Effect of Pore Filling on Properties of Nanocomposites LiClO4–MIL–101(Cr) with High Ionic Conductivity
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. X-ray Diffraction and Morphology Studies
3.2. Pore Size Distribution in the Nanocomposites
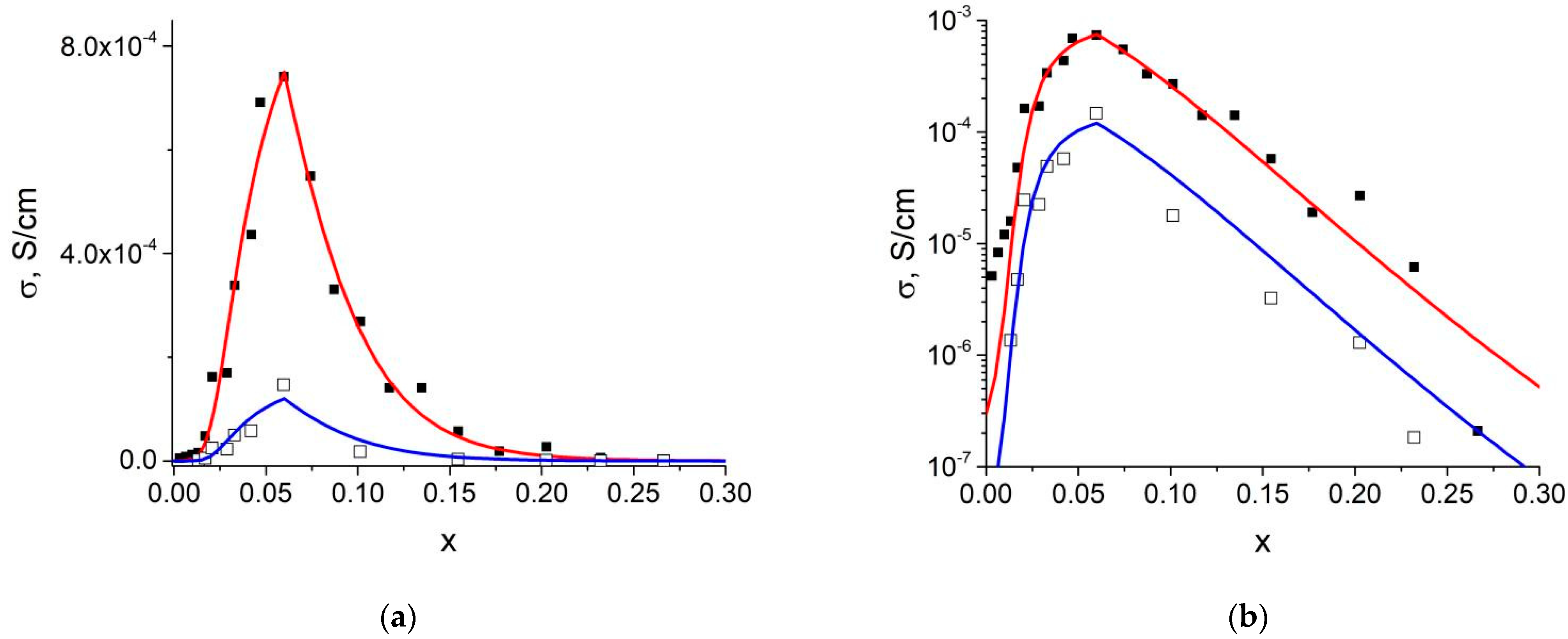
3.3. Transport Properties of Composites
4. Interpretation of the Data
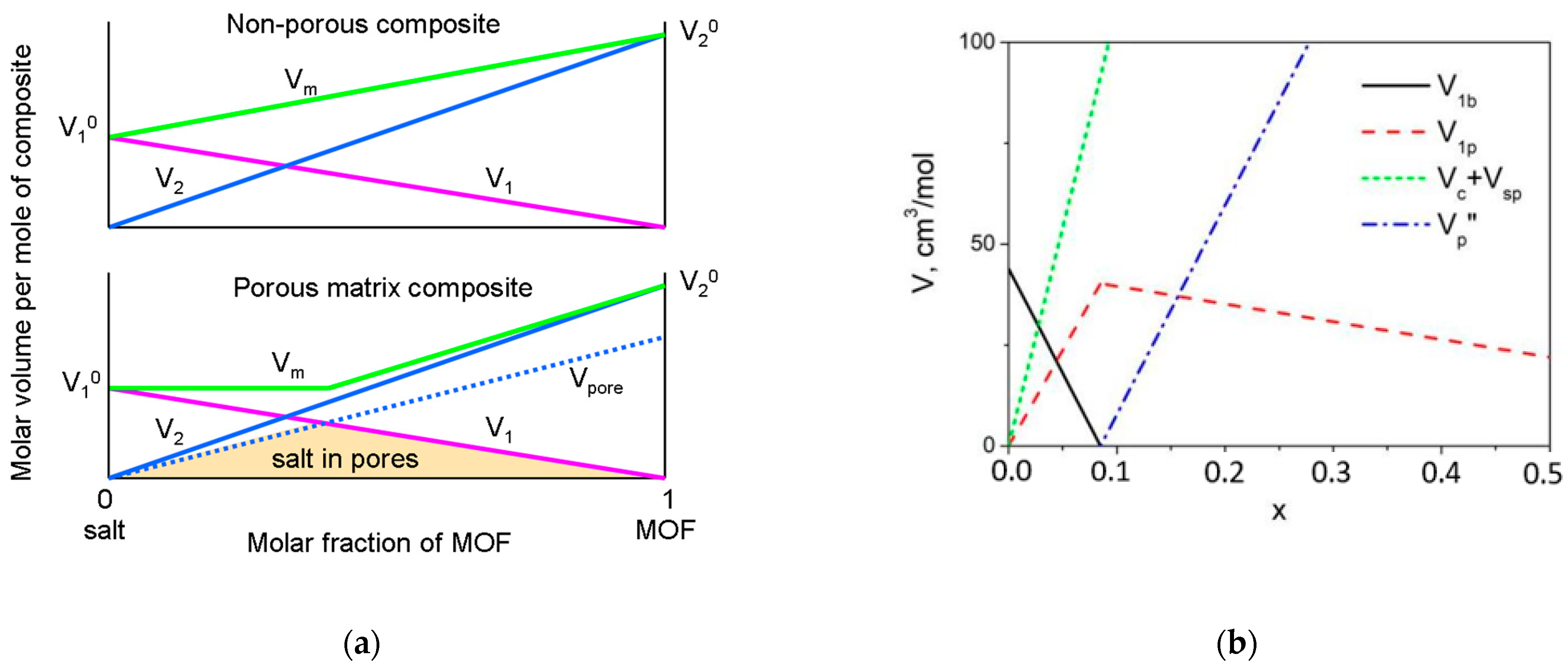
4.1. The Model of the Composite Morphology
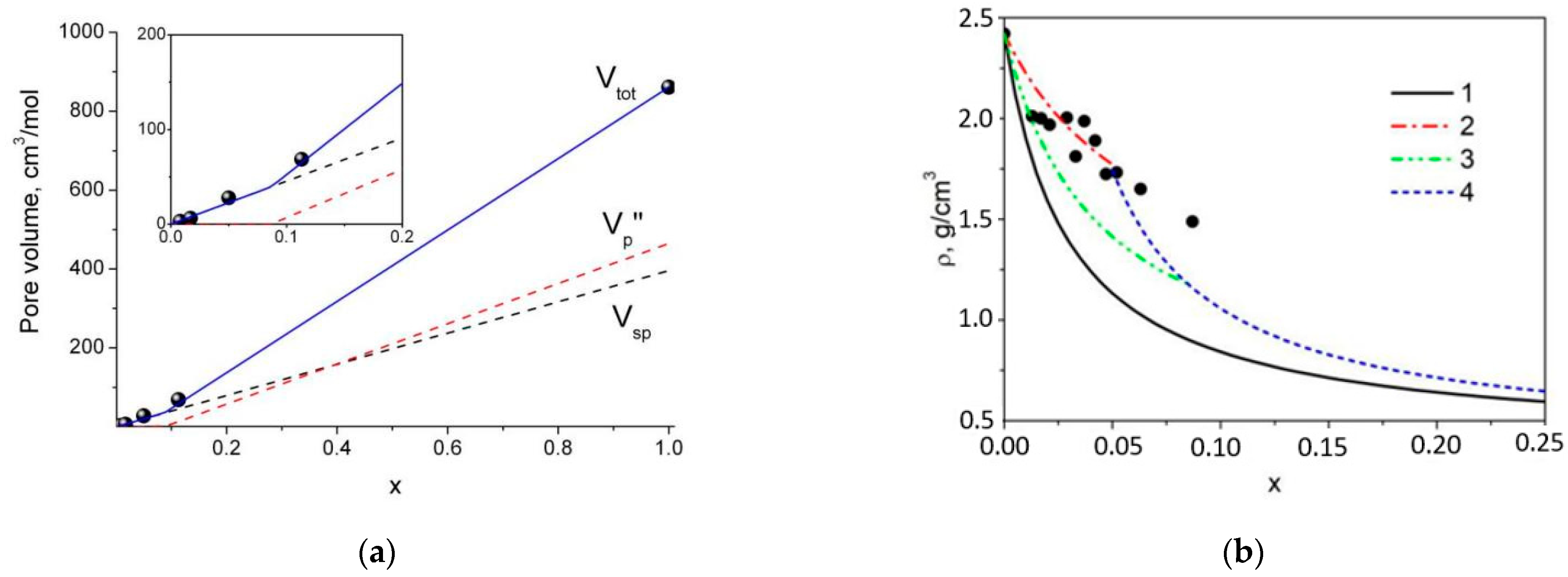
4.2. Estimation of the Pore Volume in Composites: Comparison with Adsorption Data
4.3. Calculation of Composites’ Density
4.4. Calculation of the Ionic Conductivity
- 1.
- At x < xmax the composite may be regarded as a two-phase mixture of bulk LiClO4 and the MOF cage with the pores completely filled with LiClO4. It can be shown that relative volume fractions of the bulk LiClO4 and MIL-101(Cr) with filled pores, φ1 and φ2, respectively, are given by relations:
- 2.
- At x > xmax the composite may be considered as the mixture of lithium perchlorate located in the pores and the cage of MIL-101(Cr), including the empty space in the pores or unfilled pores. Relative volume fractions of such components are given by relations:
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Parameter | Description |
|---|---|
| x | Molar fraction of MIL-101(Cr) |
| M, M1 and M2 | Molecular mass of the composite (M), the first component, LiClO4, and the second component MIL-101(Cr) in composite, respectively [g/mol] |
| Vm | Total molar volume of composite [cm3/mol] |
| V1 | The effective partial molar volume of the 1-st component (LiClO4) [cm3/mol] |
| V2 | The effective partial molar volume of the 2-nd component (MIL-101(Cr)) in composite [cm3/mol] |
| V10 | The molar volume of the 1-st component, the pristine LiClO4 [cm3/mol] |
| V20 | The molar volume of the 2-nd component, the pristine MIL-101(Cr) [cm3/mol] |
| Vc0 | The molar volume of the framework walls in pristine MIL-101(Cr) [cm3/mol] |
| Vsp0 | The molar volume of microporous cages (small pores) inaccessible to the salt in pristine MIL-101(Cr) [cm3/mol] |
| Vp0 | The molar volume of mesocages accessible to the salt in pristine MIL-101(Cr) [cm3/mol] |
| Vc | The volume of the framework walls of MIL-101(Cr) [cm3/mol] per mole of the composite |
| Vsp | The volume of microporous cages (small pores) in MIL-101(Cr), which are inaccessible to the salt, per mole of the composite [cm3/mol] |
| Vp | The volume of mesocages in MIL-101(Cr) accessible to the salt in composite per mole of the composite [cm3/mol] |
| V1p | The volume of the salt located inside the (accessible) pores of MIL-101(Cr) per mole of the composite [cm3/mol] |
| V1b | The volume of the salt located in the bulk state outside the pores of MIL-101(Cr) per mole of the composite [cm3/mol] |
| Vp′ | The volume of mesocages filled with the salt per mole of the composite, [cm3/mol] |
| Vp″ | The volume of empty mesocages per mole of the composite [cm3/mol] |
| Vtot | The total molar volume of pores in MIL-101(Cr) [cm3/mol] |
| Vpore | The total volume of pores in MIL-101(Cr) [cm3/g] |
| xmax | The molar fraction of MIL-101(Cr) at which the volume of the ionic salt located in the pores reaches a maximum value corresponding to the volume of accessible pores |
| ρ | The density of the composite [g/cm3] |
| σ, A, Ea | The conductivity of the composite [S/cm], the pre-exponential factor [S·K/cm] and the activation energy [kJ/mol] for conductivity of composite, respectively. |
| σ1, σ2 | Conductivities of the components 1 and 2 in Equation (15); conductivities [S/cm] of pure LiClO4 and MIL-101(Cr) in Equations (18) and (21), respectively. |
| f | The volume fraction of the component 2 in the mixing Equation (15) |
| σ1p | Conductivity of LiClO4 located in the pores of MIL-101(Cr) [S/cm]. |
| α1, α2 | Parameters of the mixing equations, Equations (15) and (18); −1 ≤ α1, α2 ≤ 1 |
| φ1, φ2 | Relative volume fractions of the components used in Equations (16)–(18) |
| ϕ1, ϕ2 β1, β2 | Relative volume fractions of the components used in Equations (19)–(21) Parameters of the mixing equation, Equation (21); −1 ≤ β1, β2 ≤ 1 |
| Vc* | The parameter of Equations (19) and (20) defined by a relation: Vc* = V10·(Vc0 + Vsp0)/Vp0 |
References
- Thangadurai, V.; Weppner, W. Recent progress in solid oxide and lithium ion conducting electrolytes research. Ionics 2006, 12, 81–92. [Google Scholar] [CrossRef]
- Minami, T.; Hayashi, A.; Tatsumisago, M. Recent progress of glass and glass-ceramics as solid electrolytes for lithium secondary batteries. Solid State Ion. 2006, 177, 2715–2720. [Google Scholar] [CrossRef]
- Knauth, P. Inorganic solid Li ion conductors: An overview. Solid State Ion. 2009, 180, 911–916. [Google Scholar] [CrossRef]
- Zhang, X.; Fergus, J.W. Solid Electrolytes for Lithium Batteries. Int. J. Technol. 2018, 6, 1178–1186. [Google Scholar] [CrossRef]
- Dirican, M.; Yan, C.; Zhu, P.; Zhang, X. Composite solid electrolytes for all-solid-state lithium batteries. Mater. Sci. Eng. R 2019, 136, 27–46. [Google Scholar] [CrossRef]
- Uvarov, N.F.; Ulihin, A.S.; Mateyshina, Y.G. Advanced Nanomaterials for Catalysis and Energy. Sadykov, V.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 11; pp. 393–434. ISBN 978-0-12-814807-5. [Google Scholar]
- Maier, J. Ionic conduction in space charge regions. Prog. Solid State Chem. 1995, 23, 171–263. [Google Scholar] [CrossRef]
- Uvarov, N.F.; Vanek, P. Stabilization of New Phases in Ion-Conducting Nanocomposites. J. Mater. Synth. Process. 2000, 8, 319–326. [Google Scholar] [CrossRef]
- Uvarov, N.F. Composite solid electrolytes: Recent advances and design strategies. J. Solid State Electrochem. 2011, 15, 367–389. [Google Scholar] [CrossRef]
- Ulihin, A.S.; Uvarov, N.F.; Mateyshina, Y.G.; Brezhneva, L.I.; Matvienko, A.A. Composite solid electrolytes LiClO4–Al2O3. Solid State Ion. 2006, 177, 2787–2790. [Google Scholar] [CrossRef]
- Ulihin, A.S.; Uvarov, N.F. Electrochemical Properties of Composite Solid Electrolytes LiClO4–MgO. ECS Trans. 2009, 16, 445–450. [Google Scholar] [CrossRef]
- Ulihin, A.S.; Slobodyuk, A.B.; Uvarov, N.F.; Kharlamova, O.A.; Isupov, V.P.; Kavun, V.Y. Conductivity and NMR study of composite solid electrolytes based on lithium perchlorate. Solid State Ion. 2008, 179, 1740–1744. [Google Scholar] [CrossRef]
- Vinod, M.P.; Bahnemann, D. Materials for all-solid-state thin-film rechargeable lithium batteries by sol-gel processing. J. Solid State Electrochem. 2002, 6, 498–501. [Google Scholar] [CrossRef]
- Uvarov, N.F.; Bokhonov, B.B.; Ulihin, A.S.; Sharafutdinov, M.R.; Kirik, S.D. Composite Solid Electrolytes with Mesoporous Oxide Additives. ECS Trans. 2010, 25, 35–40. [Google Scholar] [CrossRef]
- Rowsell, J.L.; Yaghi, O.M. Metal–organic frameworks: A new class of porous materials. Microporous Mesoporous Mater. 2004, 73, 3–14. [Google Scholar] [CrossRef]
- Maksimchuk, N.V.; Zalomaeva, O.V.; Skobelev, I.Y.; Kovalenko, K.A.; Fedin, V.P.; Kholdeeva, O.A. Metal–organic frameworks of the MIL-101 family as heterogeneous single-site catalysts. Proc. R. Soc. A 2012, 468, 2017–2034. [Google Scholar] [CrossRef]
- Stock, N.; Biswas, S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef]
- Nandasiri, M.I.; Jambovane, S.R.; McGrail, B.P.; Schaef, H.T.; Nune, S.K. Adsorption, separation, and catalytic properties of densified metal-organic frameworks. Coord. Chem. Rev. 2016, 311, 38–52. [Google Scholar] [CrossRef]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; et al. Stable Metal–Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30, e1704303. [Google Scholar] [CrossRef]
- Ponomareva, V.G.; Kovalenko, K.A.; Chupakhin, A.P.; Dybtsev, D.N.; Shutova, E.S.; Fedin, V.P. Imparting High Proton Conductivity to a Metal–Organic Framework Material by Controlled Acid Impregnation. J. Am. Chem. Soc. 2012, 134, 15640–15643. [Google Scholar] [CrossRef]
- Ulihin, A.; Ponomareva, V.; Uvarov, N.; Kovalenko, K.; Fedin, V. Enhanced lithium ionic conductivity of lithium perchlorate in the metal-organic framework matrix. Ionics 2020, 26, 6167–6173. [Google Scholar] [CrossRef]
- Wang, M.; Pan, N. Predictions of effective physical properties of complex multiphase materials. Mater. Sci. Eng. R 2008, 63, 1–30. [Google Scholar] [CrossRef]
- Palla, P.L.; Giordano, S. Transport properties of multigrained nanocomposites with imperfect interfaces. J. Appl. Phys. 2016, 120, 184301. [Google Scholar] [CrossRef]
- Mikrajuddin; Shi, F.G.; Okuyama, K. Electrical Conduction in Insulator Particle—Solid-State Ionic and Conducting Particle-Insulator Matrix Composites A Unified Theory. J. Electrochem. Soc. 2000, 147, 3157–3165. [Google Scholar] [CrossRef]
- Hasyim, M.R.; Lanagan, M.T. A new percolation model for composite solid electrolytes and dispersed ionic conductors. Model. Simul. Mater. Sci. Eng. 2018, 26, 025011. [Google Scholar] [CrossRef]
- Zaman, W.; Hortance, N.; Dixit, M.B.; De Andradec, V.; Hatzell, K.B. Visualizing percolation and ion transport in hybrid solid electrolytes for Li–metal batteries. J. Mater. Chem. A 2019, 7, 23914–23921. [Google Scholar] [CrossRef]
- Uvarov, N.F. Estimation of composites conductivity using a general mixing rule. Solid State Ion. 2000, 136–137, 1267–1272. [Google Scholar] [CrossRef]
- Uvarov, N.F. Estimation of electrical properties of composite solid electrolytes of different morphologies. Solid State Ion. 2017, 302, 19–24. [Google Scholar] [CrossRef]
- Ferey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surblé, S.; Margiolaki, I. A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef]
- Available online: http://www.chemspider.com/Chemical-Structure.133514.html (accessed on 19 September 2022).
- Ivlev, S.I.; Akimov, D.V.; Egorov, N.B.; Kraus, F. Synthesis and characterization of LiClO4·H2O. Monatsh. Chem. 2016, 147, 279–288. [Google Scholar] [CrossRef]
- Permyakova, A.; Wang, S.; Courbon, E.; Nouar, F.; Heymans, N.; D’Ans, P.; Barrier, N.; Billemont, P.; De Weireld, G.; Steunou, N.; et al. Design of salt–metal organic framework composites for seasonal heat storage applications. J. Mater. Chem. A 2017, 5, 12889–12898. [Google Scholar] [CrossRef]
- Tan, B.; Luo, Y.; Liang, X.; Wang, S.; Gao, X.; Zhang, Z.; Fan, Y. Composite salt in MIL-101(Cr) with high water uptake and fast adsorption kinetics for adsorption heat pumps. Microporous Mesoporous Mater. 2019, 286, 141–148. [Google Scholar] [CrossRef]
- Ponomarenko, I.V.; Glaznev, I.S.; Gubar, A.V.; Aristov, Y.I.; Kirik, S.D. Synthesis and water sorption properties of a new composite “CaCl2 confined into SBA-15 pores”. Microporous Mesoporous Mater. 2010, 129, 243–250. [Google Scholar] [CrossRef]
- Lebedev, O.I.; Millange, F.; Serre, C.; Van Tendeloo, G.; Ferey, G. First Direct Imaging of Giant Pores of the Metal−Organic Framework MIL-101. Chem. Mater. 2005, 17, 6525–6527. [Google Scholar] [CrossRef]
- Li, X.; Feng, Y.; Li, M.; Li, W.; Wei, H.; Song, D. Smart Hybrids of Zn2GeO4 Nanoparticles and Ultrathin g-C3N4 Layers: Synergistic Lithium Storage and Excellent Electrochemical Performance. Adv. Funct. Mater. 2015, 25, 6858–6866. [Google Scholar] [CrossRef]
- De Guzman, R.C.; Yang, J.; Cheng, M.M.C.; Salley, S.O.; Simon Ng, K.Y. High capacity silicon nitride-based composite anodes for lithium ion batteries. J. Mater. Chem. A 2014, 2, 14577–14584. [Google Scholar] [CrossRef]
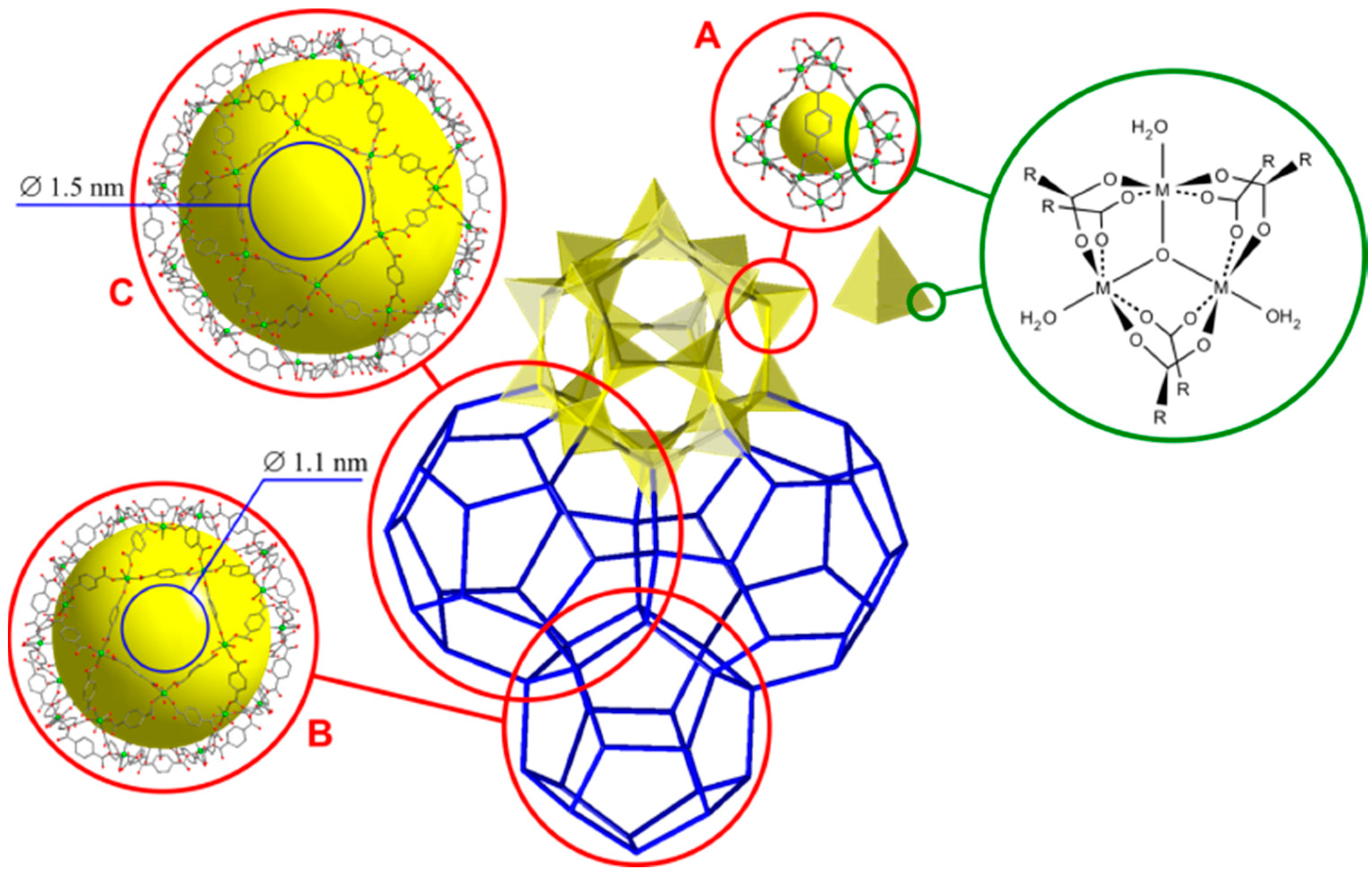
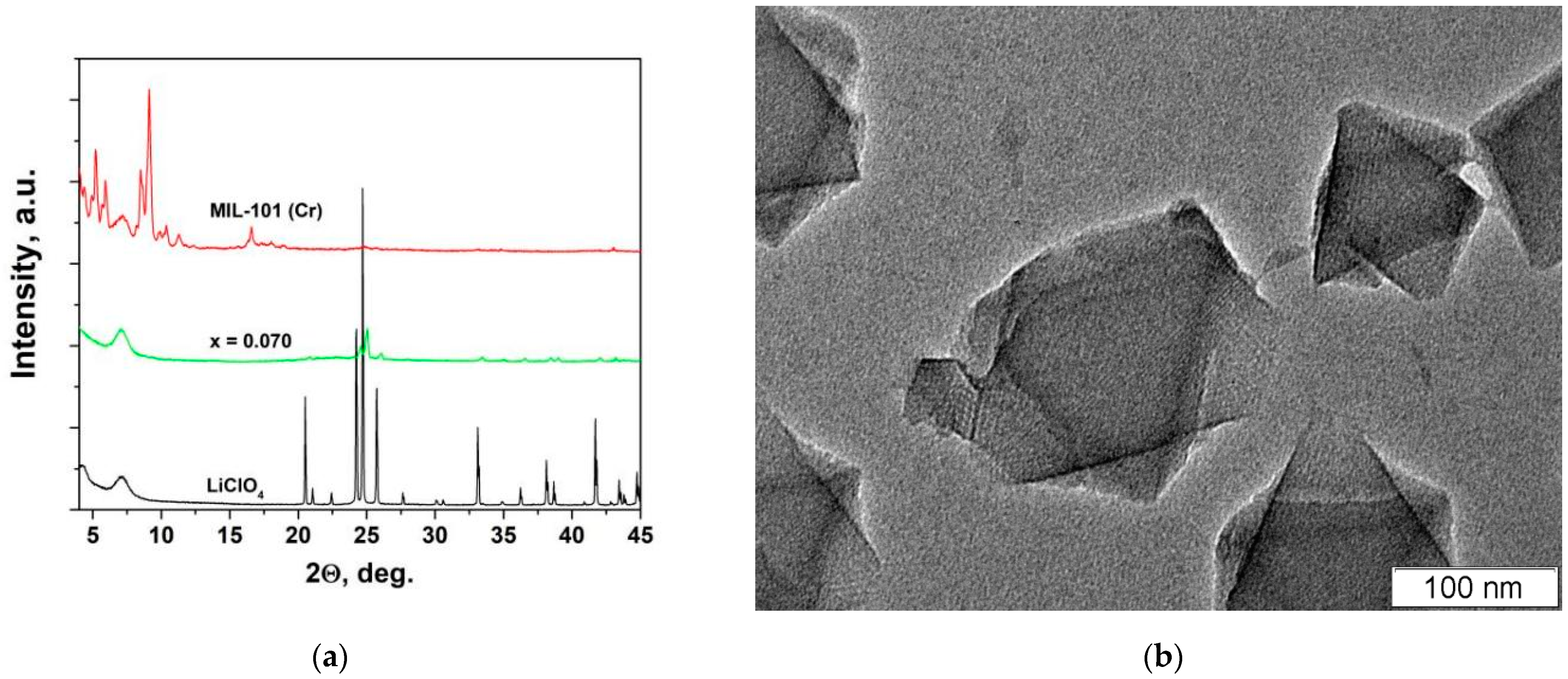

| Composition | Specific Surface Area/m2·g−1 | Vpore/cm3·g−1 | Vads(N2) */cm3·g−1 | ||
|---|---|---|---|---|---|
| BET | DFT | Total * | DFT | ||
| 0.992LiClO4–0.008MIL-101(Cr) | 39 | 25.6 | 0.028 | 0.028 | 18.1 |
| 0.983LiClO4–0.017MIL-101(Cr) | 76 | 56.7 | 0.056 | 0.054 | 36.2 |
| 0.950LiClO4–0.050MIL-101(Cr) | 413 | 265.2 | 0.212 | 0.205 | 137.3 |
| 0.887LiClO4–0.113MIL-101(Cr) | 888 | 546.1 | 0.423 | 0.399 | 273.6 |
| MIL-101(Cr) | 2200 | 1776 | 1.34 | 1.26 | 889 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uvarov, N.; Ulihin, A.; Ponomareva, V.; Kovalenko, K.; Fedin, V. Effect of Pore Filling on Properties of Nanocomposites LiClO4–MIL–101(Cr) with High Ionic Conductivity. Nanomaterials 2022, 12, 3263. https://doi.org/10.3390/nano12193263
Uvarov N, Ulihin A, Ponomareva V, Kovalenko K, Fedin V. Effect of Pore Filling on Properties of Nanocomposites LiClO4–MIL–101(Cr) with High Ionic Conductivity. Nanomaterials. 2022; 12(19):3263. https://doi.org/10.3390/nano12193263
Chicago/Turabian StyleUvarov, Nikolai, Artem Ulihin, Valentina Ponomareva, Konstantin Kovalenko, and Vladimir Fedin. 2022. "Effect of Pore Filling on Properties of Nanocomposites LiClO4–MIL–101(Cr) with High Ionic Conductivity" Nanomaterials 12, no. 19: 3263. https://doi.org/10.3390/nano12193263
APA StyleUvarov, N., Ulihin, A., Ponomareva, V., Kovalenko, K., & Fedin, V. (2022). Effect of Pore Filling on Properties of Nanocomposites LiClO4–MIL–101(Cr) with High Ionic Conductivity. Nanomaterials, 12(19), 3263. https://doi.org/10.3390/nano12193263









