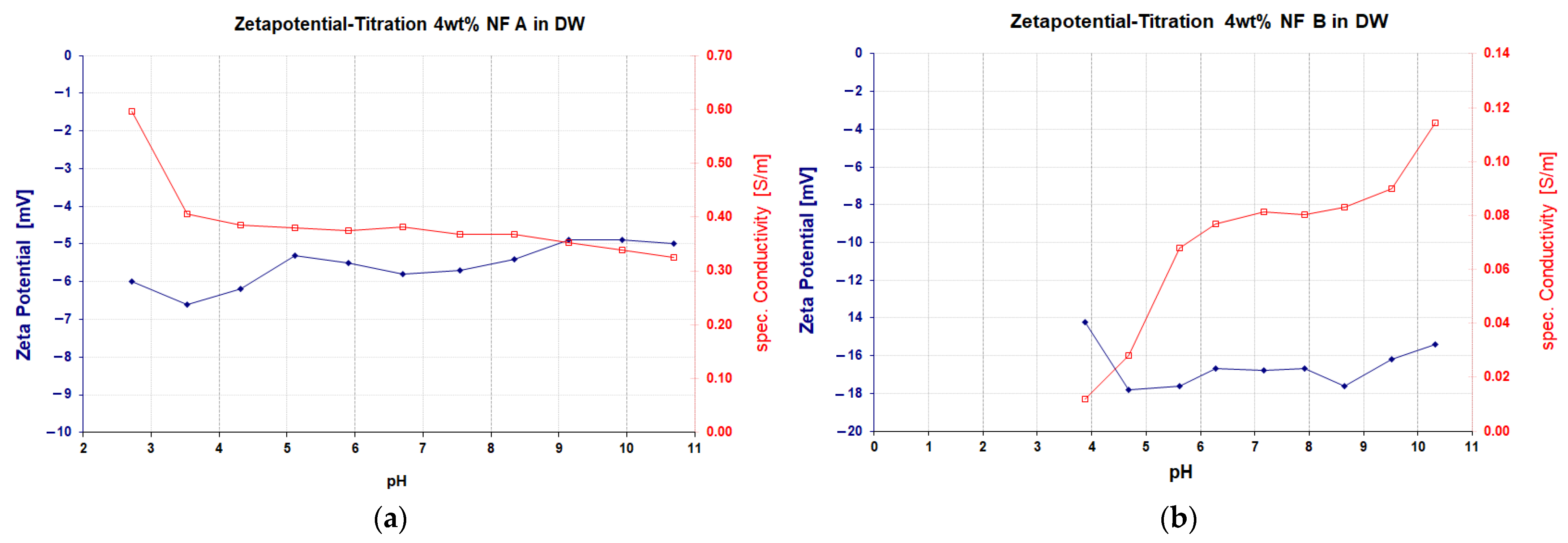
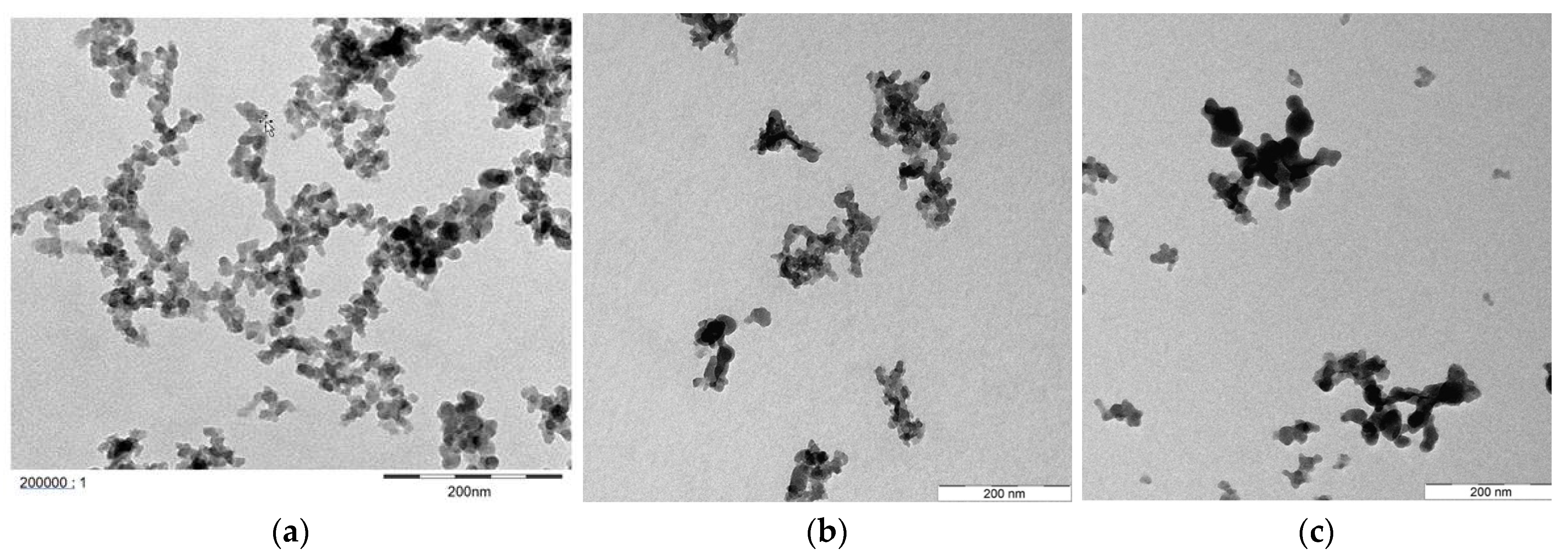
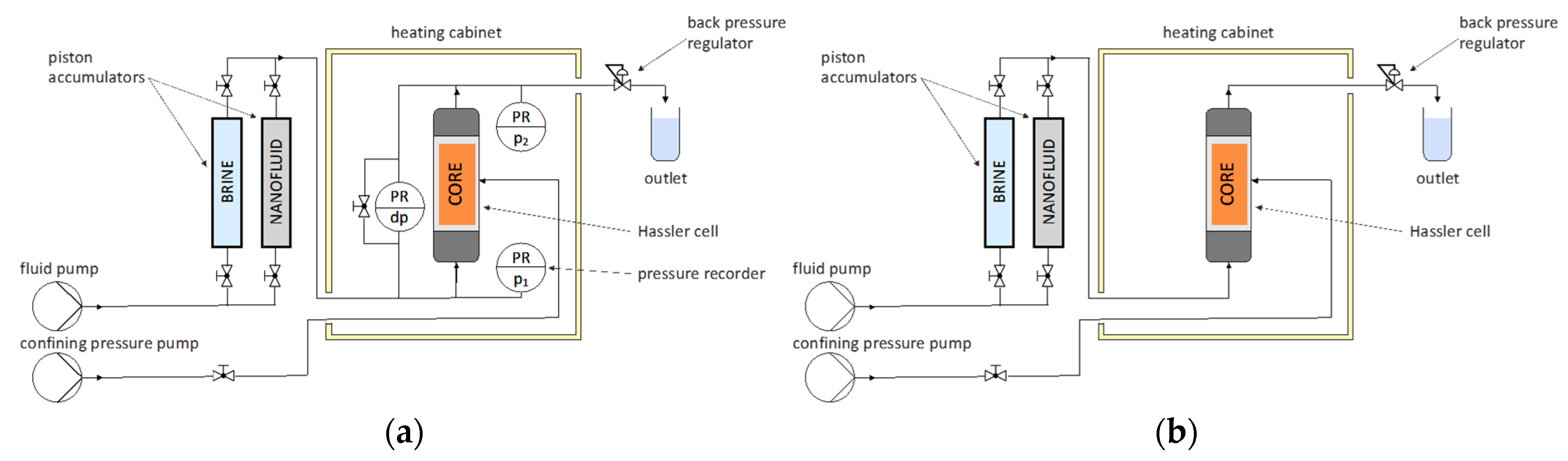
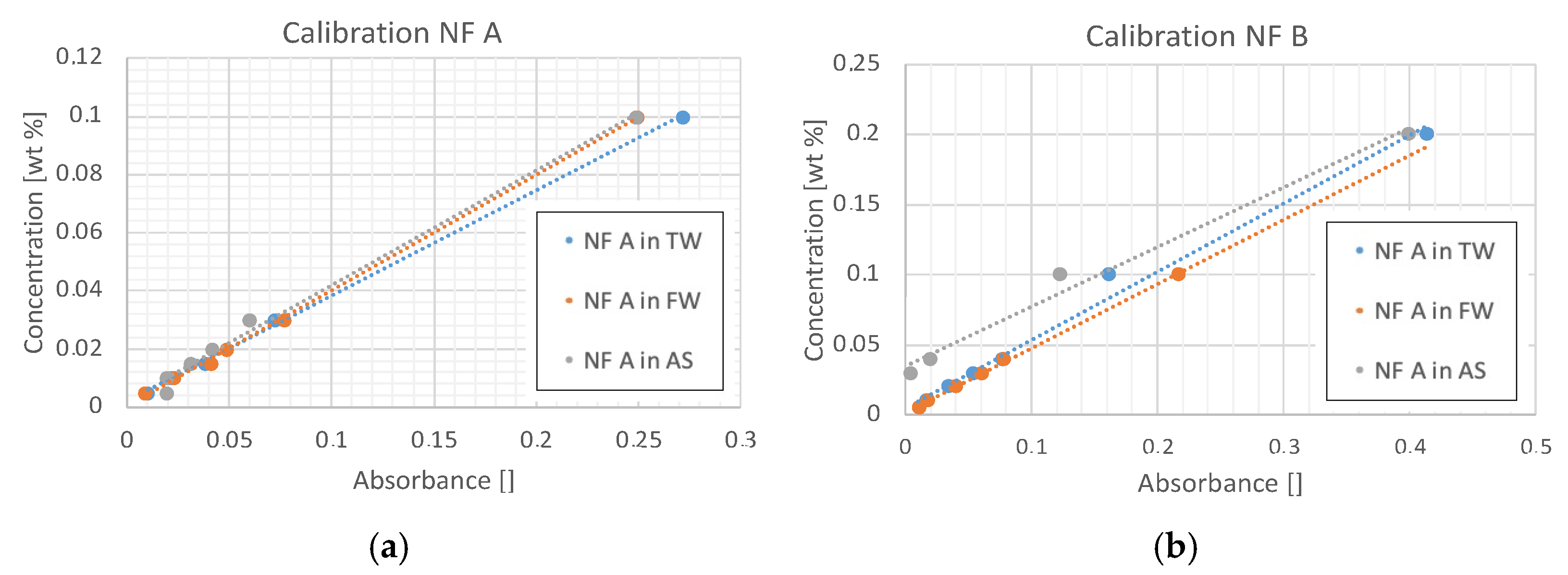





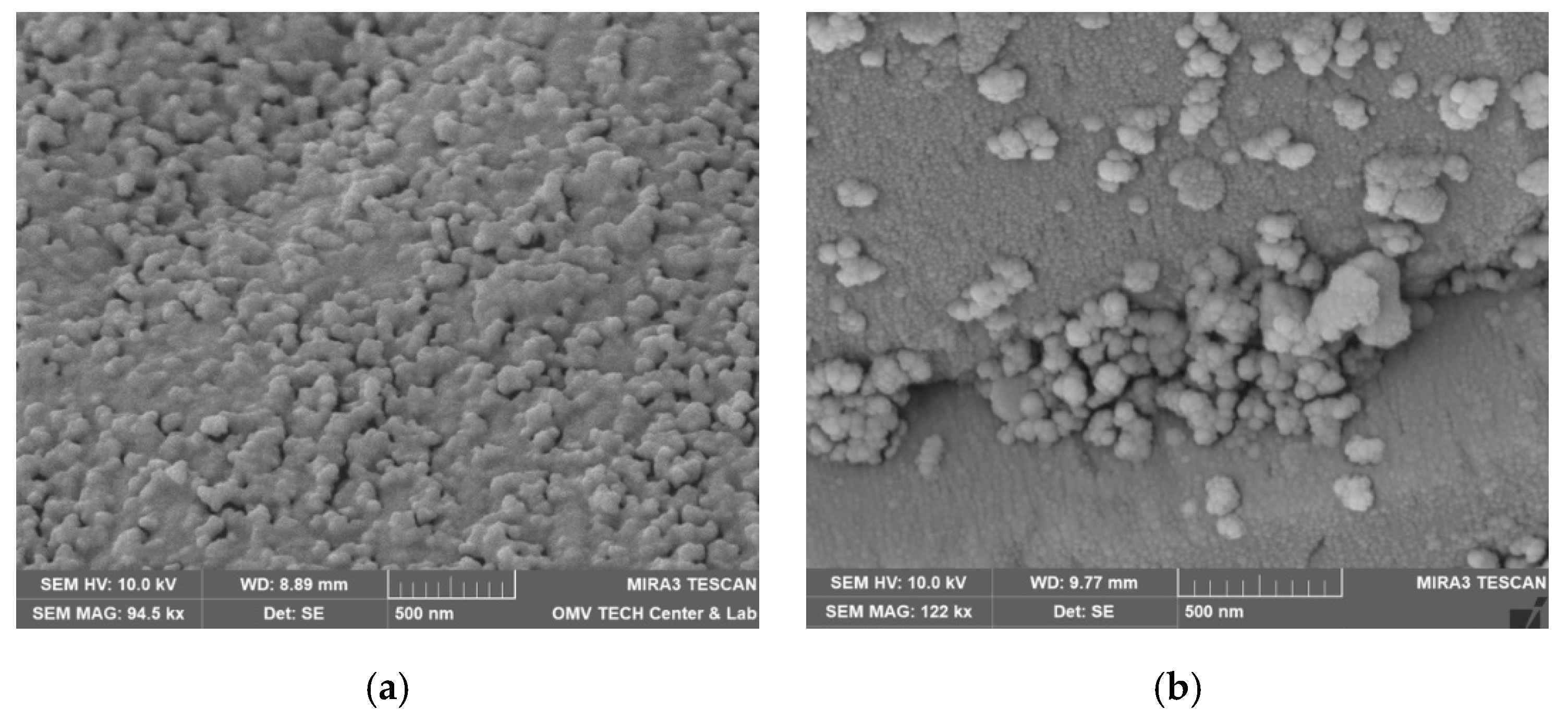
| Parameter | Units | Berea 1 | Keuper 2 | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Length | cm | 6.95 | 0.02 | 8.12 | 0.09 |
| Diameter | 2.97 | 0.01 | 2.98 | 0.01 | |
| Grain Volume | kg/cm3 | 37.01 | 0.31 | 42.98 | 0.67 |
| Porosity | % | 21.92 | 0.121 | 23.54 | 0.794 |
| N2 permeability (kg) | mD | 485.00 | 32.00 | 1424.00 | 172.00 |
| Water (Test Water) permeability (kw) | 314.00 | 66.00 | 890.00 | 193.90 | |
| BET 3—Core Plug 60 °C | (m2/g) | 1.4364 | 0.0051 | 0.9896 | 0.0028 |
| BET 3—Core Plug 110 °C | 1.6184 | 0.0064 | - | - | |
| BET 3—Crushed Cores 60 °C | 1.5621 | 0.0032 | 1.5645 | 0.0055 | |
| Formulation | TW (g/L) Softened Injection Brine | FW (g/L) Synthetic Formation Brine |
|---|---|---|
| NaCl (g/L) | 18.96 | 19.75 |
| NaHCO3 (g/L) | 1.85 | - |
| CaCl2 ·2H2O (g/L) | - | 0.400 |
| MgCl2 ·6 H2O (g/L) | - | 0.660 |
| NH4Cl (g/L) | - | 0.170 |
| pH (23 °C) | 8.46 | 6.53 |
| Ionic Strength (M) | 0.346 | 0.373 |
| Property | Nanofluid A | Nanofluid B |
|---|---|---|
| Density at 25 °C (g/cm3) | 1.15 ± 8 × 10−4 | 1.14 ± 1 × 10−3 |
| Density at 60 °C (g/cm3) | 1.13 ± 2 × 10−3 | 1.11 ± 4 × 10−4 |
| Solid content (%) (loss on drying at 105 °C) | 24.9 | 27.8 |
| Viscosity at 10 1/s (mPa.s) | 19 | 48 |
| Viscosity at 100 1/s (mPa.s) | 18 | 37 |
| Particle size (d50) DLS (nm) 1 | 128 | 140 |
| Particle size (d50) SLS (nm) 1 | 111 | 117 |
| Rg (nm) 2 | 60 3 | 96 4 |
| Rhyd (nm) 5 | 52 | 61 |
| pH at 22 °C 6 | 8.99 | 2.82 |
| pH at 22 °C 6 for 0.1 wt% in TW | 8.53 | 8.49 |
| pH at 22 °C 6 for 0.03 wt% in TW | 8.56 | 8.55 |
| pH at 22 °C 6 for 0.1 wt% in FW | 7.14 | 4.91 |
| pH at 22 °C 6 for 0.03 wt% in FW | 6.96 | 6.16 |
| pH at 22 °C 6 for 0.03 wt% in AS | 9.90 | 9.87 |
| Material | Brine | ki | di | R2 | Abscorr,i | |
|---|---|---|---|---|---|---|
| 22 °C | 60 °C | |||||
| Nanofluid A | TW | 0.3624 | 0.0020 | 0.9992 | - | - |
| FW | 0.3984 | 0.0002 | 0.9991 | - | - | |
| AS | 0.3962 | 0.0023 | 0.9931 | - | - | |
| Nanofluid B | TW | 0.4871 | 0.0046 | 0.9880 | - | - |
| FW | 0.4579 | 0.0020 | 0.9982 | - | - | |
| AS | 0.4248 | 0.0346 | 0.9868 | - | - | |
| Berea | TW | - | - | - | 0.031 | 0.047 |
| FW | - | - | - | 0.019 | 0.027 | |
| AS | - | - | - | 0.048 | 0.091 | |
| Keuper | TW | - | - | - | 0.054 | 0.133 |
| FW | - | - | - | 0.023 | 0.044 | |
| AS | - | - | - | 0.088 | 0.237 | |
| Core | Initial Conc. (wt%) | Brine | T | Residual Conc. cNF,i | Adsorption | Specific Adsorption | pH | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (°C) | (wt%) | (wt%) | (%) | (mg/g) | (mg/m2) | B-R 1 | NF-B-R 2 | NF-B 3 | |||
| Berea | 0.1 | TW | 22 | 0.0118 | 0.0882 | 88 | 3.53 | 2.26 | 8.29 | 8.26 | 8.45 |
| 60 | 0.0107 | 0.0893 | 89 | 3.57 | 2.39 | 8.24 | 8.29 | 8.58 | |||
| FW | 22 | 0.0083 | 0.0917 | 92 | 3.67 | 2.35 | 6.77 | 6.75 | 6.94 | ||
| 60 | 0.0097 | 0.0903 | 90 | 3.61 | 2.31 | 6.87 | 6.78 | 7.14 | |||
| AS | 22 | 0.0088 | 0.0912 | 91 | 3.65 | 2.34 | 9.82 | 9.88 | 9.89 | ||
| 0.03 | TW | 22 | 0.0102 | 0.0198 | 66 | 0.79 | 0.51 | 8.29 | 8.30 | 8.41 | |
| 60 | 0.0070 | 0.0230 | 77 | 0.96 | 0.61 | 8.24 | 8.37 | 8.63 | |||
| FW | 22 | 0.0050 | 0.0250 | 83 | 1.00 | 0.64 | 6.77 | 6.79 | 6.80 | ||
| 60 | 0.0050 | 0.0250 | 83 | 1.00 | 0.64 | 6.87 | 6.73 | 6.99 | |||
| Keuper | 0.1 | TW | 22 | 0.0227 | 0.0773 | 77 | 3.09 | 1.98 | 8.40 | 8.40 | 8.45 |
| 60 | 0.0133 | 0.0867 | 87 | 3.57 | 2.29 | 8.49 | 8.45 | 8.58 | |||
| FW | 22 | 0.0198 | 0.0802 | 80 | 3.21 | 2.05 | 7.03 | 7.15 | 6.94 | ||
| 60 | 0.0119 | 0.0881 | 88 | 3.52 | 2.25 | 7.18 | 7.07 | 7.14 | |||
| AS | 22 | 0.0233 | 0.0767 | 77 | 3.07 | 1.96 | 9.85 | 9.88 | 9.89 | ||
| 0.03 | TW | 22 | 0.0116 | 0.0184 | 61 | 0.74 | 0.47 | 8.42 | 8.38 | 8.41 | |
| FW | 22 | 0.0070 | 0.0230 | 77 | 0.92 | 0.59 | 7.03 | 7.11 | 6.80 | ||
| 60 | 0.0073 | 0.0227 | 76 | 0.91 | 0.58 | 7.18 | 7.15 | 6.99 | |||
| Core | Initial Conc. (wt%) | Brine | T | Residual Conc. cNF,i | Adsorption | Specific Adsorption | pH | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (°C) | (wt%) | (wt%) | (%) | (mg/g) | (mg/m2) | B-R 1 | NF-B-R 2 | NF-B 3 | |||
| Berea | 0.1 | TW | 22 | 0.0125 | 0.0875 | 88 | 3.50 | 2.27 | 8.29 | 8.22 | 8.44 |
| 60 | 0.0146 | 0.0854 | 85 | 3.42 | 2.19 | 8.24 | 8.27 | 8.61 | |||
| FW | 22 | 0.0057 | 0.0943 | 94 | 3.77 | 2.41 | 6.77 | 6.21 | 4.96 | ||
| 60 | 0.0068 | 0.0932 | 93 | 3.73 | 2.39 | 6.87 | 6.36 | 4.71 | |||
| AS | 22 | 0.0387 | 0.0613 | 61 | 2.45 | 1.57 | 9.82 | 9.83 | 9.86 | ||
| 0.03 | FW | 22 | 0.0051 | 0.0249 | 83 | 1.00 | 0.64 | 6.77 | 6.53 | 6.24 | |
| 60 | 0.0125 | 0.0243 | 81 | 1.01 | 0.65 | - | 6.74 | - | |||
| Keuper | 0.1 | TW | 22 | 0.0172 | 0.0828 | 83 | 3.31 | 2.12 | 8.40 | 8.30 | 8.44 |
| FW | 22 | 0.0065 | 0.0935 | 93 | 3.74 | 2.39 | 7.03 | 6.64 | 4.96 | ||
| 60 | 0.0066 | 0.0934 | 93 | 3.74 | 2.39 | 7.18 | 6.73 | 4.71 | |||
| AS | 22 | 0.0468 | 0.0532 | 53 | 2.13 | 1.36 | - | 9.86 | - | ||
| FW | 22 | 0.0062 | 0.0238 | 79 | 0.95 | 0.61 | 7.03 | 6.89 | 6.24 | ||
| Material | 0.1 wt% NF A in TW | 1 wt% NF A in TW | ||
|---|---|---|---|---|
| Berea | Keuper | Berea | Keuper | |
| NF Recovery (%) | 22.46 | 104.20 | 79.31 | 104.98 |
| NF Adsorption (mg/m2) | 0.317 | - | 0.846 | - |
| NF Adsorption (mg/g) | 0.455 | - | 1.215 | - |
| Tracer Recovery (%) | 85.49 | 84.72 | 84.37 | 90.05 |
| Material | 0.1 wt% NF A in TW | 1 wt% NF A in TW | ||
|---|---|---|---|---|
| Berea | Keuper | Berea | Keuper | |
| NF Recovery (%) | 77.21 | 108.10 | 112.65 | 113.10 |
| NF Adsorption (mg/m2) | 0.090 | - | - | - |
| NF Adsorption (mg/g) | 0.130 | - | - | - |
| Tracer Recovery (%) | 90.35 | 89.85 | 89.50 | 85.87 |
| Material | Brine | Concent. (wt%) | Rg (nm) | Rh (nm) |
|---|---|---|---|---|
| Nanofluid A | TW | 0.1 | 48 ± 1.6% | 56 ± 2.7% |
| 1 | 48 ± 0.5% | 54 ± 1.4% | ||
| FW | 0.1 | 49 ± 0.1% | 54 ± 0.5% | |
| DIW | - | 60 | 52 | |
| Nanofluid B | TW | 0.1 | 64 ± 0.8% | 62 ± 1.6% |
| 1 | 66 ± 0.4% | 67 ± 3% | ||
| FW | 0.1 | 68 ± 1.6% | 64 ± 0.8% | |
| DIW | - | 96 | 61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheurer, C.; Hincapie, R.E.; Neubauer, E.; Metz, A.; Ness, D. Sorption of Nanomaterials to Sandstone Rock. Nanomaterials 2022, 12, 200. https://doi.org/10.3390/nano12020200
Scheurer C, Hincapie RE, Neubauer E, Metz A, Ness D. Sorption of Nanomaterials to Sandstone Rock. Nanomaterials. 2022; 12(2):200. https://doi.org/10.3390/nano12020200
Chicago/Turabian StyleScheurer, Christian, Rafael E. Hincapie, Elisabeth Neubauer, Astrid Metz, and Daniel Ness. 2022. "Sorption of Nanomaterials to Sandstone Rock" Nanomaterials 12, no. 2: 200. https://doi.org/10.3390/nano12020200
APA StyleScheurer, C., Hincapie, R. E., Neubauer, E., Metz, A., & Ness, D. (2022). Sorption of Nanomaterials to Sandstone Rock. Nanomaterials, 12(2), 200. https://doi.org/10.3390/nano12020200








