Micro- and Nanoscale Imaging of Fluids in Water Using Refractive-Index-Matched Materials
Abstract
1. Introduction
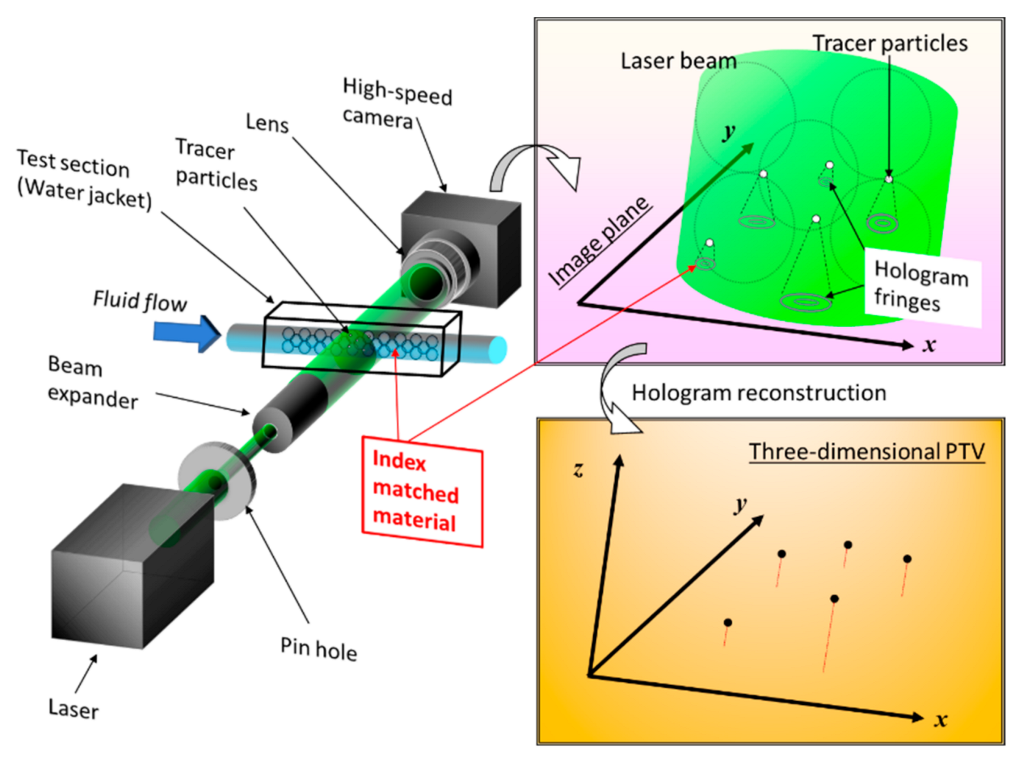
2. Three-Dimensional Visualization Technique Using Holography in a Microflow
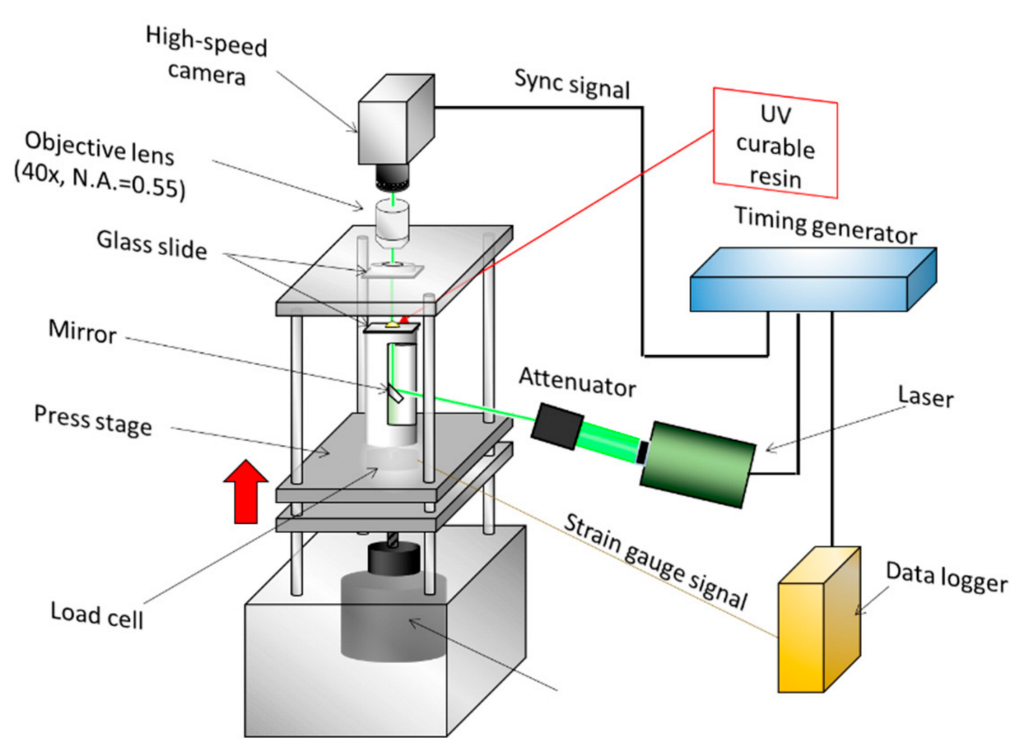
3. Three-Dimensional Visualization in a Nanoflow
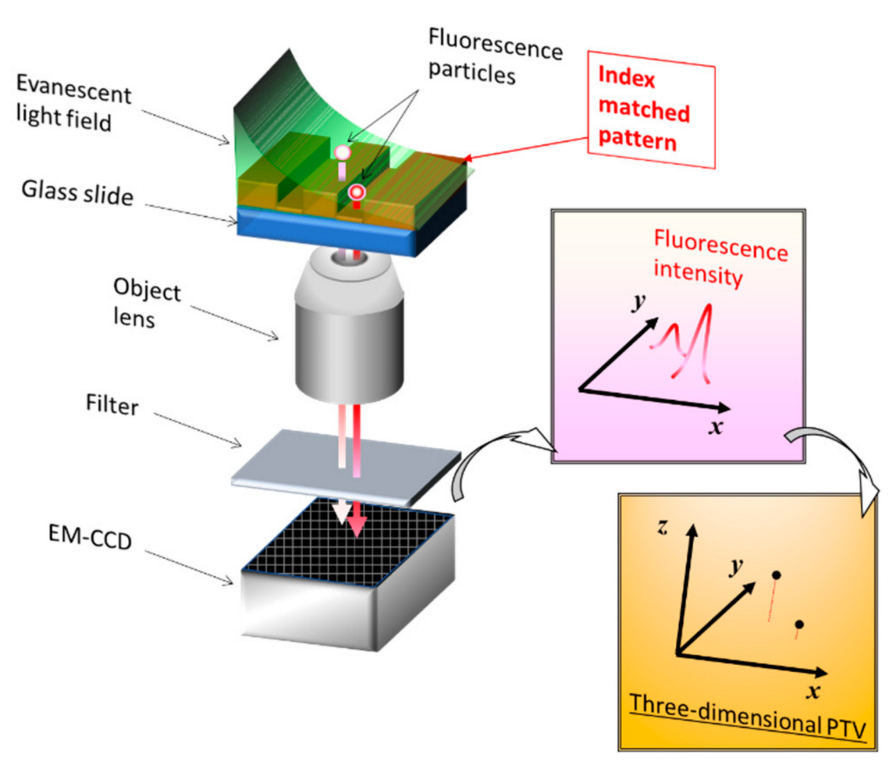
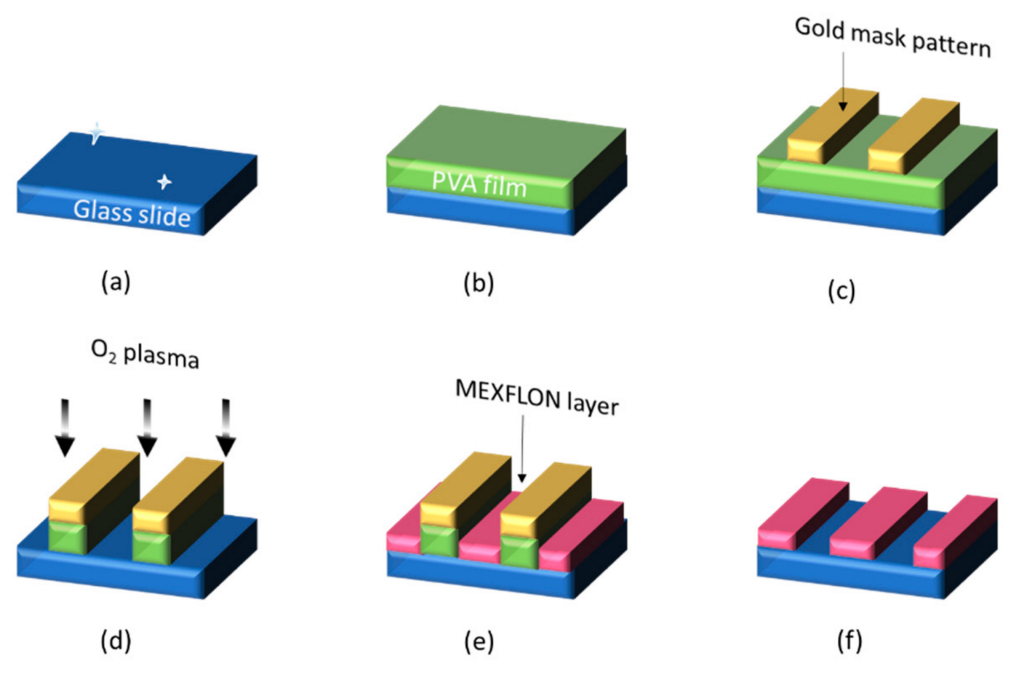
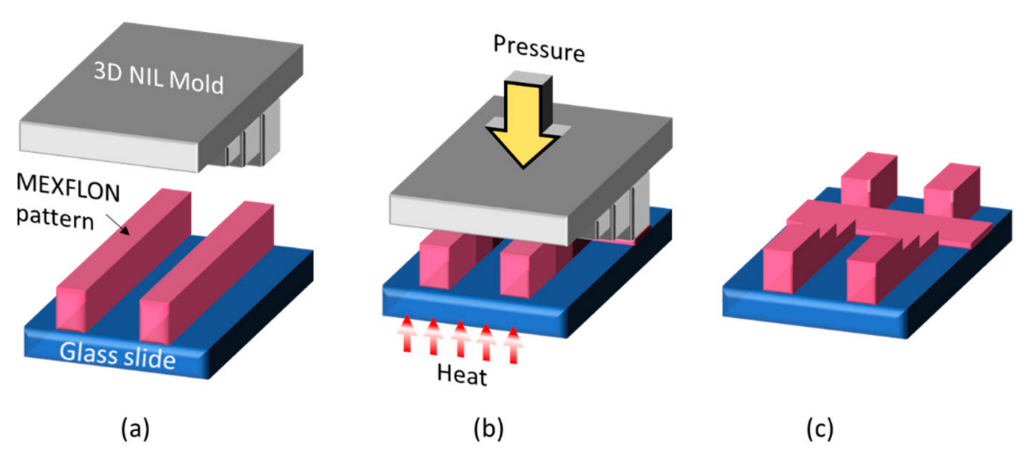
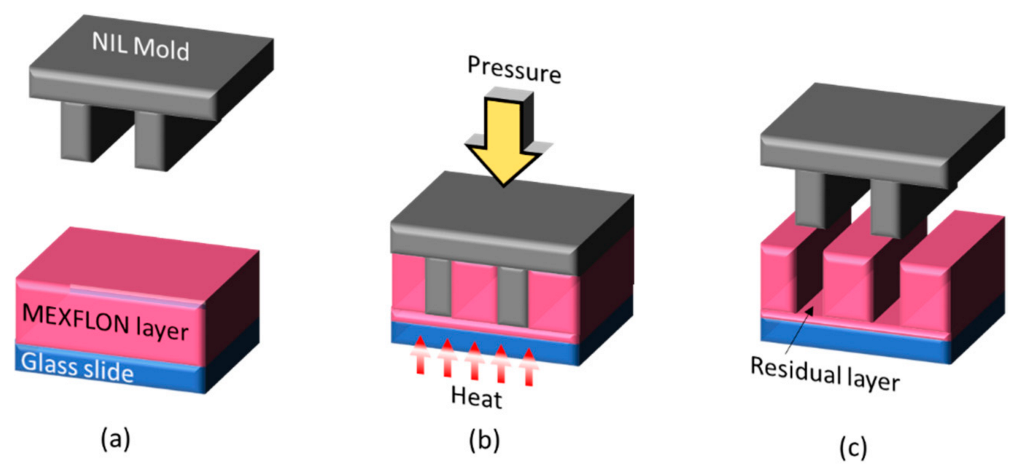
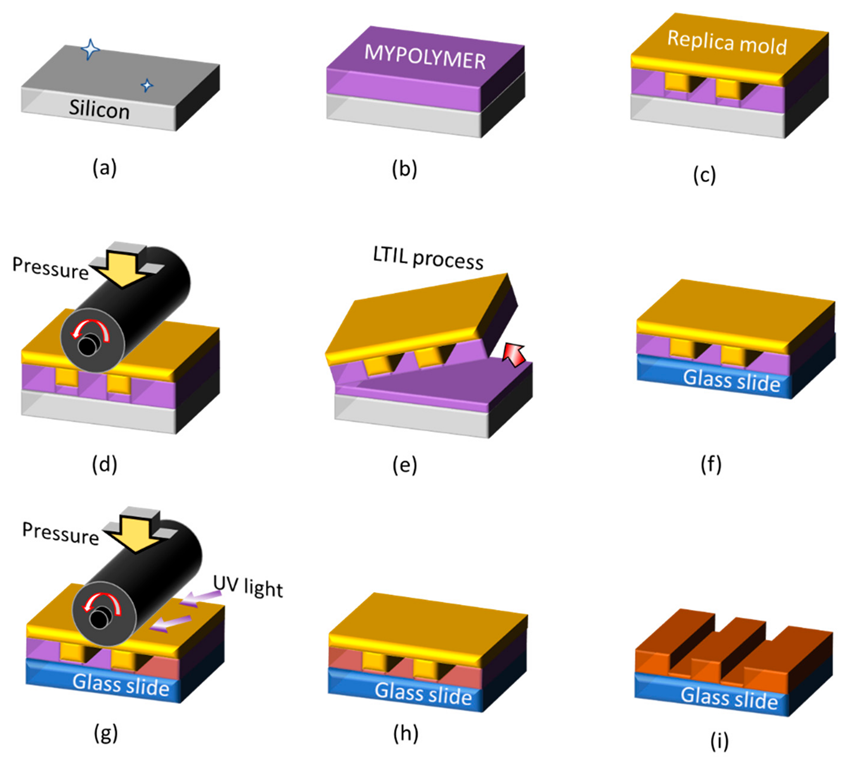
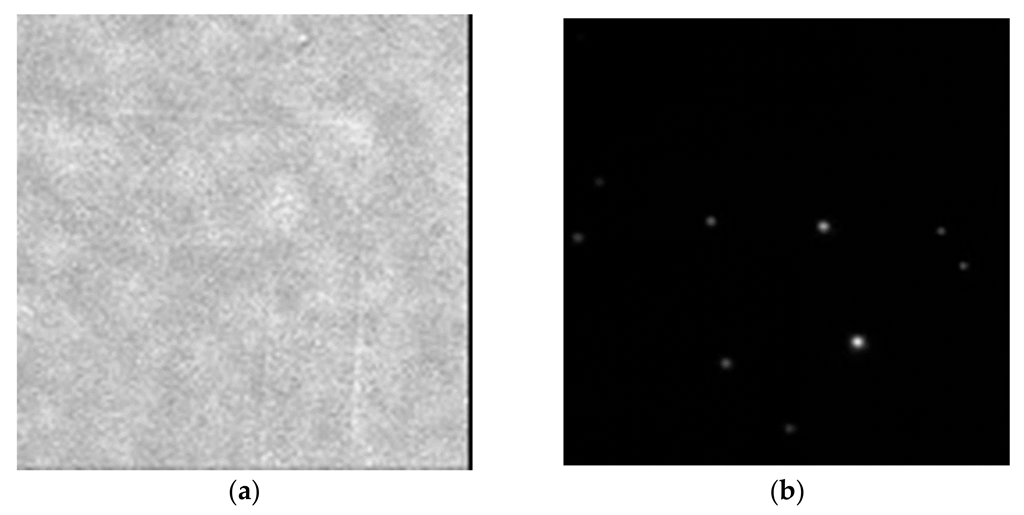
4. Imprint Technology for Nano-Obstacles for TIRF Measurements
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Adrian, L.; Adrian, R.J.; Westerweel, J. Particle Image Velocimetry; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Raffel, M.; Kähler, C.J.; Willert, C.E.; Wereley, S.T.; Scarano, F.; Kompenhans, J. Particle Image Velocimetry: A Practical Guide, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Budwig, R. Refractive-Index Matching Methods for Liquid Flow Investigations. Exp. Fluids 1994, 17, 350–355. [Google Scholar] [CrossRef]
- Wright, S.F.; Zadrazil, I.; Markides, C.N. A review of solid-fluid selection options for optical-based measurements in single-phase liquid, two-phase liquid-liquid and multiphase solid-liquid flows. Exp. Fluids 2017, 58, 108. [Google Scholar] [CrossRef]
- Satake, S.; Aoyagi, Y.; Tsuda, T.; Unno, N.; Yuki, K. Three-dimensional flow measurement of a sphere-packed pipe by a digital hologram and refractive index-matching method. Fusion Eng. Des. 2014, 89, 1064–1067. [Google Scholar] [CrossRef]
- Yuki, K.; Okurnura, M.; Hashizume, H.; Toda, S.; Morley, N.B.; Sagara, A. Flow Visualization and Heat Transfer Characteristics for Sphere-Packed Pipes. J. Thermophys. Heat Tranfer 2008, 22, 632–648. [Google Scholar] [CrossRef]
- Takeuchi, J.; Satake, S.I.; Morley, N.B.; Kunugi, T.; Yokomine, T.; Abdou, M.A. Experimental study of MHD effects on turbulent flow of Flibe simulant fluid in circular pipe. Fusion Eng. Des. 2008, 83, 1082–1086. [Google Scholar] [CrossRef]
- Fort, C.; Bardet, P.M. Refractive-index-matched polymer for experimental fluid dynamics in water. Exp. Fluids 2021, 62, 1–8. [Google Scholar] [CrossRef]
- Byron, M.L.; Variano, E.A. Refractive-index-matched hydrogel materials for measuring flow-structure interactions. Exp. Fluids 2013, 54, 1–6. [Google Scholar] [CrossRef]
- Someya, S.; Ochi, D.; Li, Y.; Tominaga, K.; Ishii, K.; Okamoto, K. Combined two-dimensional velocity and temperature measurements using a high-speed camera and luminescent particles. Appl. Phys. B-Lasers O 2010, 99, 325–332. [Google Scholar] [CrossRef]
- Ravasio, A.; Vaishnavi, S.; Ladoux, B.; Viasnoff, V. High-resolution imaging of cellular processes across textured surfaces using an indexed-matched elastomer. Acta Biomater. 2015, 14, 53–60. [Google Scholar] [CrossRef]
- Katz, J.; Sheng, J. Applications of Holography in Fluid Mechanics and Particle Dynamics. Annu. Rev. Fluid Mech. 2010, 42, 531–555. [Google Scholar] [CrossRef]
- Wereley, S.T.; Meinhart, C.D. Recent Advances in Micro-Particle Image Velocimetry. Annu. Rev. Fluid Mech. 2010, 42, 557–576. [Google Scholar] [CrossRef]
- Barnhart, D.H.; Adrian, R.J.; Papen, G.C. Phase-Conjugate Holographic System for High-Resolution Particle-Image Velocimetry. Appl. Opt. 1994, 33, 7159–7170. [Google Scholar] [CrossRef] [PubMed]
- Murata, S.; Yasuda, N. Potential of digital holography in particle measurement. Opt. Laser Technol. 2000, 32, 567–574. [Google Scholar] [CrossRef]
- Xu, W.B.; Jericho, M.H.; Meinertzhagen, I.A.; Kreuzer, H.J. Digital in-line holography for biological applications. Proc. Natl. Acad. Sci. USA 2001, 98, 11301–11305. [Google Scholar] [CrossRef]
- Owen, R.B.; Zozulya, A.A. In-line digital holographic sensor for monitoring and characterizing marine particulates. Opt. Eng. 2000, 39, 2187–2197. [Google Scholar] [CrossRef]
- Meng, H.; Pan, G.; Pu, Y.; Woodward, S.H. Holographic particle image velocimetry: From film to digital recording. Meas. Sci. Technol. 2004, 15, 673–685. [Google Scholar] [CrossRef]
- Malkiel, E.; Sheng, I.; Katz, J.; Strickler, J.R. The three-dimensional flow field generated by a feeding calanoid copepod measured using digital holography. J. Exp. Biol. 2003, 206, 3657–3666. [Google Scholar] [CrossRef]
- Kebbel, V.; Adams, M.; Hartmann, H.J.; Juptner, W. Digital holography as a versatile optical diagnostic method for microgravity experiments. Meas. Sci. Technol. 1999, 10, 893–899. [Google Scholar] [CrossRef]
- Schnars, U.; Juptner, W.P.O. Digital recording and numerical reconstruction of holograms. Meas. Sci. Technol. 2002, 13, R85–R101. [Google Scholar] [CrossRef]
- Soria, J.; Atkinson, C. Towards 3C-3D digital holographic fluid velocity vector field measurement-tomographic digital holographic PIV (Tomo-HPIV). Meas. Sci. Technol. 2008, 19, 074002. [Google Scholar] [CrossRef]
- Satake, S.I.; Kunugi, T.; Sato, K.; Ito, T. Digital holographic particle tracking velocimetry for 3-D transient flow around an obstacle in a narrow channel. Opt. Rev. 2004, 11, 162–164. [Google Scholar] [CrossRef]
- Xu, W.; Jericho, M.H.; Meinertzhagen, I.A.; Kreuzer, H.J. Digital in-line holography of microspheres. Appl. Opt. 2002, 41, 5367–5375. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Murata, S.; Morihara, T. Three-dimensional flow measurement by digital holographic particle image velocimetry with spatio-temporal derivative method (Evaluation of the measurement accuracy by numerical simulation). JSME Int. J. Ser. B-Fluids Therm. Eng. 2006, 49, 1133–1140. [Google Scholar] [CrossRef]
- Hinsch, K.D. 3-Dimensional Particle Velocimetry. Meas. Sci. Technol. 1995, 6, 742–753. [Google Scholar] [CrossRef]
- Sheng, J.; Malkiel, E.; Katz, J. Using digital holographic microscopy for simultaneous measurements of 3D near wall velocity and wall shear stress in a turbulent boundary layer. Exp. Fluids 2008, 45, 1023–1035. [Google Scholar] [CrossRef]
- Ooms, T.A.; Lindken, R.; Westerweel, J. Digital holographic microscopy applied to measurement of a flow in a T-shaped micromixer. Exp. Fluids 2009, 47, 941–955. [Google Scholar] [CrossRef]
- Satake, S.; Kunugi, T.; Sato, K.; Ito, T.; Taniguchi, J. Three-dimensional flow tracking in a micro channel with high time resolution using micro digital-holographic particle-tracking velocimetry. Opt. Rev. 2005, 12, 442–444. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.J. Measurement of 3D laminar flow inside a micro tube using micro digital holographic particle tracking velocimetry. J. Micromech. Microeng. 2007, 17, 2157–2162. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.J. Effect of particle number density in in-line digital holographic particle velocimetry. Exp. Fluids 2008, 44, 623–631. [Google Scholar] [CrossRef]
- Satake, S.; Kunugi, T.; Sato, K.; Ito, T.; Kanamori, H.; Taniguchi, J. Measurements of 3D flow in a micro-pipe via micro digital holographic particle tracking velocimetry. Meas. Sci. Technol. 2006, 17, 1647–1651. [Google Scholar] [CrossRef]
- Satake, S.I.; Anraku, T.; Kanamori, H.; Kunugi, T.; Sato, K.; Ito, T. Study on high speed parallel algorithm using PC grid environment for visualization measurements by Digital Holographic Particle Tracking Velocimetry. Comput. Phys. Commun. 2008, 178, 1–7. [Google Scholar] [CrossRef]
- Satake, S.I.; Kanamori, H.; Kunugi, T.; Sato, K.; Ito, T.; Yamamoto, K. Parallel computing of a digital hologram and particle searching for microdigital-holographic particle-tracking velocimetry. Appl. Opt. 2007, 46, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Masuda, N.; Wakabayashi, H.; Kazo, Y.; Ito, T.; Satake, S.; Kunugi, T.; Sato, K. Special purpose computer system for flow visualization using holography technology. Opt. Express 2008, 16, 7686–7692. [Google Scholar] [CrossRef] [PubMed]
- Masuda, N.; Sugie, T.; Ito, T.; Tanaka, S.; Hamada, Y.; Satake, S.; Kunugi, T.; Sato, K. Special purpose computer system with highly parallel pipelines for flow visualization using holography technology. Comput. Phys. Commun. 2010, 181, 1986–1989. [Google Scholar] [CrossRef]
- Masuda, N.; Ito, T.; Kayama, K.; Kono, H.; Satake, S.; Kunugi, T.; Sato, K. Special purpose computer for digital holographic particle tracking velocimetry. Opt. Express 2006, 14, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Satake, S.; Anraku, T.; Kanamori, H.; Kunugi, T.; Sato, K.; Ito, T. Measurements of three-dimensional flow in microchannel with complex shape by micro-digital-holographic particle-tracking-velocimetry. J. Heat Trans.-T Asme 2008, 130, 042413. [Google Scholar] [CrossRef]
- Satake, S.; Yonemoto, Y.; Kikuchi, T.; Kunugi, T. Detection of microbubble position by a digital hologram. Appl. Opt. 2011, 50, 5999–6005. [Google Scholar] [CrossRef]
- Ooms, T.; Koek, W.; Westerweel, J. Digital holographic particle image velocimetry: Eliminating a sign-ambiguity error and a bias error from the measured particle field displacement. Meas. Sci. Technol. 2008, 19, 074003. [Google Scholar] [CrossRef]
- Yoshida, S.; Itakura, K. Compressed holographic particle tracking velocimetry for microflow measurements. Opt. Rev. 2020, 27, 441–446. [Google Scholar] [CrossRef]
- Zhou, Z.; Santosh, K.S.; Mallery, K.; Jiang, W.S.; Hong, J.R. Holographic astigmatic particle tracking velocimetry (HAPTV). Meas. Sci. Technol. 2020, 31, 065202. [Google Scholar] [CrossRef]
- Satake, S.; Aoyagi, Y.; Unno, N.; Yuki, K.; Seki, Y.; Enoeda, M. Three-dimensional flow measurement of a water flow in a sphere-packed pipe by digital holographic PTV. Fusion Eng. Des. 2015, 98–99, 1864–1867. [Google Scholar] [CrossRef]
- Satake, S.; Taniguchi, J.; Kanai, T.; Unno, N. Three-dimensional measurements of UV-imprint process by micro-digital holographic-PTV. Microelectron Eng. 2012, 97, 51–54. [Google Scholar] [CrossRef]
- Satake, S.I.; Anraku, T.; Tamoto, F.; Sato, K.; Kunugi, T. Three-dimensional simultaneous measurements of micro-fluorescent-particle position and temperature field via digital hologram. Microelectron Eng. 2011, 88, 1875–1877. [Google Scholar] [CrossRef]
- Axelrod, D. Evanescent Excitation and Emission in Fluorescence Microscopy. Biophys. J. 2013, 104, 1401–1409. [Google Scholar] [CrossRef]
- Huang, P.; Breuer, K.S.; Guasto, J.S. Evanescent Wave Microscopy. In Encyclopedia of Microfluidics and Nanofluidics; Li, D., Ed.; Springer: New York, NY, USA, 2015; pp. 1050–1059. [Google Scholar]
- Li, H.F.; Sadr, R.; Yoda, M. Multilayer nano-particle image velocimetry. Exp. Fluids 2006, 41, 185–194. [Google Scholar] [CrossRef]
- Yoda, M. Super-Resolution Imaging in Fluid Mechanics Using New Illumination Approaches. Annu. Rev. Fluid Mech. 2020, 52, 369–393. [Google Scholar] [CrossRef]
- Parikesit, G.O.F.; Guasto, J.S.; Girardo, S.; Mele, E.; Stabile, R.; Pisignano, D.; Lindken, R.; Westerweel, J. Nanoparticle image velocimetry at topologically structured surfaces. Biomicrofluidics 2009, 3, 044111. [Google Scholar] [CrossRef] [PubMed]
- Unno, N.; Maeda, A.; Satake, S.; Tsuji, T.; Taniguchi, J. Fabrication of nanostep for total internal reflection fluorescence microscopy to calibrate in water. Microelectron Eng. 2015, 133, 98–103. [Google Scholar] [CrossRef]
- Taniguchi, J.; Idea, S.; Unno, N.; Sakaguchi, H. Nanoprint lithography of gold nanopatterns on polyethylene terephthalate. Microelectron Eng. 2009, 86, 590–595. [Google Scholar] [CrossRef]
- Satake, S.; Unno, N.; Nakata, S.; Taniguchi, J. 3D measurement of the position of gold particles via evanescent digital holographic particle tracking velocimetry. Meas. Sci. Technol. 2016, 27, 084009. [Google Scholar] [CrossRef]
- Nakata, S.; Unno, N.; Satake, S.; Taniguchi, J. Three-dimensional thermal nanoimprinting by using lift-off patterns made of polymer resin. Microelectron Eng. 2016, 160, 81–86. [Google Scholar] [CrossRef]
- Unno, N.; Nakata, S.; Satake, S.; Taniguchi, J. Three-dimensional particle tracking around microstructures in water via total internal reflection fluorescence microscopy and refractive-index-matching method. Exp. Fluids 2016, 57, 120. [Google Scholar] [CrossRef]
- Merino, S.; Retolaza, A.; Juarros, A.; Schift, H. The influence of stamp deformation on residual layer homogeneity in thermal nanoimprint lithography. Microelectron. Eng. 2008, 85, 1892–1896. [Google Scholar] [CrossRef]
- Taylor, H.; Smistrup, K.; Boning, D. Modeling and simulation of stamp deflections in nanoimprint lithography: Exploiting backside grooves to enhance residual layer thickness uniformity. Microelectron. Eng. 2011, 88, 2154–2157. [Google Scholar] [CrossRef]
- Fernandez, A.; Medina, J.; Benkel, C.; Guttmann, M.; Bilenberg, B.; Thamdrup, L.H.; Nielsen, T.; Torres, C.M.S.; Kehagias, N. Residual layer-free Reverse Nanoimprint Lithography on silicon and metal-coated substrates. Microelectron. Eng. 2015, 141, 56–61. [Google Scholar] [CrossRef]
- Kolli, V.; Woidt, C.; Hillmer, H. Residual-layer-free 3D nanoimprint using hybrid soft templates. Microelectron. Eng. 2016, 149, 159–165. [Google Scholar] [CrossRef]
- Unno, N.; Kigami, H.; Fujinami, T.; Nakata, S.; Satake, S.; Taniguchi, J. Fabrication of calibration plate for total internal reflection fluorescence microscopy using roll-type liquid transfer imprint lithography. Microelectron. Eng. 2017, 180, 86–92. [Google Scholar] [CrossRef]
- Fujinami, T.; Kigami, H.; Unno, N.; Taniguchi, J.; Satake, S. Three-dimensional particle tracking in concave structures made by ultraviolet nanoimprint via total internal reflection fluorescence microscopy and refractive-index-matching method. Opt. Rev. 2018, 25, 437–443. [Google Scholar] [CrossRef]
- Matsuda, Y.; Kigami, H.; Unno, N.; Satake, S.; Taniguchi, J. Three-dimensional Flow Measurements around Micro-pillars Made by UV-NIL in Water via Micro-digital Holographic Particle Tracking Velocimetry (Micro-DHPTV). J. Photopolym. Sci. Technol. 2020, 33, 557–562. [Google Scholar] [CrossRef]
- Tadashi Ando, Y.H.; Yanagawa, T.; Unno, N.; Taniguchi, J.; Satake, S. A 3D-Simulation and Experimental Study of the Fluid Flow Around a Nano-Step Structure Formed by UV-NIL. J. Photopolym. Sci. Technol. 2022; in press. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satake, S.-i. Micro- and Nanoscale Imaging of Fluids in Water Using Refractive-Index-Matched Materials. Nanomaterials 2022, 12, 3203. https://doi.org/10.3390/nano12183203
Satake S-i. Micro- and Nanoscale Imaging of Fluids in Water Using Refractive-Index-Matched Materials. Nanomaterials. 2022; 12(18):3203. https://doi.org/10.3390/nano12183203
Chicago/Turabian StyleSatake, Shin-ichi. 2022. "Micro- and Nanoscale Imaging of Fluids in Water Using Refractive-Index-Matched Materials" Nanomaterials 12, no. 18: 3203. https://doi.org/10.3390/nano12183203
APA StyleSatake, S.-i. (2022). Micro- and Nanoscale Imaging of Fluids in Water Using Refractive-Index-Matched Materials. Nanomaterials, 12(18), 3203. https://doi.org/10.3390/nano12183203






