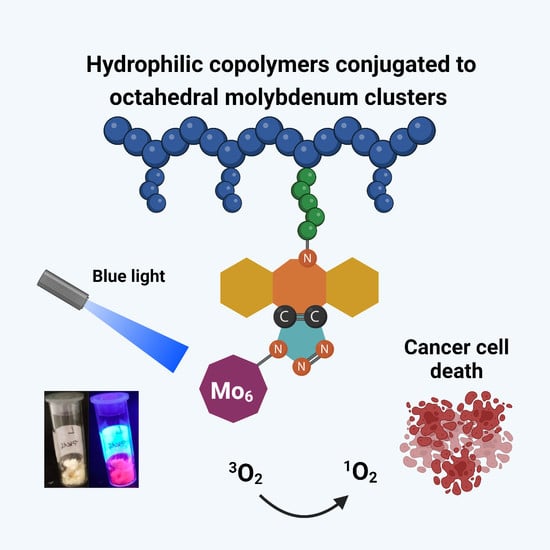
Octahedral Molybdenum Cluster-Based Nanomaterials for Potential Photodynamic Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Monomers
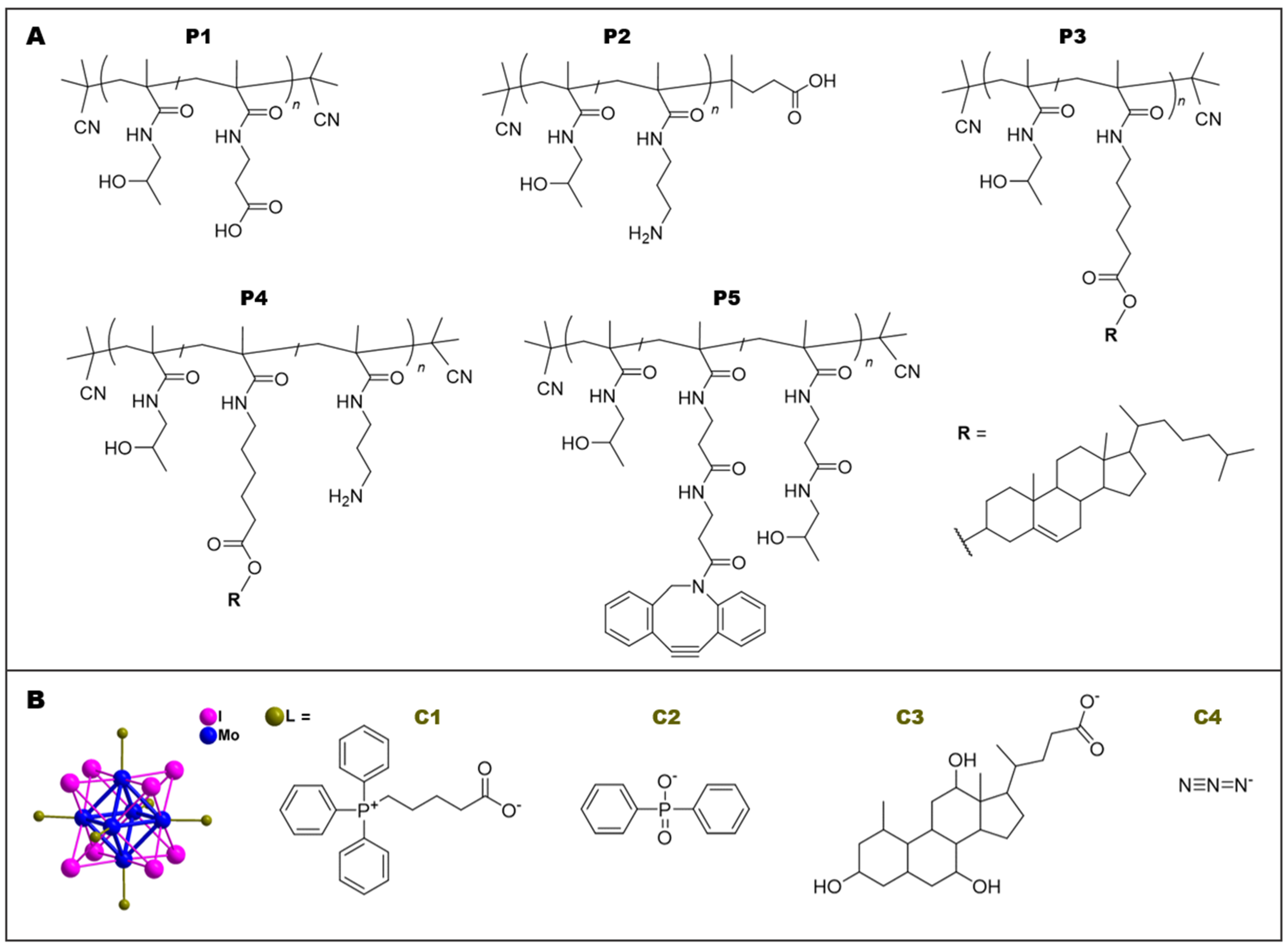
2.3. Synthesis of Polymer Precursors P1–P5
2.4. Synthesis of Mo6 Clusters C1–C4
2.5. Synthesis of Polymer-Cluster Constructs POL1–POL6
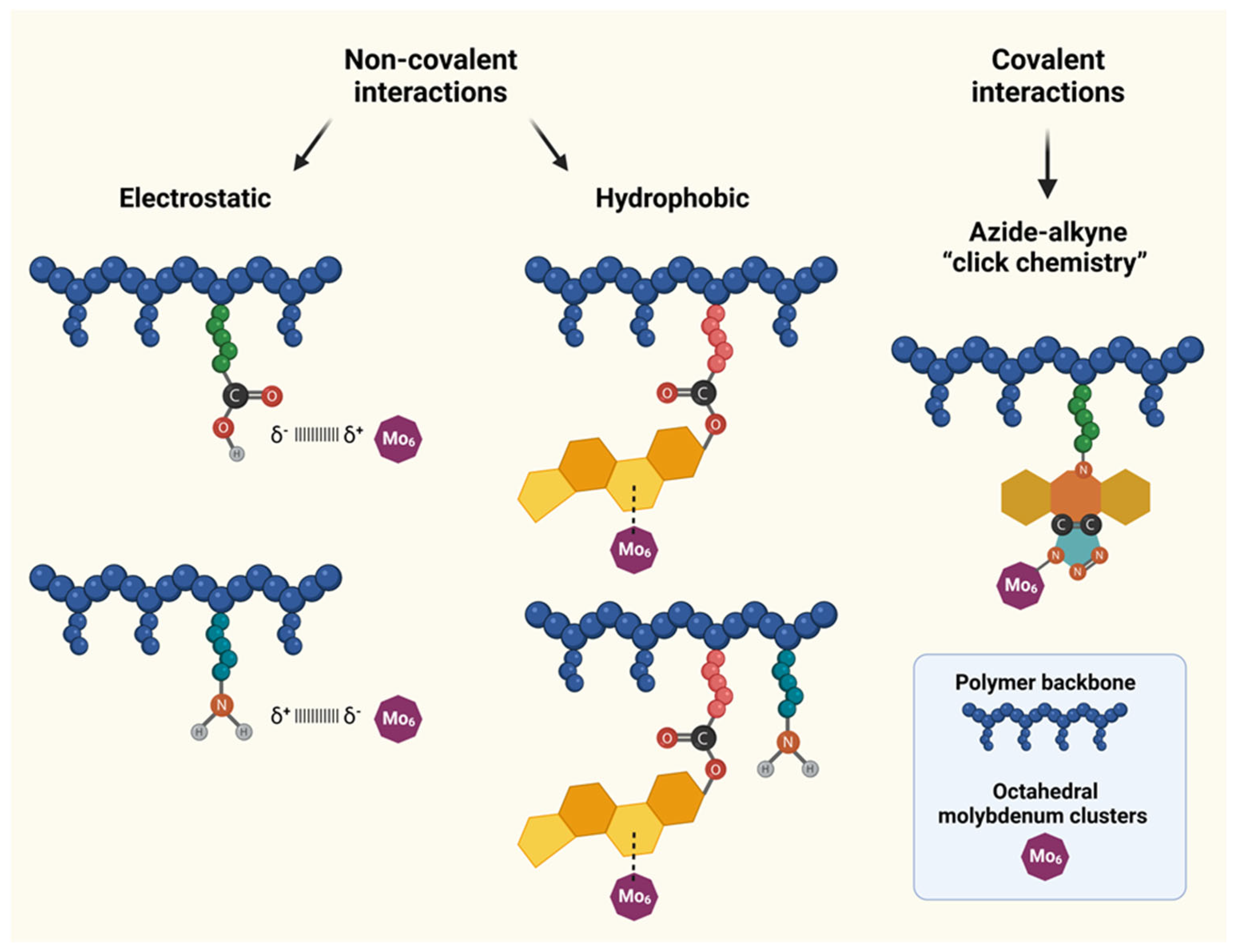
2.5.1. Electrostatic Non-Covalent–POL1 and POL2
2.5.2. Hydrophobic Non-Covalent Complexes–POL3 and POL4
2.5.3. Covalent Conjugates–POL5–POL6
2.6. Physico-Chemical and Photophysical Characterization
2.6.1. Size Exclusion Chromatography (SEC)
2.6.2. Dynamic Light Scattering (DLS)
2.6.3. UV–VIS Spectrophotometry
2.6.4. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.6.5. Attenuated Total Reflectance (ATR) Fourier-Transform Infrared (FTIR) Spectroscopy
2.6.6. Luminescence Spectroscopy
3. Results and Discussion
3.1. Synthesis of Polymer Precursors
3.2. Preparation of Polymer-Cluster Constructs
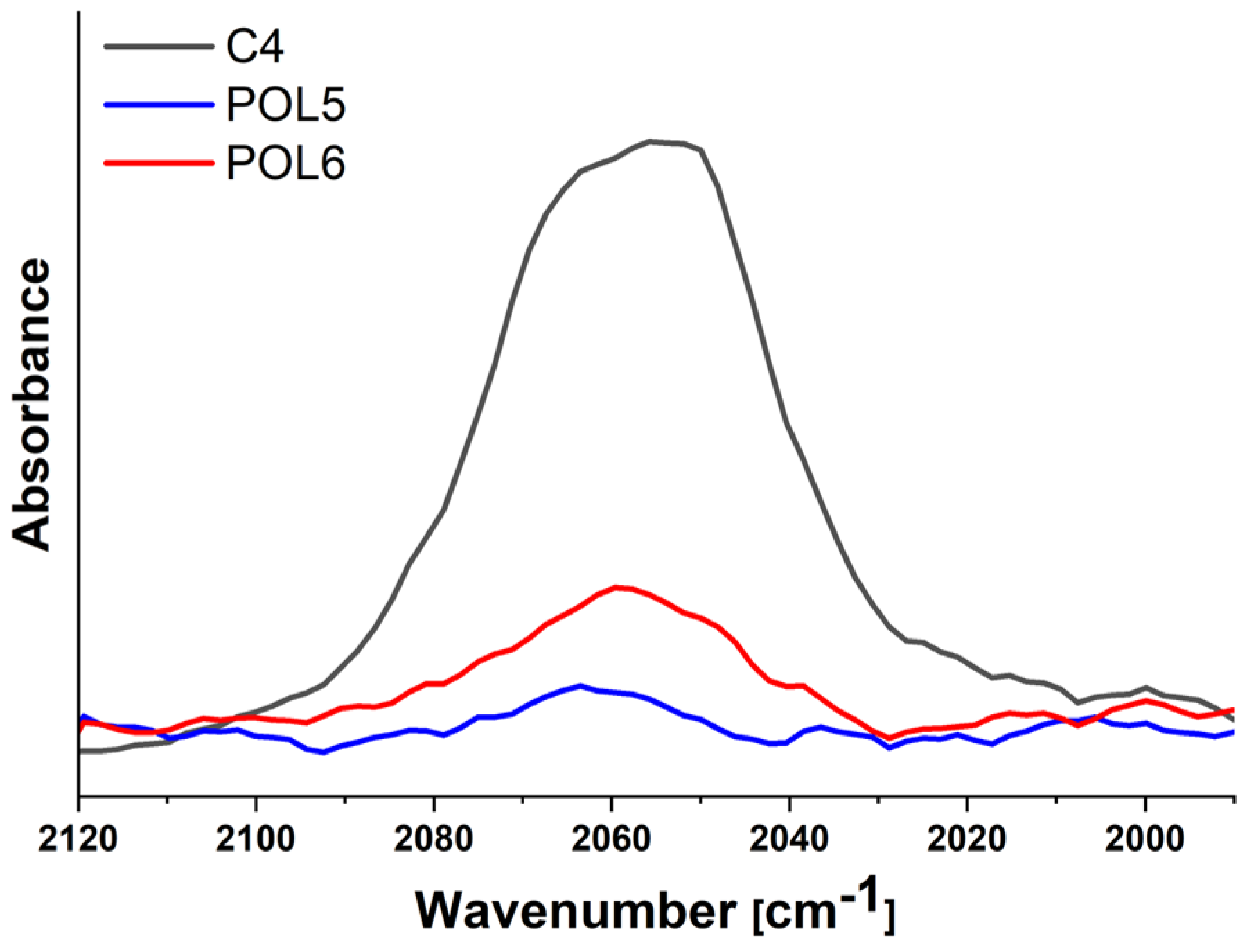
3.3. Stability and Photophysical Properties of the Polymer-Cluster Constructs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maeda, H.; Nakamura, H.; Fang, J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Šubr, V.; Islam, W.; Hackbarth, S.; Islam, R.; Etrych, T.; Ulbrich, K.; Maeda, H. N-(2-hydroxypropyl)methacrylamide polymer conjugated pyropheophorbide-a, a promising tumor-targeted theranostic probe for photodynamic therapy and imaging. Eur. J. Pharm. Biopharm. 2018, 130, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.; Selbo, P.K.; Weyergang, A.; Dietze, A.; Prasmickaite, L.; Bonsted, A.; Engesaeter, B.Ø.; Angell-Petersen, E.; Warloe, T.; Frandsen, N.; et al. Porphyrin-related photosensitizers for cancer imaging and therapeutic applications. J. Microsc. 2005, 218 Pt 2, 133–147. [Google Scholar] [CrossRef]
- Li, L.; Huh, K.M. Polymeric nanocarrier systems for photodynamic therapy. Biomater. Res. 2014, 18, 19. [Google Scholar] [CrossRef] [PubMed]
- Chepurna, O.M.; Yakovliev, A.; Ziniuk, R.; Nikolaeva, O.A.; Levchenko, S.M.; Xu, H.; Losytskyy, M.Y.; Bricks, J.L.; Slominskii, Y.L.; Vretik, L.O.; et al. Core–shell polymeric nanoparticles co-loaded with photosensitizer and organic dye for photodynamic therapy guided by fluorescence imaging in near and short-wave infrared spectral regions. J. Nanobiotechnol. 2020, 18, 19. [Google Scholar] [CrossRef] [PubMed]
- Gibot, L.; Lemelle, A.; Till, U.; Moukarzel, B.; Mingotaud, A.-F.; Pimienta, V.; Saint-Aguet, P.; Rols, M.-P.; Gaucher, M.; Violleau, F.; et al. Polymeric Micelles Encapsulating Photosensitizer: Structure/Photodynamic Therapy Efficiency Relation. Biomacromolecules 2014, 15, 1443–1455. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-E.K.; Kopelman, R. Polymeric Nanoparticles for Photodynamic Therapy. In Methods in Molecular Biology; Springer: Clifton, NJ, USA, 2011; Volume 726, pp. 151–178. [Google Scholar]
- Ricci-Júnior, E.; Marchetti, J.M. Preparation, characterization, photocytotoxicity assay of PLGA nanoparticles containing zinc (II) phthalocyanine for photodynamic therapy use. J. Microencapsul. 2006, 23, 523–538. [Google Scholar] [CrossRef]
- Weiss, G.J.; Chao, J.; Neidhart, J.D.; Ramanathan, R.K.; Bassett, D.; Neidhart, J.A.; Choi, C.H.J.; Chow, W.; Chung, V.; Forman, S.J.; et al. First-in-human phase 1/2a trial of CRLX101, a cyclodextrin-containing polymer-camptothecin nanopharmaceutical in patients with advanced solid tumor malignancies. Investig. New Drugs 2013, 31, 986–1000. [Google Scholar] [CrossRef]
- Brandhonneur, N.; Hatahet, T.; Amela-Cortes, M.; Molard, Y.; Cordier, S.; Dollo, G. Molybdenum cluster loaded PLGA nanoparticles: An innovative theranostic approach for the treatment of ovarian cancer. Eur. J. Pharm. Biopharm. 2018, 125, 95–105. [Google Scholar] [CrossRef]
- Kirakci, K.; Demel, J.; Hynek, J.; Zelenka, J.; Rumlová, M.; Ruml, T.; Lang, K. Phosphinate Apical Ligands: A Route to a Water-Stable Octahedral Molybdenum Cluster Complex. Inorg. Chem. 2019, 58, 16546–16552. [Google Scholar] [CrossRef]
- Kirakci, K.; Zelenka, J.; Rumlová, M.; Cvačka, J.; Ruml, T.; Lang, K. Cationic octahedral molybdenum cluster complexes functionalized with mitochondria-targeting ligands: Photodynamic anticancer and antibacterial activities. Biomater. Sci. 2019, 7, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Brandhonneur, N.; Boucaud, Y.; Verger, A.; Dumait, N.; Molard, Y.; Cordier, S.; Dollo, G. Molybdenum cluster loaded PLGA nanoparticles as efficient tools against epithelial ovarian cancer. Int. J. Pharm. 2021, 592, 120079. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.B.; Days, L.C.; Alajroush, D.R.; Faye, K.; Khodour, Y.; Beebe, S.J.; Holder, A.A. Photodynamic Therapy of Inorganic Complexes for the Treatment of Cancer†. Photochem. Photobiol. 2022, 98, 17–41. [Google Scholar] [CrossRef] [PubMed]
- Kirakci, K.; Kubát, P.; Fejfarová, K.; Martinčík, J.; Nikl, M.; Lang, K. X-ray Inducible Luminescence and Singlet Oxygen Sensitization by an Octahedral Molybdenum Cluster Compound: A New Class of Nanoscintillators. Inorg. Chem. 2016, 55, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Kirakci, K.; Zelenka, J.; Rumlová, M.; Martinčík, J.; Nikl, M.; Ruml, T.; Lang, K. Octahedral molybdenum clusters as radiosensitizers for X-ray induced photodynamic therapy. J. Mater. Chem. B 2018, 6, 4301–4307. [Google Scholar] [CrossRef] [PubMed]
- Kirakci, K.; Pozmogova, T.N.; Protasevich, A.Y.; Vavilov, G.D.; Stass, D.V.; Shestopalov, M.A.; Lang, K. A water-soluble octahedral molybdenum cluster complex as a potential agent for X-ray induced photodynamic therapy. Biomater. Sci. 2021, 9, 2893–2902. [Google Scholar] [CrossRef] [PubMed]
- Koncošová, M.; Rumlová, M.; Mikyšková, R.; Reiniš, M.; Zelenka, J.; Ruml, T.; Kirakci, K.; Lang, K. Avenue to X-ray-induced photodynamic therapy of prostatic carcinoma with octahedral molybdenum cluster nanoparticles. J. Mater. Chem. B 2022, 10, 3303–3310. [Google Scholar] [CrossRef]
- Felip-León, C.; Arnau del Valle, C.; Pérez-Laguna, V.; Isabel Millán-Lou, M.; Miravet, J.F.; Mikhailov, M.; Sokolov, M.N.; Rezusta-López, A.; Galindo, F. Superior performance of macroporous over gel type polystyrene as a support for the development of photo-bactericidal materials. J. Mater. Chem. B 2017, 5, 6058–6064. [Google Scholar] [CrossRef]
- Vorotnikova, N.A.; Alekseev, A.Y.; Vorotnikov, Y.A.; Evtushok, D.V.; Molard, Y.; Amela-Cortes, M.; Cordier, S.; Smolentsev, A.I.; Burton, C.G.; Kozhin, P.M.; et al. Octahedral molybdenum cluster as a photoactive antimicrobial additive to a fluoroplastic. Mater. Sci. Eng. C 2019, 105, 110150. [Google Scholar] [CrossRef] [PubMed]
- Kirakci, K.; Nguyen, T.K.N.; Grasset, F.; Uchikoshi, T.; Zelenka, J.; Kubát, P.; Ruml, T.; Lang, K. Electrophoretically Deposited Layers of Octahedral Molybdenum Cluster Complexes: A Promising Coating for Mitigation of Pathogenic Bacterial Biofilms under Blue Light. ACS Appl. Mater. Interfaces 2020, 12, 52492–52499. [Google Scholar] [CrossRef]
- López-López, N.; Muñoz Resta, I.; de Llanos, R.; Miravet, J.F.; Mikhaylov, M.; Sokolov, M.N.; Ballesta, S.; García-Luque, I.; Galindo, F. Photodynamic Inactivation of Staphylococcus aureus Biofilms Using a Hexanuclear Molybdenum Complex Embedded in Transparent polyHEMA Hydrogels. ACS Biomater. Sci. Eng. 2020, 6, 6995–7003. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.A.; Turro, C.; Newsham, M.D.; Nocera, D.G. Oxygen quenching of electronically excited hexanuclear molybdenum and tungsten halide clusters. J. Phys. Chem. 1990, 94, 4500–4507. [Google Scholar] [CrossRef]
- Kirakci, K.; Kubát, P.; Langmaier, J.; Polívka, T.; Fuciman, M.; Fejfarová, K.; Lang, K. A comparative study of the redox and excited state properties of (nBu4N)2[Mo6X14] and (nBu4N)2[Mo6X8(CF3COO)6] (X = Cl, Br, or I). Dalt. Trans. 2013, 42, 7224. [Google Scholar] [CrossRef]
- Kirakci, K.; Kubáňová, M.; Přibyl, T.; Rumlová, M.; Zelenka, J.; Ruml, T.; Lang, K. A Cell Membrane Targeting Molybdenum-Iodine Nanocluster: Rational Ligand Design toward Enhanced Photodynamic Activity. Inorg. Chem. 2022, 61, 5076–5083. [Google Scholar] [CrossRef] [PubMed]
- Aubert, T.; Burel, A.; Esnault, M.A.; Cordier, S.; Grasset, F.; Cabello-Hurtado, F. Root uptake and phytotoxicity of nanosized molybdenum octahedral clusters. J. Hazard. Mater. 2012, 219–220, 111–118. [Google Scholar] [CrossRef]
- Nakamura, H.; Liao, L.; Hitaka, Y.; Tsukigawa, K.; Subr, V.; Fang, J.; Ulbrich, K.; Maeda, H. Micelles of zinc protoporphyrin conjugated to N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer for imaging and light-induced antitumor effects in vivo. J. Control. Release 2013, 165, 191–198. [Google Scholar] [CrossRef]
- Ulbrich, K.; Šubr, V.; Strohalm, J.; Plocová, D.; Jelínková, M.; Říhová, B. Polymeric drugs based on conjugates of synthetic and natural macromolecules. I. Synthesis and physico-chemical characterisation. J. Control. Release 2000, 64, 63–79. [Google Scholar] [CrossRef]
- Šubr, V.; Ulbrich, K. Synthesis and properties of new N-(2-hydroxypropyl)-methacrylamide copolymers containing thiazolidine-2-thione reactive groups. React. Funct. Polym. 2006, 66, 1525–1538. [Google Scholar] [CrossRef]
- Chytil, P.; Etrych, T.; Koňák, Č.; Šírová, M.; Mrkvan, T.; Bouček, J.; Říhová, B.; Ulbrich, K. New HPMA copolymer-based drug carriers with covalently bound hydrophobic substituents for solid tumour targeting. J. Control. Release 2008, 127, 121–130. [Google Scholar] [CrossRef]
- Bojarová, P.; Tavares, M.R.; Laaf, D.; Bumba, L.; Petrásková, L.; Konefał, R.; Bláhová, M.; Pelantová, H.; Elling, L.; Etrych, T.; et al. Biocompatible glyconanomaterials based on HPMA-copolymer for specific targeting of galectin-3. J. Nanobiotechnol. 2018, 16, 73. [Google Scholar] [CrossRef]
- Chytil, P.; Etrych, T.; Kříž, J.; Subr, V.; Ulbrich, K. N-(2-Hydroxypropyl)methacrylamide-based polymer conjugates with pH-controlled activation of doxorubicin for cell-specific or passive tumour targeting. Synthesis by RAFT polymerisation and physicochemical characterisation. Eur. J. Pharm. Sci. 2010, 41, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Perrier, S.; Takolpuckdee, P.; Mars, C.A. Reversible Addition−Fragmentation Chain Transfer Polymerization: End Group Modification for Functionalized Polymers and Chain Transfer Agent Recovery. Macromolecules 2005, 38, 2033–2036. [Google Scholar] [CrossRef]
- Machová, D.; Koziolová, E.; Chytil, P.; Venclíková, K.; Etrych, T.; Janoušková, O. Nanotherapeutics with suitable properties for advanced anticancer therapy based on HPMA copolymer-bound ritonavir via pH-sensitive spacers. Eur. J. Pharm. Biopharm. 2018, 131, 141–150. [Google Scholar] [CrossRef]
- Kirakci, K.; Zelenka, J.; Křížová, I.; Ruml, T.; Lang, K. Octahedral Molybdenum Cluster Complexes with Optimized Properties for Photodynamic Applications. Inorg. Chem. 2020, 59, 9287–9293. [Google Scholar] [CrossRef] [PubMed]
- Kirakci, K.; Kubát, P.; Kučeráková, M.; Šícha, V.; Gbelcová, H.; Lovecká, P.; Grznárová, P.; Ruml, T.; Lang, K. Water-soluble octahedral molybdenum cluster compounds Na2[Mo6I8(N3)6] and Na2[Mo6I8(NCS)6]: Syntheses, luminescence, and in vitro studies. Inorganica Chim. Acta 2016, 441, 42–49. [Google Scholar] [CrossRef]
- Etrych, T.; Mrkvan, T.; Chytil, P.; Koňák, Č.; Říhová, B.; Ulbrich, K. N-(2-hydroxypropyl)methacrylamide-based polymer conjugates with pH-controlled activation of doxorubicin. I. New synthesis, physicochemical characterization and preliminary biological evaluation. J. Appl. Polym. Sci. 2008, 109, 3050–3061. [Google Scholar] [CrossRef]
- Fang, J.; Islam, W.; Maeda, H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv. Drug Deliv. Rev. 2020, 157, 142–160. [Google Scholar] [CrossRef]
- Pola, R.; Braunová, A.; Laga, R.; Pechar, M.; Ulbrich, K. Click chemistry as a powerful and chemoselective tool for the attachment of targeting ligands to polymer drug carriers. Polym. Chem. 2014, 5, 1340–1350. [Google Scholar] [CrossRef]
- Filippov, S.K.; Chytil, P.; Konarev, P.V.; Dyakonova, M.; Papadakis, C.; Zhigunov, A.; Plestil, J.; Stepanek, P.; Etrych, T.; Ulbrich, K.; et al. Macromolecular HPMA-based nanoparticles with cholesterol for solid-tumor targeting: Detailed study of the inner structure of a highly efficient drug delivery system. Biomacromolecules 2012, 13, 2594–2604. [Google Scholar] [CrossRef]
- Chytil, P.; Etrych, T.; Kostka, L.; Ulbrich, K. Hydrolytically Degradable Polymer Micelles for Anticancer Drug Delivery to Solid Tumors. Macromol. Chem. Phys. 2012, 213, 858–867. [Google Scholar] [CrossRef]
- Filippov, S.K.; Vishnevetskaya, N.S.; Niebuur, B.J.; Koziolová, E.; Lomkova, E.A.; Chytil, P.; Etrych, T.; Papadakis, C.M. Influence of molar mass, dispersity, and type and location of hydrophobic side chain moieties on the critical micellar concentration and stability of amphiphilic HPMA-based polymer drug carriers. Colloid Polym. Sci. 2017, 295, 1313–1325. [Google Scholar] [CrossRef]
- Chytil, P.; Šírová, M.; Kudláčová, J.; Říhová, B.; Ulbrich, K.; Etrych, T. Bloodstream Stability Predetermines the Antitumor Efficacy of Micellar Polymer–Doxorubicin Drug Conjugates with pH-Triggered Drug Release. Mol. Pharm. 2018, 15, 3654–3663. [Google Scholar] [CrossRef] [PubMed]
- Braunová, A.; Chytil, P.; Laga, R.; Šírová, M.; Machová, D.; Parnica, J.; Říhová, B.; Janoušková, O.; Etrych, T. Polymer nanomedicines based on micelle-forming amphiphilic or water-soluble polymer-doxorubicin conjugates: Comparative study of in vitro and in vivo properties related to the polymer carrier structure, composition, and hydrodynamic properties. J. Control. Release 2020, 321, 718–733. [Google Scholar] [CrossRef] [PubMed]
- Lieber, E.; Rao, C.N.R.; Hoffman, C.W.W.; Chao, T.S. Infrared Spectra of Organic Azides. Anal. Chem. 1957, 29, 916–918. [Google Scholar] [CrossRef]
- Agrell, I.; Klæboe, P.; Pettersson, B.; Svensson, S.; Koskikallio, J.; Kachi, S. The Infra-red Spectra of Some Inorganic Azide Compounds. Acta Chem. Scand. 1971, 25, 2965–2974. [Google Scholar] [CrossRef][Green Version]
- Diana, E.; Gatterer, K.; Kettle, S.F.A. The vibrational spectroscopy of the coordinated azide anion; A theoretical study. Phys. Chem. Chem. Phys. 2016, 18, 414–425. [Google Scholar] [CrossRef]
- Gai, X.S.; Coutifaris, B.A.; Brewer, S.H.; Fenlon, E.E. A direct comparison of azide and nitrile vibrational probes. Phys. Chem. Chem. Phys. 2011, 13, 5926–5930. [Google Scholar] [CrossRef]

| Polymer Precursor | Structure | Functional Group | Functional Groups (mol. %) a | Mw (g/mol) b | Ð b | DH (nm) c |
|---|---|---|---|---|---|---|
| P0a P0b | poly(HPMA-co- MA-AP-TT) | TT |
| 18,500 39,200 | 1.03 1.04 | 5.9 ± 0.7 |
| 7.4 ± 0.4 | ||||||
| P1 | poly(HPMA-co- MA-AP-COOH) | COOH | 1.9 | 18,700 | 1.05 | 5.3 ± 0.2 |
| P2 | poly(HPMA-co-APMA) | NH2 | 5.1 | 24,100 | 1.04 | 4.9 ± 0.1 |
| P3 | poly(HPMA-co- MA-Acap-cholesterol) | cholesterol | 2.3 | 26,400 | 1.06 | 39.9 ± 1.1 |
| P4 | poly(HPMA-co-APMA-co- MA-AH-cholesterol) | cholesterol + NH2 | 2.5 cholesterol 2.3 NH2 | 24,600 | 1.07 | 26.8 ± 0.7 |
| P5 | poly(HPMA-co- MA-AP-DBCO) | DBCO | 8 | 40,000 | 1.06 | 11.5 ± 0.8 |
| Cluster | Formula | Mw (g/mol) | DH (nm) a | ZP (mV) a | Charge |
|---|---|---|---|---|---|
| C1 | [Mo6I8(OCOC4H8PPh3)6]Br4 | 4084.9 | 48.4 ± 5.3 | 13 | 4 |
| C2 | Na2[Mo6I8(OPOCPh2)6] | 2939.9 | 20.2 ± 11.6 | −67 | −2 |
| C3 | Na2[Mo6I8(cholate)6] | 4082.0 | 5.9 ± 1.4 | −9 | −2 |
| C4 | Na2[Mo6I8(N3)6] | 1889.0 | 60.6 ± 15.3 | −16 | −2 |
| Polymer-Cluster Constructs | Prepared From | Type of Interaction | Mo Cluster (wt%) | DH (nm) a | ZP (mV) a | λL (nm) b | ΦL(Ar) b | ΦL(air) b | FT(air) b |
|---|---|---|---|---|---|---|---|---|---|
| POL1 | P1 + C1 | Electrostatic | 11.5 | 5.1 ± 1.1 | 9 | 695 | 0.16 | 0.04 | 0.75 |
| POL2 | P2 + C2 | Electrostatic | 25.9 | 29.2 ± 9.0 | 4 | 690 | 0.39 | 0.08 | 0.79 |
| POL3 | P3 + C3 | Hydrophobic | 20.0 | 8.4 ± 2.3 | −14 | 690 | 0.25 | 0.05 | 0.80 |
| POL4 | P4 + C3 | Hydrophobic | 20.0 | 12.0 ± 3.1 | 1 | 695 | 0.49 | 0.09 | 0.82 |
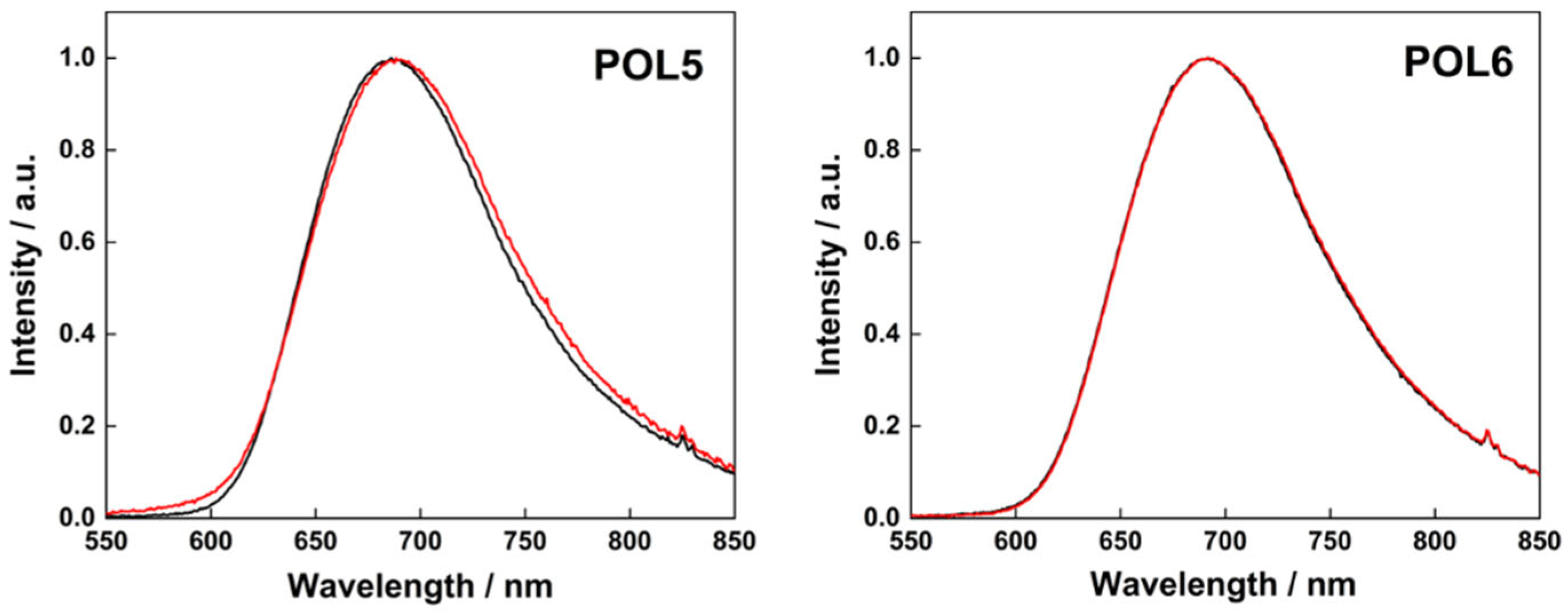
| POL5 | P5 + C4 | Covalent | 14.2 | 7.3 ± 1.1 | −17 | 685 | 0.25 | 0.06 | 0.76 |
| POL6 | P5 + C4 + azide-NH2 | Covalent | 14.2 | 11.0 ± 0.9 | −7 | 685 | 0.25 | 0.06 | 0.76 |
| Polymer-Cluster Constructs | DH (nm) a | ZP (mV) a | λL (nm) b | ΦL(Ar) | ΦL(air) | FT(air) |
|---|---|---|---|---|---|---|
| POL5, fresh | 7.3 ± 1.1 | −17 | 688 | 0.27 | 0.06 | 0.78 |
| POL5, 5 days old | 7.9 ± 1.4 | −15 | 690 | 0.25 | 0.06 | 0.76 |
| POL6, fresh | 11.0 ± 0.9 | −7 | 689 | 0.27 | 0.06 | 0.78 |
| POL6, 5 days old | 14.2 ± 0.1 | −1 | 690 | 0.27 | 0.06 | 0.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavares, M.R.; Kirakci, K.; Kotov, N.; Pechar, M.; Lang, K.; Pola, R.; Etrych, T. Octahedral Molybdenum Cluster-Based Nanomaterials for Potential Photodynamic Therapy. Nanomaterials 2022, 12, 3350. https://doi.org/10.3390/nano12193350
Tavares MR, Kirakci K, Kotov N, Pechar M, Lang K, Pola R, Etrych T. Octahedral Molybdenum Cluster-Based Nanomaterials for Potential Photodynamic Therapy. Nanomaterials. 2022; 12(19):3350. https://doi.org/10.3390/nano12193350
Chicago/Turabian StyleTavares, Marina Rodrigues, Kaplan Kirakci, Nikolay Kotov, Michal Pechar, Kamil Lang, Robert Pola, and Tomáš Etrych. 2022. "Octahedral Molybdenum Cluster-Based Nanomaterials for Potential Photodynamic Therapy" Nanomaterials 12, no. 19: 3350. https://doi.org/10.3390/nano12193350
APA StyleTavares, M. R., Kirakci, K., Kotov, N., Pechar, M., Lang, K., Pola, R., & Etrych, T. (2022). Octahedral Molybdenum Cluster-Based Nanomaterials for Potential Photodynamic Therapy. Nanomaterials, 12(19), 3350. https://doi.org/10.3390/nano12193350