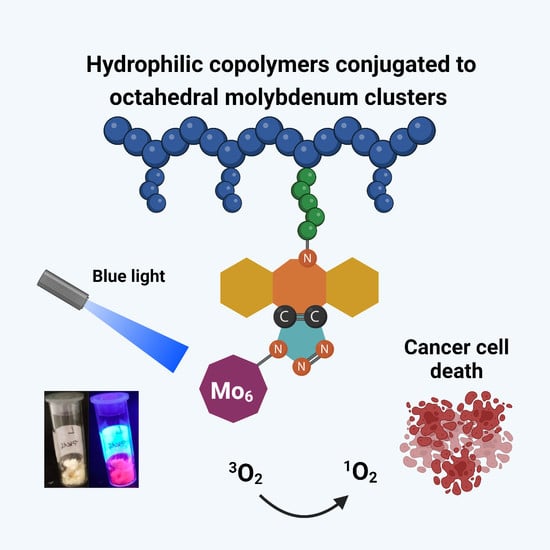
Octahedral Molybdenum Cluster-Based Nanomaterials for Potential Photodynamic Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Monomers
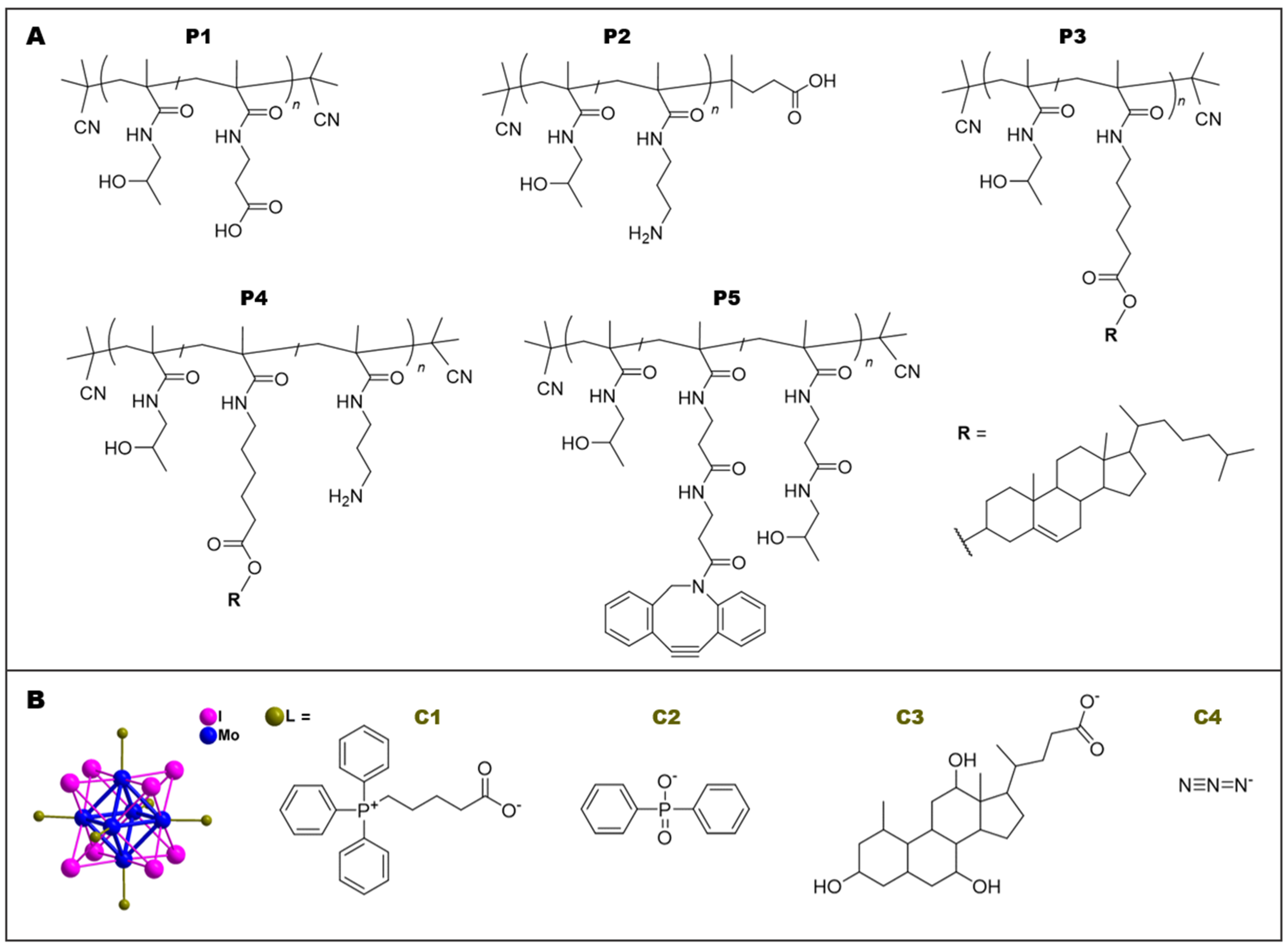
2.3. Synthesis of Polymer Precursors P1–P5
2.4. Synthesis of Mo6 Clusters C1–C4
2.5. Synthesis of Polymer-Cluster Constructs POL1–POL6
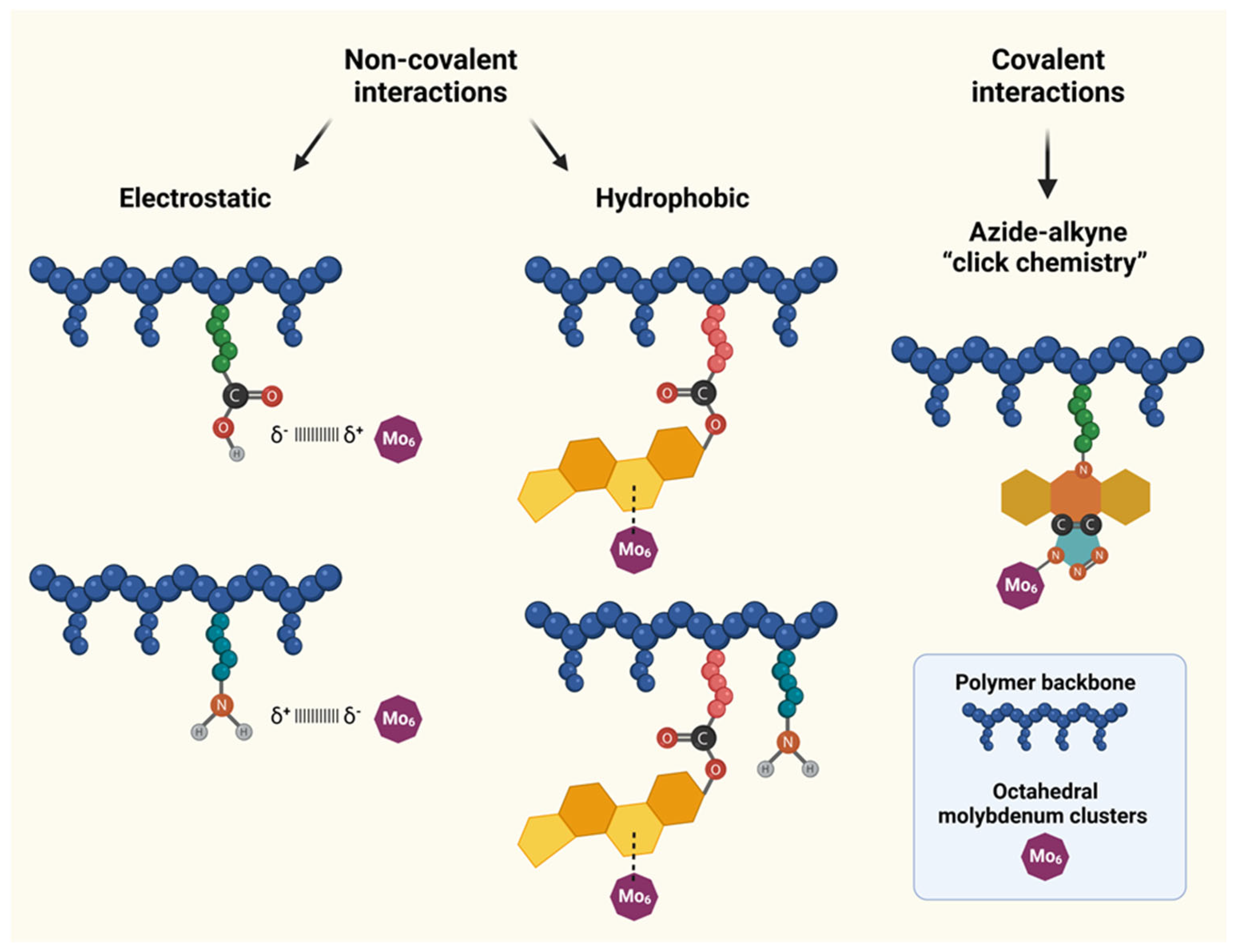
2.5.1. Electrostatic Non-Covalent–POL1 and POL2
2.5.2. Hydrophobic Non-Covalent Complexes–POL3 and POL4
2.5.3. Covalent Conjugates–POL5–POL6
2.6. Physico-Chemical and Photophysical Characterization
2.6.1. Size Exclusion Chromatography (SEC)
2.6.2. Dynamic Light Scattering (DLS)
2.6.3. UV–VIS Spectrophotometry
2.6.4. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.6.5. Attenuated Total Reflectance (ATR) Fourier-Transform Infrared (FTIR) Spectroscopy
2.6.6. Luminescence Spectroscopy
3. Results and Discussion
3.1. Synthesis of Polymer Precursors
3.2. Preparation of Polymer-Cluster Constructs
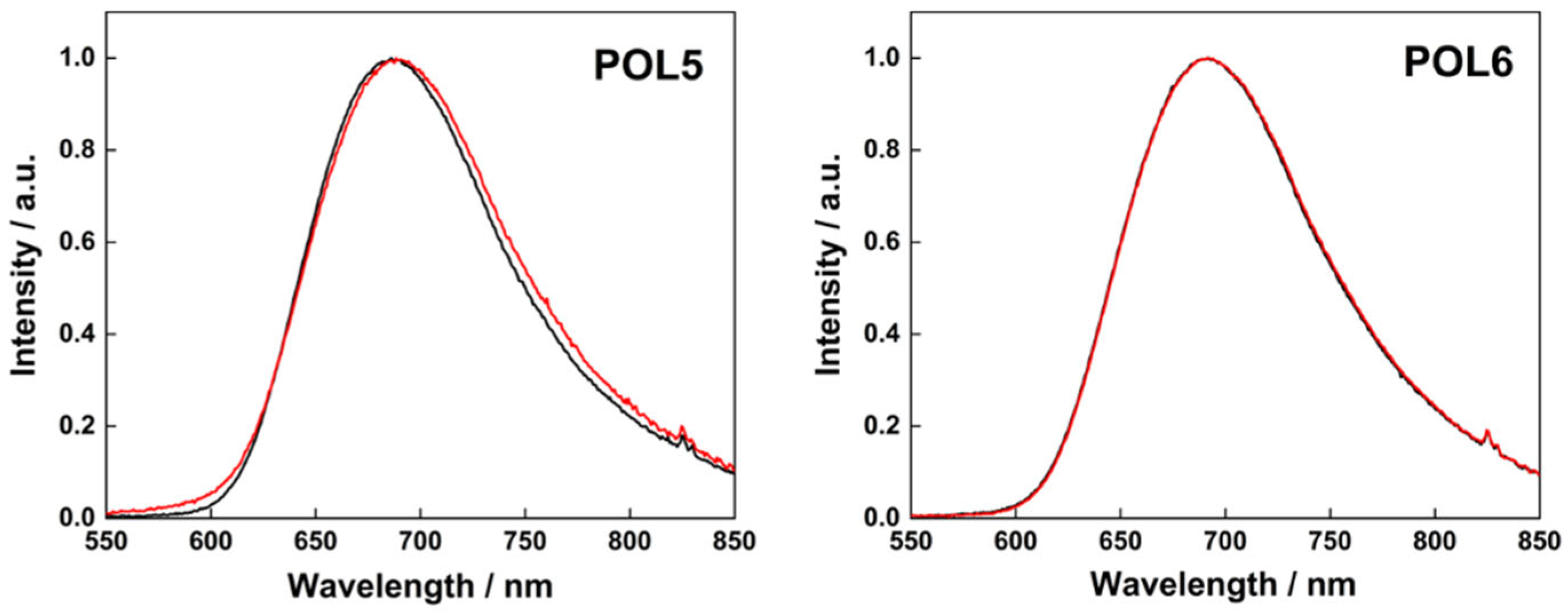
3.3. Stability and Photophysical Properties of the Polymer-Cluster Constructs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maeda, H.; Nakamura, H.; Fang, J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Šubr, V.; Islam, W.; Hackbarth, S.; Islam, R.; Etrych, T.; Ulbrich, K.; Maeda, H. N-(2-hydroxypropyl)methacrylamide polymer conjugated pyropheophorbide-a, a promising tumor-targeted theranostic probe for photodynamic therapy and imaging. Eur. J. Pharm. Biopharm. 2018, 130, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.; Selbo, P.K.; Weyergang, A.; Dietze, A.; Prasmickaite, L.; Bonsted, A.; Engesaeter, B.Ø.; Angell-Petersen, E.; Warloe, T.; Frandsen, N.; et al. Porphyrin-related photosensitizers for cancer imaging and therapeutic applications. J. Microsc. 2005, 218 Pt 2, 133–147. [Google Scholar] [CrossRef]
- Li, L.; Huh, K.M. Polymeric nanocarrier systems for photodynamic therapy. Biomater. Res. 2014, 18, 19. [Google Scholar] [CrossRef] [PubMed]
- Chepurna, O.M.; Yakovliev, A.; Ziniuk, R.; Nikolaeva, O.A.; Levchenko, S.M.; Xu, H.; Losytskyy, M.Y.; Bricks, J.L.; Slominskii, Y.L.; Vretik, L.O.; et al. Core–shell polymeric nanoparticles co-loaded with photosensitizer and organic dye for photodynamic therapy guided by fluorescence imaging in near and short-wave infrared spectral regions. J. Nanobiotechnol. 2020, 18, 19. [Google Scholar] [CrossRef] [PubMed]
- Gibot, L.; Lemelle, A.; Till, U.; Moukarzel, B.; Mingotaud, A.-F.; Pimienta, V.; Saint-Aguet, P.; Rols, M.-P.; Gaucher, M.; Violleau, F.; et al. Polymeric Micelles Encapsulating Photosensitizer: Structure/Photodynamic Therapy Efficiency Relation. Biomacromolecules 2014, 15, 1443–1455. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-E.K.; Kopelman, R. Polymeric Nanoparticles for Photodynamic Therapy. In Methods in Molecular Biology; Springer: Clifton, NJ, USA, 2011; Volume 726, pp. 151–178. [Google Scholar]
- Ricci-Júnior, E.; Marchetti, J.M. Preparation, characterization, photocytotoxicity assay of PLGA nanoparticles containing zinc (II) phthalocyanine for photodynamic therapy use. J. Microencapsul. 2006, 23, 523–538. [Google Scholar] [CrossRef]
- Weiss, G.J.; Chao, J.; Neidhart, J.D.; Ramanathan, R.K.; Bassett, D.; Neidhart, J.A.; Choi, C.H.J.; Chow, W.; Chung, V.; Forman, S.J.; et al. First-in-human phase 1/2a trial of CRLX101, a cyclodextrin-containing polymer-camptothecin nanopharmaceutical in patients with advanced solid tumor malignancies. Investig. New Drugs 2013, 31, 986–1000. [Google Scholar] [CrossRef]
- Brandhonneur, N.; Hatahet, T.; Amela-Cortes, M.; Molard, Y.; Cordier, S.; Dollo, G. Molybdenum cluster loaded PLGA nanoparticles: An innovative theranostic approach for the treatment of ovarian cancer. Eur. J. Pharm. Biopharm. 2018, 125, 95–105. [Google Scholar] [CrossRef]
- Kirakci, K.; Demel, J.; Hynek, J.; Zelenka, J.; Rumlová, M.; Ruml, T.; Lang, K. Phosphinate Apical Ligands: A Route to a Water-Stable Octahedral Molybdenum Cluster Complex. Inorg. Chem. 2019, 58, 16546–16552. [Google Scholar] [CrossRef]
- Kirakci, K.; Zelenka, J.; Rumlová, M.; Cvačka, J.; Ruml, T.; Lang, K. Cationic octahedral molybdenum cluster complexes functionalized with mitochondria-targeting ligands: Photodynamic anticancer and antibacterial activities. Biomater. Sci. 2019, 7, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Brandhonneur, N.; Boucaud, Y.; Verger, A.; Dumait, N.; Molard, Y.; Cordier, S.; Dollo, G. Molybdenum cluster loaded PLGA nanoparticles as efficient tools against epithelial ovarian cancer. Int. J. Pharm. 2021, 592, 120079. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.B.; Days, L.C.; Alajroush, D.R.; Faye, K.; Khodour, Y.; Beebe, S.J.; Holder, A.A. Photodynamic Therapy of Inorganic Complexes for the Treatment of Cancer†. Photochem. Photobiol. 2022, 98, 17–41. [Google Scholar] [CrossRef] [PubMed]
- Kirakci, K.; Kubát, P.; Fejfarová, K.; Martinčík, J.; Nikl, M.; Lang, K. X-ray Inducible Luminescence and Singlet Oxygen Sensitization by an Octahedral Molybdenum Cluster Compound: A New Class of Nanoscintillators. Inorg. Chem. 2016, 55, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Kirakci, K.; Zelenka, J.; Rumlová, M.; Martinčík, J.; Nikl, M.; Ruml, T.; Lang, K. Octahedral molybdenum clusters as radiosensitizers for X-ray induced photodynamic therapy. J. Mater. Chem. B 2018, 6, 4301–4307. [Google Scholar] [CrossRef] [PubMed]
- Kirakci, K.; Pozmogova, T.N.; Protasevich, A.Y.; Vavilov, G.D.; Stass, D.V.; Shestopalov, M.A.; Lang, K. A water-soluble octahedral molybdenum cluster complex as a potential agent for X-ray induced photodynamic therapy. Biomater. Sci. 2021, 9, 2893–2902. [Google Scholar] [CrossRef] [PubMed]
- Koncošová, M.; Rumlová, M.; Mikyšková, R.; Reiniš, M.; Zelenka, J.; Ruml, T.; Kirakci, K.; Lang, K. Avenue to X-ray-induced photodynamic therapy of prostatic carcinoma with octahedral molybdenum cluster nanoparticles. J. Mater. Chem. B 2022, 10, 3303–3310. [Google Scholar] [CrossRef]
- Felip-León, C.; Arnau del Valle, C.; Pérez-Laguna, V.; Isabel Millán-Lou, M.; Miravet, J.F.; Mikhailov, M.; Sokolov, M.N.; Rezusta-López, A.; Galindo, F. Superior performance of macroporous over gel type polystyrene as a support for the development of photo-bactericidal materials. J. Mater. Chem. B 2017, 5, 6058–6064. [Google Scholar] [CrossRef]
- Vorotnikova, N.A.; Alekseev, A.Y.; Vorotnikov, Y.A.; Evtushok, D.V.; Molard, Y.; Amela-Cortes, M.; Cordier, S.; Smolentsev, A.I.; Burton, C.G.; Kozhin, P.M.; et al. Octahedral molybdenum cluster as a photoactive antimicrobial additive to a fluoroplastic. Mater. Sci. Eng. C 2019, 105, 110150. [Google Scholar] [CrossRef] [PubMed]
- Kirakci, K.; Nguyen, T.K.N.; Grasset, F.; Uchikoshi, T.; Zelenka, J.; Kubát, P.; Ruml, T.; Lang, K. Electrophoretically Deposited Layers of Octahedral Molybdenum Cluster Complexes: A Promising Coating for Mitigation of Pathogenic Bacterial Biofilms under Blue Light. ACS Appl. Mater. Interfaces 2020, 12, 52492–52499. [Google Scholar] [CrossRef]
- López-López, N.; Muñoz Resta, I.; de Llanos, R.; Miravet, J.F.; Mikhaylov, M.; Sokolov, M.N.; Ballesta, S.; García-Luque, I.; Galindo, F. Photodynamic Inactivation of Staphylococcus aureus Biofilms Using a Hexanuclear Molybdenum Complex Embedded in Transparent polyHEMA Hydrogels. ACS Biomater. Sci. Eng. 2020, 6, 6995–7003. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.A.; Turro, C.; Newsham, M.D.; Nocera, D.G. Oxygen quenching of electronically excited hexanuclear molybdenum and tungsten halide clusters. J. Phys. Chem. 1990, 94, 4500–4507. [Google Scholar] [CrossRef]
- Kirakci, K.; Kubát, P.; Langmaier, J.; Polívka, T.; Fuciman, M.; Fejfarová, K.; Lang, K. A comparative study of the redox and excited state properties of (nBu4N)2[Mo6X14] and (nBu4N)2[Mo6X8(CF3COO)6] (X = Cl, Br, or I). Dalt. Trans. 2013, 42, 7224. [Google Scholar] [CrossRef]
- Kirakci, K.; Kubáňová, M.; Přibyl, T.; Rumlová, M.; Zelenka, J.; Ruml, T.; Lang, K. A Cell Membrane Targeting Molybdenum-Iodine Nanocluster: Rational Ligand Design toward Enhanced Photodynamic Activity. Inorg. Chem. 2022, 61, 5076–5083. [Google Scholar] [CrossRef] [PubMed]
- Aubert, T.; Burel, A.; Esnault, M.A.; Cordier, S.; Grasset, F.; Cabello-Hurtado, F. Root uptake and phytotoxicity of nanosized molybdenum octahedral clusters. J. Hazard. Mater. 2012, 219–220, 111–118. [Google Scholar] [CrossRef]
- Nakamura, H.; Liao, L.; Hitaka, Y.; Tsukigawa, K.; Subr, V.; Fang, J.; Ulbrich, K.; Maeda, H. Micelles of zinc protoporphyrin conjugated to N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer for imaging and light-induced antitumor effects in vivo. J. Control. Release 2013, 165, 191–198. [Google Scholar] [CrossRef]
- Ulbrich, K.; Šubr, V.; Strohalm, J.; Plocová, D.; Jelínková, M.; Říhová, B. Polymeric drugs based on conjugates of synthetic and natural macromolecules. I. Synthesis and physico-chemical characterisation. J. Control. Release 2000, 64, 63–79. [Google Scholar] [CrossRef]
- Šubr, V.; Ulbrich, K. Synthesis and properties of new N-(2-hydroxypropyl)-methacrylamide copolymers containing thiazolidine-2-thione reactive groups. React. Funct. Polym. 2006, 66, 1525–1538. [Google Scholar] [CrossRef]
- Chytil, P.; Etrych, T.; Koňák, Č.; Šírová, M.; Mrkvan, T.; Bouček, J.; Říhová, B.; Ulbrich, K. New HPMA copolymer-based drug carriers with covalently bound hydrophobic substituents for solid tumour targeting. J. Control. Release 2008, 127, 121–130. [Google Scholar] [CrossRef]
- Bojarová, P.; Tavares, M.R.; Laaf, D.; Bumba, L.; Petrásková, L.; Konefał, R.; Bláhová, M.; Pelantová, H.; Elling, L.; Etrych, T.; et al. Biocompatible glyconanomaterials based on HPMA-copolymer for specific targeting of galectin-3. J. Nanobiotechnol. 2018, 16, 73. [Google Scholar] [CrossRef]
- Chytil, P.; Etrych, T.; Kříž, J.; Subr, V.; Ulbrich, K. N-(2-Hydroxypropyl)methacrylamide-based polymer conjugates with pH-controlled activation of doxorubicin for cell-specific or passive tumour targeting. Synthesis by RAFT polymerisation and physicochemical characterisation. Eur. J. Pharm. Sci. 2010, 41, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Perrier, S.; Takolpuckdee, P.; Mars, C.A. Reversible Addition−Fragmentation Chain Transfer Polymerization: End Group Modification for Functionalized Polymers and Chain Transfer Agent Recovery. Macromolecules 2005, 38, 2033–2036. [Google Scholar] [CrossRef]
- Machová, D.; Koziolová, E.; Chytil, P.; Venclíková, K.; Etrych, T.; Janoušková, O. Nanotherapeutics with suitable properties for advanced anticancer therapy based on HPMA copolymer-bound ritonavir via pH-sensitive spacers. Eur. J. Pharm. Biopharm. 2018, 131, 141–150. [Google Scholar] [CrossRef]
- Kirakci, K.; Zelenka, J.; Křížová, I.; Ruml, T.; Lang, K. Octahedral Molybdenum Cluster Complexes with Optimized Properties for Photodynamic Applications. Inorg. Chem. 2020, 59, 9287–9293. [Google Scholar] [CrossRef] [PubMed]
- Kirakci, K.; Kubát, P.; Kučeráková, M.; Šícha, V.; Gbelcová, H.; Lovecká, P.; Grznárová, P.; Ruml, T.; Lang, K. Water-soluble octahedral molybdenum cluster compounds Na2[Mo6I8(N3)6] and Na2[Mo6I8(NCS)6]: Syntheses, luminescence, and in vitro studies. Inorganica Chim. Acta 2016, 441, 42–49. [Google Scholar] [CrossRef]
- Etrych, T.; Mrkvan, T.; Chytil, P.; Koňák, Č.; Říhová, B.; Ulbrich, K. N-(2-hydroxypropyl)methacrylamide-based polymer conjugates with pH-controlled activation of doxorubicin. I. New synthesis, physicochemical characterization and preliminary biological evaluation. J. Appl. Polym. Sci. 2008, 109, 3050–3061. [Google Scholar] [CrossRef]
- Fang, J.; Islam, W.; Maeda, H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv. Drug Deliv. Rev. 2020, 157, 142–160. [Google Scholar] [CrossRef]
- Pola, R.; Braunová, A.; Laga, R.; Pechar, M.; Ulbrich, K. Click chemistry as a powerful and chemoselective tool for the attachment of targeting ligands to polymer drug carriers. Polym. Chem. 2014, 5, 1340–1350. [Google Scholar] [CrossRef]
- Filippov, S.K.; Chytil, P.; Konarev, P.V.; Dyakonova, M.; Papadakis, C.; Zhigunov, A.; Plestil, J.; Stepanek, P.; Etrych, T.; Ulbrich, K.; et al. Macromolecular HPMA-based nanoparticles with cholesterol for solid-tumor targeting: Detailed study of the inner structure of a highly efficient drug delivery system. Biomacromolecules 2012, 13, 2594–2604. [Google Scholar] [CrossRef]
- Chytil, P.; Etrych, T.; Kostka, L.; Ulbrich, K. Hydrolytically Degradable Polymer Micelles for Anticancer Drug Delivery to Solid Tumors. Macromol. Chem. Phys. 2012, 213, 858–867. [Google Scholar] [CrossRef]
- Filippov, S.K.; Vishnevetskaya, N.S.; Niebuur, B.J.; Koziolová, E.; Lomkova, E.A.; Chytil, P.; Etrych, T.; Papadakis, C.M. Influence of molar mass, dispersity, and type and location of hydrophobic side chain moieties on the critical micellar concentration and stability of amphiphilic HPMA-based polymer drug carriers. Colloid Polym. Sci. 2017, 295, 1313–1325. [Google Scholar] [CrossRef]
- Chytil, P.; Šírová, M.; Kudláčová, J.; Říhová, B.; Ulbrich, K.; Etrych, T. Bloodstream Stability Predetermines the Antitumor Efficacy of Micellar Polymer–Doxorubicin Drug Conjugates with pH-Triggered Drug Release. Mol. Pharm. 2018, 15, 3654–3663. [Google Scholar] [CrossRef] [PubMed]
- Braunová, A.; Chytil, P.; Laga, R.; Šírová, M.; Machová, D.; Parnica, J.; Říhová, B.; Janoušková, O.; Etrych, T. Polymer nanomedicines based on micelle-forming amphiphilic or water-soluble polymer-doxorubicin conjugates: Comparative study of in vitro and in vivo properties related to the polymer carrier structure, composition, and hydrodynamic properties. J. Control. Release 2020, 321, 718–733. [Google Scholar] [CrossRef] [PubMed]
- Lieber, E.; Rao, C.N.R.; Hoffman, C.W.W.; Chao, T.S. Infrared Spectra of Organic Azides. Anal. Chem. 1957, 29, 916–918. [Google Scholar] [CrossRef]
- Agrell, I.; Klæboe, P.; Pettersson, B.; Svensson, S.; Koskikallio, J.; Kachi, S. The Infra-red Spectra of Some Inorganic Azide Compounds. Acta Chem. Scand. 1971, 25, 2965–2974. [Google Scholar] [CrossRef]
- Diana, E.; Gatterer, K.; Kettle, S.F.A. The vibrational spectroscopy of the coordinated azide anion; A theoretical study. Phys. Chem. Chem. Phys. 2016, 18, 414–425. [Google Scholar] [CrossRef]
- Gai, X.S.; Coutifaris, B.A.; Brewer, S.H.; Fenlon, E.E. A direct comparison of azide and nitrile vibrational probes. Phys. Chem. Chem. Phys. 2011, 13, 5926–5930. [Google Scholar] [CrossRef] [Green Version]

| Polymer Precursor | Structure | Functional Group | Functional Groups (mol. %) a | Mw (g/mol) b | Ð b | DH (nm) c |
|---|---|---|---|---|---|---|
| P0a P0b | poly(HPMA-co- MA-AP-TT) | TT |
| 18,500 39,200 | 1.03 1.04 | 5.9 ± 0.7 |
| 7.4 ± 0.4 | ||||||
| P1 | poly(HPMA-co- MA-AP-COOH) | COOH | 1.9 | 18,700 | 1.05 | 5.3 ± 0.2 |
| P2 | poly(HPMA-co-APMA) | NH2 | 5.1 | 24,100 | 1.04 | 4.9 ± 0.1 |
| P3 | poly(HPMA-co- MA-Acap-cholesterol) | cholesterol | 2.3 | 26,400 | 1.06 | 39.9 ± 1.1 |
| P4 | poly(HPMA-co-APMA-co- MA-AH-cholesterol) | cholesterol + NH2 | 2.5 cholesterol 2.3 NH2 | 24,600 | 1.07 | 26.8 ± 0.7 |
| P5 | poly(HPMA-co- MA-AP-DBCO) | DBCO | 8 | 40,000 | 1.06 | 11.5 ± 0.8 |
| Cluster | Formula | Mw (g/mol) | DH (nm) a | ZP (mV) a | Charge |
|---|---|---|---|---|---|
| C1 | [Mo6I8(OCOC4H8PPh3)6]Br4 | 4084.9 | 48.4 ± 5.3 | 13 | 4 |
| C2 | Na2[Mo6I8(OPOCPh2)6] | 2939.9 | 20.2 ± 11.6 | −67 | −2 |
| C3 | Na2[Mo6I8(cholate)6] | 4082.0 | 5.9 ± 1.4 | −9 | −2 |
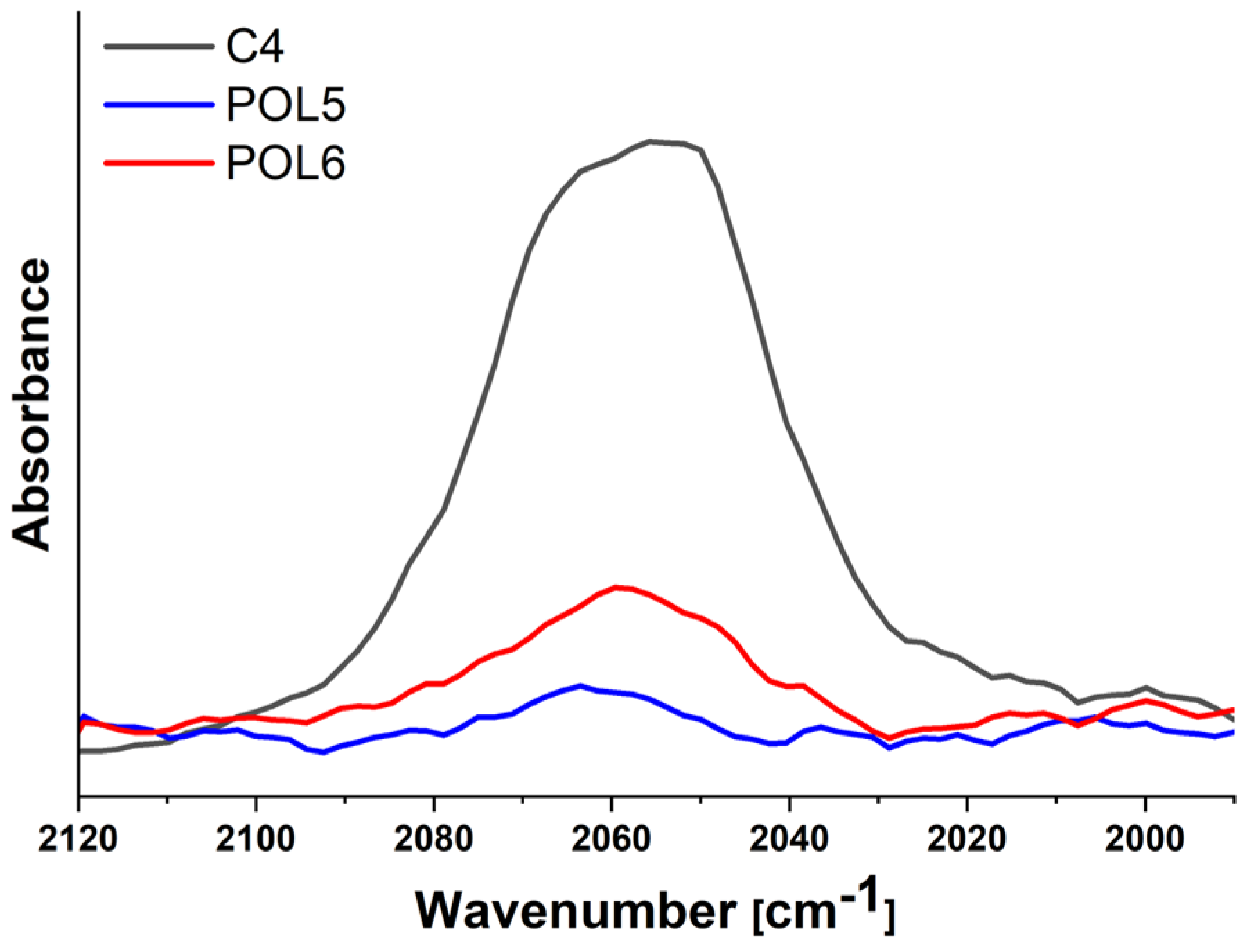
| C4 | Na2[Mo6I8(N3)6] | 1889.0 | 60.6 ± 15.3 | −16 | −2 |
| Polymer-Cluster Constructs | Prepared From | Type of Interaction | Mo Cluster (wt%) | DH (nm) a | ZP (mV) a | λL (nm) b | ΦL(Ar) b | ΦL(air) b | FT(air) b |
|---|---|---|---|---|---|---|---|---|---|
| POL1 | P1 + C1 | Electrostatic | 11.5 | 5.1 ± 1.1 | 9 | 695 | 0.16 | 0.04 | 0.75 |
| POL2 | P2 + C2 | Electrostatic | 25.9 | 29.2 ± 9.0 | 4 | 690 | 0.39 | 0.08 | 0.79 |
| POL3 | P3 + C3 | Hydrophobic | 20.0 | 8.4 ± 2.3 | −14 | 690 | 0.25 | 0.05 | 0.80 |
| POL4 | P4 + C3 | Hydrophobic | 20.0 | 12.0 ± 3.1 | 1 | 695 | 0.49 | 0.09 | 0.82 |
| POL5 | P5 + C4 | Covalent | 14.2 | 7.3 ± 1.1 | −17 | 685 | 0.25 | 0.06 | 0.76 |
| POL6 | P5 + C4 + azide-NH2 | Covalent | 14.2 | 11.0 ± 0.9 | −7 | 685 | 0.25 | 0.06 | 0.76 |
| Polymer-Cluster Constructs | DH (nm) a | ZP (mV) a | λL (nm) b | ΦL(Ar) | ΦL(air) | FT(air) |
|---|---|---|---|---|---|---|
| POL5, fresh | 7.3 ± 1.1 | −17 | 688 | 0.27 | 0.06 | 0.78 |
| POL5, 5 days old | 7.9 ± 1.4 | −15 | 690 | 0.25 | 0.06 | 0.76 |
| POL6, fresh | 11.0 ± 0.9 | −7 | 689 | 0.27 | 0.06 | 0.78 |
| POL6, 5 days old | 14.2 ± 0.1 | −1 | 690 | 0.27 | 0.06 | 0.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavares, M.R.; Kirakci, K.; Kotov, N.; Pechar, M.; Lang, K.; Pola, R.; Etrych, T. Octahedral Molybdenum Cluster-Based Nanomaterials for Potential Photodynamic Therapy. Nanomaterials 2022, 12, 3350. https://doi.org/10.3390/nano12193350
Tavares MR, Kirakci K, Kotov N, Pechar M, Lang K, Pola R, Etrych T. Octahedral Molybdenum Cluster-Based Nanomaterials for Potential Photodynamic Therapy. Nanomaterials. 2022; 12(19):3350. https://doi.org/10.3390/nano12193350
Chicago/Turabian StyleTavares, Marina Rodrigues, Kaplan Kirakci, Nikolay Kotov, Michal Pechar, Kamil Lang, Robert Pola, and Tomáš Etrych. 2022. "Octahedral Molybdenum Cluster-Based Nanomaterials for Potential Photodynamic Therapy" Nanomaterials 12, no. 19: 3350. https://doi.org/10.3390/nano12193350
APA StyleTavares, M. R., Kirakci, K., Kotov, N., Pechar, M., Lang, K., Pola, R., & Etrych, T. (2022). Octahedral Molybdenum Cluster-Based Nanomaterials for Potential Photodynamic Therapy. Nanomaterials, 12(19), 3350. https://doi.org/10.3390/nano12193350