Design and Application of Electrochemical Sensors with Metal–Organic Frameworks as the Electrode Materials or Signal Tags
Abstract
:1. Introduction
2. MOFs as the Electrode Materials of Electrochemical Sensors
2.1. Pristine MOFs
| Electrode Material | Analyte | Linear Range | LOD | Ref. |
|---|---|---|---|---|
| Zn4O(BDC)3 (MOF-5) | Pb2+ | 10 nM~1.0 μM | 4.9 nM | [33] |
| TMU-16-NH2(Zn) | Cd2+ | 62.5 nM~1.1 μM | 1.8 nM | [34] |
| [H2N(CH3)2]4[Zn3(Hdpa)2]•4DMF | Cu2+ | 5.0 pM~900 nM | 1.0 pM | [35] |
| Co−MOFs | glucose | 1.0 μM~3.0 mM | 1.3 nM | [37] |
| Cu−MOFs | BPA | 50 nM~3.0 μM | 13 nM | [38] |
| Cu−BTC | methocarbamol | 80 μM~800 μM | 50 nM | [39] |
| Cr−MOFs | H2O2 | 25 μM~500 μM | 3.52 μM | [40] |
| Cu−BTC | DA and UA | 50 nM~500 μM and 0.5 μM~600 μM | 30 and 200 nM | [45] |
| Cu3(BTC)2 | 2,4-dichlorophenol | 40 nM~1.0 μM | 9.0 nM | [54] |
| Co−MOFs | H2O2 | 5.0 μM~9.0 mM | 3.76 μM | [47] |
| Ni−MOFs | Pb2+ | 0.5 μM~6.0 μM | 0.508 μM | [57] |
| MIL−101(Cr) | DA and UA | 5.0~250 μM and 30~200 μM | Not reported | [46] |
| Co–Ni−MOFs | Trp | 10 nM~300 μM | 8.7 nM | [65] |
| Ni−MOFs | AA | 0.5 μM~8.1 μM | 0.25 μM | [66] |
| (Co−TCPP(Fe))5 | H2O2 | 0.4 μM~50 μM | 0.15 μM | [67] |
| MOF−52(Zr) | NO3− | 20 μM~800 μM | 2.1 μM | [76] |
| PcFe@ZIF-8 | TCAA | 20 nM~1.0 μM | 1.89 nM | [68] |
2.2. Carbon Materials-Modified MOFs
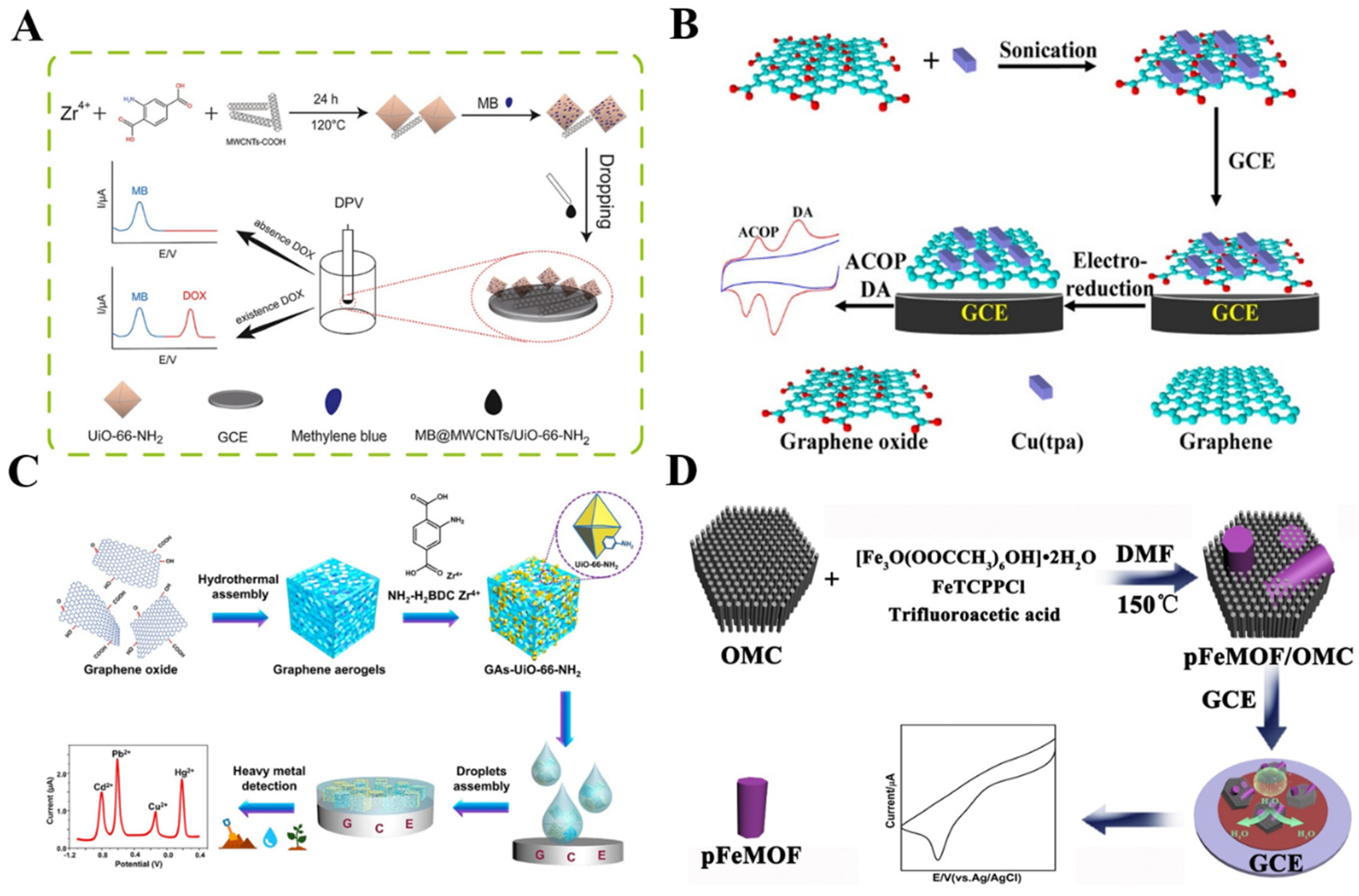
2.3. Noble Metal Nanomaterials
| Electrode Material | Analyte | Linear Range | LOD | Ref. |
|---|---|---|---|---|
| Pt@UiO-66 | H2O2 | 5.0 μM~14.75 mM | 3.06 μM | [115] |
| Cu−MOF/Au NPs | NO3− | 0.1 μM~4.0 mM | 8.2 nM | [116] |
| AuNPs@Cu−MOF | BPA | 0.2~1.0 mM | 37.8 μM | [121] |
| Cu−MOF/ZnTe NRs/Au NPs | catechol | 250 nM~0.3 mM | 16 nM | [122] |
| AuNPs/MMPF-6(Fe) | hydroxylamine | 0.01~1.0 μM and 1.0~20 μM | 4.0 nM | [123] |
| AgNPs@ZIF-67 | H2O2 | 5.0 μM~275 μM | 1.5 μM | [126] |
| AgNPs/MIL-101(Fe) | Trp | 1.0~50 μM | 0.14 μM | [130] |
| Ag/Cu−TCPP | GSH | 1.0~100 μM | 66 nM | [131] |
| ZIF-67/Ag NPs/PDA | Cl− | 2.0~400 mM | 1.0 mM | [132] |
| Pt@PMOF(Fe) | H2O2 | 0~10 mM | 6.0 μM | [124] |
2.4. Conductive Polymers
3. MOFs as Supporting Platforms
| Type of MOFs | Analyte | Linear Range | LOD | Ref. |
|---|---|---|---|---|
| Ag@Zn−TSA | H2O2 | 0.3 μM~20000 μM | 0.08 μM | [148] |
| ZIF-8@GOx | miRNA-21 | 0.1 nM~10 μM | 29 pM | [149] |
| Cu−Hemin−GOx | Glucose | 10~1555 μM | 2.2 μM | [158] |
| PDA/ZIF-8@rGO | Glucose | 1.2 μM~1.2 mM | 0.333 μM | [159] |
| GDH/MG−Tb@MOF−CNTs | Glucose | 25 μM~17 mM | 8 μM | [161] |
| MG−ZIFs−GDH | Glucose | 0.1~2 mM | Not reported | [162] |
| GOx/Hemin@NC−ZIF | Glucose | 1~20 mM | 10 μM | [164] |
| HRP/ZIF-67(Co)/MWCNT | H2O2 | 1.86~1050 μM | 0.11 μM | [165] |
| HRP@PCN-333(Fe) | H2O2 | 0.5 μM~1.5 mM | 0.09 μM | [166] |
| ZIFs@HRP/GO | H2O2 | 20 μM~6 mM | 3.4 μM | [167] |
| MP-11-PCN-333(Al)−GO | H2O2 | 10~800 μM | 3 μM | [168] |
| MP-11-PCN-333(Al)- 3D-KSC | H2O2 | 0.387 μM~1.725 mM | 0.127 μM | [169] |
| Cyt c@ZIF-8 | H2O2 | 290 μM~3.6 mM | Not reported | [170] |
| Tyr@NiZn−MOF NSs | Phenol | 0.08 μM~58.2 μM | 6.5 nM | [171] |
| Tyr@Cu−MOFs | BPA | 50 nM~3.0 μM | 13 nM | [172] |
| Cu−BTABB−MOF@rGO | BPA | 0~100 μM | 0.208 μM | [173] |
| Tyr@Cu–TCPP | BPA | 3.5 nM~18.9 μM | 1.2 nM | [174] |
| AuPd NPs@UiO-66-NH2/CoSe2 | Sulfaquinoxaline | 1 pg/mL~100 ng/mL | 0.547 pg/mL | [175] |
| Zn−MOF-on-Zr−MOF | PTK7 | 1 pg/mL~1.0 ng/mL | 0.84 pg/mL | [177] |
| Fe3O4@TMU-21 | HER2 | 1 pg/mL~100 ng/mL | 0.3 pg/mL | [178] |
| MOF-808/CNTs | CA 125 | 0.001~0.1 and 0.1~30 ng/mL | 0.5 pg/mL | [181] |
| AuNPs@ZIF-8 | AFP | 0.1 pg/mL~100 ng/mL | 0.033 pg/mL | [182] |
| MTV polyMOF(Ti) | ZEN | 10 fg/mL~10 ng/mL | 8.9 fg/mL | [183] |
| 493-MOF | Lysozyme | 5 pg/mL~1 ng/mL | 3.6 pg/mL | [184] |
| 2D AuNCs@521−MOF | Cocaine | 1 pg/mL~1 ng/mL | 0.44 pg/mL | [185] |
| Cu−MOFs | S. aureus | 7 − 7 × 106 cfu/mL | 1.9 cfu/mL | [187] |
| Cu−MOFs | HBsAg | 1~500 ng/mL | 730 pg/mL | [188] |
| Cu−MOF/COOH−GO | Methyl jasmonate | 10 pM~100 μM | 0.35 pM | [189] |
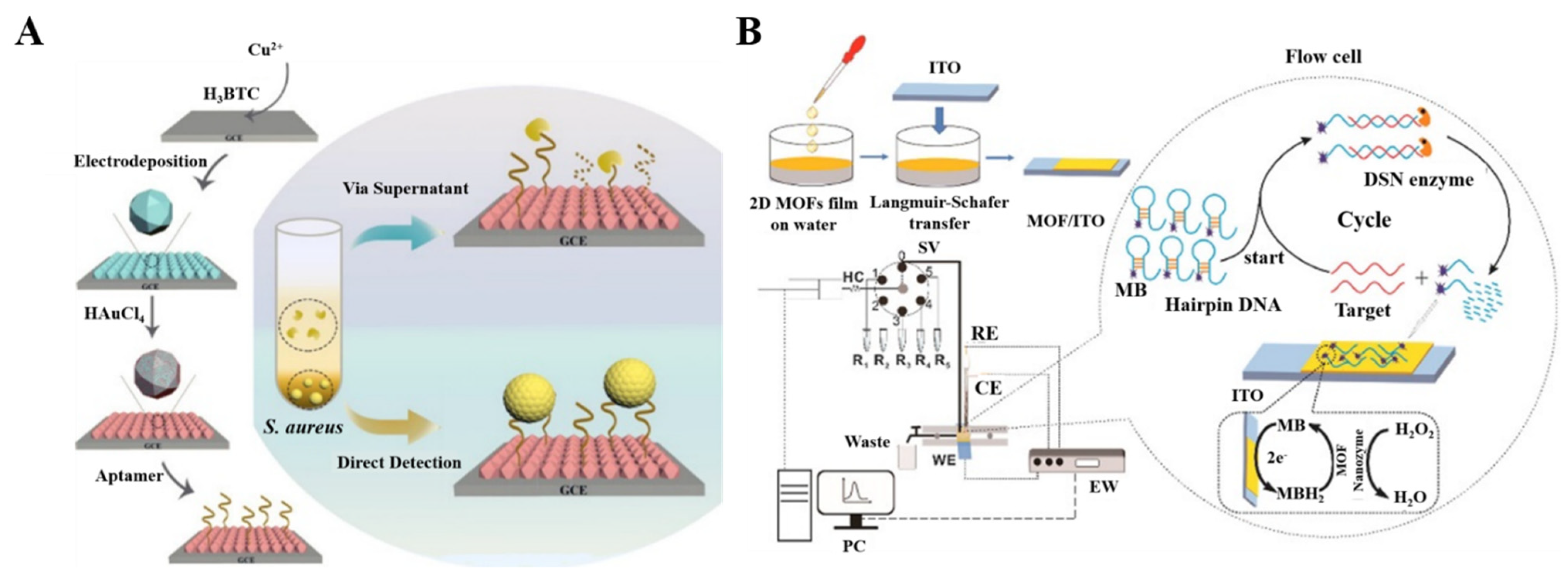
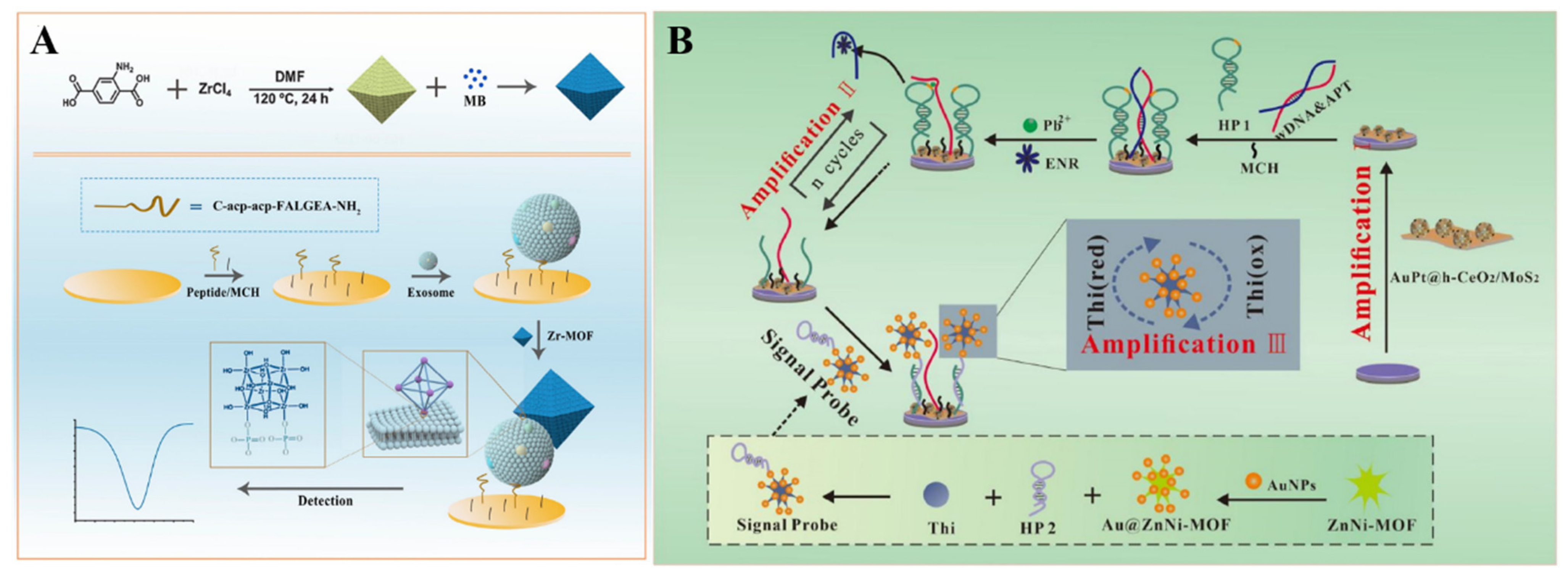
4. MOFs as Signal Labels
4.1. Nanocarriers
| Type of MOFs | Analyte | Linear Range | LOD | Ref. |
|---|---|---|---|---|
| Zn−MOF/Fe3O4–COOH/Thi | CTnI | 0.04~50 ng/mL | 0.9 pg/mL | [192] |
| MB@Zr−MOFs | patulin | 50 fg/mL~5 μg/mL | 14.6 fg/mL | [193] |
| MB@MIL-101-NH2(Cr) | p53 gene | 10 fM~100 nM | 1.4 fM | [194] |
| MB@Zr−MOFs | N6-methyladenine | 1 fM~1 nM | 0.89 fM | [195] |
| UiO-66-TB | procalcitonin | 1 pg/mL~100 ng/mL | 0.3 pg/mL | [196] |
| GDH@ZIF-8/[Fe(CN)6]3−/UiO-66 | exosome | 1.0 × 103~1.0 × 108 particles/mL | 300 particles/mL | [197] |
| Fc−Zn−MOF | amyloid−β | 0.1 pg/mL~100 ng/mL | 0.03 pg/mL | [198] |
| Cu−MOFs−TB | CRP | 0.5~200 ng/mL | 166.7 pg/mL | [199] |
| MB@Zr−MOFs | exosome | 9.5 × 103~1.9 × 107 particles/μL | 7.83 × 103 particles/μL | [200] |
| Au@ZnNi−MOF | enrofloxacin | 5 fg/mL~10 pg/mL | 0.102 fg/mL | [201] |
| Cu−TCPP–TB and PB | CEA and CA125 | 0.1~160 ng/mL and 0.5~200 U/mL | 0.03 ng/mL and 0.05 U/mL | [202] |
| HP−UIO-66-MB and Fc | KANA and CAP | 0.1 pM~50 nM | 35 fM and 21 fM | [203] |
| UiO-66-NH2-MB and TMB | let-7a and miRNA-21 | 0.01~10 and 0.02~10 pM | 3.6 fM and 8.2 fM | [204] |
| UiO-66-NH2-Cd2+ and Pb2+ | TRS and THD | 0.2~750 ng/mL | 0.07 and 0.1 ng/mL | [205] |
| hemin-MOFs/PtNPs | FGFR3 gene mutation | 0.1 fM~1 nM | 0.033 fM | [209] |
| FeTCPP@MOF-SA | DNA | 10 fM~10 nM | 0.48 fM | [210] |
| MB−GOx−ZIF-8/Au−rGO | CA 242 | 0.001~1000 U/mL | 69.34 μU/mL | [212] |
| HRP/hemin/G-quadruplex Au@Pt/MIL-53 (Al) | nucleocapsid protein | 0.025~50 ng/mL | 8.33 pg/mL | [213] |
| HRP/hemin/Gquadruplex PtNPs/PCN-224 | Cancer cells | 20~1×107 cells/mL | 6 cells/mL | [214] |
| HRP/Ab@ZIF-L | ZEN | 0.5 ng/L~0.476 μg/L | 0.5 ng/L | [215] |
| GOx/HRP/ZIF-90 | CA-125 | 0.1 pg/L~40 μg/L | 0.05 pg/mL | [216] |
| CdS@ZIF-8 | Escherichia coli O157:H7 | 10~108 CFU/mL | 3 CFU/mL | [222] |
| AgNPs/PCN-224 | telomerase activity | 1 × 10−7~1 × 10−1 IU/L | 5.4 × 10−8 IU/L | [223] |
| Cu2O@Cu−MOF@AuNPs | CEA | 50 fg/mL~80 ng/mL | 17 fg/mL | [224] |
| AgPt/PCN-223-Fe | ochratoxin A | 20 fg/mL~2 ng/mL | 14 fg/mL | [225] |
| Pd/MIL101-NH2 | telomerase activity | 5 × 102~1.62 × 107 HeLa cells/mL | 11.25 HeLa cells/mL | [226] |
| Pd@UiO-66 | miRNA-21 | 20 fM~600 pM | 0.713 fM | [227] |
| PdNPs@Fe−MOFs | miRNA-122 | 0.01 fM~10 pM | 0.003 fM | [228] |
| Pd@PCN-222 | ochratoxin A | 10 fg/mL~10 ng/mL | 6.79 fg/mL | [229] |
| Pt@UiO-66-NH2 | telomerase activity | 5 × 102~1 × 107 HeLa cells/mL | 2.0 × 10−11 IU/L | [230] |
| Fe−MOFs/PdPt NPs | Pb2+ | 5 pM~1 μM | 2 pM | [231] |

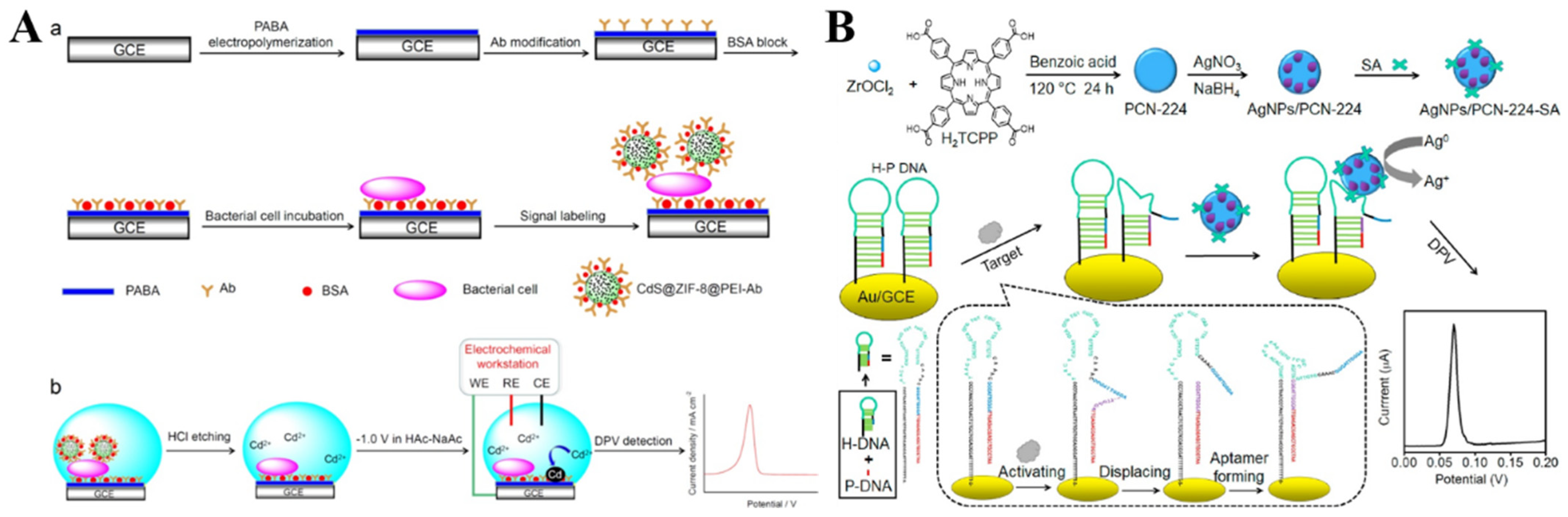
4.2. Electroactive Labels
| Type of MOFs | Analyte | Linear Range | LOD | Ref. |
|---|---|---|---|---|
| Cu−BTC MOFs | lipopolysaccharide | 1.0 pg/mL~1.0 ng/mL | 0.29 pg/mL | [232] |
| Ag−MOFs | CEA | 0.05~120 ng/mL | 8 fg/mL | [233] |
| PtPd NPs/Co−MOFs | thrombin | 1 pM~30 nM | 0.32 pM | [234] |
| AuNPs/Cu−MOFs | miRNA-155 | 1.0 fM~10 nM | 0.35 fM | [235] |
| ZIF-67/ZIF-8 | PSA | 5 pg/mL~50 ng/mL | 0.78 pg/mL | [236] |
| Cd−MOFs | ochratoxin A | 0.05~100 ng/mL | 10 pg/mL | [237] |
| Cd−MOFs-74 | p53 gene | 0.01~30 pM | 6.3 fM | [238] |
| Cu−MOFs@PtPd NPs | Hg2+ | 0.001~100 nM | 0.52 pM | [239] |
| Co−MOFs@AuNPs | Mucin 1 | 0.004~400 pM | 1.34 fM | [240] |
| Cu−MOFs@AuNPs | CRP | 1~400 ng/mL | 0.2 ng/mL | [241] |
| PtNPs@Cu−MOF | Pb2+ | 3.0 pM~5 μM | 0.2 pM | [242] |
| Invertase/Cu−MOF | DNA methyltransferase activity | 0.002~1 U/mL | 0.001 U/mL | [243] |
| MIL-101(Fe) | telomerase activity | 1 × 10−6~5 × 10−2 IU/L | 1.8 × 10−7 IU/L | [244] |
| UiO-66-NH2 | Mucin 1 | 5 pg/mL~ 50 ng/mL | 0.72 pg/mL | [245] |
| Fe−MOFs/MB−GA−UiO-66-NH2 | miRNA | 1 fM~100 nM | 50 aM | [246] |
| Fe−MOFs@AuNPs | thrombin | 0.298~29.8 pM | 59.6 fM | [247] |
4.3. Electrocatalysts
| Type of MOFs | Analytes | Linear Ranges | LOD | Ref. |
|---|---|---|---|---|
| Cu2+−NMOFs | lipopolysaccharide | 0.0015~750 ng/mL | 0.61 pg/mL | [248] |
| CuMOF | hantavirus | 1 fM~1 nM | 0.74 fM | [249] |
| Cu−MOFs@GOx | CA15-3 | 10 μU/mL~10 mU/mL | 5.06 μU/mL | [250] |
| pSC4−AuNPs/Cu−MOFs | Fractalkine | 10 pg/mL~10 μg/mL | 7.4 pg/mL | [251] |
| AuNPs/Ce−MOFs | lipopolysaccharide | 10 fg/mL~100 ng/mL | 3.3 fg/mL | [252] |
| Thi/AuNPs/Ce(III, IV)−MOF | thrombin | 0.1 fM~10 nM | 0.06 fM | [253] |
| AuNPs/Ce−MOF | telomerase activity | 2 × 102~2 × 106 cells/mL | 27 cells/mL | [254] |
| PorMOF@SA | telomerase activity | 1 × 102~1 × 107 cells/mL | 30 cells/mL | [255] |
| PCN-222@SA | DNA | 10 fM~100 nM | 0.29 fM | [256] |
| L/(Fe-P)n-MOF | T4 polynucleotide kinase | 1.0 mU/mL~1.0 U/mL | 0.62 mU/mL | [257] |
| GR−5/(Fe−P)n−MOF | Pb2+ | 0.05~200 nM | 0.034 nM | [260] |
4.4. Sacrificial Templates
5. Conclusions and Future Aspects
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, L.; Ying, Y.; Li, Y.; Fu, Y. Integration and synergy in protein-nanomaterial hybrids for biosensing: Strategies and in-field detection applications. Biosens. Bioelectron. 2020, 154, 112036–112045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Jiang, C.; Lan, L.; Ping, J.; Ye, Z.; Ying, Y. Nanomaterial-based biosensors for agro-product safety. TrAC Trends Anal. Chem. 2021, 143, 116369–116381. [Google Scholar] [CrossRef]
- Kaur, H.; Shorie, M. Nanomaterial based aptasensors for clinical and environmental diagnostic applications. Nanoscale Adv. 2019, 1, 2123–2138. [Google Scholar] [CrossRef]
- Zhao, Q.; Lu, D.; Zhang, G.; Zhang, D.; Shi, X. Recent improvements in enzyme-linked immunosorbent assays based on nanomaterials. Talanta 2021, 223, 121722–121737. [Google Scholar] [CrossRef]
- Abolhasan, R.; Mehdizadeh, A.; Rashidi, M.R.; Aghebati-Maleki, L.; Yousefi, M. Application of hairpin DNA-based biosensors with various signal amplification strategies in clinical diagnosis. Biosens. Bioelectron. 2019, 129, 164–174. [Google Scholar] [CrossRef]
- Chai, H.; Cheng, W.; Jin, D.; Miao, P. Recent progress in DNA hybridization chain reaction strategies for amplified biosensing. ACS Appl. Mater. Interfaces 2021, 13, 38931–38946. [Google Scholar] [CrossRef]
- Ju, H. Functional nanomaterials and nanoprobes for amplified biosensing. Appl. Mater. Today 2018, 10, 51–71. [Google Scholar] [CrossRef]
- Olorunyomi, J.F.; Geh, S.T.; Caruso, R.A.; Doherty, C.M. Metal-organic frameworks for chemical sensing devices. Mater. Horiz. 2021, 8, 2387–2419. [Google Scholar] [CrossRef]
- Yuan, R.; Li, H.K.; He, H. Recent advances in metal/covalent organic framework-based electrochemical aptasensors for biosensing applications. Dalton. Trans. 2021, 50, 14091–14104. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, W.; Yin, Y.; Fang, W.; Xue, H. Metal-organic framework-based sensors for the detection of toxins and foodborne pathogens. Food Control 2022, 133, 108684–108696. [Google Scholar] [CrossRef]
- Cai, W.; Liu, X.; Wang, L.; Wang, B. Design and synthesis of noble metal–based electrocatalysts using metal–organic frameworks and derivatives. Mater. Today Nano 2022, 17, 100144–100164. [Google Scholar] [CrossRef]
- Wu, H.B.; Lou, X.W. Metal-organic frameworks and their derived materials for electrochemical energy storage and conversion: Promises and challenges. Sci. Adv. 2017, 3, 9252–9268. [Google Scholar] [CrossRef] [PubMed]
- Al Sharabati, M.; Sabouni, R.; Husseini, G.A. Biomedical applications of metal-organic frameworks for disease diagnosis and drug delivery: A review. Nanomaterials 2022, 12, 277. [Google Scholar] [CrossRef]
- Yi, F.Y.; Chen, D.X.; Wu, M.K.; Han, L.; Jiang, H.L. Chemical sensors based on metal-organic frameworks. ChemPlusChem 2016, 81, 675–690. [Google Scholar] [CrossRef]
- Pandey, M.D. Luminescent metal–organic frameworks as biosensors. Mater. Lett. 2022, 308, 131230. [Google Scholar] [CrossRef]
- Marimuthu, M.; Arumugam, S.S.; Jiao, T.; Sabarinathan, D.; Li, H.; Chen, Q. Metal organic framework based sensors for the detection of food contaminants. TrAC Trends Anal. Chem. 2022, 154, 116642–116666. [Google Scholar] [CrossRef]
- Kempahanumakkagari, S.; Vellingiri, K.; Deep, A.; Kwon, E.E.; Bolan, N.; Kim, K.H. Metal-organic framework composites as electrocatalysts for electrochemical sensing applications. Coordin. Chem. Rev. 2018, 357, 105–129. [Google Scholar] [CrossRef]
- Carrasco, S. Metal-organic frameworks for the development of biosensors: A current overview. Biosensors 2018, 8, 92. [Google Scholar] [CrossRef]
- Lv, M.; Zhou, W.; Tavakoli, H.; Bautista, C.; Xia, J.; Wang, Z.; Li, X. Aptamer-functionalized metal-organic frameworks (MOFs) for biosensing. Biosens. Bioelectron. 2021, 176, 112947–112963. [Google Scholar] [CrossRef]
- Karimzadeh, Z.; Mahmoudpour, M.; Guardia, M.d.l.; Ezzati Nazhad Dolatabadi, J.; Jouyban, A. Aptamer-functionalized metal organic frameworks as an emerging nanoprobe in the food safety field: Promising development opportunities and translational challenges. TrAC Trends Anal. Chem. 2022, 152, 116622–116641. [Google Scholar] [CrossRef]
- Zhang, X.L.; Li, G.L.; Wu, D.; Li, X.L.; Hu, N.; Chen, J.; Chen, G.; Wu, Y.N. Recent progress in the design fabrication of metal-organic frameworks-based nanozymes and their applications to sensing and cancer therapy. Biosens. Bioelectron. 2019, 137, 178–198. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, L.; Liu, D.; Ma, W.; Dramou, P.; He, H. Nanozymes based on metal-organic frameworks: Construction and prospects. TrAC Trends Anal. Chem. 2020, 133, 116080–116096. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, S.; Tang, Y.; Zhang, X.; Bai, Y.; Pang, H. Advances in metal–organic framework-based nanozymes and their applications. Coordin. Chem. Rev. 2021, 449, 214216–214239. [Google Scholar] [CrossRef]
- Mohan, B.; Kumar, S.; Xi, H.; Ma, S.; Tao, Z.; Xing, T.; You, H.; Zhang, Y.; Ren, P. Fabricated metal-organic frameworks (MOFs) as luminescent and electrochemical biosensors for cancer biomarkers detection. Biosens. Bioelectron. 2022, 197, 113738. [Google Scholar] [CrossRef] [PubMed]
- Afreen, S.; He, Z.; Xiao, Y.; Zhu, J.J. Nanoscale metal-organic frameworks in detecting cancer biomarkers. J. Mater. Chem. B 2020, 8, 1338–1349. [Google Scholar] [CrossRef]
- Anik, U.; Timur, S.; Dursun, Z. Metal organic frameworks in electrochemical and optical sensing platforms: A review. Microchim. Acta 2019, 186, 196–210. [Google Scholar] [CrossRef]
- Sahoo, J.; Krishnaraj, C.; Sun, J.; Bihari Panda, B.; Subramanian, P.S.; Sekhar Jena, H. Lanthanide based inorganic phosphates and biological nucleotides sensor. Coordin. Chem. Rev. 2022, 466, 214583. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.; He, Q.; Yan, J.; Xiong, H.; Wen, N.; Cai, S.; Peng, D.; Liu, Y.; Liu, Z. Metal-organic frameworks for virus detection. Biosens. Bioelectron. 2020, 169, 112604–112625. [Google Scholar] [CrossRef]
- Gorle, D.B.; Ponnada, S.; Kiai, M.S.; Nair, K.K.; Nowduri, A.; Swart, H.C.; Ang, E.H.; Nanda, K.K. Review on recent progress in metal-organic framework-based materials for fabricating electrochemical glucose sensors. J. Mater. Chem. B 2021, 9, 7927–7954. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Ying, Y. Recent advances in sensing applications of metal nanoparticle/metal–organic framework composites. TrAC Trends Anal. Chem. 2021, 143, 116395–116415. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Garkani Nejad, F.; Sheikhshoaie, I.; Nugraha, A.S.; Jang, H.W.; Yamauchi, Y.; Shokouhimehr, M. Performance of metal–organic frameworks in the electrochemical sensing of environmental pollutants. J. Mater. Chem. A 2021, 9, 8195–8220. [Google Scholar] [CrossRef]
- Liu, S.; Lai, C.; Liu, X.; Li, B.; Zhang, C.; Qin, L.; Huang, D.; Yi, H.; Zhang, M.; Li, L.; et al. Metal-organic frameworks and their derivatives as signal amplification elements for electrochemical sensing. Coordin. Chem. Rev. 2020, 424, 213520–213537. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.C.; Xie, J.; Hu, X.Y. Metal-organic framework modified carbon paste electrode for lead sensor. Sens. Actuators B Chem. 2013, 177, 1161–1166. [Google Scholar] [CrossRef]
- Roushani, M.; Valipour, A.; Saedi, Z. Electroanalytical sensing of Cd2+ based on metal-organic framework modified carbon paste electrode. Sens. Actuators B Chem. 2016, 233, 419–425. [Google Scholar] [CrossRef]
- Jin, J.C.; Wu, J.; Yang, G.P.; Wu, Y.L.; Wang, Y.Y. A microporous anionic metal-organic framework for a highly selective and sensitive electrochemical sensor of Cu2+ ions. Chem. Commun. 2016, 52, 8475–8478. [Google Scholar] [CrossRef]
- Kajal, N.; Singh, V.; Gupta, R.; Gautam, S. Metal organic frameworks for electrochemical sensor applications: A review. Environ. Res. 2022, 204, 112320–112338. [Google Scholar] [CrossRef]
- Li, Y.; Xie, M.; Zhang, X.; Liu, Q.; Lin, D.; Xu, C.; Xie, F.; Sun, X. Co-MOF nanosheet array: A high-performance electrochemical sensor for non-enzymatic glucose detection. Sens. Actuators B Chem. 2019, 278, 126–132. [Google Scholar] [CrossRef]
- Zhao, F.; Sun, T.; Geng, F.; Chen, P.; Gao, Y. Metal-organic frameworks-based electrochemical sensors and biosensors. Int. J. Electrochem. Sci. 2019, 14, 5287–5304. [Google Scholar] [CrossRef]
- Karimi-Harandi, M.-H.; Shabani-Nooshabadi, M.; Darabi, R. Cu-BTC metal-organic frameworks as catalytic modifier for ultrasensitive electrochemical determination of methocarbamol in the presence of methadone. J. Electrochem. Soc. 2021, 168, 097507–097516. [Google Scholar] [CrossRef]
- Lopa, N.S.; Rahman, M.M.; Ahmed, F.; Sutradhar, S.C.; Ryu, T.; Kim, W. A base-stable metal-organic framework for sensitive and non-enzymatic electrochemical detection of hydrogen peroxide. Electrochim. Acta 2018, 274, 49–56. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, X.; Bu, Y.; Zhang, J.; Zhang, R. Copper cobalt sulfide structures derived from MOF precursors with enhanced electrochemical glucose sensing properties. Nanomaterials 2022, 12, 1394. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.-L.; Gao, E.-Q. Metal–organic frameworks for electrochemical sensors of neurotransmitters. Coordin. Chem. Rev. 2021, 434, 213784–213795. [Google Scholar] [CrossRef]
- Adeel, M.; Asif, K.; Rahman, M.M.; Daniele, S.; Canzonieri, V.; Rizzolio, F. Glucose detection devices and methods based on metal–organic frameworks and related materials. Adv. Funct. Mater. 2021, 31, 2106023–2106050. [Google Scholar] [CrossRef]
- Daniel, M.; Mathew, G.; Anpo, M.; Neppolian, B. MOF based electrochemical sensors for the detection of physiologically relevant biomolecules: An overview. Coordin. Chem. Rev. 2022, 468, 214627–214661. [Google Scholar] [CrossRef]
- Azizpour Moallem, Q.; Beitollahi, H. Electrochemical sensor for simultaneous detection of dopamine and uric acid based on a carbon paste electrode modified with nanostructured Cu-based metal-organic frameworks. Microchem. J. 2022, 177, 107261–107267. [Google Scholar] [CrossRef]
- Li, Y.; Huangfu, C.; Du, H.; Liu, W.; Li, Y.; Ye, J. Electrochemical behavior of metal–organic framework MIL-101 modified carbon paste electrode: An excellent candidate for electroanalysis. J. Electroanal. Chem. 2013, 709, 65–69. [Google Scholar] [CrossRef]
- Yang, L.; Xu, C.; Ye, W.; Liu, W. An electrochemical sensor for H2O2 based on a new Co-metal-organic framework modified electrode. Sens. Actuators B Chem. 2015, 215, 489–496. [Google Scholar] [CrossRef]
- Sohrabi, H.; Salahshour Sani, P.; Orooji, Y.; Majidi, M.R.; Yoon, Y.; Khataee, A. MOF-based sensor platforms for rapid detection of pesticides to maintain food quality and safety. Food Chem. Toxicol. 2022, 165, 113176–113192. [Google Scholar] [CrossRef]
- Fang, X.; Zong, B.Y.; Mao, S. Metal-organic framework-based sensors for environmental contaminant sensing. Nano-Micro Lett. 2018, 10, 64–82. [Google Scholar] [CrossRef]
- Manoj, D.; Rajendran, S.; Hoang, T.K.A.; Soto-Moscoso, M. The role of MOF based nanocomposites in the detection of phenolic compounds for environmental remediation- a review. Chemosphere 2022, 300, 134516–134529. [Google Scholar] [CrossRef]
- Jiang, R.; Pang, Y.-H.; Yang, Q.-Y.; Wan, C.-Q.; Shen, X.-F. Copper porphyrin metal-organic framework modified carbon paper for electrochemical sensing of glyphosate. Sens. Actuators B Chem. 2022, 358, 131492–131500. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, L.; Shen, C.; Wang, C.; Hu, X.; Wang, G. An electrochemical sensor on the hierarchically porous Cu-BTC MOF platform for glyphosate determination. Sens. Actuators B Chem. 2019, 283, 487–494. [Google Scholar] [CrossRef]
- Hu, Y.-w. Preparation of a Cu-MOF as an electrode modifier for the determination of carbendazim in water. Int. J. Electrochem. Sci. 2018, 13, 5031–5040. [Google Scholar] [CrossRef]
- Dong, S.; Suo, G.; Li, N.; Chen, Z.; Peng, L.; Fu, Y.; Yang, Q.; Huang, T. A simple strategy to fabricate high sensitive 2,4-dichlorophenol electrochemical sensor based on metal organic framework Cu3(BTC)2. Sens. Actuators B Chem. 2016, 222, 972–979. [Google Scholar] [CrossRef]
- Garg, N.; Deep, A.; Sharma, A.L. Recent trends and advances in porous metal-organic framework nanostructures for the electrochemical and optical sensing of heavy metals in water. Crit. Rev. Anal. Chem. 2022, 52, 1–25. [Google Scholar] [CrossRef]
- Devaraj, M.; Sasikumar, Y.; Rajendran, S.; Ponce, L.C. Review—metal organic framework based nanomaterials for electrochemical sensing of toxic heavy metal ions: Progress and their prospects. J. Electrochem. Soc. 2021, 168, 037513–037522. [Google Scholar] [CrossRef]
- Guo, H.X.; Zheng, Z.S.; Zhang, Y.H.; Lin, H.B.; Xu, Q.B. Highly selective detection of Pb2+ by a nanoscale Ni-based metal-organic framework fabricated through one-pot hydrothermal reaction. Sens. Actuators B Chem. 2017, 248, 430–436. [Google Scholar] [CrossRef]
- Niu, B.; Yao, B.; Zhu, M.; Guo, H.; Ying, S.; Chen, Z. Carbon paste electrode modified with fern leave-like MIL-47(as) for electrochemical simultaneous detection of Pb(ii), Cu(ii) and Hg(ii). J. Electroanal. Chem. 2021, 886, 115121–115129. [Google Scholar] [CrossRef]
- Zhou, Y.; Abazari, R.; Chen, J.; Tahir, M.; Kumar, A.; Ikreedeegh, R.R.; Rani, E.; Singh, H.; Kirillov, A.M. Bimetallic metal–organic frameworks and MOF-derived composites: Recent progress on electro- and photoelectrocatalytic applications. Coordin. Chem. Rev. 2022, 451, 214264–214306. [Google Scholar] [CrossRef]
- Rezki, M.; Septiani, N.L.W.; Iqbal, M.; Adhika, D.R.; Wenten, I.G.; Yuliarto, B. Review—recent advance in multi-metallic metal organic frameworks (MM-MOFs) and their derivatives for electrochemical biosensor application. J. Electrochem. Soc. 2022, 169, 017504–017524. [Google Scholar] [CrossRef]
- Du, Q.; Liao, Y.; Shi, N.; Sun, S.; Liao, X.; Yin, G.; Huang, Z.; Pu, X.; Wang, J. Facile synthesis of bimetallic metal–organic frameworks on nickel foam for a high performance non-enzymatic glucose sensor. J. Electroanal. Chem. 2022, 904, 115887–115894. [Google Scholar] [CrossRef]
- Zou, H.; Tian, D.; Lv, C.; Wu, S.; Lu, G.; Guo, Y.; Liu, Y.; Yu, Y.; Ding, K. The synergistic effect of Co/Ni in ultrathin metal-organic framework nanosheets for the prominent optimization of non-enzymatic electrochemical glucose detection. J. Mater. Chem. B 2020, 8, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Ma, Y.; Liu, Y.; Wang, M.; Guo, C.; He, L.; Song, Y.; Zhang, Z.; Du, M. Hierarchically structured hollow bimetallic ZnNi MOF microspheres as a sensing platform for adenosine detection. Sens. Actuators B Chem. 2020, 303, 127199–127207. [Google Scholar] [CrossRef]
- Janjani, P.; Bhardwaj, U.; Gupta, R.; Singh Kushwaha, H. Bimetallic Mn/Fe MOF modified screen-printed electrodes for non-enzymatic electrochemical sensing of organophosphate. Anal. Chim. Acta 2022, 1202, 339676–339686. [Google Scholar] [CrossRef]
- Huang, W.; Chen, Y.; Wu, L.; Long, M.; Lin, Z.; Su, Q.; Zheng, F.; Wu, S.; Li, H.; Yu, G. 3D Co-doped Ni-based conductive MOFs modified electrochemical sensor for highly sensitive detection of l-tryptophan. Talanta 2022, 247, 123596–123605. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, S.; Hu, X.; Shao, Z.; Zheng, M.; Pang, H. Ultrathin nanosheet Ni-metal organic framework assemblies for high-efficiency ascorbic acid electrocatalysis. ChemElectroChem 2018, 5, 3859–3865. [Google Scholar] [CrossRef]
- Wang, Y.X.; Zhao, M.T.; Ping, J.F.; Chen, B.; Cao, X.H.; Huang, Y.; Tan, C.L.; Ma, Q.L.; Wu, S.X.; Yu, Y.F.; et al. Bioinspired design of ultrathin 2D bimetallic metal-organic-framework nanosheets used as biomimetic enzymes. Adv. Mater. 2016, 28, 4149–4155. [Google Scholar] [CrossRef]
- Zeng, Z.R.; Fang, X.; Miao, W.; Liu, Y.; Maiyalagan, T.; Mao, S. Electrochemically sensing of trichloroacetic acid with iron(ii) phthalocyanine and Zn-based metal organic framework nanocomposites. ACS Sens. 2019, 4, 1934–1941. [Google Scholar] [CrossRef]
- Ko, M.; Mendecki, L.; Eagleton, A.M.; Durbin, C.G.; Stolz, R.M.; Meng, Z.; Mirica, K.A. Employing conductive metal-organic frameworks for voltammetric detection of neurochemicals. J. Am. Chem. Soc. 2020, 142, 11717–11733. [Google Scholar] [CrossRef]
- Wei, C.; Zhou, H.; Liu, Q. PCN-222 MOF decorated conductive pedot films for sensitive electrochemical determination of chloramphenicol. Mater. Chem. Phys. 2021, 270, 124831–124839. [Google Scholar] [CrossRef]
- Zhou, Z.; Mukherjee, S.; Hou, S.; Li, W.; Elsner, M.; Fischer, R.A. Porphyrinic MOF film for multifaceted electrochemical sensing. Angew. Chem. Int. Ed. 2021, 60, 20551–20557. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-H.; Kung, C.-W.; Chang, T.-H.; Lu, H.-C.; Ho, K.-C.; Liao, Y.-C. Inkjet-printed porphyrinic metal–organic framework thin films for electrocatalysis. J. Mater. Chem. A 2016, 4, 11094–11102. [Google Scholar] [CrossRef]
- Liu, J.; Bo, X.; Li, M.; Yin, D.; Guo, L. Contrastive study on porphyrinic iron metal-organic framework supported on various carbon matrices as efficient electrocatalysts. J. Colloid Interface Sci. 2018, 513, 438–447. [Google Scholar] [CrossRef]
- Xu, X.; Li, C.H.; Zhang, H.; Guo, X.M. Construction of electrochemical and photoelectrochemical sensing platform based on porphyrinic metal-organic frameworks for determination of ascorbic acid. Nanomaterials 2022, 12, 482. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, H.; Li, C.-H.; Guo, X.-M. Multimode determination of uric acid based on porphyrinic MOFs thin films by electrochemical and photoelectrochemical methods. Microchem. J. 2022, 175, 107198–107208. [Google Scholar] [CrossRef]
- Kung, C.-W.; Chang, T.-H.; Chou, L.-Y.; Hupp, J.T.; Farha, O.K.; Ho, K.-C. Porphyrin-based metal–organic framework thin films for electrochemical nitrite detection. Electrochem. Commun. 2015, 58, 51–56. [Google Scholar] [CrossRef]
- Meng, W.; Xu, S.; Dai, L.; Li, Y.H.; Zhu, J.; Wang, L. An enhanced sensitivity towards H2O2 reduction based on a novel Cu metal-organic framework and acetylene black modified electrode. Electrochim. Acta 2017, 230, 324–332. [Google Scholar] [CrossRef]
- Song, Y.H.; Xu, M.L.; Gong, C.C.; Shen, Y.; Wang, L.Y.; Xie, Y.; Wang, L. Ratiometric electrochemical glucose biosensor based on GOD/AuNPs/Cu-BTC MOFs/macroporous carbon integrated electrode. Sens. Actuators B Chem. 2018, 257, 792–799. [Google Scholar] [CrossRef]
- Mokhtar, N.A.I.M.; Zawawi, R.M.; Khairul, W.M.; Yusof, N.A. Electrochemical and optical sensors made of composites of metal–organic frameworks and carbon-based materials. A review. Environ. Chem. Lett. 2022. [Google Scholar] [CrossRef]
- Wang, M.Q.; Zhang, Y.; Bao, S.J.; Yu, Y.N.; Ye, C. Ni(ii)-based metal-organic framework anchored on carbon nanotubes for highly sensitive non-enzymatic hydrogen peroxide sensing. Electrochim. Acta 2016, 190, 365–370. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.; Li, S.; Yang, J.; Dong, J. In-situ growth of cerium-based metal organic framework on multi-walled carbon nanotubes for electrochemical detection of gallic acid. Colloid Surface. A 2022, 650, 129318–129327. [Google Scholar] [CrossRef]
- Mousaabadi, K.Z.; Ensafi, A.A.; Rezaei, B. Simultaneous determination of some opioid drugs using Cu-hemin MOF@MWCNTs as an electrochemical sensor. Chemosphere 2022, 303, 135149–135155. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Huang, J.; Ye, H.; Xu, H.; Cai, D.; Wang, D. A high-performance electrochemical sensor for sensitive detection of tetracycline based on a Zr-UiO-66/MWCNTs/AuNPs composite electrode. Anal Methods 2022, 14, 3000–3010. [Google Scholar] [CrossRef]
- Du, Y.; Wang, B.; Kang, K.; Ji, X.; Wang, L.; Zhao, W.; Ren, J. Signal synergistic amplification strategy based on functionalized cemofs for highly sensitive electrochemical detection of phenolic isomers. Microchem. J. 2022, 177, 107285–107294. [Google Scholar] [CrossRef]
- Yan, Y.; Bo, X.; Guo, L. MOF-818 metal-organic framework-reduced graphene oxide/multiwalled carbon nanotubes composite for electrochemical sensitive detection of phenolic acids. Talanta 2020, 218, 121123–121130. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shen, Y.; Zhang, Y.; Zeng, T.; Wan, Q.; Lai, G.; Yang, N. A UiO-66-NH2/carbon nanotube nanocomposite for simultaneous sensing of dopamine and acetaminophen. Anal. Chim. Acta 2021, 1158, 338419–338428. [Google Scholar] [CrossRef]
- Wang, M.Q.; Ye, C.; Bao, S.J.; Zhang, Y.; Yu, Y.N.; Xu, M.W. Carbon nanotubes implanted manganese-based MOFs for simultaneous detection of biomolecules in body fluids. Analyst. 2016, 141, 1279–1285. [Google Scholar] [CrossRef]
- Xu, C.; Liu, L.; Wu, C.; Wu, K. Unique 3D heterostructures assembled by quasi-2D Ni-MOF and CNTs for ultrasensitive electrochemical sensing of bisphenol A. Sens. Actuators B Chem. 2020, 310, 127885–127892. [Google Scholar] [CrossRef]
- Rong, S.; Zou, L.; Meng, L.; Yang, X.; Dai, J.; Wu, M.; Qiu, R.; Tian, Y.; Feng, X.; Ren, X.; et al. Dual function metal-organic frameworks based ratiometric electrochemical sensor for detection of doxorubicin. Anal. Chim. Acta 2022, 1196, 339545–339552. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Yan, P.; Wan, Q.J.; Yang, N.J. Integration of chromium terephthalate metal-organic frameworks with reduced graphene oxide for voltammetry of 4-nonylphenol. Carbon 2018, 134, 540–547. [Google Scholar] [CrossRef]
- Wang, L.; Yang, H.; He, J.; Zhang, Y.Y.; Yu, J.; Song, Y.H. Cu-hemin metal-organic-frameworks/chitosan-reduced graphene oxide nanocomposites with peroxidase-like bioactivity for electrochemical sensing. Electrochim. Acta 2016, 213, 691–697. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.; Wang, Q.; Gao, F.; Gao, F.; Yang, Y.; Guo, H. Highly dispersible and stable copper terephthalate metal-organic framework-graphene oxide nanocomposite for an electrochemical sensing application. ACS Appl. Mater. Interfaces 2014, 6, 11573–11580. [Google Scholar] [CrossRef] [PubMed]
- Mariyappan, V.; Chen, S.-M.; Jeyapragasam, T.; Devi, J.M. Designing and construction of a cobalt-metal-organic framework/heteroatoms co-doped reduced graphene oxide mesoporous nanocomposite based efficient electrocatalyst for chlorogenic acid detection. J. Alloy. Compd. 2022, 898, 163028–163039. [Google Scholar] [CrossRef]
- Xie, Y.; Tu, X.L.; Ma, X.; Xiao, M.Q.; Liu, G.B.; Qu, F.L.; Dai, R.Y.; Lu, L.M.; Wang, W.M. In-situ synthesis of hierarchically porous polypyrrole@ZIF-8/graphene aerogels for enhanced electrochemical sensing of 2, 2-methylenebis (4-chlorophenol). Electrochim. Acta 2019, 311, 114–122. [Google Scholar] [CrossRef]
- Lu, M.; Deng, Y.; Luo, Y.; Lv, J.; Li, T.; Xu, J.; Chen, S.W.; Wang, J. Graphene aerogel-metal-organic framework-based electrochemical method for simultaneous detection of multiple heavy-metal ions. Anal. Chem. 2019, 91, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bo, X.; Nsabimana, A.; Han, C.; Li, M.; Guo, L. Electrocatalytically active cobalt-based metal–organic framework with incorporated macroporous carbon composite for electrochemical applications. J. Mater. Chem. A 2015, 3, 732–738. [Google Scholar] [CrossRef]
- Tang, J.; Liu, R.; Li, J.; Hui, Z.; Zheng, S.; Wu, J.; Guo, J.; Wang, X.; Wang, J. In situ growth of zeolitic imidazolate frameworks nanocrystals on ordered macroporous carbon for hydroquinone and catechol simultaneous determination. J. Electrochem. Soc. 2020, 167, 067504–067512. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, S.; Zhou, M.; Guo, L. A novel electrochemical sensor for detection of baicalein in human serum based on DUT-9/mesoporous carbon composite. Electroanal. 2019, 32, 648–655. [Google Scholar] [CrossRef]
- Liu, J.; Bo, X.J.; Yang, J.; Yin, D.D.; Guo, L.P. One-step synthesis of porphyrinic iron-based metal-organic framework/ordered mesoporous carbon for electrochemical detection of hydrogen peroxide in living cells. Sens. Actuators B Chem. 2017, 248, 207–213. [Google Scholar] [CrossRef]
- Wang, L.; Teng, Q.Q.; Sun, X.T.; Chen, Y.T.; Wang, Y.M.; Wang, H.; Zhang, Y.F. Facile synthesis of metal-organic frameworks/ordered mesoporous carbon composites with enhanced electrocatalytic ability for hydrazine. J. Colloid Interface Sci. 2018, 512, 127–133. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, J.; Zhang, Z.; Yu, H.; Rong, S.; Gao, H.; Pan, H.; Chang, D. Electrochemical strategy with zeolitic imidazolate framework-8 and ordered mesoporous carbon for detection of xanthine. IET Nanobiotechnol. 2020, 14, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Li, C.L.; Wu, C.; Wu, K.B. Strategy for highly sensitive electrochemical sensing: In situ coupling of a metal-organic framework with ball-mill-exfoliated graphene. Anal. Chem. 2019, 91, 6043–6050. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Camarada, M.B.; Lu, X.; Tang, K.; Li, W.; Qiu, D.; Wen, Y.; Wu, G.; Luo, Q.; Bai, L. Detection and electrocatalytic mechanism of zearalenone using nanohybrid sensor based on copper-based metal-organic framework/magnetic Fe3O4-graphene oxide modified electrode. Food Chem. 2022, 370, 131024–131033. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yu, Y.; Xue, S.; Ma, X.; Tao, H. Innovative electrochemical sensor based on graphene oxide aerogel wrapped copper centered metal-organic framework to detect catechol. J. Electroanal. Chem. 2021, 899, 115686–115694. [Google Scholar] [CrossRef]
- Li, C.; Wu, R.J.; Zou, J.C.; Zhang, T.T.; Zhang, S.F.; Zhang, Z.Q.; Hu, X.; Yan, Y.Q.; Ling, X.M. MNPs@anionic MOFs/ERGO with the size selectivity for the electrochemical determination of H2O2 released from living cells. Biosens. Bioelectron. 2018, 116, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.R.; Lau, D.; Cao, G.J.; Tang, Y.; Wu, C. In situ synthesis of a sandwich-like graphene@ZIF-67 heterostructure for highly sensitive nonenzymatic glucose sensing in human serums. ACS Appl. Mater. Interfaces 2019, 11, 9374–9384. [Google Scholar] [CrossRef]
- Zhao, P.; Chen, S.; Zhou, J.; Zhang, S.; Huo, D.; Hou, C. A novel Fe-hemin-metal organic frameworks supported on chitosan-reduced graphene oxide for real-time monitoring of H2O2 released from living cells. Anal. Chim. Acta 2020, 1128, 90–98. [Google Scholar] [CrossRef]
- Wachholz Junior, D.; Deroco, P.B.; Kubota, L.T. A copper-based metal-organic framework/reduced graphene oxide-modified electrode for electrochemical detection of paraquat. Microchim. Acta 2022, 189, 278–289. [Google Scholar] [CrossRef]
- Lin, J.; Hassan, M.; Bo, X.; Guo, L. Synthesis of iron-based metal-organic framework@large mesoporous carbon composites and their electrocatalytic properties. J. Electroanal. Chem. 2017, 801, 373–380. [Google Scholar] [CrossRef]
- Yuan, S.; Bo, X.; Guo, L. In-situ growth of iron-based metal-organic framework crystal on ordered mesoporous carbon for efficient electrocatalysis of p-nitrotoluene and hydrazine. Anal. Chim. Acta 2018, 1024, 73–83. [Google Scholar] [CrossRef]
- Rajpurohit, A.S.; Bora, D.K.; Srivastava, A.K. Simultaneous determination of amlodipine and losartan using an iron metal–organic framework/mesoporous carbon nanocomposite-modified glassy carbon electrode by differential pulse voltammetry. Anal. Methods 2018, 10, 5423–5438. [Google Scholar] [CrossRef]
- Yan, Y.; Ma, J.; Bo, X.; Guo, L. Rod-like Co based metal-organic framework embedded into mesoporous carbon composite modified glassy carbon electrode for effective detection of pyrazinamide and isonicotinyl hydrazide in biological samples. Talanta 2019, 205, 120138–120147. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Lin, S.; Bo, X.; Guo, L. Simultaneous and sensitive electrochemical detection of dihydroxybenzene isomers with UiO-66 metal-organic framework/mesoporous carbon. Talanta 2017, 174, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bo, X.; Guo, L. A novel electrochemical sensing platform of JUC-62 metal-organic framework / platelet ordered mesoporous carbon for high selective detection of nitro-aromatic compounds. Sens. Actuators B Chem. 2019, 297, 126741–126752. [Google Scholar] [CrossRef]
- Xu, Z.D.; Yang, L.Z.; Xu, C.L. Pt@UiO-66 heterostructures for highly selective detection of hydrogen peroxide with an extended linear range. Anal. Chem. 2015, 87, 3438–3444. [Google Scholar] [CrossRef]
- Chen, H.Y.; Yang, T.; Liu, F.Q.; Li, W.H. Electrodeposition of gold nanoparticles on Cu-based metal-organic framework for the electrochemical detection of nitrite. Sens. Actuators B Chem. 2019, 286, 401–407. [Google Scholar] [CrossRef]
- Liu, Y.; Weerasooriya, R.; Chen, X. The metal-organic framework supported gold nanoparticles as a highly sensitive platform for electrochemical detection of methyl mercury species in the aqueous environment. J. Hazard. Mater. 2022, 431, 128608–128617. [Google Scholar] [CrossRef]
- Rani, R.; Deep, A.; Mizaikoff, B.; Singh, S. Zirconium metal organic framework based opto-electrochemical sensor for nitrofurazone detection. J. Electroanal. Chem. 2022, 909, 116124–116133. [Google Scholar] [CrossRef]
- Chai, C.; Gao, J.; Zhao, G.; Li, L.; Tang, Y.; Wu, C.; Wan, C. In-situ synthesis of ultrasmall Au nanoparticles on bimetallic metal-organic framework with enhanced electrochemical activity for estrone sensing. Anal. Chim. Acta 2021, 1152, 338242–338248. [Google Scholar] [CrossRef]
- Qiu, Z.; Yang, T.; Gao, R.; Jie, G.; Hou, W. An electrochemical ratiometric sensor based on 2D MOF nanosheet/Au/polyxanthurenic acid composite for detection of dopamine. J. Electroanal. Chem. 2019, 835, 123–129. [Google Scholar] [CrossRef]
- da Silva, C.T.P.; Veregue, F.R.; Aguiar, L.W.; Meneguin, J.G.; Moisés, M.P.; Fávaro, S.L.; Radovanovic, E.; Girotto, E.M.; Rinaldi, A.W. Aunp@MOF composite as electrochemical material for determination of bisphenol A and its oxidation behavior study. New J. Chem. 2016, 40, 8872–8877. [Google Scholar] [CrossRef]
- Mollarasouli, F.; Kurbanoglu, S.; Asadpour-Zeynali, K.; Ozkan, S.A. Preparation of porous Cu metal organic framework/ZnTe nanorods/Au nanoparticles hybrid platform for nonenzymatic determination of catechol. J. Electroanal. Chem. 2020, 856, 113672–113682. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Chen, H.H.; Hu, X.Y.; Ma, S.Q. Fabrication of highly sensitive and stable hydroxylamine electrochemical sensor based on gold nanoparticles and metal-metalloporphyrin framework modified electrode. ACS Appl. Mater. Interfaces 2016, 8, 18173–18181. [Google Scholar] [CrossRef] [PubMed]
- Ling, P.; Cheng, S.; Chen, N.; Qian, C.; Gao, F. Nanozyme-modified metal-organic frameworks with multienzymes activity as biomimetic catalysts and electrocatalytic interfaces. ACS Appl. Mater. Interfaces 2020, 12, 17185–17192. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Chen, Y.-C.; Chuang, W.-S.; Li, J.-H.; Wang, Y.-S.; Chuang, C.-H.; Chen, C.-Y.; Kung, C.-W. Pore-confined silver nanoparticles in a porphyrinic metal–organic framework for electrochemical nitrite detection. ACS Appl. Nano Mater. 2020, 3, 9440–9448. [Google Scholar] [CrossRef]
- Dong, Y.H.; Duan, C.Q.; Sheng, Q.L.; Zheng, J.B. Preparation of Ag@zeolitic imidazolate framework-67 at room temperature for electrochemical sensing of hydrogen peroxide. Analyst 2019, 144, 521–529. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, T.; Sun, X.; Zhou, A.; Li, M. Determination of peracetic acid by an Ag nanoparticle decorated Cu-organic framework modified electrode. Microchem. J. 2021, 171, 106856–106863. [Google Scholar] [CrossRef]
- Peng, X.; Wan, Y.; Wang, Y.; Liu, T.; Zou, P.; Wang, X.; Zhao, Q.; Ding, F.; Rao, H. Flower-like Ni(ii)-based metal-organic framework-decorated Ag nanoparticles: Fabrication, characterization and electrochemical detection of glucose. Electroanalysis 2019, 31, 2179–2186. [Google Scholar] [CrossRef]
- Ma, J.; Bai, W.; Zheng, J. Non-enzymatic electrochemical hydrogen peroxide sensing using a nanocomposite prepared from silver nanoparticles and copper (ii)-porphyrin derived metal-organic framework nanosheets. Microchim. Acta 2019, 186, 482–489. [Google Scholar] [CrossRef]
- Peng, Z.; Jiang, Z.; Huang, X.; Li, Y. A novel electrochemical sensor of tryptophan based on silver nanoparticles/metal–organic framework composite modified glassy carbon electrode. RSC Adv. 2016, 6, 13742–13748. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, M.; Pu, Y.; Liu, L.; Li, F.; Li, M.; Zhang, M. Silver nanoparticle-functionalized 3D flower-like copper (ii)-porphyrin framework nanocomposites as signal enhancers for fabricating a sensitive glutathione electrochemical sensor. Sens. Actuators B Chem. 2021, 342, 130047–130054. [Google Scholar] [CrossRef]
- Fan, J.; Wang, H.; Zeng, X.; Su, L.; Zhang, X. An electrochemical sensor based on ZIF-67/Ag nanoparticles (NPs)/polydopamine (PDA) nanocomposites for detecting chloride ion with good reproducibility. J. Electroanal. Chem. 2022, 914, 116323–116329. [Google Scholar] [CrossRef]
- Chen, Y.L.; Sun, X.; Biswas, S.; Xie, Y.; Wang, Y.; Hu, X.Y. Integrating polythiophene derivates to PCN-222(Fe) for electrocatalytic sensing of L-dopa. Biosens. Bioelectron. 2019, 141, 111470–111477. [Google Scholar] [CrossRef]
- Vinodh, R.; Babu, R.S.; Sambasivam, S.; Gopi, C.; Alzahmi, S.; Kim, H.J.; de Barros, A.L.F.; Obaidat, I.M. Recent advancements of polyaniline/metal organic framework (PANI/MOF) composite electrodes for supercapacitor applications: A critical review. Nanomaterials 2022, 12, 1511. [Google Scholar] [CrossRef] [PubMed]
- Alsafrani, A.E.; Adeosun, W.A.; Marwani, H.M.; Khan, I.; Jawaid, M.; Asiri, A.M.; Khan, A. Efficient synthesis and characterization of polyaniline@aluminium-succinate metal-organic frameworks nanocomposite and its application for Zn(ii) ion sensing. Polymers 2021, 13, 3383. [Google Scholar] [CrossRef]
- Zhou, K.; Shen, D.; Li, X.; Chen, Y.; Hou, L.; Zhang, Y.; Sha, J. Molybdenum oxide-based metal-organic framework/polypyrrole nanocomposites for enhancing electrochemical detection of dopamine. Talanta 2020, 209, 120507–120514. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, F.; Lin, H.; Jiang, C.; Guo, W.; Fan, S.; Qu, F. An innovative sensor for hydroxylamine determination: Using molybdenum hybrid zeolitic imidazolate framework–conducting polymer composite as electrocatalyst. Electrochim. Acta 2019, 327, 134945–134954. [Google Scholar] [CrossRef]
- Chen, Y.L.; Huang, W.; Chen, K.J.; Zhang, T.; Wang, Y.; Wang, J.M. Facile fabrication of electrochemical sensor based on novel core-shell PPy@ZIF-8 structures: Enhanced charge collection for quercetin in human plasma samples. Sens. Actuators B Chem. 2019, 290, 434–442. [Google Scholar] [CrossRef]
- Huang, T.Y.; Kung, C.W.; Liao, Y.T.; Kao, S.Y.; Cheng, M.; Chang, T.H.; Henzie, J.; Alamri, H.R.; Alothman, Z.A.; Yamauchi, Y.; et al. Enhanced charge collection in MOF-525-pedot nanotube composites enable highly sensitive biosensing. Adv. Sci. 2017, 4, 1700261–1700268. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Huang, W.; Zhang, T.; Hu, X.; Perman, J.A.; Ma, S. A metal–organic framework and conducting polymer based electrochemical sensor for high performance cadmium ion detection. J. Mater. Chem. A 2017, 5, 8385–8393. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, H.; Zheng, J. Polypyrrole microsphere modified porous UiO-66 for electrochemical nitrite sensing. J. Electrochem. Soc. 2022, 169, 047515–047520. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, W.; Shen, Z.; Sun, S.; Dai, H.; Ma, H.; Lin, M. Sensitive and selective detection of Pb (ii) and Cu (ii) using a metal-organic framework/polypyrrole nanocomposite functionalized electrode. Sens. Actuators B Chem. 2020, 304, 127286–127292. [Google Scholar] [CrossRef]
- Wang, H.; Feng, X.; Bo, X.; Zhou, M.; Guo, L. Nickel-based metal-organic framework/crosslinked tubular poly(3,4-ethylenedioxythiophene) composite as an electrocatalyst for the detection of gallic acid and tinidazole. ChemElectroChem 2020, 7, 4031–4037. [Google Scholar] [CrossRef]
- Liu, X.; Gao, X.; Yang, L.; Zhao, Y.; Li, F. Metal-organic framework-functionalized paper-based electrochemical biosensor for ultrasensitive exosome assay. Anal. Chem. 2021, 93, 11792–11799. [Google Scholar] [CrossRef] [PubMed]
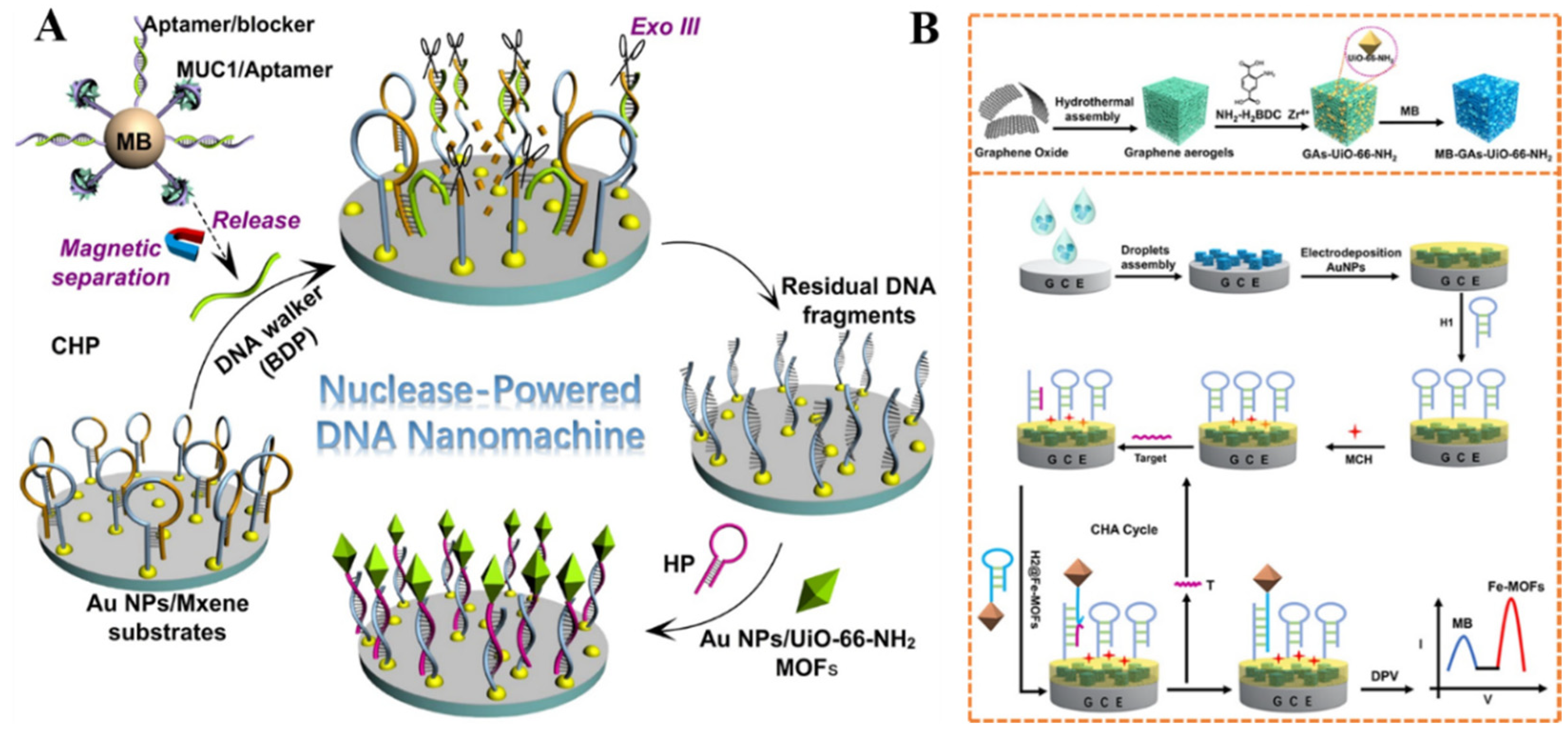
- Wang, Z.; Dong, P.; Sun, Z.X.; Sun, C.; Bu, H.Y.; Han, J.; Chen, S.P.; Xie, G. NH2-Ni-MOF electrocatalysts with tunable size/morphology for ultrasensitive C-reactive protein detection via an aptamer binding induced DNA walker-antibody sandwich assay. J. Mater. Chem. B 2018, 6, 2426–2431. [Google Scholar] [CrossRef]
- Wang, F.; Gui, Y.; Liu, W.; Li, C.; Yang, Y. Precise molecular profiling of circulating exosomes using a metal-organic framework-based sensing interface and an enzyme-based electrochemical logic platform. Anal. Chem. 2022, 94, 875–883. [Google Scholar] [CrossRef]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Enzyme immobilized nanomaterials as electrochemical biosensors for detection of biomolecules. Enzyme Microb. Technol. 2022, 156, 110006–110016. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, D.; Suo, G.; Wei, W.; Huang, T. Exploiting multi-function metal-organic framework nanocomposite Ag@Zn-TSA as highly efficient immobilization matrixes for sensitive electrochemical biosensing. Anal. Chim. Acta 2016, 934, 203–211. [Google Scholar] [CrossRef]
- Kong, L.; Lv, S.; Qiao, Z.; Yan, Y.; Zhang, J.; Bi, S. Metal-organic framework nanoreactor-based electrochemical biosensor coupled with three-dimensional DNA walker for label-free detection of microRNA. Biosens. Bioelectron. 2022, 207, 114188–114195. [Google Scholar] [CrossRef]
- Liang, W.; Wied, P.; Carraro, F.; Sumby, C.J.; Nidetzky, B.; Tsung, C.K.; Falcaro, P.; Doonan, C.J. Metal-organic framework-based enzyme biocomposites. Chem. Rev. 2021, 121, 1077–1129. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.B.; Wang, X.; Wu, L.D.; Wu, L.X.; Dhanjai; Fu, L.; Gao, Y.; Chen, J.P. Response characteristics of bisphenols on a metal-organic framework-based tyrosinase nanosensor. ACS Appl. Mater. Interfaces 2016, 8, 16533–16539. [Google Scholar] [CrossRef]
- Feng, Y.; Xu, Y.; Liu, S.; Wu, D.; Su, Z.; Chen, G.; Liu, J.; Li, G. Recent advances in enzyme immobilization based on novel porous framework materials and its applications in biosensing. Coordin. Chem. Rev. 2022, 459, 214414–214440. [Google Scholar] [CrossRef]
- Wang, Q.; Lian, X.Z.; Fang, Y.; Zhou, H.C. Applications of immobilized bio-catalyst in metal-organic frameworks. Catalysts 2018, 8, 166. [Google Scholar] [CrossRef]
- Mehta, J.; Bhardwaj, N.; Bhardwaj, S.K.; Kim, K.-H.; Deep, A. Recent advances in enzyme immobilization techniques: Metal-organic frameworks as novel substrates. Coordin. Chem. Rev. 2016, 322, 30–40. [Google Scholar] [CrossRef]
- Huang, W.; Huang, S.; Chen, G.; Ouyang, G. Biocatalytic metal-organic frameworks: Promising materials for biosensing. ChemBioChem 2022, 23, e202100567. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhou, J.; Chen, J.; Xie, Z.; Kuang, Q.; Zheng, L. One-step synthesis of thermally stable artificial multienzyme cascade system for efficient enzymatic electrochemical detection. Nano Res. 2019, 12, 3031–3036. [Google Scholar] [CrossRef]
- Singh, R.; Musameh, M.; Gao, Y.; Ozcelik, B.; Mulet, X.; Doherty, C.M. Stable MOF@enzyme composites for electrochemical biosensing devices. J. Mater. Chem. C 2021, 9, 7677–7688. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, L.; Dai, H.; Li, Z.; Fu, Y.; Li, Y. Biomineralization-mimetic preparation of robust metal-organic frameworks biocomposites film with high enzyme load for electrochemical biosensing. J. Electroanal. Chem. 2018, 823, 40–46. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, C.; Zhang, Y.; He, F.; Liu, M.Z.; Li, X.L. Preparation of graphene nano-sheet bonded PDA/MOF microcapsules with immobilized glucose oxidase as a mimetic multi-enzyme system for electrochemical sensing of glucose. J. Mater. Chem. B 2016, 4, 3695–3702. [Google Scholar] [CrossRef]
- Paul, A.; Vyas, G.; Paul, P.; Srivastava, D.N. Gold-nanoparticle-encapsulated ZIF-8 for a mediator-free enzymatic glucose sensor by amperometry. ACS Appl. Nano Mater. 2018, 1, 3600–3607. [Google Scholar] [CrossRef]
- Song, Y.; Shen, Y.; Gong, C.; Chen, J.; Xu, M.; Wang, L.; Wang, L. A novel glucose biosensor based on Tb@mesoporous metal-organic frameworks/carbon nanotube nanocomposites. ChemElectroChem 2017, 4, 1457–1462. [Google Scholar] [CrossRef]
- Ma, W.; Jiang, Q.; Yu, P.; Yang, L.; Mao, L. Zeolitic imidazolate framework-based electrochemical biosensor for in vivo electrochemical measurements. Anal. Chem. 2013, 85, 7550–7557. [Google Scholar] [CrossRef] [PubMed]
- Lyu, F.; Zhang, Y.; Zare, R.N.; Ge, J.; Liu, Z. One-pot synthesis of protein-embedded metal-organic frameworks with enhanced biological activities. Nano Lett. 2014, 14, 5761–5765. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, M.; Xiong, C.; Zhu, X.; Chen, C.; Zhou, F.; Dong, Y.; Wang, Y.; Xu, J.; Li, Y.; et al. Dual confinement of high-loading enzymes within metal-organic frameworks for glucose sensor with enhanced cascade biocatalysis. Biosens. Bioelectron. 2022, 196, 113695–113701. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, W.; Lian, M.; Chen, X.; Lu, Y.; Yang, W. Enzyme immobilization on ZIF-67/MWCNT composite engenders high sensitivity electrochemical sensing. J. Electroanal. Chem. 2019, 833, 505–511. [Google Scholar] [CrossRef]
- Chen, W.; Yang, W.; Lu, Y.; Zhu, W.; Chen, X. Encapsulation of enzyme into mesoporous cages of metal–organic frameworks for the development of highly stable electrochemical biosensors. Anal. Methods 2017, 9, 3213–3220. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, J.; Nie, Y.; Ren, L.; Liu, B.; Liu, G. Metal-organic frameworks/graphene oxide composite: A new enzymatic immobilization carrier for hydrogen peroxide biosensors. J. Electrochem. Soc. 2015, 163, B32–B37. [Google Scholar] [CrossRef]
- Xu, M. A novel H2O2 biosensor based on the composite of MP-11 encapasulated in PCN-333(Al)-graphene oxide. Int. J. Electrochem. Sci. 2017, 12, 10390–10401. [Google Scholar] [CrossRef]
- Gong, C.; Shen, Y.; Chen, J.; Song, Y.; Chen, S.; Song, Y.; Wang, L. Microperoxidase-11@PCN-333 (Al)/three-dimensional macroporous carbon electrode for sensing hydrogen peroxide. Sens. Actuators B Chem. 2017, 239, 890–897. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.R.; Hou, M.; Li, X.Y.; Wu, X.L.; Ge, J. Immobilization on metal-organic framework engenders high sensitivity for enzymatic electrochemical detection. ACS Appl. Mater. Interfaces 2017, 9, 13831–13836. [Google Scholar] [CrossRef]
- Wen, Y.; Li, R.; Liu, J.; Zhang, X.; Wang, P.; Zhang, X.; Zhou, B.; Li, H.; Wang, J.; Li, Z.; et al. Promotion effect of Zn on 2D bimetallic NiZn metal organic framework nanosheets for tyrosinase immobilization and ultrasensitive detection of phenol. Anal. Chim. Acta 2020, 1127, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, X.; Wu, L.; Chen, J. 3D metal-organic framework as highly efficient biosensing platform for ultrasensitive and rapid detection of bisphenol A. Biosens. Bioelectron. 2015, 65, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Ponnada, S.; Gorle, D.B.; Kiai, M.S.; Raju, C.V.; Faraji, M.; Sharma, R.K.; Nowduri, A. Understanding the endocrine disruptor and determination of bisphenol A by functional Cu-BTABB-MOF/rGO composite as facile rapid electrochemical sensor: An experimental and DFT investigation. Anal Methods 2022, 14, 560–573. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yuan, J.; Xu, Y.; Jiang, Y.; Bai, W.; Zheng, J. Ultrasensitive electrochemical determination of bisphenol A in food samples based on a strategy for activity enhancement of enzyme: Layer-by-layer self-assembly of tyrosinase between two-dimensional porphyrin metal–organic framework nanofilms. Chem. Eng. J. 2022, 446, 137001–137012. [Google Scholar] [CrossRef]
- Li, S.; He, B.; Liang, Y.; Wang, J.; Jiao, Q.; Liu, Y.; Guo, R.; Wei, M.; Jin, H. Sensitive electrochemical aptasensor for determination of sulfaquinoxaline based on AuPd NPs@UiO-66-NH2/CoSe2 and RecJf exonuclease-assisted signal amplification. Anal. Chim. Acta 2021, 1182, 338948–338957. [Google Scholar] [CrossRef]
- Qin, X.; Wang, B.; Li, X.; Ding, Y.; Yang, X.; Zhou, Y.; Xu, W.; Xu, M.; Gu, C. Toluidine blue-assisted synthesis of functionalized M (M = Cu, Co, Zn)-metal-organic frameworks for electrochemical immunoassay of proteins. J. Electroanal. Chem. 2022, 911, 116186–116193. [Google Scholar] [CrossRef]
- Zhou, N.; Su, F.F.; Guo, C.P.; He, L.H.; Jia, Z.K.; Wang, M.H.; Jia, Q.J.; Zhang, Z.H.; Lu, S.Y. Two-dimensional oriented growth of Zn-MOF-on-Zr-MOF architecture: A highly sensitive and selective platform for detecting cancer markers. Biosens. Bioelectron. 2019, 123, 51–58. [Google Scholar] [CrossRef]
- Ehzari, H.; Samimi, M.; Safari, M.; Gholivand, M.B. Label-free electrochemical immunosensor for sensitive HER2 biomarker detection using the core-shell magnetic metal-organic frameworks. J. Electroanal. Chem. 2020, 877, 114722–114731. [Google Scholar] [CrossRef]
- Hu, M.; Hu, X.F.; Zhang, Y.P.; Teng, M.; Deng, R.G.; Xing, G.X.; Tao, J.Z.; Xu, G.R.; Chen, J.; Zhang, Y.J.; et al. Label-free electrochemical immunosensor based on AuNPs/Zn/Ni-ZIF-8-800@graphene composites for sensitive detection of monensin in milk. Sens. Actuators B Chem. 2019, 288, 571–578. [Google Scholar] [CrossRef]
- Liu, C.S.; Sun, C.X.; Tian, J.Y.; Wang, Z.W.; Ji, H.F.; Song, Y.P.; Zhang, S.; Zhang, Z.H.; He, L.H.; Du, M. Highly stable aluminum-based metal-organic frameworks as biosensing platforms for assessment of food safety. Biosens. Bioelectron. 2017, 91, 804–810. [Google Scholar] [CrossRef]
- Biswas, S.; Lan, Q.; Xie, Y.; Sun, X.; Wang, Y. Label-free electrochemical immunosensor for ultrasensitive detection of carbohydrate antigen 125 based on antibody-immobilized biocompatible MOF-808/CNT. ACS Appl. Mater. Interfaces 2021, 13, 3295–3302. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, X.; Su, B.; Zhang, H.; Li, R.; Liu, Z.; Chen, Q.; Huang, T.; Cao, H. An innovative electrochemical immunosensor based on nanobody heptamer and AuNPs@ZIF-8 nanocomposites as support for the detection of alpha fetoprotein in serum. Microchem. J. 2022, 179, 107463–107471. [Google Scholar] [CrossRef]
- Duan, F.; Rong, F.; Guo, C.; Chen, K.; Wang, M.; Zhang, Z.; Pettinari, R.; Zhou, L.; Du, M. Electrochemical aptasensing strategy based on a multivariate polymertitanium-metal-organic framework for zearalenone analysis. Food Chem. 2022, 385, 132654–132663. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.S.; Zhang, Z.H.; Chen, M.; Zhao, H.; Duan, F.H.; Chen, D.M.; Wang, M.H.; Zhang, S.; Du, M. Pore modulation of zirconium-organic frameworks for high-efficiency detection of trace proteins. Chem. Commun. 2017, 53, 3941–3944. [Google Scholar] [CrossRef]
- Su, F.F.; Zhang, S.; Ji, H.F.; Zhao, H.; Tian, J.Y.; Liu, C.S.; Zhang, Z.H.; Fang, S.M.; Zhu, X.L.; Du, M. Two-dimensional zirconium-based metal organic framework nanosheet composites embedded with Au nanoclusters: A highly sensitive electrochemical aptasensor toward detecting cocaine. ACS Sens. 2017, 2, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Duan, F.H.; Tian, J.Y.; He, J.Y.; Yang, L.Y.; Zhao, H.; Zhang, S.; Liu, C.S.; He, L.H.; Chen, M.; et al. Aptamer-embedded zirconium-based metal-organic framework composites prepared by de novo bio-inspired approach with enhanced biosensing for detecting trace analytes. ACS Sens. 2017, 2, 982–989. [Google Scholar] [CrossRef]
- Sun, Z.; Peng, Y.; Wang, M.; Lin, Y.; Jalalah, M.; Alsareii, S.A.; Harraz, F.A.; Yang, J.; Li, G. Electrochemical deposition of Cu metal-organic framework films for the dual analysis of pathogens. Anal. Chem. 2021, 93, 8994–9001. [Google Scholar] [CrossRef]
- Rezki, M.; Septiani, N.L.W.; Iqbal, M.; Harimurti, S.; Sambegoro, P.; Adhika, D.R.; Yuliarto, B. Amine-functionalized Cu-MOF nanospheres towards label-free hepatitis B surface antigen electrochemical immunosensors. J. Mater. Chem. B 2021, 9, 5711–5721. [Google Scholar] [CrossRef]
- Xing, G.; Wang, C.; Liu, K.; Luo, B.; Hou, P.; Wang, X.; Dong, H.; Wang, J.; Li, A. A probe-free electrochemical immunosensor for methyl jasmonate based on a Cu-MOF-carboxylated graphene oxide platform. RSC Adv. 2022, 12, 16688–16695. [Google Scholar] [CrossRef]
- Li, H.; Qi, H.; Chang, J.; Gai, P.; Li, F. Recent progress in homogeneous electrochemical sensors and their designs and applications. TrAC Trends Anal. Chem. 2022, 156, 116622–116636. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Wang, X.; Han, L. Flow-homogeneous electrochemical sensing system based on 2D metal-organic framework nanozyme for successive microRNA assay. Biosens. Bioelectron. 2022, 206, 114120–114128. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Khoshfetrat, S.M.; Mirzaeizadeh, Z.; Kabiri, S.; Rezaie, J.; Omidfar, K. Electrochemical immunosensor for determination of cardiac troponin I using two-dimensional metal-organic framework/Fe3O4-COOH nanosheet composites loaded with thionine and pCTAB/DES modified electrode. Talanta 2022, 237, 122911–122918. [Google Scholar] [CrossRef]
- He, B.S.; Dong, X.Z. Hierarchically porous Zr-MOFs labelled methylene blue as signal tags for electrochemical patulin aptasensor based on ZnO nano flower. Sens. Actuators B Chem. 2019, 294, 192–198. [Google Scholar] [CrossRef]
- Lv, M.; Cao, X.; Tian, M.; Jiang, R.; Gao, C.; Xia, J.; Wang, Z. A novel electrochemical biosensor based on MIL-101-NH2 (Cr) combining target-responsive releasing and self-catalysis strategy for p53 detection. Biosens. Bioelectron. 2022, 214, 114518–114524. [Google Scholar] [CrossRef]
- Zheng, Q.; Wang, T.; Li, X.; Qian, H.; Bian, X.; Li, X.; Bai, H.; Ding, S.; Yan, Y. Femtomolar and locus-specific detection of N(6)-methyladenine in DNA by integrating double-hindered replication and nucleic acid-functionalized MB@Zr-MOF. J. Nanobiotechnol. 2021, 19, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Du, K.; Li, X.; Xu, X.; Dong, X.; Fang, J.; Cao, W.; Wei, Q. Ratiometric electrochemical immunosensor for the detection of procalcitonin based on the ratios of SiO2-Fc–COOH–Au and UiO-66-TB complexes. Biosen. Bioelectron. 2021, 171, 112713–112719. [Google Scholar] [CrossRef]
- Gu, C.; Bai, L.; Pu, L.; Gai, P.; Li, F. Highly sensitive and stable self-powered biosensing for exosomes based on dual metal-organic frameworks nanocarriers. Biosens. Bioelectron. 2021, 176, 112907–112912. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, M.F.; Chen, G.J.; Zhang, Y.Q.; Wei, Q.; Zhuo, Y.; Xie, G.; Yuan, R.; Chen, S.P. Ferrocene covalently confined in porous MOF as signal tag for highly sensitive electrochemical immunoassay of amyloid-beta. J. Mater. Chem. B 2017, 5, 8330–8336. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xia, X.; Meng, S.; Ma, Y.; Yang, T.; Yang, Y.; Hu, R. An electrochemical immunosensor coupling a bamboo-like carbon nanostructure substrate with toluidine blue-functionalized Cu(ii)-MOFs as signal probes for a C-reactive protein assay. RSC Adv. 2021, 11, 6699–6708. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, L.; Wu, S.; Pan, Y.; Dong, Y.; Zhu, S.; Yang, J.; Yin, Y.; Li, G. An electrochemical biosensor designed by using Zr-based metal-organic frameworks for the detection of glioblastoma-derived exosomes with practical application. Anal. Chem. 2020, 92, 3819–3826. [Google Scholar] [CrossRef]
- Zhang, B.; Lv, L.; Ma, X.; Xie, L.; Lin, M.; Chen, H.; He, B. Au@ZnNi-MOF labeled electrochemical aptasensor for detection of enrofloxacin based on AuPt@h-CeO2/MoS2 and DNAzyme-driven DNA walker triple amplification signal strategy. Biosens. Bioelectron. 2022, 210, 114296–114303. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, J.; Shang, Y.; Xu, J.; Wang, X.; Zheng, J. An electrochemical immunosensor for simultaneous detection of two lung cancer markers based on electroactive probes. J. Electroanal. Chem. 2022, 919, 116559–116565. [Google Scholar] [CrossRef]
- Huang, S.; Gan, N.; Li, T.; Zhou, Y.; Cao, Y.; Dong, Y. Electrochemical aptasensor for multi-antibiotics detection based on endonuclease and exonuclease assisted dual recycling amplification strategy. Talanta 2018, 179, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Wang, X.; Wang, J.; Li, H.; Li, F. Nucleic acid-functionalized metal-organic framework-based homogeneous electrochemical biosensor for simultaneous detection of multiple tumor biomarkers. Anal. Chem. 2019, 91, 3604–3610. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cheng, J.; Wang, B.; Guo, Y.; Dong, X.; Zhao, J. An amino-modified metal-organic framework (type UiO-66-NH2) loaded with cadmium(ii) and lead(ii) ions for simultaneous electrochemical immunosensing of triazophos and thiacloprid. Microchim. Acta 2019, 186, 101–110. [Google Scholar] [CrossRef]
- Chen, M.; Gan, N.; Zhou, Y.; Li, T.; Xu, Q.; Cao, Y.; Chen, Y. An electrochemical aptasensor for multiplex antibiotics detection based on metal ions doped nanoscale MOFs as signal tracers and RecJf exonuclease-assisted targets recycling amplification. Talanta 2016, 161, 867–874. [Google Scholar] [CrossRef]
- Chen, M.; Gan, N.; Zhou, Y.; Li, T.; Xu, Q.; Cao, Y.; Chen, Y. A novel aptamer- metal ions- nanoscale MOF based electrochemical biocodes for multiple antibiotics detection and signal amplification. Sens. Actuators B Chem. 2017, 242, 1201–1209. [Google Scholar] [CrossRef]
- Zhou, Y.; Wei, L.; Chen, L. Application of metal-organic framework (MOF)-based enzymatic amplification strategy for the sensitive electrochemical detection of tuberculosis. Sens. Actuators B Chem. 2020, 324, 128724–128732. [Google Scholar] [CrossRef]
- Chen, J.; Yu, C.; Zhao, Y.L.; Niu, Y.Z.; Zhang, L.; Yu, Y.J.; Wu, J.; He, J.L. A novel non-invasive detection method for the FGFR3 gene mutation in maternal plasma for a fetal achondroplasia diagnosis based on signal amplification by hemin-MOFs/PtNPs. Biosens. Bioelectron. 2017, 91, 892–899. [Google Scholar] [CrossRef]
- Ling, P.; Lei, J.; Zhang, L.; Ju, H. Porphyrin-encapsulated metal-organic frameworks as mimetic catalysts for electrochemical DNA sensing via allosteric switch of hairpin DNA. Anal. Chem. 2015, 87, 3957–3963. [Google Scholar] [CrossRef]
- Xie, S.; Ye, J.; Yuan, Y.; Chai, Y.; Yuan, R. A multifunctional hemin@metal-organic framework and its application to construct an electrochemical aptasensor for thrombin detection. Nanoscale 2015, 7, 18232–18238. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ma, Z. Multifunctionalized zifs nanoprobe-initiated tandem reaction for signal amplified electrochemical immunoassay of carbohydrate antigen 24-2. Biosens. Bioelectron. 2019, 129, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Liang, Z.; Hu, O.; He, Q.; Sun, D.; Chen, Z. An electrochemical dual-aptamer biosensor based on metal-organic frameworks MIL-53 decorated with Au@Pt nanoparticles and enzymes for detection of COVID-19 nucleocapsid protein. Electrochim. Acta 2021, 387, 138553–138560. [Google Scholar] [CrossRef]
- Ou, D.; Sun, D.; Liang, Z.; Chen, B.; Lin, X.; Chen, Z. A novel cytosensor for capture, detection and release of breast cancer cells based on metal organic framework PCN-224 and DNA tetrahedron linked dual-aptamer. Sens. Actuators B Chem. 2019, 285, 398–404. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X.; Dong, F.; Li, Y.; Guo, Y.; Liu, X.; Xu, J.; Wu, X.; Zheng, Y. Ultrasensitive immunoassay for detection of zearalenone in agro-products using enzyme and antibody co-embedded zeolitic imidazolate framework as labels. J. Hazard. Mater. 2021, 412, 125276–125284. [Google Scholar] [CrossRef]
- Li, W.; Chen, S.; Yang, Y.; Song, Y.; Ma, C.; Qiao, X.; Hong, C. Ultrasensitive electrochemical immunosensor based on the signal amplification strategy of the competitive reaction of Zn2+ and ATP ions to construct a “signal on” mode GOx-HRP enzyme cascade reaction. Microchim. Acta 2021, 188, 61–69. [Google Scholar] [CrossRef]
- Feng, J.; Liang, X.; Ma, Z. New immunoprobe: Dual-labeling ZIF-8 embellished with multifunctional bovine serum albumin lamella for electrochemical immunoassay of tumor marker. Biosens. Bioelectron. 2021, 175, 112853–112858. [Google Scholar] [CrossRef]
- Zhang, C.L.; He, J.L.; Zhang, Y.C.; Chen, J.; Zhao, Y.L.; Niu, Y.Z.; Yu, C. Cerium dioxide-doped carboxyl fullerene as novel nanoprobe and catalyst in electrochemical biosensor for amperometric detection of the CYP2C19*2 allele in human serum. Biosens. Bioelectron. 2018, 102, 94–100. [Google Scholar] [CrossRef]
- Zhao, H.; Du, X.; Dong, H.; Jin, D.; Tang, F.; Liu, Q.; Wang, P.; Chen, L.; Zhao, P.; Li, Y. Electrochemical immunosensor based on Au/Co-BDC/MoS2 and DPCN/MoS2 for the detection of cardiac troponin I. Biosens. Bioelectron. 2021, 175, 112883–112890. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, H.; Han, J.; Xie, G.; Chen, S.P. Rare Co/Fe-MOFs exhibiting high catalytic activity in electrochemical aptasensors for ultrasensitive detection of ochratoxin A. Chem. Commun. 2017, 53, 9926–9929. [Google Scholar] [CrossRef]
- Chen, Y.H.; Liu, X.Z.; Guo, S.L.; Cao, J.; Zhou, J.; Zuo, J.L.; Bai, L.J. A sandwich-type electrochemical aptasensor for mycobacterium tuberculosis MPT64 antigen detection using C(60)NPs decorated N-CNTs/GO nanocomposite coupled with conductive pei-functionalized metal-organic framework. Biomaterials. 2019, 216, 119253–119262. [Google Scholar] [CrossRef]
- Zhong, M.; Yang, L.; Yang, H.; Cheng, C.; Deng, W.; Tan, Y.; Xie, Q.; Yao, S. An electrochemical immunobiosensor for ultrasensitive detection of Escherichia coli o157:H7 using CdS quantum dots-encapsulated metal-organic frameworks as signal-amplifying tags. Biosens. Bioelectron. 2019, 126, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, P.; Huang, J.; Xu, H.; Lei, J.; Zhang, L. Direct electrochemistry of silver nanoparticles-decorated metal-organic frameworks for telomerase activity sensing via allosteric activation of an aptamer hairpin. Anal. Chim. Acta 2021, 1184, 339036–339045. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, Y.; Ma, C.; Song, Y.; Hong, C.; Qiao, X. A sandwich-type electrochemical immunosensor for ultrasensitive detection of CEA based on core–shell Cu2O@Cu-MOF@Au NPs nanostructure attached with HRP for triple signal amplification. J. Mater. Sci. 2020, 55, 13980–13994. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.; Qiang, Y. Ultrasensitive electrochemical aptasensor for ochratoxin A detection using AgPt bimetallic nanoparticles decorated iron-porphyrinic metal-organic framework for signal amplification. Sens. Actuators B Chem. 2020, 312, 127964–127971. [Google Scholar] [CrossRef]
- Wang, L.; Meng, T.; Liang, L.; Sun, J.; Wu, S.; Wang, H.; Yang, X.; Zhang, Y. Fabrication of amine-functionalized metal-organic frameworks with embedded palladium nanoparticles for highly sensitive electrochemical detection of telomerase activity. Sens. Actuators B Chem. 2019, 278, 133–139. [Google Scholar] [CrossRef]
- Meng, T.; Shang, N.; Nsabimana, A.; Ye, H.; Wang, H.; Wang, C.; Zhang, Y. An enzyme-free electrochemical biosensor based on target-catalytic hairpin assembly and Pd@UiO-66 for the ultrasensitive detection of microRNA-21. Anal. Chim. Acta 2020, 1138, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Yu, C.; Yang, B.; Liu, Z.R.; Xia, P.Y.; Wang, Q. Target-catalyzed hairpin assembly and metal-organic frameworks mediated nonenzymatic co-reaction for multiple signal amplification detection of miR-122 in human serum. Biosens. Bioelectron. 2018, 102, 307–315. [Google Scholar] [CrossRef]
- Wu, C.; Wu, X.; Hou, F.; Wu, L.; Liu, G. An ultrasensitive electrochemical aptasensor based on Pd@PCN-222 as a signal probe coupled with exonuclease III-assisted cycling amplification for the detection of ochratoxin A. Food Control 2022, 139, 109066–109074. [Google Scholar] [CrossRef]
- Ling, P.; Lei, J.; Jia, L.; Ju, H. Platinum nanoparticles encapsulated metal-organic frameworks for the electrochemical detection of telomerase activity. Chem. Commun. 2016, 52, 1226–1229. [Google Scholar] [CrossRef]
- Yu, Y.J.; Yu, C.; Niu, Y.Z.; Chen, J.; Zhao, Y.L.; Zhang, Y.C.; Gao, R.F.; He, J.L. Target triggered cleavage effect of DNAzyme: Relying on Pd-Pt alloys functionalized Fe-MOFs for amplified detection of Pb2+. Biosens. Bioelectron. 2018, 101, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Wang, N.; Huang, Z.; Dai, H.; Xu, L.; Sun, S.; Ma, H.; Lin, M. Electrochemical endotoxin aptasensor based on a metal-organic framework labeled analytical platform. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110501–110507. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.B.; Shang, Y.H.; Zhu, Q.Y.; Zhang, X.X.; Zheng, J.B. A voltammetric immunoassay for the carcinoembryonic antigen using silver(i)-terephthalate metal-organic frameworks containing gold nanoparticles as a signal probe. Microchim. Acta 2019, 186, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lv, J.J.; Yang, Z.H.; Yuan, R.; Chai, Y.Q. A sensitive electrochemical aptasensor for thrombin detection based on electroactive Co-based metal-organic frameworks with target-triggering nesa strategy. Anal. Chem. 2017, 89, 11636–11640. [Google Scholar] [CrossRef]
- Wang, H.; Jian, Y.N.; Kong, Q.K.; Liu, H.Y.; Lan, F.F.; Liang, L.L.; Ge, S.G.; Yu, J.H. Ultrasensitive electrochemical paper-based biosensor for microRNA via strand displacement reaction and metal-organic frameworks. Sens. Actuators B Chem. 2018, 257, 561–569. [Google Scholar] [CrossRef]
- Zhou, C.; Cui, K.; Liu, Y.; Hao, S.; Zhang, L.; Ge, S.; Yu, J. Ultrasensitive microfluidic paper-based electrochemical/visual analytical device via signal amplification of Pd@hollow Zn/Co core-shell ZIF67/ZIF8 nanoparticles for prostate-specific antigen detection. Anal. Chem. 2021, 93, 5459–5467. [Google Scholar] [CrossRef]
- Li, D.-l.; Zhang, X.; Ma, Y.; Deng, Y.; Hu, R.; Yang, Y. Preparation of an OTA aptasensor based on a metal–organic framework. Anal. Methods 2018, 10, 3273–3279. [Google Scholar] [CrossRef]
- Liu, J.L.; Ma, Y.C.; Yang, T.; Hu, R.; Yang, Y.H. A single nucleotide polymorphism electrochemical sensor based on DNA-functionalized Cd-MOFs-74 as cascade signal amplification probes. Microchim. Acta 2021, 188, 266–275. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Jin, H.; Wei, M.; Ren, W.; Zhang, Y.; Wu, L.; He, B. Electrochemical biosensor for sensitive detection of Hg2+ baesd on clustered peonylike copper-based metal-organic frameworks and DNAzyme-driven DNA walker dual amplification signal strategy. Sens. Actuators B Chem. 2021, 329, 129215–129224. [Google Scholar] [CrossRef]
- Xie, F.-T.; Li, Y.-L.; Guan, Y.; Liu, J.-W.; Yang, T.; Mao, G.-J.; Wu, Y.; Yang, Y.-H.; Hu, R. Ultrasensitive dual-signal electrochemical ratiometric aptasensor based on Co-MOFs with intrinsic self-calibration property for Mucin 1. Anal. Chim. Acta 2022, 1225, 340219–340227. [Google Scholar] [CrossRef]
- Liu, T.Z.; Hu, R.; Zhang, X.; Zhang, K.L.; Liu, Y.; Zhang, X.B.; Bai, R.Y.; Li, D.; Yang, Y.H. Metal-organic framework nanomaterials as novel signal probes for electron transfer mediated ultrasensitive electrochemical immunoassay. Anal. Chem. 2016, 88, 12516–12523. [Google Scholar] [CrossRef] [PubMed]
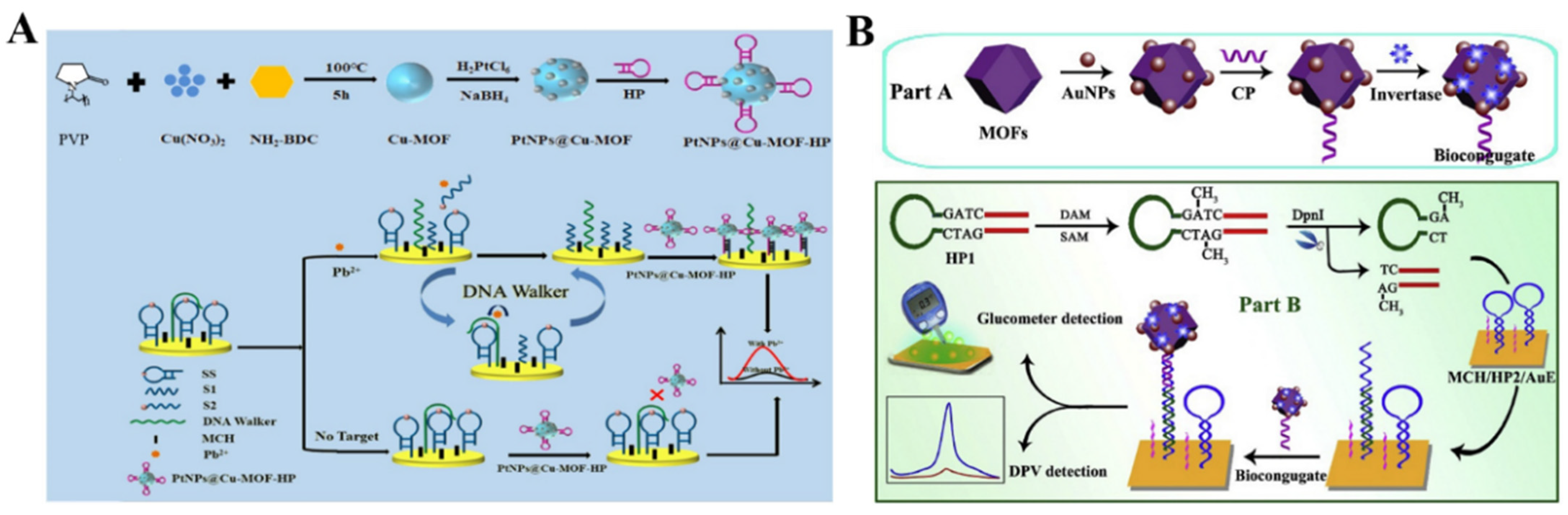
- Dong, J.; Zhang, D.; Li, C.; Bai, T.; Jin, H.; Suo, Z. A sensitive electrochemical sensor based on PtNPs@Cu-MOF signal probe and DNA walker signal amplification for Pb2+ detection. Bioelectrochemistry 2022, 146, 108134–108141. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Meng, X.Z.; Gu, H.W.; Yi, H.C.; Sun, W.Y. A dual-response biosensor for electrochemical and glucometer detection of DNA methyltransferase activity based on functionalized metal-organic framework amplification. Biosens. Bioelectron. 2019, 134, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Dong, P.; Wang, Y.; Liu, T.; Huang, Y.; Lei, J. A stepwise recognition strategy for the detection of telomerase activity via direct electrochemical analysis of metal-organic frameworks. Analyst 2021, 146, 1859–1864. [Google Scholar] [CrossRef]
- Li, Z.Y.; Li, Y.; Huang, L.; Hu, R.; Yang, T.; Yang, Y.H. An electrochemical aptasensor based on intelligent walking DNA nanomachine with cascade signal amplification powered by nuclease for Mucin 1 assay. Anal. Chim. Acta 2022, 1214, 339964–339971. [Google Scholar] [CrossRef]
- Dong, J.; Wen, L.; Yang, H.; Zhao, J.; He, C.; Hu, Z.; Peng, L.; Hou, C.; Huo, D. Catalytic hairpin assembly-driven ratiometric dual-signal electrochemical biosensor for ultrasensitive detection of MicroRNA based on the ratios of Fe-MOFs and MB-GA-UiO-66-NH2. Anal. Chem. 2022, 94, 5846–5855. [Google Scholar] [CrossRef]
- Xie, F.T.; Zhao, X.L.; Chi, K.N.; Yang, T.; Hu, R.; Yang, Y.H. Fe-MOFs as signal probes coupling with DNA tetrahedral nanostructures for construction of ratiometric electrochemical aptasensor. Anal. Chim. Acta 2020, 1135, 123–131. [Google Scholar] [CrossRef]
- Li, Z.; Dai, G.; Luo, F.; Lu, Y.; Zhang, J.; Chu, Z.; He, P.; Zhang, F.; Wang, Q. An electrochemical sensor for bacterial lipopolysaccharide detection based on dual functional Cu2+-modified metal-organic framework nanoparticles. Microchim. Acta 2020, 187, 415–424. [Google Scholar] [CrossRef]
- Yiwei, X.; Yahui, L.; Weilong, T.; Jiyong, S.; Xiaobo, Z.; Wen, Z.; Xinai, Z.; Yanxiao, L.; Changqiang, Z.; Lele, A.; et al. Electrochemical determination of hantavirus using gold nanoparticle-modified graphene as an electrode material and Cu-based metal-organic framework assisted signal generation. Microchim. Acta 2021, 188, 112–121. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, D.; Ma, Z.; Han, H. Cascade catalysis-initiated radical polymerization amplified impedimetric immunosensor for ultrasensitive detection of carbohydrate antigen 15-3. Biosens. Bioelectron. 2019, 137, 1–7. [Google Scholar] [CrossRef]
- Dong, H.B.; Hu, X.J.; Zhao, J.L.; Li, H.J.; Koh, K.; Gao, L.; Shao, M.; Chen, H.X. Sensitive detection of fractalkine based on AuNPs and metal-organic frameworks composite at para-sulfonatocalix 4 arene-AuNPs assembled multilayer interface. Sens. Actuators B Chem. 2018, 276, 150–157. [Google Scholar] [CrossRef]
- Shen, W.J.; Zhuo, Y.; Chai, Y.Q.; Yuan, R. Ce-based metal-organic frameworks and DNAzyme-assisted recycling as dual signal amplifiers for sensitive electrochemical detection of lipopolysaccharide. Biosens. Bioelectron. 2016, 83, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Han, J.; An, S.J.; Xie, G.; Chen, S.P. Ce(III, IV)-MOF electrocatalyst as signal-amplifying tag for sensitive electrochemical aptasensing. Biosens. Bioelectron. 2018, 109, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Zhu, L.; Huang, J.; Ren, J.; Lei, J. Electrocatalysis of cerium metal-organic frameworks for ratiometric electrochemical detection of telomerase activity. Biosens. Bioelectron. 2019, 138, 111313–111320. [Google Scholar] [CrossRef]
- Ling, P.; Lei, J.; Ju, H. Nanoscaled porphyrinic metal-organic frameworks for electrochemical detection of telomerase activity via telomerase triggered conformation switch. Anal. Chem. 2016, 88, 10680–10686. [Google Scholar] [CrossRef]
- Ling, P.; Lei, J.; Ju, H. Porphyrinic metal-organic framework as electrochemical probe for DNA sensing via triple-helix molecular switch. Biosens. Bioelectron. 2015, 71, 373–379. [Google Scholar] [CrossRef]
- Song, W.; Yin, W.; Zhang, Z.; He, P.; Yang, X.; Zhang, X. A DNA functionalized porphyrinic metal-organic framework as a peroxidase mimicking catalyst for amperometric determination of the activity of T4 polynucleotide kinase. Microchim. Acta 2019, 186, 149–156. [Google Scholar] [CrossRef]
- Zhang, G.; Shan, D.; Dong, H.; Cosnier, S.; Al-Ghanim, K.A.; Ahmad, Z.; Mahboob, S.; Zhang, X. DNA-mediated nanoscale metal-organic frameworks for ultrasensitive photoelectrochemical enzyme-free immunoassay. Anal. Chem. 2018, 90, 12284–12291. [Google Scholar] [CrossRef]
- Zhang, X.; Wasson, M.C.; Shayan, M.; Berdichevsky, E.K.; Ricardo-Noordberg, J.; Singh, Z.; Papazyan, E.K.; Castro, A.J.; Marino, P.; Ajoyan, Z.; et al. A historical perspective on porphyrin-based metal-organic frameworks and their applications. Coord. Chem. Rev. 2021, 429, 213615–213636. [Google Scholar] [CrossRef]
- Cui, L.; Wu, J.; Li, J.; Ju, H. Electrochemical sensor for lead cation sensitized with a DNA functionalized porphyrinic metal-organic framework. Anal. Chem. 2015, 87, 10635–10641. [Google Scholar] [CrossRef]
- Bao, T.; Fu, R.; Jiang, Y.; Wen, W.; Zhang, X.; Wang, S. Metal-mediated polydopamine nanoparticles-DNA nanomachine coupling electrochemical conversion of metal-organic frameworks for ultrasensitive MicroRNA sensing. Anal. Chem. 2021, 93, 13475–13484. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Ifraemov, R.; Raslin, A.; Hod, I. Room-temperature electrochemical conversion of metal-organic frameworks into porous amorphous metal sulfides with tailored composition and hydrogen evolution activity. Adv. Funct. Mater. 2018, 28, 1707244–1707252. [Google Scholar] [CrossRef]
- Guo, L.; Mu, Z.; Yan, B.; Wang, J.; Zhou, J.; Bai, L. A novel electrochemical biosensor for sensitive detection of non-small cell lung cancer ctDNA using NG-PEI-COFTAPB-TFPB as sensing platform and Fe-MOF for signal enhancement. Sens. Actuators B Chem. 2022, 350, 130874–130883. [Google Scholar] [CrossRef]
- Tang, J.; Liu, L.; Qin, J.; Lv, X.; Li, J.; Tang, D.; Zhuang, J. Biocatalysis-mediated MOF-to-prussian blue transformation enabling sensitive detection of NSCLC-associated miRNAs with dual-readout signals. Biosens. Bioelectron. 2022, 206, 114139–114147. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Li, Y.; Teng, H.; Ding, C.; Xu, G.; Luo, X. Designed zwitterionic peptide combined with sacrificial Fe-MOF for low fouling and highly sensitive electrochemical detection of T4 polynucleotide kinase. Sens. Actuators B Chem. 2020, 305, 127329–127337. [Google Scholar] [CrossRef]
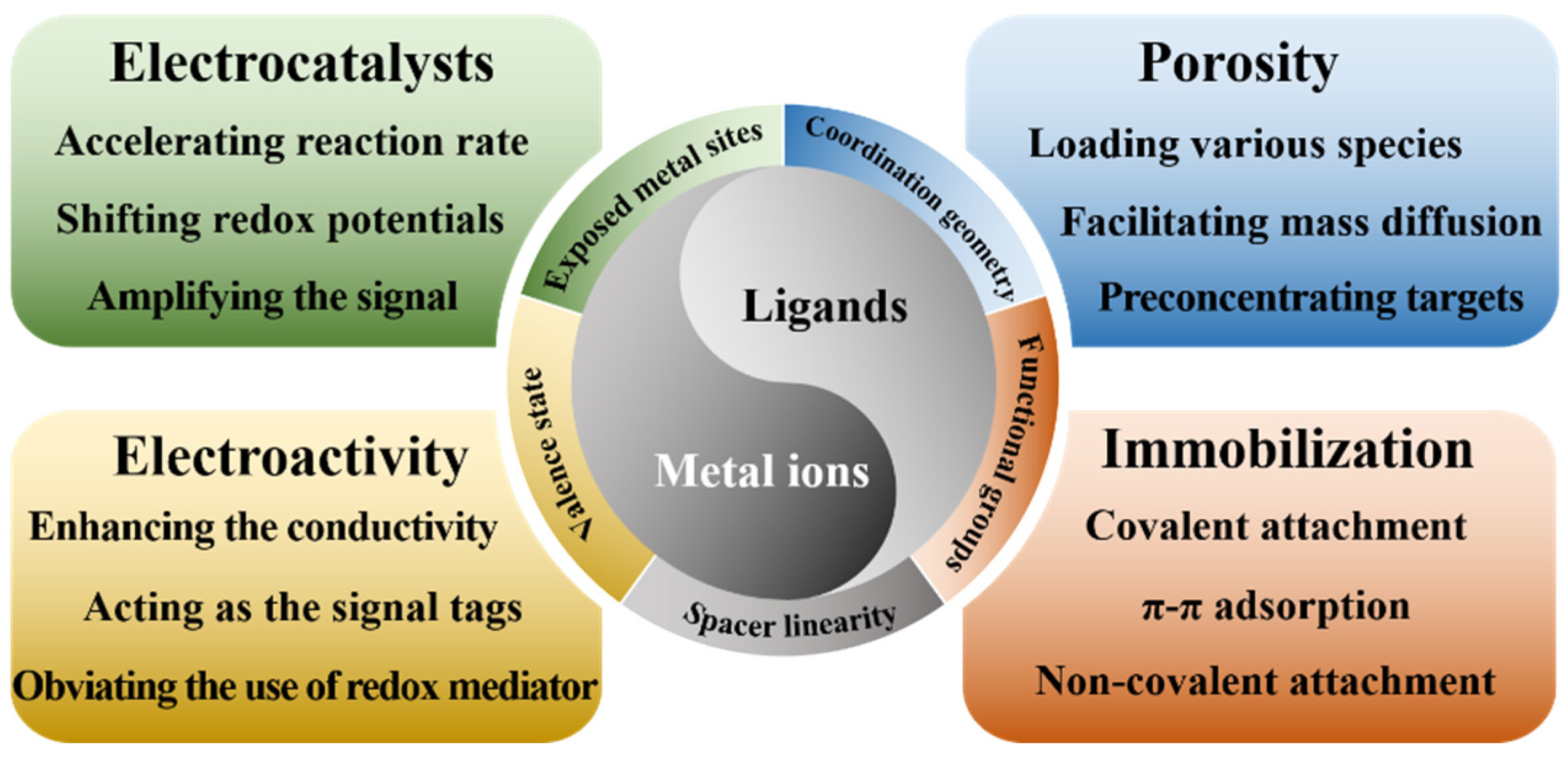

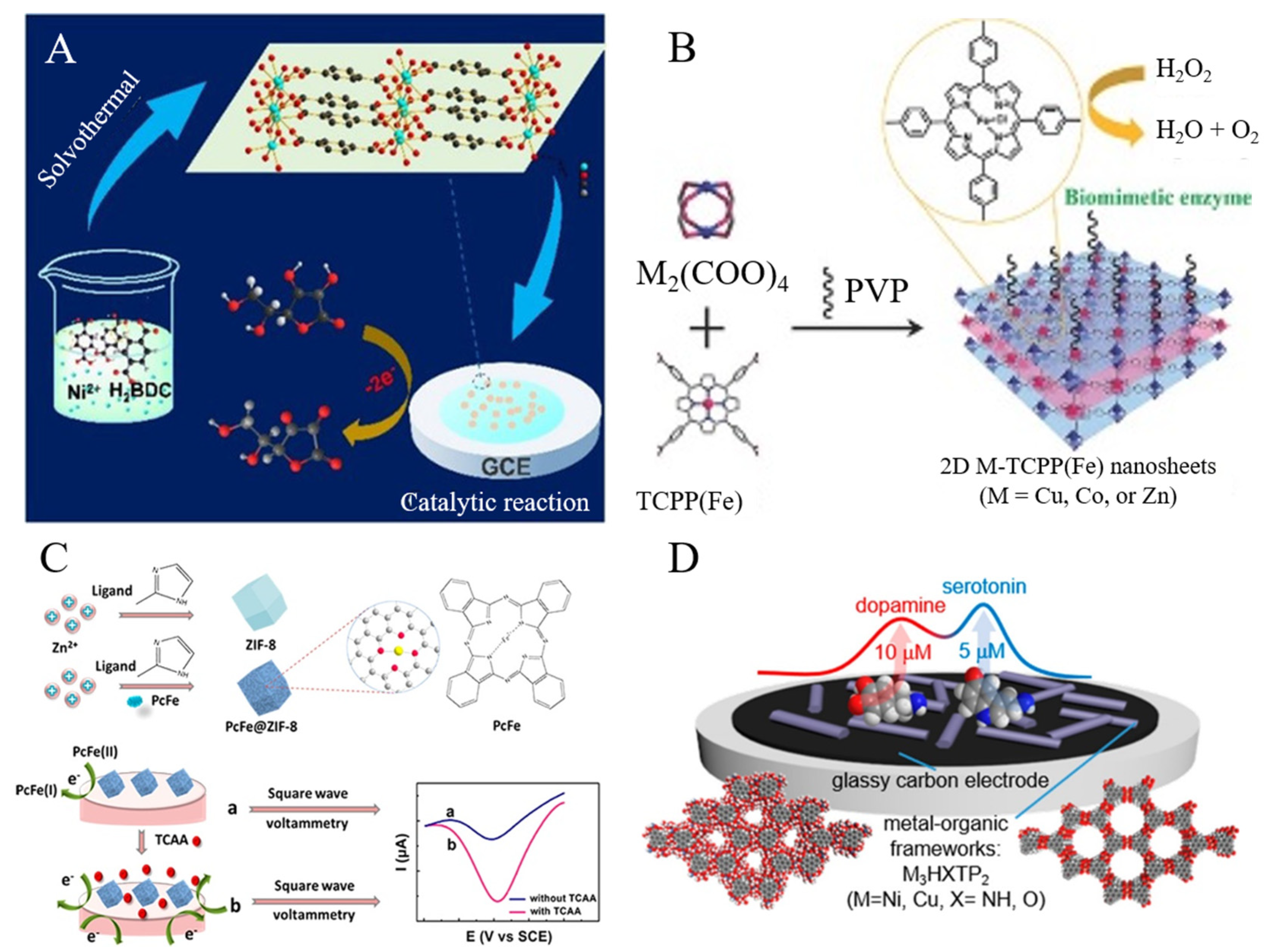
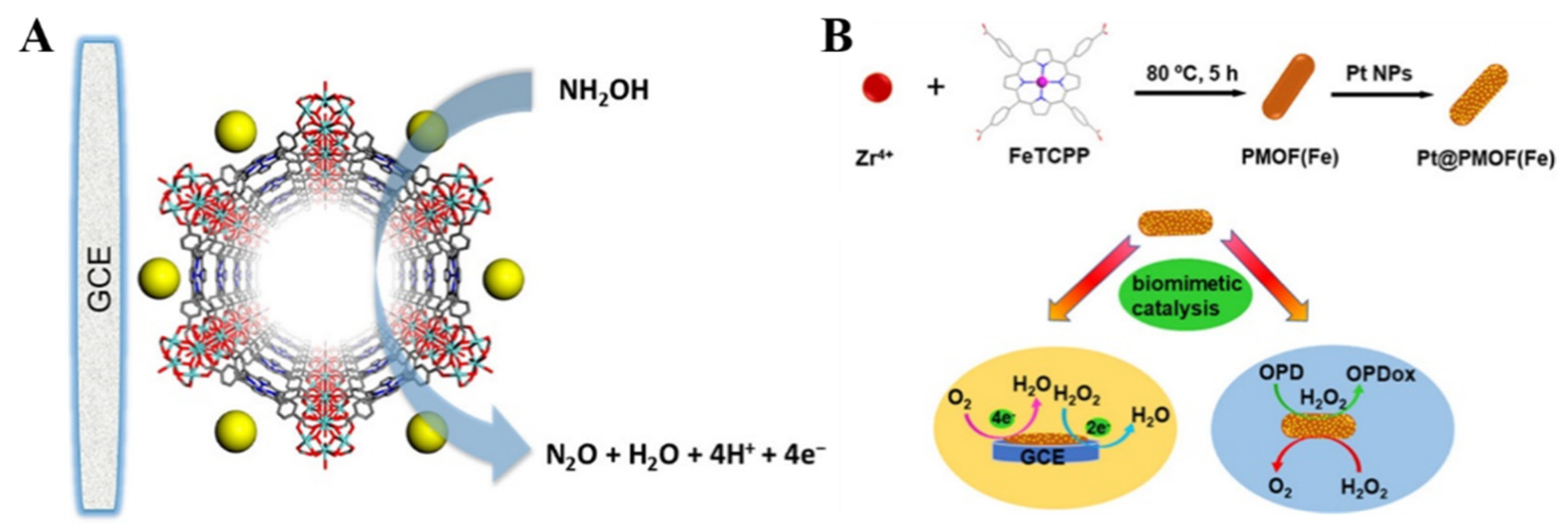

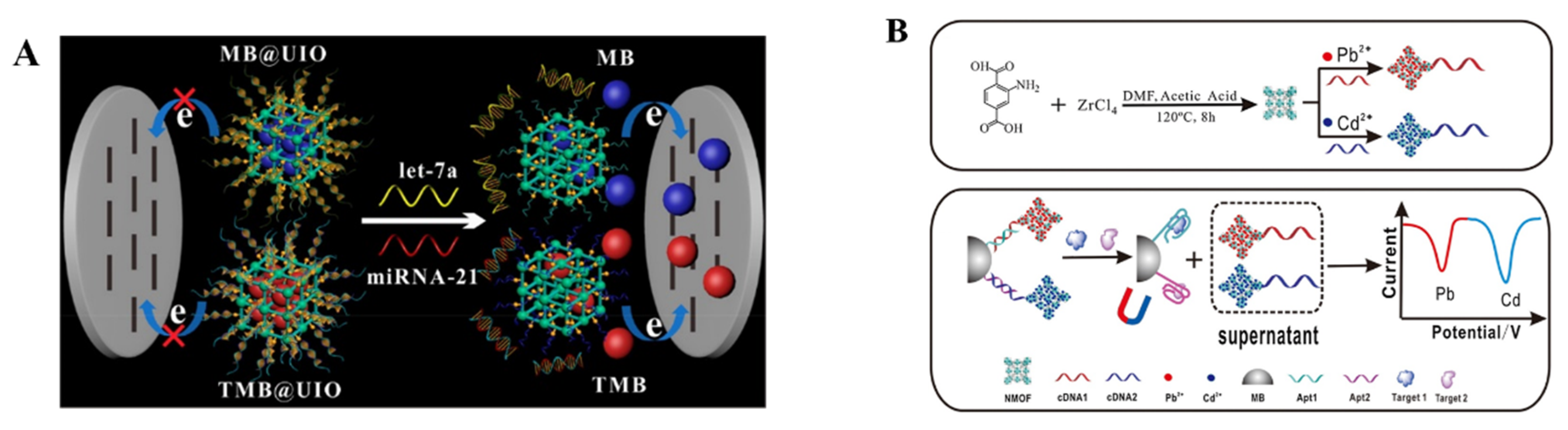

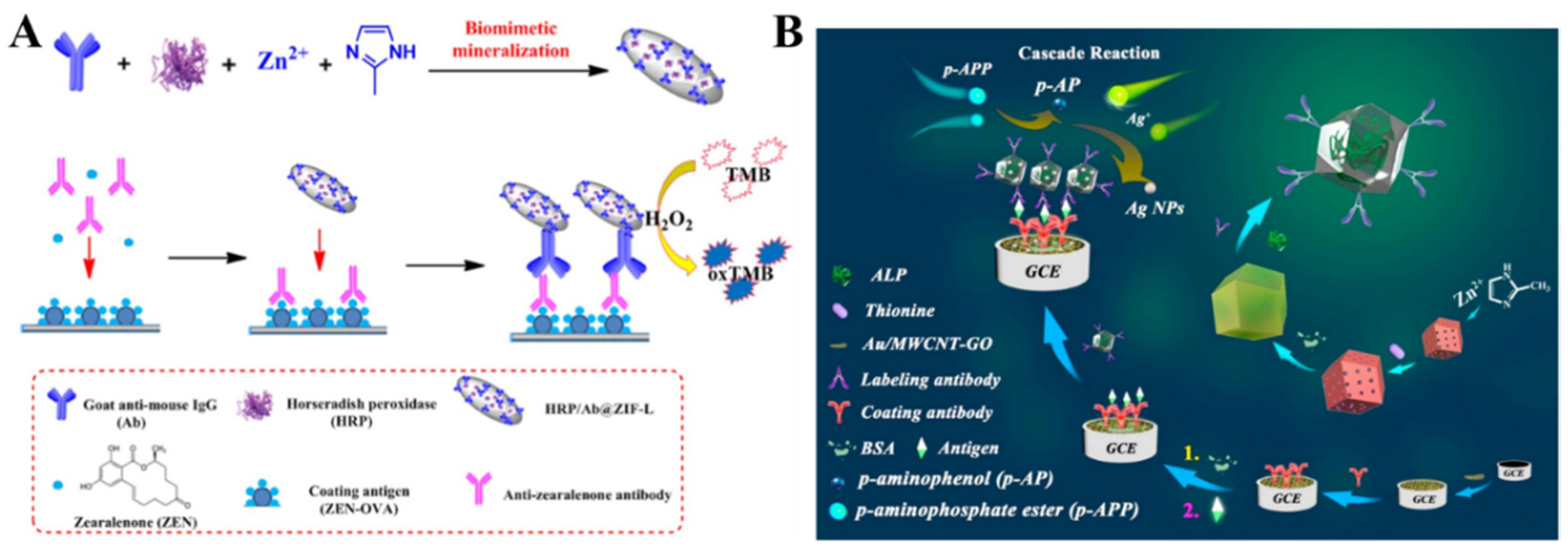

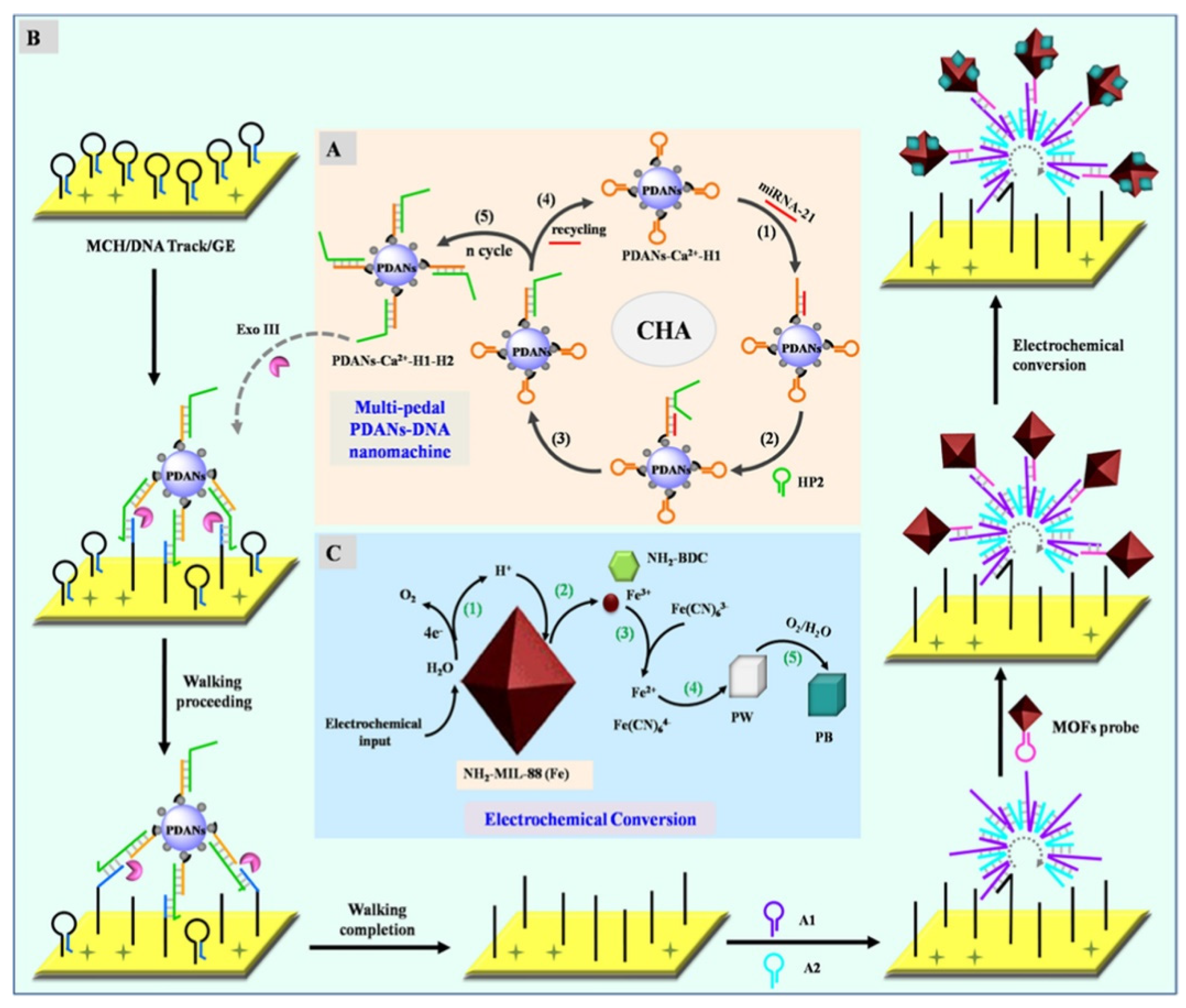
| Electrode Material | Analyte | Linear Range | LOD | Ref. |
|---|---|---|---|---|
| MIL-101(Cr)@rGO | 4-nonylphenol | 100 nM~12.5 μM | 33 nM | [90] |
| Cu−hemin MOF/CS−rGO | H2O2 | 65 nM~0.41 mM | 19 nM | [91] |
| Cu(tpa)−EGR | ACOP and DA | 1.0 μM~100 μM and 1.0 μM~50 μM | 0.36 and 0.21 μM | [92] |
| Co−MOF/BP−RGO | chlorogenic acid | 1.0 nM~391 μM | 14 nM | [93] |
| PPy@ZIF-8/GAs | dichlorophenol | 10 nM~10 μM | 0.1 nM | [94] |
| GA-UiO-66-NH2 | Cd2+, Pb2+, Cu2+, and Hg2+ | 10 nM~1.5 μM, 1.0 nM~2.0 μM, 10 nM~1.6 μM and 1.0 nM~22 μM | 9.0, 1.0, 8.0 and 0.9 nM | [95] |
| Ni−MOFs/CNTs | H2O2 | 10 μM~51.6 mM | 2.1 μM | [80] |
| UiO-66-NH2/CNTs | DA and AC | 30 nM~2.0 μM | 15 and 9.0 nM | [86] |
| Mn−BDC@MWCNT | AA, DA and UA | 0.1 μM~1.15 mM, 10 nM~0.5 mM, and 20 nM~1.1 mM | 10, 2.0 and 5.0 nM | [87] |
| 3D Ni−MOF@CNTs | BPA | 1.0 nM~1.0 μM | 0.35 nM | [88] |
| MB@MWCNTs/UiO-66-NH2 | doxorubicin | 0.1 μM~75 μM | 51 nM | [89] |
| Co−MOF@MPC | hydrazine and nitrobenzene | 5.0 μM~0.63 mM and 0.5 μM~15 μM | 1.75 and 0.21 μM | [96] |
| ZIF67−OMC | HQ and catechol | 0.1 μM~100 μM | 52 and 36 nM | [97] |
| DUT-9/MC | Baicalein | 50 nM~20 μM | 15 nM | [98] |
| pFeMOF/OMC | H2O2 | 0.5~70.5 μM | 0.45 μM | [99] |
| Cu−MOFs/OMC | hydrazine | 0.5 μM~0.711 mM | 0.35 μM | [100] |
| Ag−ZIF-8/OMC | xanthine | 1.0 μM~0.28 mM | 0.167 μM | [101] |
| Electrode Material | Analyte | Linear Range | LOD | Ref. |
|---|---|---|---|---|
| PANI@Al−SA | Zn2+ | 2.8~228.6 μM | 0.59 μM | [135] |
| CuTRZMoO4@PPy | DA | 1.0~100 μM | 80 nM | [136] |
| ZIF−PEDOT | hydroxylamine | 0.1~0.6922 mM | 40 nM | [137] |
| PPy@ZIF-8 | quercetin | 0.01~7.0 μM and 7.0~150 μM | 7.0 nM | [138] |
| UiO-66-NH2@PANI | Cd2+ | 4.5 nM~5.4 μM | 2.7 nM | [140] |
| PPy/UiO-66 | NO3– | 50 nM~1.0555 mM | 37 nM | [141] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.; Lou, J.; Yang, L.; Liu, M.; Xia, N.; Liu, L. Design and Application of Electrochemical Sensors with Metal–Organic Frameworks as the Electrode Materials or Signal Tags. Nanomaterials 2022, 12, 3248. https://doi.org/10.3390/nano12183248
Chang Y, Lou J, Yang L, Liu M, Xia N, Liu L. Design and Application of Electrochemical Sensors with Metal–Organic Frameworks as the Electrode Materials or Signal Tags. Nanomaterials. 2022; 12(18):3248. https://doi.org/10.3390/nano12183248
Chicago/Turabian StyleChang, Yong, Jiaxin Lou, Luyao Yang, Miaomiao Liu, Ning Xia, and Lin Liu. 2022. "Design and Application of Electrochemical Sensors with Metal–Organic Frameworks as the Electrode Materials or Signal Tags" Nanomaterials 12, no. 18: 3248. https://doi.org/10.3390/nano12183248
APA StyleChang, Y., Lou, J., Yang, L., Liu, M., Xia, N., & Liu, L. (2022). Design and Application of Electrochemical Sensors with Metal–Organic Frameworks as the Electrode Materials or Signal Tags. Nanomaterials, 12(18), 3248. https://doi.org/10.3390/nano12183248








