Nanocone-Array-Based Platinum-Iridium Oxide Neural Microelectrodes: Structure, Electrochemistry, Durability and Biocompatibility Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microelectrode Fabrication
2.2. Electrodeposition of Pt and IrOx
2.3. Electrochemical Characterization
2.4. Stability Evaluation
2.5. Morphology Characterization
2.6. Biocompatibility Study
3. Results and Discussion
3.1. Electrodeposition Process and Evaluation of Pt Nanocone
3.2. Optimization and Batch Production of Pt Nanocone
3.3. IrOx/Pt Nanocone for Better Neural Interface
3.4. Chronic Stability Evaluation
3.5. Biocompatibility Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yue, L.; Weiland, J.D.; Roska, B.; Humayun, M.S. Retinal stimulation strategies to restore vision: Fundamentals and systems. Prog. Retin. Eye Res. 2016, 53, 21–47. [Google Scholar] [CrossRef] [Green Version]
- Zeng, F.-G.; Rebscher, S.J.; Fu, Q.-J.; Chen, H.; Sun, X.; Yin, L.; Ping, L.; Feng, H.; Yang, S.; Gong, S.; et al. Development and evaluation of the Nurotron 26-electrode cochlear implant system. Hear. Res. 2015, 322, 188–199. [Google Scholar] [CrossRef] [Green Version]
- Sarica, C.; Iorio-Morin, C.; Aguirre-Padilla, D.H.; Najjar, A.; Paff, M.; Fomenko, A.; Yamamoto, K.; Zemmar, A.; Lipsman, N.; Ibrahim, G.M.; et al. Implantable Pulse Generators for Deep Brain Stimulation: Challenges, Complications, and Strategies for Practicality and Longevity. Front. Hum. Neurosci. 2021, 15, 708481. [Google Scholar] [CrossRef]
- Cuttaz, E.A.; Chapman, C.A.R.; Syed, O.; Goding, J.A.; Green, R.A. Stretchable, Fully Polymeric Electrode Arrays for Peripheral Nerve Stimulation. Adv. Sci. 2021, 8, 2004033. [Google Scholar] [CrossRef]
- Wang, J.; He, T.; Lee, C. Development of neural interfaces and energy harvesters towards self-powered implantable systems for healthcare monitoring and rehabilitation purposes. Nano Energy 2019, 65, 104039. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, H.; Li, S.; Liang, X.; Zhang, M.; Dai, X.; Zhang, Y. Flexible Electrodes for In Vivo and In Vitro Electrophysiological Signal Recording. Adv. Healthc. Mater. 2021, 10, 2100646. [Google Scholar] [CrossRef]
- Cho, Y.; Park, S.; Lee, J.; Yu, K.J. Emerging Materials and Technologies with Applications in Flexible Neural Implants: A Comprehensive Review of Current Issues with Neural Devices. Adv. Mater. 2021, 33, 2005786. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, X.; Zhang, S.; Deng, C.; Wu, T. Think big, see small—A review of nanomaterials for neural interfaces. Nano Sel. 2022, 3, 903–918. [Google Scholar] [CrossRef]
- Woods, G.A.; Rommelfanger, N.J.; Hong, G. Bioinspired Materials for In Vivo Bioelectronic Neural Interfaces. Matter 2020, 3, 1087–1113. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhao, S.; Yang, H.; Zhang, Y.; Wu, T. Micro/nano technologies for high-density retinal implant. Micromachines 2019, 10, 419. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.S.; Tan, C.; Castagnola, E.; Cui, X.T. Electrode Materials for Chronic Electrical Microstimulation. Adv. Healthc. Mater. 2021, 10, 2100119. [Google Scholar] [CrossRef]
- Chen, N.; Tian, L.; Patil, A.C.; Peng, S.; Yang, I.H.; Thakor, N.V.; Ramakrishna, S. Neural interfaces engineered via micro- and nanostructured coatings. Nano Today 2017, 14, 59–83. [Google Scholar] [CrossRef]
- Oldroyd, P.; Malliaras, G.G. Achieving long-term stability of thin-film electrodes for neurostimulation. Acta Biomater. 2022, 139, 65–81. [Google Scholar] [CrossRef]
- Goding, J.A.; Gilmour, A.D.; Aregueta-Robles, U.A.; Hasan, E.A.; Green, R.A. Living Bioelectronics: Strategies for Developing an Effective Long-Term Implant with Functional Neural Connections. Adv. Funct. Mater. 2018, 28, 1702969. [Google Scholar] [CrossRef]
- Gori, M.; Vadalà, G.; Giannitelli, S.M.; Denaro, V.; Pino, G.D. Biomedical and Tissue Engineering Strategies to Control Foreign Body Reaction to Invasive Neural Electrodes. Front. Bioeng. Biotechnol. 2021, 9, 659033. [Google Scholar] [CrossRef]
- Yuk, H.; Lu, B.; Zhao, X. Hydrogel bioelectronics. Chem. Soc. Rev. 2019, 48, 1642–1667. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Wang, J.; Fang, Y. Bioinspired flexible electronics for seamless neural interfacing and chronic recording. Nanoscale Adv. 2020, 2, 3095–3102. [Google Scholar] [CrossRef]
- Chen, C.; Sun, X.; Peng, H. The Rise of Soft Neural Electronics. Giant 2021, 8, 100075. [Google Scholar] [CrossRef]
- Ayton, L.N.; Blamey, P.J.; Guymer, R.H.; Luu, C.D.; Nayagam, D.A.; Sinclair, N.C.; Shivdasani, M.N.; Yeoh, J.; McCombe, M.F.; Briggs, R.J. First-in-human trial of a novel suprachoroidal retinal prosthesis. PLoS ONE 2014, 9, e115239. [Google Scholar] [CrossRef] [Green Version]
- Petoe, M.A.; Titchener, S.A.; Kolic, M.; Kentler, W.G.; Abbott, C.J.; Nayagam, D.A.; Baglin, E.K.; Kvansakul, J.; Barnes, N.; Walker, J.G.; et al. A second-generation (44-channel) suprachoroidal retinal prosthesis: Interim clinical trial results. Transl. Vis. Sci. Technol. 2021, 10, 12. [Google Scholar] [CrossRef]
- Titchener, S.A.; Nayagam, D.A.; Kvansakul, J.; Kolic, M.; Baglin, E.K.; Abbott, C.J.; McGuinness, M.B.; Ayton, L.N.; Luu, C.D.; Greenstein, S. A Second-Generation (44-Channel) Suprachoroidal Retinal Prosthesis: Long-Term Observation of the Electrode–Tissue Interface. Transl. Vis. Sci. Technol. 2022, 11, 12. [Google Scholar] [CrossRef]
- Arevalo, J.F.; Al Rashaed, S.; Alhamad, T.A.; Kahtani, E.A.; Al-Dhibi, H.A.; Mura, M.; Nowilaty, S.; Al-Zahrani, Y.A.; Kozak, I.; Al-Sulaiman, S.; et al. Argus II retinal prosthesis for retinitis pigmentosa in the Middle East: The 2015 Pan-American Association of Ophthalmology Gradle Lecture. Int. J. Retin. Vitr. 2021, 7, 65. [Google Scholar] [CrossRef]
- Rizzo, S.; Barale, P.-O.; Ayello-Scheer, S.; Devenyi, R.G.; Delyfer, M.-N.; Korobelnik, J.-F.; Rachitskaya, A.; Yuan, A.; Jayasundera, K.T.; Zacks, D.N.; et al. Adverse Events of the Argus II Retinal Prosthesis: Incidence, Causes, and Best Practices for Managing and Preventing Conjunctival Erosion. Retina 2020, 40, 303–311. [Google Scholar] [CrossRef]
- Luo, Y.H.-L.; da Cruz, L. The Argus® II Retinal Prosthesis System. Prog. Retin. Eye Res. 2016, 50, 89–107. [Google Scholar] [CrossRef]
- Zhou, D.M. Platinum Electrode Surface Coating and Method for Manufacturing the Same. U.S. Patent 6974533 B2, 13 December 2005. [Google Scholar]
- Zeng, Q.; Xia, K.; Sun, B.; Yin, Y.; Wu, T.; Humayun, M.S. Electrodeposited iridium oxide on platinum nanocones for improving neural stimulation microelectrodes. Electrochim. Acta 2017, 237, 152–159. [Google Scholar] [CrossRef]
- Schuettler, M.; Doerge, T.; Wien, S.L.; Becker, S.; Stieglitz, T. Cytotoxicity of Platinum Black. In Proceedings of the 10th Annual Conference of the International Functional Electrical Stimulation Society: IFESS, Montreal, QC, Canada, 5–8 July 2005; pp. 343–345. [Google Scholar]
- Green, R.A.; Toor, H.; Dodds, C.; Lovell, N.H. Variation in Performance of Platinum Electrodes with Size and Surface Roughness. Sens. Mater. 2012, 24, 165–180. [Google Scholar]
- Weremfo, A.; Carter, P.; Hibbert, D.B.; Zhao, C. Investigating the Interfacial Properties of Electrochemically Roughened Platinum Electrodes for Neural Stimulation. Langmuir 2015, 31, 2593–2599. [Google Scholar] [CrossRef]
- Zeng, Q.; Huang, Z.; Cai, G.; Wu, T. Platinum Nanocrystal Assisted by Low-Content Iridium for High-Performance Flexible Electrode: Applications on Neural Interface, Water Oxidation, and Anti-Microbial Contamination. Adv. Mater. Interfaces 2021, 8, 2100965. [Google Scholar] [CrossRef]
- Lu, Y.; Cai, Z.; Cao, Y.; Yang, H.; Duan, Y.Y. Activated iridium oxide films fabricated by asymmetric pulses for electrical neural microstimulation and recording. Electrochem. Commun. 2008, 10, 778–782. [Google Scholar] [CrossRef]
- Zeng, Q.; Wu, T. Enhanced electrochemical performance of neural electrodes based on PEDOT:PSS hydrogel. J. Appl. Polym. Sci. 2022, 139, 51804. [Google Scholar] [CrossRef]
- Boehler, C.; Oberueber, F.; Schlabach, S.; Stieglitz, T.; Asplund, M. Long-Term Stable Adhesion for Conducting Polymers in Biomedical Applications: IrOx and Nanostructured Platinum Solve the Chronic Challenge. ACS Appl. Mater. Interfaces 2017, 9, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Q.; Xia, K.; Zhang, Y.; Wu, T. Well Controlled 3D Iridium Oxide/Platinum Nanocomposites with Greatly Enhanced Electrochemical Performances. Adv. Mater. Interfaces 2019, 6, 1900356. [Google Scholar] [CrossRef]
- Su, Z.; Yang, C.; Xie, B.; Lin, Z.; Zhang, Z.; Liu, J.; Li, B.; Kang, F.; Wong, C.P. Scalable fabrication of MnO2 nanostructure deposited on free-standing Ni nanocone arrays for ultrathin, flexible, high-performance micro-supercapacitor. Energy Environ. Sci. 2014, 7, 2652–2659. [Google Scholar] [CrossRef]
- Cogan, S.F. Neural stimulation and recording electrodes. Annu. Rev. Biomed. Eng. 2008, 10, 275–309. [Google Scholar] [CrossRef] [Green Version]
- Lian, H.; Roy, E.; Zheng, H. Protocol for primary microglial culture preparation. Bio-Protocol 2016, 6, e1989. [Google Scholar] [CrossRef] [Green Version]
- Yamagiwa, S.; Fujishiro, A.; Sawahata, H.; Numano, R.; Ishida, M.; Kawano, T. Layer-by-layer assembled nanorough iridium-oxide/platinum-black for low-voltage microscale electrode neurostimulation. Sens. Actuators B Chem. 2015, 206, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Boehler, C.; Stieglitz, T.; Asplund, M. Nanostructured platinum grass enables superior impedance reduction for neural microelectrodes. Biomaterials 2015, 67, 346–353. [Google Scholar] [CrossRef]
- Fan, B.; Rodriguez, A.V.; Vercosa, D.G.; Kemere, C.; Robinson, J.T. Sputtered porous Pt for wafer-scale manufacture of low-impedance flexible microelectrodes. J. Neural Eng. 2020, 17, 036029. [Google Scholar] [CrossRef]
- Elyahoodayan, S.; Jiang, W.; Lee, C.D.; Shao, X.; Weiland, G.; Whalen, J.J.I.; Petrossians, A.; Song, D. Stimulation and Recording of the Hippocampus Using the Same Pt-Ir Coated Microelectrodes. Front. Neurosci. 2021, 15, 616063. [Google Scholar] [CrossRef]
- Chen, C.; Ruan, S.; Bai, X.; Lin, C.; Xie, C.; Lee, I.S. Patterned iridium oxide film as neural electrode interface: Biocompatibility and improved neurite outgrowth with electrical stimulation. Mater. Sci. Eng. C 2019, 103, 109865. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System during Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441. [Google Scholar] [CrossRef] [PubMed]
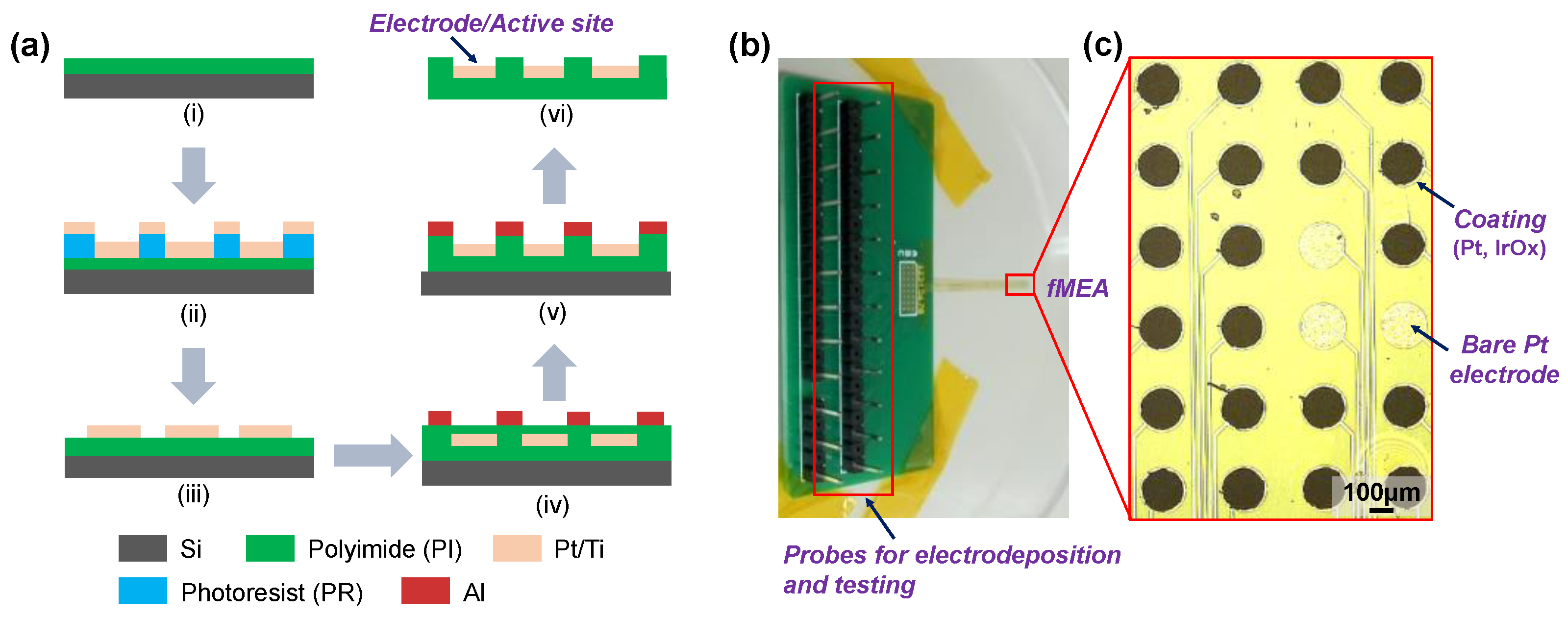
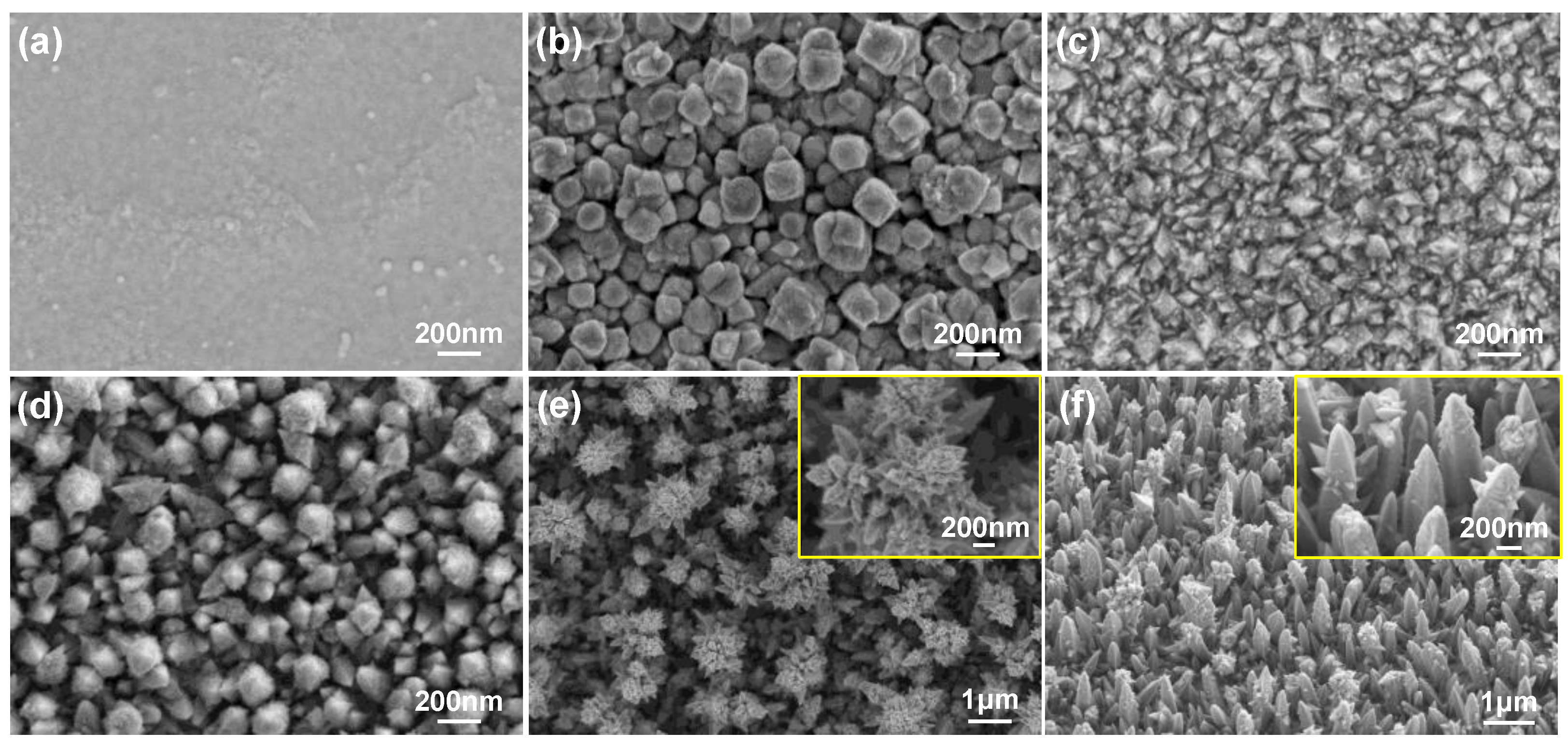
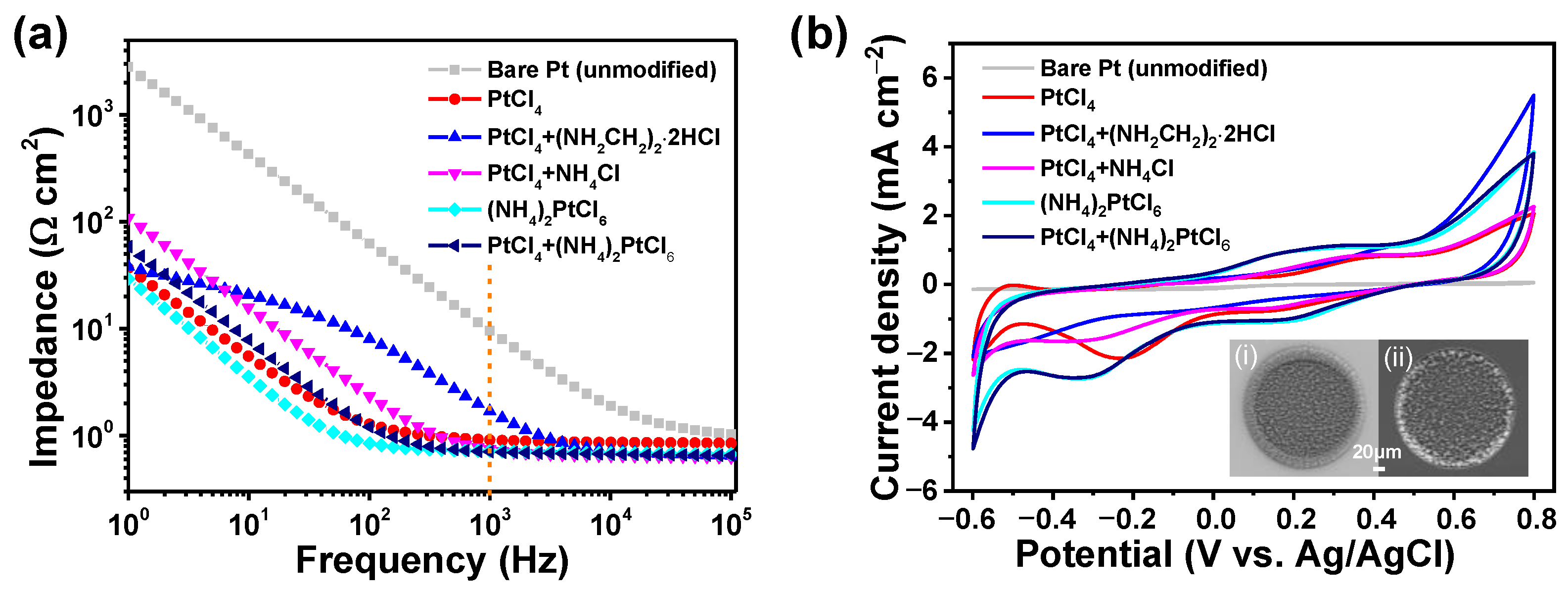
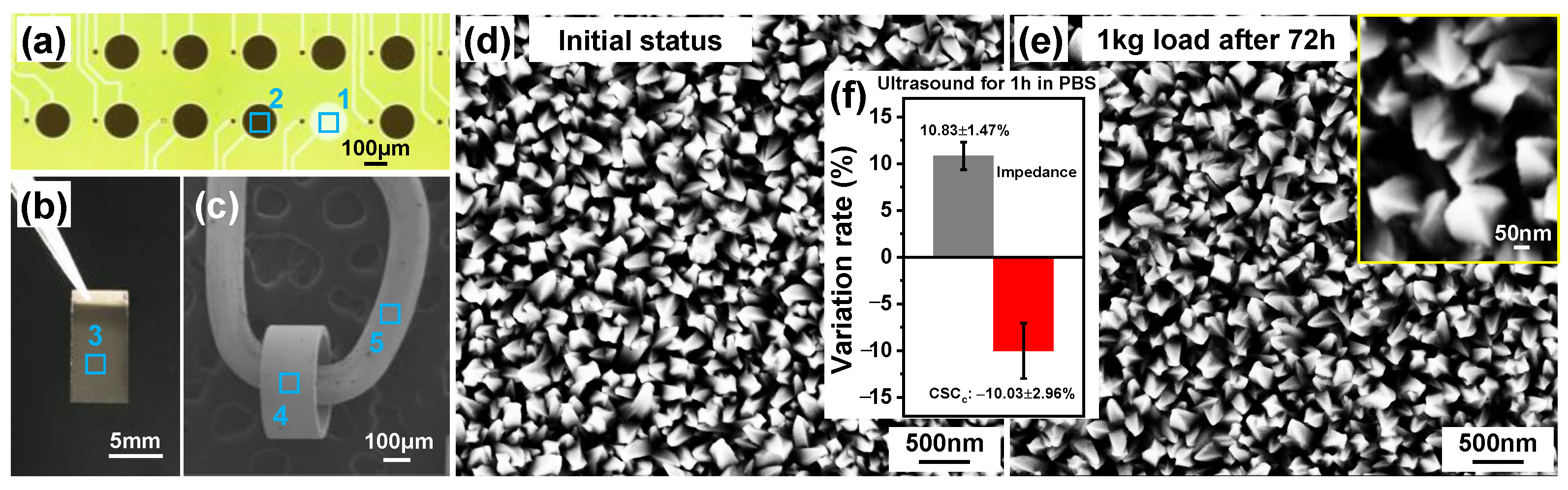


| Materials | Impedance at 1 kHz/Ω cm2 | CSCc/ mC cm−2 | CIC/ mC cm−2 | Comments | References |
|---|---|---|---|---|---|
| IrOx/Pt black | 32 (40-fold lower) | 46.7 | - | Pb additive, unsafe | [38] |
| Roughed Pt | Two orders of magnitude lower | 44-fold increase | 1 | Low CIC | [29] |
| Pt nanograss | Two orders of magnitude lower at 100 Hz | 40-fold increase | <0.5 | Low CIC | [39] |
| Sputtered Pt | 0.52 (9-fold lower) | 11.4 | - | Low CSCc | [40] |
| Deposited Pt-Ir | 7.8-fold reduction | 12.5 ± 0.75 | 3.58 | Low CSCc | [41] |
| Activated IrOx | 15.25 (reduction of 20%) | 58.57 ± 0.96 | - | High CSCc, but no significant reduction of impedance | [42] |
| IrOx/Pt gray | Reduction of 93.32% | 22.29 | ~0.83 | Low CIC, disordered structure | [26] |
| Nanocone-array-based Pt-IrOx | 0.72 ± 0.04 (reduction of 92.95%) | 52.44 ± 2.53 | 4.39 ± 0.36 | Without template, low impedance, high CSCc and CIC, superior stability and biocompatibility | This work |
| Electrostimulation Time | 0 Day | 1 Day | 2 Days | 5 Days | 15 Days | 30 Days |
|---|---|---|---|---|---|---|
| CSCc/mC cm−2 | 52.44 | 52.05 | 50.96 | 50 | 44.91 | 40.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Q.; Yu, S.; Fan, Z.; Huang, Y.; Song, B.; Zhou, T. Nanocone-Array-Based Platinum-Iridium Oxide Neural Microelectrodes: Structure, Electrochemistry, Durability and Biocompatibility Study. Nanomaterials 2022, 12, 3445. https://doi.org/10.3390/nano12193445
Zeng Q, Yu S, Fan Z, Huang Y, Song B, Zhou T. Nanocone-Array-Based Platinum-Iridium Oxide Neural Microelectrodes: Structure, Electrochemistry, Durability and Biocompatibility Study. Nanomaterials. 2022; 12(19):3445. https://doi.org/10.3390/nano12193445
Chicago/Turabian StyleZeng, Qi, Shoujun Yu, Zihui Fan, Yubin Huang, Bing Song, and Tian Zhou. 2022. "Nanocone-Array-Based Platinum-Iridium Oxide Neural Microelectrodes: Structure, Electrochemistry, Durability and Biocompatibility Study" Nanomaterials 12, no. 19: 3445. https://doi.org/10.3390/nano12193445
APA StyleZeng, Q., Yu, S., Fan, Z., Huang, Y., Song, B., & Zhou, T. (2022). Nanocone-Array-Based Platinum-Iridium Oxide Neural Microelectrodes: Structure, Electrochemistry, Durability and Biocompatibility Study. Nanomaterials, 12(19), 3445. https://doi.org/10.3390/nano12193445






