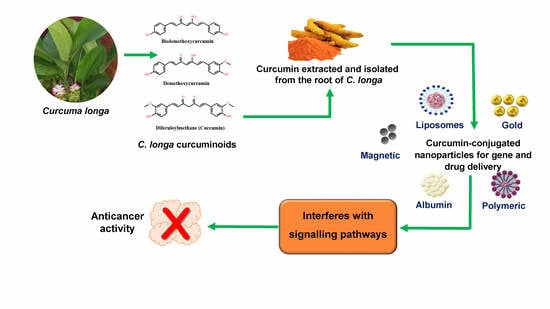
The Potential of Curcumin-Capped Nanoparticle Synthesis in Cancer Therapy: A Green Synthesis Approach
Abstract
:1. Introduction
2. Cancer Epidemiology
3. The Promise of Cancer Nanotherapeutics
3.1. The Limitations of Conventional Cancer Therapy
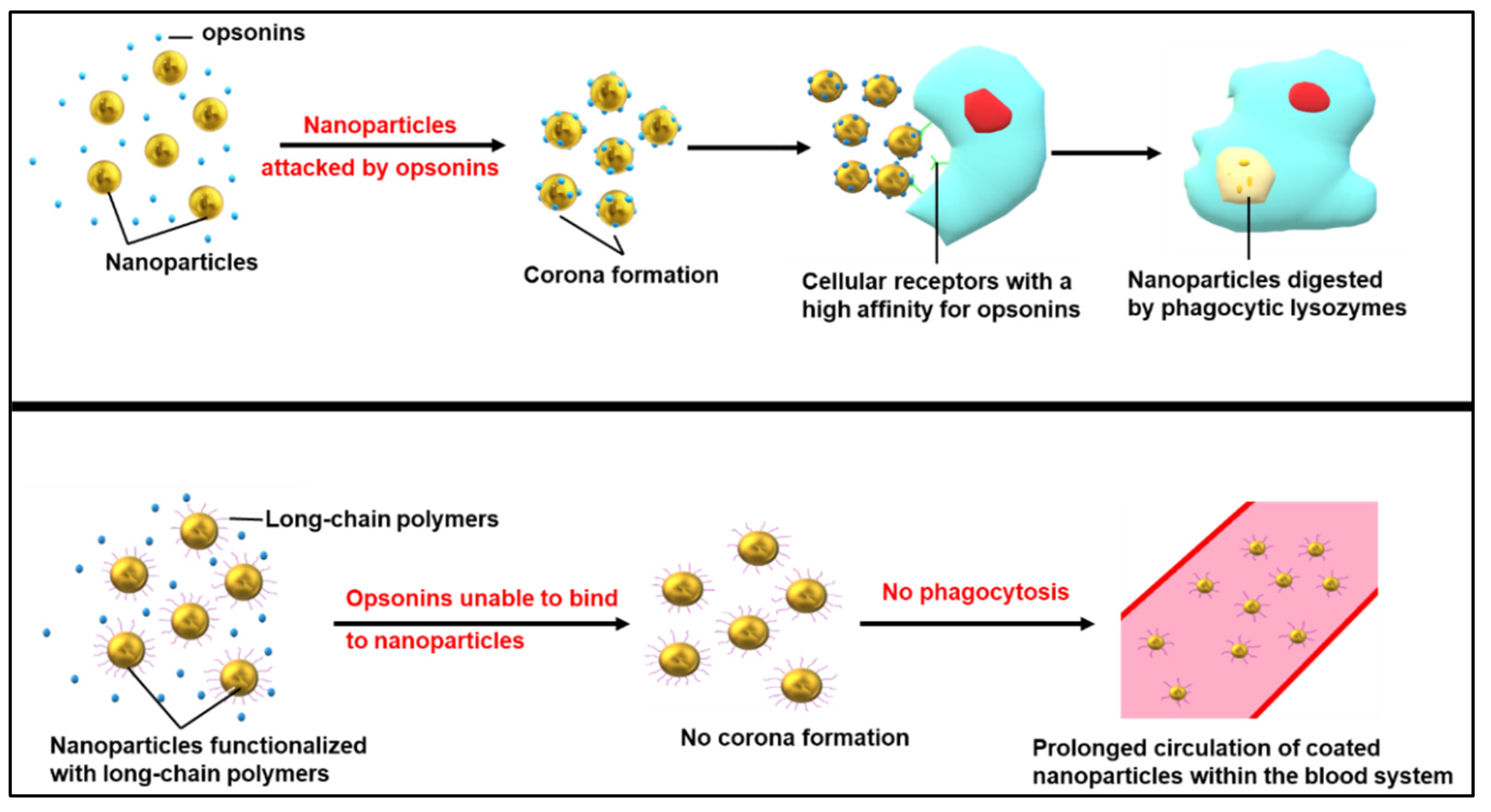
3.2. Overcoming Cancer Therapy Limitations with Nanomedicine
4. Green Synthesis of NPs
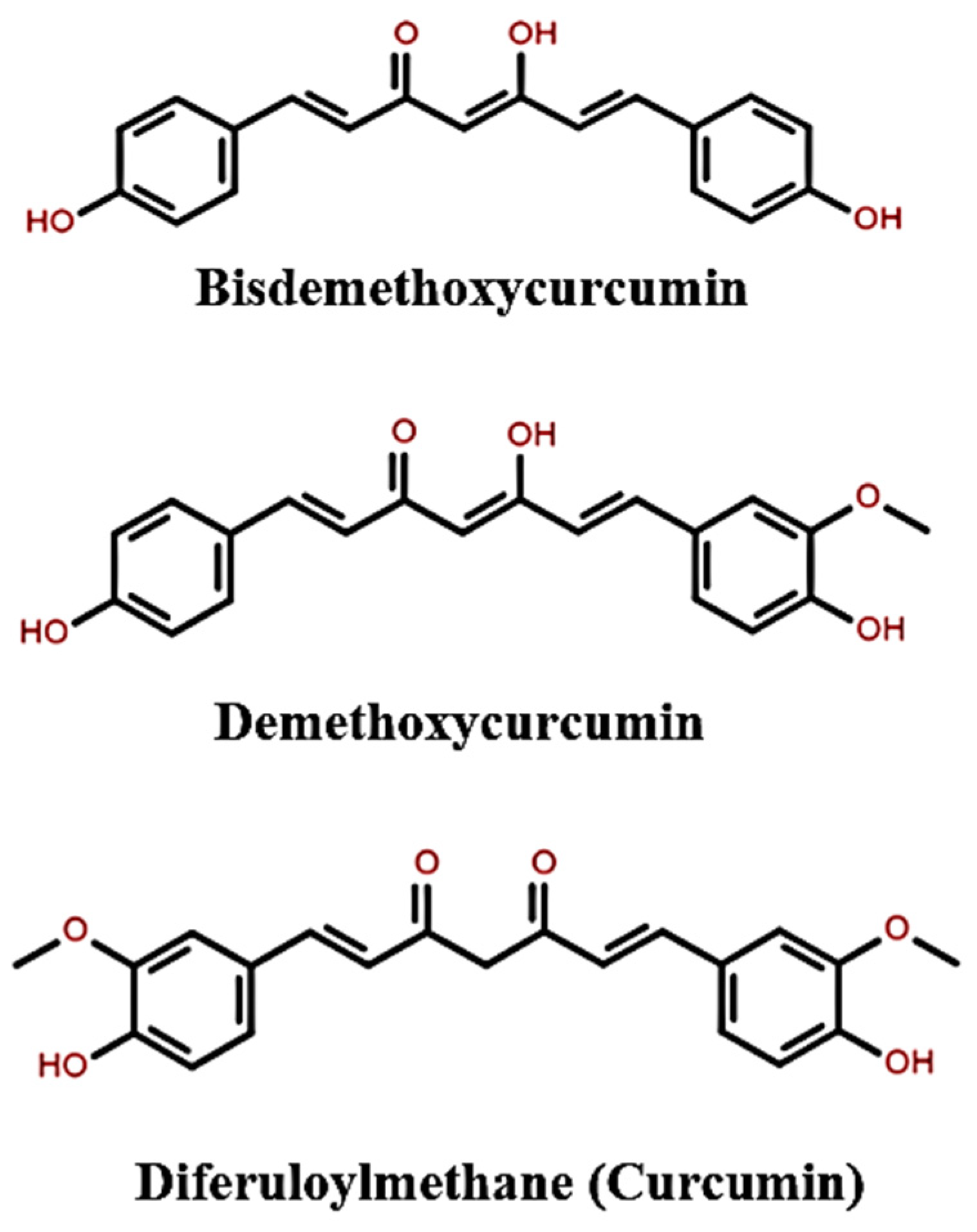
5. The Properties of Curcumin
5.1. Biological and Pharmaceutical Properties of Curcumin
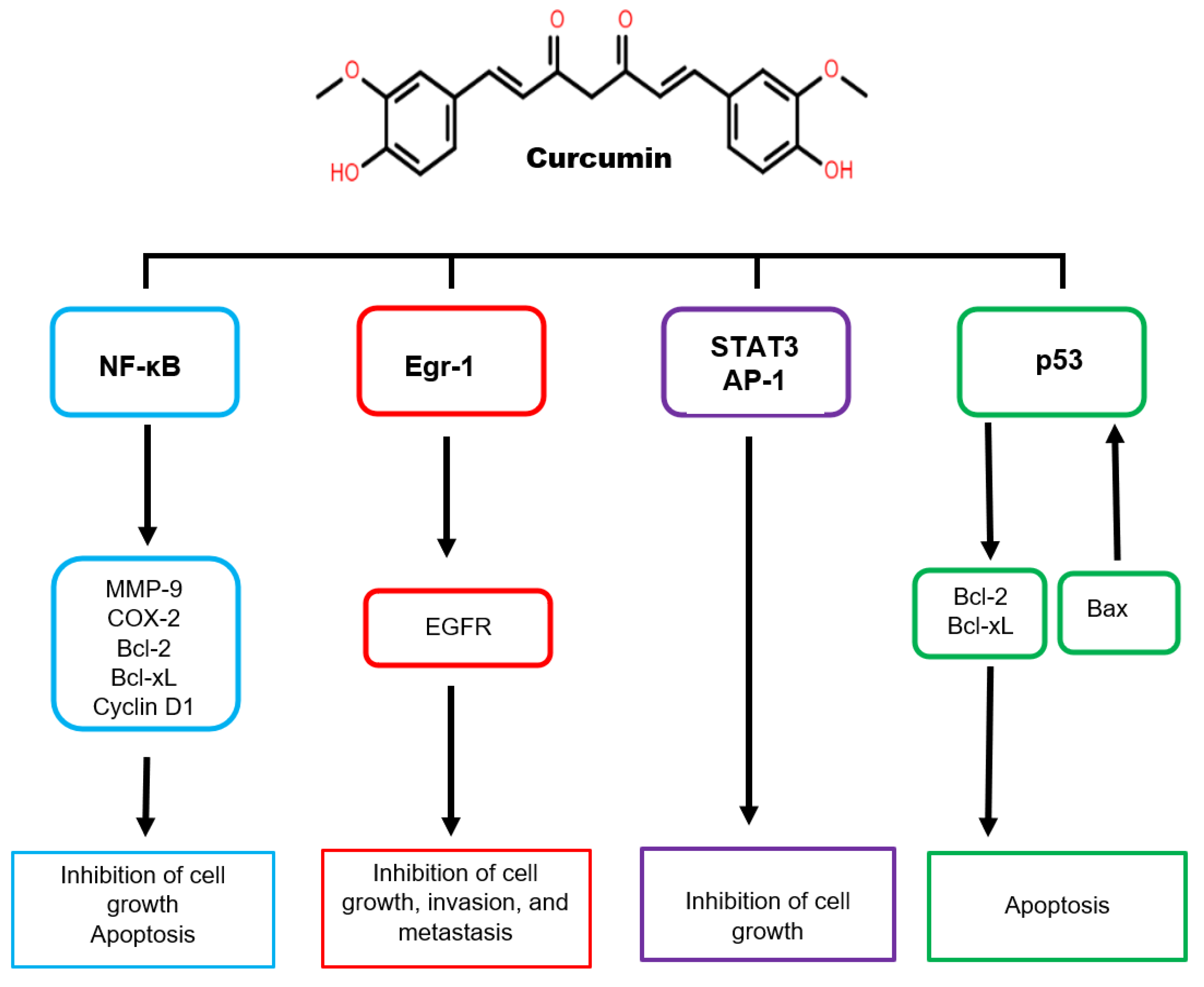
5.2. The Anticancer Properties of Curcumin
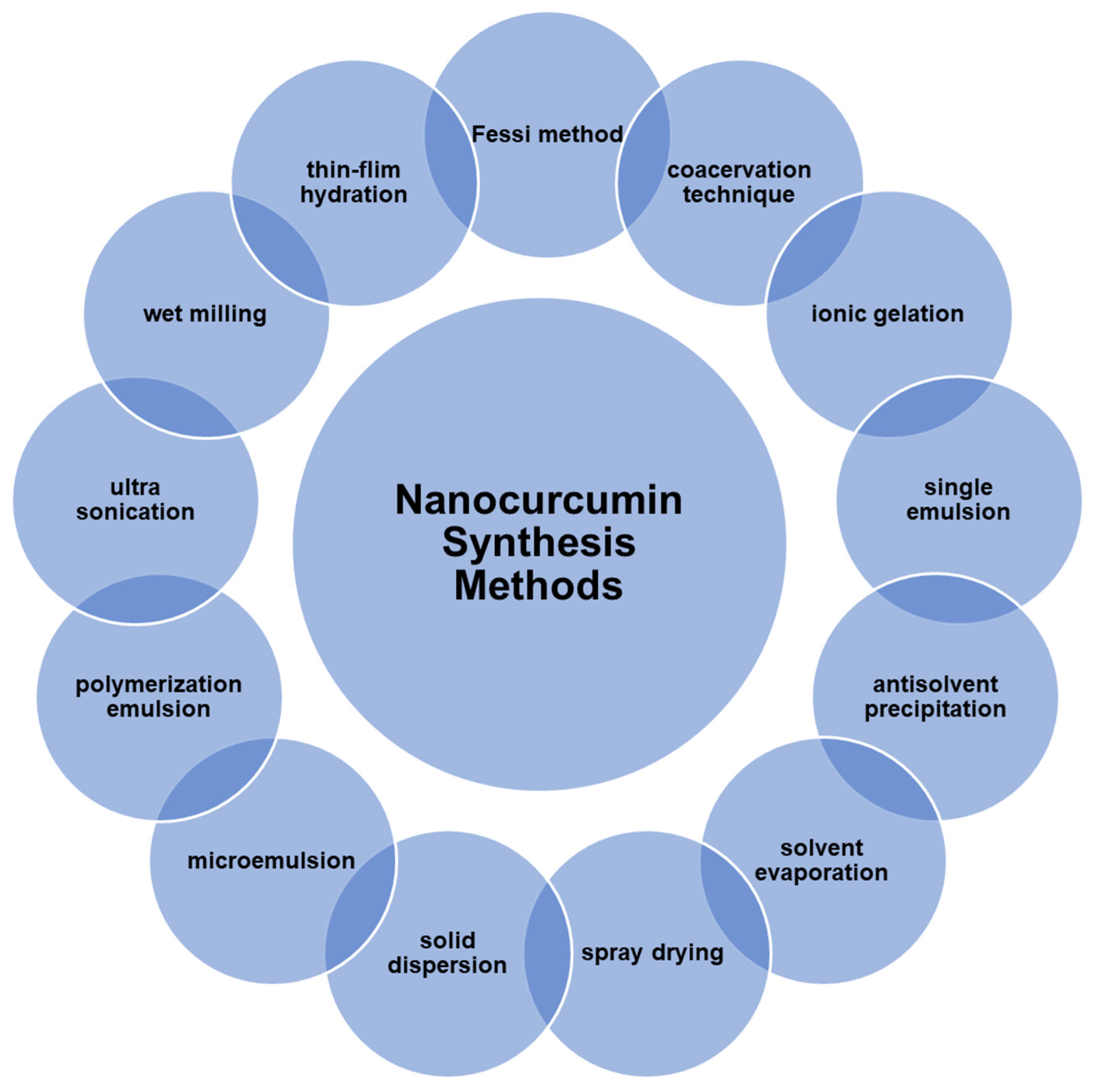
6. Nanocurcumin Synthesis
7. Curcumin-Capped NPs in Cancer Therapy
8. Clinical Trials Involving Curcumin
9. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krasteva, N.; Georgieva, M. Promising Therapeutic Strategies for Colorectal Cancer Treatment Based on Nanomaterials. Pharmaceutics 2022, 14, 1213. [Google Scholar] [CrossRef] [PubMed]
- Venkatas, J.; Singh, M. Nanomedicine-mediated optimization of immunotherapeutic approaches in cervical cancer. Nanomedicine 2021, 16, 1311–1328. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ward, Z.J.; Scott, A.M.; Hricak, H.; Atun, R. Global costs, health benefits, and economic benefits of scaling up treatment and imaging modalities for the survival of 11 cancers: A simulation-based analysis. Lancet Oncol. 2021, 22, 341–350. [Google Scholar] [CrossRef]
- Venkatas, J.; Singh, M. Cervical cancer: A meta-analysis, therapy, and future of nanomedicine. Ecancermedicalscience 2020, 14, 1111–1128. [Google Scholar] [CrossRef]
- Juárez, A.A.S.; Alvarado, E.M.; Gallegos, E.R. Cell death induced by photodynamic therapy with the conjugate of gold nanoparticles-PpIX in HeLa cell line. AIP Conf. Proc. 2019, 209, 4008–4012. [Google Scholar]
- Sun, Q.; Barz, M.; De Geest, B.G.; Diken, M.; Hennink, W.E.; Kiessling, F.; Lammers, T.; Shi, Y. Nanomedicine and macroscale materials in immuno-oncology. Chem. Soc. Rev. 2019, 48, 351–381. [Google Scholar]
- Muniyappan, N.; Pandeeswaran, M.; Amalraj, A. Green synthesis of gold nanoparticles using Curcuma pseudomontana isolated curcumin: Its characterization, antimicrobial, antioxidant, and anti-inflammatory activities. Environ. Chem. Ecotoxicol. 2021, 3, 117–124. [Google Scholar] [CrossRef]
- Rajput, N. Methods of preparation of nanoparticles-a review. Int. J. Adv. Eng. Technol. 2015, 7, 1806. [Google Scholar]
- Menon, S.; Shanmugam, R.; Kumar, V. A review on biogenic synthesis of gold nanoparticles, characterization, and its applications. Resour. Effic. Technol. 2017, 3, 516–527. [Google Scholar] [CrossRef]
- Olawale, F.; Oladimeji, O.; Ariatti, M.; Singh, M. Emerging Roles of green synthesized Chalcogen and Chalcogenide nanoparticles in Cancer theranostics. J. Nanotechnol. 2022, 2022, 6176610. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Yusoff, M.; Maniam, G.P.; Govindan, N. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications-an updated report. Saudi Pharm. J. 2016, 24, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, A.; Gholami, L.; Ghasemi, H.; Kheiripour, N. Effects of nano-curcumin and curcumin on the oxidant and antioxidant system of the liver mitochondria in aluminum phosphide-induced experimental toxicity. Nanomed. J. 2020, 7, 58–64. [Google Scholar]
- Saranya, T.S.; Rajan, V.K.; Biswas, R.; Jayakumar, R.; Sathianarayanan, S. Synthesis, characterization, and biomedical applications of curcumin conjugated chitosan microspheres. Int. J. Biol. Macromol. 2018, 110, 227–233. [Google Scholar] [CrossRef]
- Shen, L.; Liu, C.-C.; An, C.-Y.; Ji, H.-F. How does curcumin work with poor bioavailability? Clues from experimental and theoretical studies. Sci. Rep. 2016, 6, 20872. [Google Scholar] [CrossRef]
- Ubeyitogullari, A.; Ciftci, O.N. A novel and green nanoparticle formation approach to forming low-crystallinity curcumin nanoparticles to improve curcumin’s bioaccessibility. Sci. Rep. 2019, 9, 19112. [Google Scholar] [CrossRef]
- Chen, S.; Wu, J.; Tang, Q.; Xu, C.; Huang, Y.; Huang, D.; Wang, S. Nano-micelles based on hydroxyethyl starch-curcumin conjugates improve curcumin’s stability, antioxidant, and anticancer activity. Carbohydr. Polym. 2020, 228, 115398. [Google Scholar] [CrossRef]
- Al Bostami, R.D.; Abuwatfa, W.H.; Husseini, G.A. Recent Advances in Nanoparticle-Based Co-Delivery Systems for Cancer Therapy. Nanomaterials 2022, 12, 2672. [Google Scholar] [CrossRef]
- Wong, K.E.; Ngai, S.C.; Chan, K.G.; Lee, L.H.; Goh, B.H.; Chuah, L.H. Curcumin nanoformulations for colorectal cancer: A review. Front. Pharmacol. 2019, 10, 152. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Health Estimates 2020: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2020. 2021. Available online: https://gco.iarc.fr/today/home (accessed on 29 June 2021).
- Lin, L.; Yan, L.; Liu, Y.; Yuan, F.; Li, H.; Ni, J. Incidence, and death in 29 cancer groups in 2017 and trend analysis from 1990 to 2017 from the Global Burden of Disease Study. J. Hematol. Oncol. 2019, 12, 96–113. [Google Scholar] [CrossRef]
- Rivenbark, A.G. An Overview of Cancer Genes. In The Molecular Basis of Human Cancer; Coleman, W.E., Tsongalis, G.J., Eds.; Springer: New York, NY, USA, 2017; pp. 121–142. [Google Scholar]
- Padayachee, J.; Daniels, A.N.; Balgobind, A.; Ariatti, M.; Singh, M. HER-2/neu and MYC gene silencing in breast cancer: Therapeutic potential and advancement in non-viral nanocarrier systems. Nanomedicine 2020, 15, 1437–1452. [Google Scholar] [CrossRef] [PubMed]
- Momenimovahed, Z.; Salehiniya, H. Incidence, mortality, and risk factors of cervical cancer in the world. Biomed. Res. Ther. 2017, 4, 1795–1811. [Google Scholar] [CrossRef]
- Maistro, S.; Teixeira, N.; Encinas, G.; Katayama, M.L.H.; Niewiadonski, V.D.T.; Cabral, L.G.; Sabino, E.C. Germline mutations in BRCA1 and BRCA2 in epithelial ovarian cancer patients in Brazil. BMC Cancer 2016, 16, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Sample, K.M. DNA repair gene expression is associated with differential prognosis between HPV16 and HPV18 positive cervical cancer patients following radiation therapy. Sci. Rep. 2020, 10, 2774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.; Husain, S.; Marathe, A.; Haq, M. Molecular Genetics of Cancer. Int. J. Hum. Health Sci. 2018, 2, 199–208. [Google Scholar]
- Roy, N.K.; Bordoloi, D.; Monisha, J.; Anip, A.; Padmavathi, G.; Kunnumakkara, A.B. Cancer—An Overview and Molecular Alterations in Cancer. In Fusion Genes and Cancer; Kunnumakkara, A.B., Padmavathi, G., Roy, N.K., Eds.; World Scientific: Singapore, 2017; pp. 1–15. [Google Scholar]
- Rajabi, M.; Mousa, S.A. The role of angiogenesis in cancer treatment. Biomedicines 2017, 5, 34. [Google Scholar] [CrossRef]
- Tomasetti, C.; Li, L.; Vogelstein, B. Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention. Science 2017, 355, 1330–1334. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Singh, A.; Chien, C.Y.; Warnakulasuriya, S. Tobacco related oral cancer. BMJ 2019, 365, 2142–2149. [Google Scholar] [CrossRef]
- Miura, K.; Olsen, C.M.; Rea, S.; Marsden, J.; Green, A.C. Melanoma and skin cancers in airline pilots and cabin crew. Br. J. Dermatol. 2019, 181, 6–16. [Google Scholar] [CrossRef]
- Murtono, M.; Ndii, M.Z.; Sugiyanto, S. Mathematical model of cervical cancer treatment using chemotherapy drug. Biol. Med. Nat. Prod. Chem. 2019, 8, 11–15. [Google Scholar] [CrossRef]
- Kong, S.Y.; Huang, K.; Zeng, C.; Ma, X.; Wang, S. The association between short-term response and long-term survival for cervical cancer patients undergoing neoadjuvant chemotherapy: A system review and meta-analysis. Sci. Rep. 2018, 8, 1545. [Google Scholar] [CrossRef] [PubMed]
- Lohitesh, K.; Chowdhury, R.; Mukherjee, S. Resistance a major hindrance to chemotherapy in hepatocellular carcinoma: An insight. Cancer Cell Int. 2018, 18, 44. [Google Scholar] [CrossRef]
- Feynman, R.P. There’s Plenty of Room at the Bottom. Eng. Sci. 1960, 23, 22–36. [Google Scholar]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, properties, and regulatory issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef] [PubMed]
- Roacho-Pérez, J.A.; Ruiz-Hernandez, F.G.; Chapa-Gonzalez, C.; Martínez-Rodríguez, H.G.; Flores-Urquizo, I.A.; Pedroza-Montoya, F.E.; Garza-Treviño, E.N.; Bautista-Villareal, M.; García-Casillas, P.E.; Sánchez-Domínguez, C.N. Magnetite Nanoparticles Coated with PEG 3350-Tween 80: In vitro Characterization Using Primary Cell Cultures. Polymers 2020, 12, 300. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.; Singh, M. Recent advances in lipid-based nanosystems for gemcitabine and gemcitabine–combination therapy. Nanomaterials 2021, 11, 597. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Huang, D.; Peppas, N.A. Advanced engineered nanoparticulate platforms to address key biological barriers for delivering chemotherapeutic agents to target sites. Adv. Drug Deliv. Rev. 2020, 167, 170–188. [Google Scholar] [CrossRef]
- El-Readi, M.Z.; Althubiti, M.A. Cancer nanomedicine: A new era of successful targeted therapy. J. Nanomater. 2019, 2019, 4927312. [Google Scholar] [CrossRef]
- Oladimeji, O.; Akinyelu, J.; Daniels, A.; Singh, M. Modified Gold Nanoparticles for efficient Delivery of Betulinic Acid to Cancer Cell Mitochondria. Int. J. Mol. Sci. 2021, 22, 5072. [Google Scholar] [CrossRef]
- Joseph, C.; Daniels, A.; Singh, S.; Singh, M. Histidine-tagged Folate-Targeted Gold Nanoparticles for enhanced transgene expression in Breast Cancer Cells in vitro. Pharmaceutics 2022, 14, 53. [Google Scholar] [CrossRef]
- Maiyo, F.; Singh, M. Polymerized Selenium nanoparticles for Folate-Receptor Targeted Delivery of anti-Luc-siRNA: Potential for Gene Silencing. Biomedicines 2020, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Moitra, K. Overcoming multidrug resistance in cancer stem cells. Bio. Med. Res. Int. 2015, 2015, 635745. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lane, L.A. Probing the biological obstacles of nanomedicine with gold nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, 1542. [Google Scholar] [CrossRef]
- Naicker, K.; Ariatti, M.; Singh, M. Active targeting of asiaglycoprotein receptors using sterically stabilized lipoplexes. Eur. J. Lipid Sci. Technol. 2016, 118, 1730–1742. [Google Scholar] [CrossRef]
- Ragelle, H.; Danhier, F.; Préat, V.; Langer, R.; Anderson, D.G. Nanoparticle-based drug delivery systems: A commercial and regulatory outlook as the field matures. Exp. Opin. Drug Deliv. 2017, 14, 851–864. [Google Scholar] [CrossRef]
- Moodley, T.; Singh, M. Sterically Stabilized Polymeric Mesoporous Silica Nanoparticles Improve Doxorubicin Efficiency: Tailored Cancer Therapy. Molecules 2020, 25, 742. [Google Scholar] [CrossRef] [Green Version]
- Daniels, A.; Singh, M.; Ariatti, M. Pegylated and Non-Pegylated siRNA lipoplexes formulated with cholesteryl cytofectins promote efficient Luciferase knockdown in HeLa tat luc cells. Nucleos. Nucleot. Nucl. 2013, 32, 206–220. [Google Scholar] [CrossRef]
- Omidi, Y.; Barar, J. Targeting tumor microenvironment: Crossing tumor interstitial fluid by multifunctional nanomedicines. BioImpacts B 2014, 4, 55. [Google Scholar]
- Shanker, U.; Jassal, V.; Rani, M.; Kaith, B.S. Towards Green Synthesis of Nanoparticles: From Bio-Assisted Sources to Benign Solvents. A Review. Int. J. Environ. Anal. Chem. 2016, 96, 801–835. [Google Scholar]
- Srivastava, S.; Usmani, Z.; Atanasov, A.G.; Singh, V.K.; Singh, N.P.; Abdel-Azeem, A.M.; Prasad, R.; Gupta, G.; Sharma, M.; Bhargava, A. Biological nanofactories: Using living forms for metal nanoparticle synthesis. Mini Rev. Med. Chem. 2021, 21, 245–265. [Google Scholar] [CrossRef]
- Peralta-Videa, J.R.; Huang, Y.; Parsons, J.G. Plant-based green synthesis of metallic nanoparticles: Scientific curiosity or a realistic alternative to chemical synthesis? Nanotechnol. Environ. Eng. 2016, 1, 4. [Google Scholar] [CrossRef]
- Rafique, M.; Tahir, R.; Gillani, S.S.A.; Tahir, M.B.; Shakil, M.; Iqbal, T.; Abdellahi, M.O. Plant-mediated green synthesis of zinc oxide nanoparticles from Syzygium Cumini for seed germination and wastewater purification. Int. J. Environ. Anal. Chem. 2022, 102, 23–38. [Google Scholar] [CrossRef]
- Guan, Z.; Ying, S.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 1, 102336. [Google Scholar]
- Kumari, S.C.; Dhand, V.; Padma, N. Green synthesis of metallic nanoparticles: A review. In Nanomaterials, Application in Biofuels and Bioenergy Production Systems, 1st ed.; Kumar, R.P., Bharathiraja, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 259–281. [Google Scholar]
- Dahoumane, S.A.; Yéprémian, C.; Djédiat, C. Improvement of kinetics, yield, and colloidal stability of biogenic gold nanoparticles using living cells of Euglena gracilis microalga. J. Nanoparticl. Res. 2016, 18, 79. [Google Scholar] [CrossRef]
- Khan, M.; Al-Marri, A.H.; Khan, M. Green approach for the effective reduction of graphene oxide using Salvadora persica L. root (Miswak) extract. Nanoscale Res Lett. 2015, 10, 281. [Google Scholar] [CrossRef]
- Hano, C.; Abbasi, B.H. Plant-Based Green Synthesis of Nanoparticles: Production, Characterization and Applications. Biomolecules 2021, 12, 31. [Google Scholar] [CrossRef]
- Ijaz, I.; Gilani, E.; Nazir, A.; Bukhari, A. Detail review on chemical, physical and green synthesis, classification, characterizations, and applications of nanoparticles. Green Chem. Lett. Rev. 2020, 13, 223–245. [Google Scholar] [CrossRef]
- Banach, M.; NPulit-Prociak, J. Proecological method for the preparation of metal nanoparticles. J. Clean. Prod. 2017, 141, 1030–1039. [Google Scholar] [CrossRef]
- Pedroza-Toscano, M.A.; Rabelero-Velasco, M.; Díaz de León, R.; Saade, H.; López, R.G.; Mendizábal, E.; Puig, J.E. Preparation of silver nanostructures from bicontinuous microemulsions. J. Nanomater. 2012, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Rashid, M.U.; Bhuiyan, M.K.H.; Quayum, M.E. Synthesis of silver nanoparticles (Ag-NPs) and their uses for quantitative analysis of vitamin C tablets. Dhaka Univ. J. Pharm. Sci. 2013, 12, 29–33. [Google Scholar] [CrossRef]
- Tsekhmistrenko, S.I.; Bityutskyy, V.S.; Tsekhmistrenko, O.S.; Horalskyi, L.P.; Tymoshok, N.O.; Spivak, M.Y. Bacterial synthesis of nanoparticles: A green approach. Biosyst. Divers. 2020, 28, 9–17. [Google Scholar] [CrossRef]
- Mareeswari, P.; Brijitta, J.; Etti, S.H.; Meganathan, C.; Kaliaraj, G.S. Rhizopus stolonifer mediated biosynthesis of biocompatible cadmium chalcogenide quantum dots. Enzyme Microb. Technol. 2016, 95, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Gholami-Shabani, M.; Shams-Ghahfarokhi, M.; Gholami-Shabani, Z.; Akbarzadeh, A.; Riazi, G.; Ajdari, S.; Amani, A.; Razzaghi-Abyaneh, M. Enzymatic synthesis of gold nanoparticles using sulfite reductase purified from Escherichia coli: A green eco-friendly approach. Process Biochem. 2015, 50, 1076–1085. [Google Scholar] [CrossRef]
- Li, J.; Tian, B.; Li, T.; Dai, S.; Weng, Y.; Lu, J.; Xu, X.; Jin, Y.; Pang, R.; Hua, Y. Biosynthesis of Au, Ag and Au–Ag bimetallic nanoparticles using protein extracts of Deinococcus radiodurans and evaluation of their cytotoxicity. Int. J. Nanomed. 2018, 13, 1411. [Google Scholar] [CrossRef] [PubMed]
- Iranmanesh, S.; Bonjar, G.H.S.; Baghizadeh, A. Study of the biosynthesis of gold nanoparticles by using several saprophytic fungi. SN Appl. Sci. 2020, 2, 1851. [Google Scholar] [CrossRef]
- Elshafei, A.M.; Othman, A.M.; Elsayed, M.A.; Al-Balakocy, N.G.; Hassan, M.M. Green synthesis of silver nanoparticles using Aspergillus oryzae NRRL447 exogenous proteins: Optimization via central composite design, characterization, and biological applications. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100553. [Google Scholar] [CrossRef]
- Dikshit, P.K.; Kumar, J.; Das, A.K.; Sadhu, S.; Sharma, S.; Singh, S.; Kim, B.S. Green Synthesis of Metallic Nanoparticles: Applications and Limitations. Catalysts 2021, 11, 902. [Google Scholar] [CrossRef]
- Deepak, P.; Amutha, V.; Kamaraj, C.; Balasubramani, G.; Aiswarya, D.; Perumal, P. Chemical and green synthesis of nanoparticles and their efficacy on cancer cells. In Micro and Nanotechnologies, Green Synthesis, Characterization and Applications of Nanoparticles; Shukla, A.K., Iravani, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 369–387. [Google Scholar]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Naidoo, C.M.; Naidoo, Y.; Dewir, Y.H.; Singh, M.; Daniels, A.N.; El-Ramady, H. In vitro investigation of the antioxidant and cytotoxic potential of Tabernaemontana ventricosa Hochst. ex A. DC. leaf, stem, and latex extracts. Horticulturae 2022, 8, 91. [Google Scholar] [CrossRef]
- Patra, D.; El Kurdi, R. Curcumin as a novel reducing and stabilizing agent for the green synthesis of metallic nanoparticles. Green Chem. Lett. Rev. 2021, 14, 474–487. [Google Scholar] [CrossRef]
- Nahari, M.H.; Al Ali, A.; Asiri, A.; Mahnashi, M.H.; Shaikh, I.A.; Shettar, A.K.; Hoskeri, J. Green Synthesis and Characterization of Iron Nanoparticles Synthesized from Aqueous Leaf Extract of Vitex leucoxylon and Its Biomedical Applications. Nanomaterials 2022, 12, 2404. [Google Scholar] [CrossRef] [PubMed]
- Latif, M.S.; Abbas, S.; Kormin, F.; Mustafa, M.K. Green synthesis of plant-mediated metal nanoparticles: The role of polyphenols. Asian J. Pharmaceut. Clin. Res. 2019, 12, 75–84. [Google Scholar] [CrossRef]
- Fabiyi, O.A.; Alabi, R.O.; Ansari, R.A. Nanoparticles’ synthesis and their application in the management of phytonematodes: An overview. In Management of Phytonematodes: Recent Advances and Future Challenges; Ansari, R., Rizvi, R., Mahmood, I., Eds.; Springer: Singapore, 2020; pp. 125–140. [Google Scholar]
- Azevedo de M. Oliveira, L.F.; de Azevedo Teles da Silva, L.V.; do Nascimento, T.G.; de Almeida, L.M.; Calumby, R.J.N.; Nunes, Á.M.; de Magalhães Oliveira, L.M.T.; da Silva Fonseca, E.J. Antioxidant, and antimicrobial activity of red propolis embedded mesoporous silica nanoparticles. Drug Dev. Ind. Pharm. 2020, 46, 1199–1208. [Google Scholar]
- Sankar, R.; Maheswari, R.; Karthik, S.; Shivashangari, K.S.; Ravikumar, V. Anticancer activity of Ficus religiosa engineered copper oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 234–239. [Google Scholar] [CrossRef]
- Dorosti, N.; Jamshidi, F. Plant-mediated gold nanoparticles by Dracocephalum kotschyi as anticholinesterase agent: Synthesis, characterization, and evaluation of anticancer and antibacterial activity. J. Appl. Biomed. 2016, 14, 235–245. [Google Scholar] [CrossRef]
- Muthukrishnan, S.; Kumar, T.S.; Rao, M.V. Anticancer activity of biogenic nanosilver and its toxicity assessment on Artemia salina-evaluation of mortality, accumulation, and elimination: An experimental report. J. Environ. Chem. Eng. 2017, 5, 1685–1695. [Google Scholar] [CrossRef]
- Seetharaman, P.; Chandrasekaran, R.; Gnanasekar, S.; Mani, I.; Sivaperumal, S. Biogenic gold nanoparticles synthesized using Crescentia cujete L. and evaluation of their different biological activities. Biocatal. Agric. Biotechnol. 2017, 11, 75–82. [Google Scholar] [CrossRef]
- Boomi, P.; Poorani, G.P.; Selvam, S.; Palanisamy, S.; Jegatheeswaran, S.; Anand, K.; Balakumar, C.; Premkumar, K.; Prabu, H.G. Green biosynthesis of gold nanoparticles using Croton sparsiflorus leaves extract and evaluation of UV protection, antibacterial and anticancer applications. Appl. Organomet. Chem. 2020, 34, e5574. [Google Scholar] [CrossRef]
- Nayak, D.; Pradhan, S.; Ashe, S.; Rauta, P.R.; Nayak, B. Biologically synthesized silver nanoparticles from three diverse family of plant extracts and their anticancer activity against epidermoid A431 carcinoma. J. Colloid Interface Sci. 2015, 457, 329–338. [Google Scholar] [CrossRef]
- Venugopal, K.; Ahmad, H.; Manikandan, E.; Arul, K.T.; Kavitha, K.; Moodley, M.K.; Rajagopal, K.; Balabhaskar, R.; Bhaskar, M. The impact of anticancer activity upon Beta vulgaris extract mediated biosynthesized silver nanoparticles (ag-NPs) against human breast (MCF-7), lung (A549) and pharynx (Hep-2) cancer cell lines. J. Photochem. Photobiol. B Biol. 2017, 173, 99–107. [Google Scholar] [CrossRef]
- Olawale, F.; Ariatti, M.; Singh, M. Ocimum tenuiflorum L. Mediated Green Synthesis of Silver and Selenium Nanoparticles: Antioxidant activity, Cytotoxicity and Density Functional Theory Studies. Adv. Nat. Sci. Nanosci. Nanotechnol. 2020, 13, 015015. [Google Scholar] [CrossRef]
- Olawale, F.; Ariatti, M.; Singh, M. Biogenic Synthesis of Silver-Core Selenium-Shell Nanoparticles Using Ocimum tenuiflorum L.: Response Surface Methodology Based Optimization and Biological Activity. Nanomaterials 2021, 11, 2516. [Google Scholar] [CrossRef] [PubMed]
- Naraginti, S.; Li, Y. Preliminary investigation of catalytic, antioxidant, anticancer and bactericidal activity of green synthesized silver and gold nanoparticles using Actinidia deliciosa. J. Photochem. Photobiol. B Biol. 2017, 170, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Bello, B.A.; Khan, S.A.; Khan, J.A.; Syed, F.Q.; Mirza, M.B.; Shah, L.; Khan, S.B. Anticancer, antibacterial and pollutant degradation potential of silver nanoparticles from Hyphaene thebaica. Biochem. Biophys. Res. Commun. 2017, 490, 889–894. [Google Scholar] [CrossRef]
- Aygun, A.; Gülbagca, F.; Ozer, L.Y.; Ustaoglu, B.; Altunoglu, Y.C.; Celik, Y.; Baloglu, M.C.; Atalar, M.N.; Alma, M.H.; Sen, F. Biogenic platinum nanoparticles using black cumin seed and their potential usage as antimicrobial and anticancer agent. J. Pharm. Biomed. Anal. 2020, 179, 112961. [Google Scholar] [CrossRef]
- Aswini, R.; Murugesan, S.; Kannan, K. Bio-engineered TiO2 nanoparticles using Ledebouria revoluta extract: Larvicidal, histopathological, antibacterial, and anticancer activity. Int. J. Environ. Anal. Chem. 2020, 101, 2926–2936. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Senthil, N.; Min, T. Nanocurcumin: A promising candidate for therapeutic applications. Front. Pharmacol. 2020, 11, 487. [Google Scholar] [CrossRef]
- Rai, M.; Pandit, R.; Gaikwad, S.; Yadav, A.; Gade, A. Potential applications of curcumin and curcumin nanoparticles: From traditional therapeutics to modern nanomedicine. Nanotechnol. Rev. 2015, 4, 161–172. [Google Scholar] [CrossRef]
- Ahmad, K.; Ansari, V.A.; Singh, K.; Kushwaha, P.; Akhtar, J. Curcuma longa: Boon for health care system with its biomedical application. Int. J. Pharm. Sci. Res. 2015, 6, 4168. [Google Scholar]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives–A review. J. Tradit. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The essential medicinal chemistry of curcumin: Miniperspective. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.S.; Dutta, P.; Chard, N.; Wu, Y.; Chen, Q.-H.; Chen, G.; Vadgama, J. A novel curcumin analog inhibits canonical and non-canonical functions of telomerase through STAT3 and NF-κB inactivation in colorectal cancer cells. Oncotarget 2019, 10, 4516–4531. [Google Scholar] [CrossRef] [PubMed]
- Den Hartogh, D.J.; Gabriel, A.; Tsiani, E. Antidiabetic Properties of Curcumin I: Evidence from In vitro Studies. Nutrients 2020, 12, 118. [Google Scholar] [CrossRef]
- Lee, S.E.; Park, H.R.; Jeon, S.; Han, D.; Park, Y.S. Curcumin attenuates acrolein-induced COX-2 expression and prostaglandin production in human umbilical vein endothelial cells. J. Lipid Atheroscl. 2020, 9, 184–194. [Google Scholar] [CrossRef]
- Da Silva, A.C.; De Freitas Santos, P.D.; Do Prado Silva, J.T.; Leimann, F.V.; Bracht, L.; Gonçalves, O.H. Impact of curcumin nanoformulation on its antimicrobial activity. Trends Food Sci. Technol. 2018, 72, 74–82. [Google Scholar] [CrossRef]
- Huang, F.; Gao, Y.; Zhang, Y.; Cheng, T.; Ou, H.; Yang, L.; Liu, J.; Shi, L.; Liu, J. Silver-decorated polymeric micelles combined with curcumin for enhanced antibacterial activity. ACS Appl. Mater. Interfaces 2017, 9, 16880–16889. [Google Scholar] [CrossRef]
- Zaharieva, M.M.; Kroumov, A.D.; Dimitrova, L.; Tsvetkova, I.; Trochopoulos, A.; Konstantinov, S.M.; Reinhold Berger, M.; Momchilova, M.; Yoncheva, K.; Miladinov Najdenski, H. Micellar curcumin improves the antibacterial activity of the alkylphosphocholines erufosine and miltefosine against pathogenic Staphyloccocus aureus strains. Biotechnol. Biotechnol. Equip. 2019, 33, 38–53. [Google Scholar] [CrossRef]
- Naseri, S.; Darroudi, M.; Aryan, E.; Gholoobi, A.; Rahimi, H.R.; Ketabi, K.; Movaqar, A.; Abdoli, M.; Gouklani, H.; Teimourpour, R.; et al. The Antiviral Effects of Curcumin Nanomicelles on the Attachment and Entry of Hepatitis C Virus. Iran. J. Virol. 2017, 11, 29–35. [Google Scholar]
- Yang, Q.Q.; Farha, A.K.; Kim, G.; Gul, K.; Gan, R.Y.; Corke, H. Antimicrobial and anticancer applications and related mechanisms of curcumin-mediated photodynamic treatments. Trends Food Sci. Technol. 2020, 97, 341–354. [Google Scholar] [CrossRef]
- Hewlings, S.; Kalman, D. Curcumin: A review of its’ effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Rafiee, Z.; Nejatian, M.; Daeihamed, M.; Jafari, S.M. Application of different nanocarriers for encapsulation of curcumin. Crit. Rev. Food Sci. Nutr. 2019, 59, 3468–3497. [Google Scholar] [CrossRef] [PubMed]
- Rajasekar, A. Facile synthesis of curcumin nanocrystals and validation of its antioxidant activity against circulatory toxicity in Wistar rats. J. Nanosci. Nanotechnol. 2015, 15, 4119–4125. [Google Scholar] [CrossRef] [PubMed]
- Farhood, B.; Mortezaee, K.; Goradel, N.H.; Khanlarkhani, N.; Salehi, E.; Nashtaei, M.S.; Najafi, M.; Sahebkar, A. Curcumin as an anti-inflammatory agent: Implications to radiotherapy and chemotherapy. J. Cell. Physiol. 2019, 234, 5728–5740. [Google Scholar] [CrossRef] [PubMed]
- Banez, M.J.; Geluz, M.I.; Chandra, A.; Hamdan, T.; Biswas, O.S.; Bryan, N.S.; Von Schwarz, E.R. A systemic review on the antioxidant and anti-inflammatory effects of resveratrol, curcumin, and dietary nitric oxide supplementation on human cardiovascular health. Nutr. Res. 2020, 78, 11–26. [Google Scholar] [CrossRef]
- Nahar, P.P.; Slitt, A.L.; Seeram, N.P. Anti-inflammatory effects of novel standardized solid lipid curcumin formulations. J. Med. Food 2015, 18, 786–792. [Google Scholar] [CrossRef]
- Suresh, S.; Sankar, P.; Telang, A.G.; Kesavan, M.; Sarkar, S.N. Nanocurcumin ameliorates Staphylococcus aureus-induced mastitis in mouse by suppressing NF-κB signaling and inflammation. Int. Immunopharmacol. 2018, 65, 408–412. [Google Scholar] [CrossRef]
- Kuttan, R.; Sudheeran, P.C.; Josph, C.D. Turmeric, and curcumin as topical agents in cancer therapy. Tumori 1987, 73, 29–31. [Google Scholar] [CrossRef]
- Perera, W.P.T.D.; Dissanayake, R.K.; Ranatunga, U.I.; Hettiarachchi, N.M.; Perera, K.D.C.; Unagolla, J.M.; DeSilva, R.T.; Pahalagedara, L.R. Curcumin loaded zinc oxide nanoparticles for activity-enhanced antibacterial and anticancer applications. RSC Adv. 2020, 10, 30785–30795. [Google Scholar] [CrossRef]
- Tan, B.; Norhaizan, M.E. Curcumin combination chemotherapy: The implication and efficacy in cancer. Molecules 2019, 24, 2527. [Google Scholar] [CrossRef]
- Hotsumi, M.; Tajiri, M.; Nikaido, Y.; Sato, T.; Makabe, K.; Konno, H. Design, synthesis, and evaluation of a water soluble C5-monoketone type curcumin analogue as a potent amyloid β aggregation inhibitor. Bioorg. Med. Chem. Lett. 2019, 29, 2157–2161. [Google Scholar] [CrossRef]
- Basniwal, R.K.; Khosla, R.; Jain, N. Improving the anticancer activity of curcumin using nanocurcumin dispersion in water. Nutr. Cancer 2014, 66, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Nagesh, P.K.B.; Jaggi, M.; Chauhan, S.C. Therapeutic applications of curcumin nanoformulations. AAPS J. 2015, 17, 1341–1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.N.; Haggag, Y.A.; Lane, M.E.; Mccarron, P.A.; Tambuwala, M.M. Polymeric nano-encapsulation of curcumin enhances its anticancer activity in breast (MDA-MB231) and lung (A549) cancer cells through reduction in expression of HIF-1α and nuclear p65 (REL A). Curr. Drug Deliv. 2018, 15, 286–295. [Google Scholar] [CrossRef]
- Baghi, N.; Bakhshinejad, B.; Keshavarz, R.; Babashah, S.; Sadeghizadeh, M. Dendrosomal nanocurcumin and exogenous p53 can act synergistically to elicit anticancer effects on breast cancer cells. Gene 2018, 670, 55–62. [Google Scholar] [CrossRef]
- Arya, G.; Das, M.; Sahoo, S.K. Evaluation of curcumin loaded chitosan/PEG blended PLGA nanoparticles for effective treatment of pancreatic cancer. Biomed. Pharmacother. 2018, 102, 555–566. [Google Scholar] [CrossRef]
- Paulraj, F.; Abas, F.; Lajis, N.H.; Othman, I.; Naidu, R. Molecular Pathways Modulated by Curcumin Analogue, Diarylpentanoids in Cancer. Biomolecules 2019, 9, 270. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; Dos Santos, A.M.; Rodero, C.F.; Gremião, M.P.D.; Chorilli, M. Design, characterization, and biological evaluation of curcumin-loaded surfactant-based systems for topical drug delivery. Int. J. Nanomed. 2016, 11, 4553. [Google Scholar] [CrossRef]
- Biswas, A.K.; Islam, M.R.; Choudhury, Z.S.; Mostafa, A.; Kadir, M.F. Nanotechnology based approaches in cancer therapeutics. Adv. Natural Sci. Nanosci. Nanotechnol. 2014, 5, 043001. [Google Scholar] [CrossRef]
- Verma, K.; Tarafdar, A.; Kumar, D.; Kumar, Y.; Rana, J.S.; Badgujar, P.C. Formulation and characterization of nano-curcumin fortified milk cream powder through microfluidization and spray drying. Food Res. Int. 2022, 160, 111705. [Google Scholar] [CrossRef]
- Atia, M.M.; Abdel-Tawab, H.S.; Mostafa, A.M.; Mobarak, S.A. Nanocurcumin and curcumin prevent N, N′-methylenebisacrylamide-induced liver damage and promotion of hepatic cancer cell growth. Sci. Rep. 2022, 12, 8319. [Google Scholar] [CrossRef]
- Zou, P.; Zhang, J.; Xia, Y.; Kanchana, K.; Guo, G.; Chen, W. ROS generation mediates the anti-cancer effects of WZ35 via activating JNK and ER stress apoptotic pathways in gastric cancer. Oncotarget 2015, 6, 5860. [Google Scholar] [CrossRef] [PubMed]
- Quispe, C.; Herrera-Bravo, J.; Khan, K.; Javed, Z.; Semwal, P.; Painuli, S.; Sharifi-Rad, J. Therapeutic applications of curcumin nanomedicine formulations in cystic fibrosis. Prog. Biomater. 2022. [Google Scholar] [CrossRef] [PubMed]
- Rajalakshmi, N.; Dhivya, S. A Review on the preparation methods of Curcumin Nanoparticles. PharmaTutor 2018, 6, 6–10. [Google Scholar] [CrossRef]
- Manikandan, S.; El Mabrouk, K.; Ballamurugan, A.M. Synthesis of Nanocurcumin and Evaluation of its Properties for Biomedical Applications. Trends Biomater. Artif. Organs 2022, 36, 241–286. [Google Scholar]
- Mukerjee, A.; Vishwanatha, J.K. Formulation, characterization and evaluation of curcumin-loaded PLGA nanospheres for cancer therapy. Anticancer Res. 2009, 29, 3867–3875. [Google Scholar] [PubMed]
- Mathew, A.; Fukuda, T.; Nagaoka, Y. Curcumin loaded-PLGA nanoparticles conjugated with Tet-1 peptide for potential use in Alzheimer’s disease. PLoS ONE. 2012, 7, 32616. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. beta-Cyclodextrin-curcumin self-assembly enhances curcumin delivery in prostate cancer cells. Colloids Surf B Biointerfaces 2010, 79, 113–125. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Ebeling, M.C.; Chauhan, N.; Jaggi, M.; Chauhan, S.C. Interaction of curcumin nanoformulations with human plasma proteins and erythrocytes. Int. J. Nanomed. 2011, 6, 2779–2790. [Google Scholar]
- He, Y.; Huang, Y.; Cheng, Y. Structure Evolution of Curcumin Nano-precipitation from a Micromixer. Cryst. Growth Des. 2010, 10, 1021–1024. [Google Scholar] [CrossRef]
- Hettiarachchi, S.S.; Dunuweera, S.P.; Dunuweera, A.N.; Rajapakse, R.G. Synthesis of curcumin nanoparticles from raw turmeric rhizome. ACS Omega 2021, 6, 8246–8252. [Google Scholar] [CrossRef]
- Maleki Dizaj, S.; Alipour, M.; Dalir Abdolahinia, E.; Ahmadian, E.; Eftekhari, A.; Forouhandeh, H.; Zununi Vahed, S. Curcumin nanoformulations: Beneficial nanomedicine against cancer. Phytother. Res. 2022, 36, 1156–1181. [Google Scholar] [CrossRef]
- Tabanelli, R.; Brogi, S.; Calderone, V. Improving curcumin bioavailability: Current strategies and future perspectives. Pharmaceutics 2021, 13, 1715. [Google Scholar] [CrossRef]
- Dutta, B.; Shelar, S.B.; Rajan, V.; Checker, S.; Barick, K.C.; Pandey, B.N.; Hassan, P.A. Gelatin grafted Fe3O4 based curcumin nanoformulation for cancer therapy. J. Drug Deliv. Sci. Technol. 2022, 67, 102974. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, L.; Peng, H.; Li, Y.; Xiong, J.; Xu, Z. The formulation and delivery of curcumin with solid lipid nanoparticles for the treatment of on non-small cell lung cancer both in vitro and in vivo. Mater. Sci. Eng. 2013, 33, 4802–4808. [Google Scholar] [CrossRef]
- Abdellah, A.M.; Sliem, M.A.; Bakr, M.; Amin, R.M. Green synthesis and biological activity of silver–curcumin nanoconjugates. Future Med. Chem. 2018, 10, 2577–2588. [Google Scholar] [CrossRef]
- Elbialy, N.S.; Abdelfatah, E.A.; Khalil, W.A. Antitumor activity of curcumin-green synthesized gold nanoparticles: In vitro study. BioNanoScience 2019, 9, 813–820. [Google Scholar] [CrossRef]
- Chaurasia, S.; Chaubey, P.; Patel, R.R.; Kumar, N.; Mishra, B. Curcumin-polymeric nanoparticles against colon-26 tumor-bearing mice: Cytotoxicity, pharmacokinetic and anticancer efficacy studies. Drug Dev. Ind. Pharm. 2016, 42, 694–700. [Google Scholar] [CrossRef]
- Liu, R.; Pei, Q.; Shou, T.; Zhang, W.; Hu, J.; Li, W. Apoptotic effect of green synthesized gold nanoparticles from Curcuma wenyujin extract against human renal cell carcinoma A498 cells. Int. J. Nanomed. 2019, 14, 4091. [Google Scholar] [CrossRef]
- Thadakapally, R.; Aafreen, A.; Aukunuru, J.; Habibuddin, M.; Jogala, S. Preparation and characterization of PEG-albumin-curcumin nanoparticles intended to treat breast cancer. Indian J. Pharm. Sci. 2016, 78, 65. [Google Scholar]
- Al-Ani, L.A.; Yehye, W.A.; Kadir, F.A.; Hashim, N.M.; AlSaadi, M.A.; Julkapli, N.M.; Hsiao, V.K. Hybrid nanocomposite curcumin-capped gold nanoparticle-reduced graphene oxide: Antioxidant potency and selective cancer cytotoxicity. PLoS ONE 2019, 14, e0216725. [Google Scholar] [CrossRef]
- Ayubi, M.; Karimi, M.; Abdpour, S.; Rostamizadeh, K.; Parsa, M.; Zamani, M.; Saedi, A. Magnetic nanoparticles decorated with PEGylated curcumin as dual targeted drug delivery: Synthesis, toxicity, and biocompatibility study. Mater. Sci. Eng. C 2019, 104, 109810. [Google Scholar] [CrossRef] [PubMed]
- Saikia, C.; Das, M.K.; Ramteke, A.; Maji, T.K. Controlled release of curcumin from thiolated starch-coated iron oxide magnetic nanoparticles: An in vitro evaluation. Int. J. Polym. Mater. Polym. Biomat. 2017, 66, 349–358. [Google Scholar] [CrossRef]
- Zhou, J.; Cao, Z.; Panwar, N.; Hu, R.; Wang, X.; Qu, J.; Yong, K.T. Functionalized gold nanorods for nanomedicine: Past, present, and future. Coord. Chem. Rev. 2017, 352, 15–66. [Google Scholar] [CrossRef]
- Daniels, A.N.; Singh, M. Sterically stabilized siRNA: Gold nanocomplexes enhance c-MYC silencing in a breast cancer cell model. Nanomedicine 2019, 14, 1387–1401. [Google Scholar] [CrossRef]
- Mbatha, L.S.; Maiyo, F.; Daniels, A.; Singh, M. Dendrimer-coated Gold Nanoparticles for Efficient Folate-Targeted mRNA Delivery in vitro. Pharmaceutics 2021, 13, 900. [Google Scholar] [CrossRef]
- Rejinold, N.S.; Thomas, R.G.; Muthiah, M.; Chennazhi, K.; Manzoor, K.; Park, I.-K.; Jeong, Y.Y.; Jayakumar, R. Anti-cancer, pharmacokinetics, and tumor localization studies of pH-, RF-and thermo-responsive nanoparticles. Int. J. Biol. Macromol. 2015, 74, 249–262. [Google Scholar] [CrossRef]
- Nambiar, S.; Osei, E.; Fleck, A.; Darko, J.; Mutsaers, A.J.; Wettig, S. Synthesis of curcumin-functionalized gold nanoparticles and cytotoxicity studies in human prostate cancer cell line. Appl. Nanosci. 2018, 8, 347–357. [Google Scholar] [CrossRef]
- Ombredane, A.S.; Silva, V.R.; Andrade, L.R.; Pinheiro, W.O.; Simonelly, M.; Oliveira, J.V.; Joanitti, G.A. In Vivo efficacy and toxicity of curcumin nanoparticles in breast cancer treatment: A systematic review. Front. Oncol. 2021, 11, 612903. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/ (accessed on 11 July 2022).





| Synthesis | Advantages | Disadvantages | Types |
|---|---|---|---|
| Physical |
|
| Radiation Sonication Laser ablation Membrane filtration Ion exchange |
| Chemical |
|
| Reduction Oxidative process Photochemical Electrochemical destruction Condensation Sol-gel method |
| Biological |
|
| Plants Bacteria Fungi Viruses |
| Curcumin-Conjugate | Morphology | Cancer Model | Anticancer Activity | Ref | ||
|---|---|---|---|---|---|---|
| Nanoparticle (Source) | Shape | Size (nm) | ||||
| Solid lipid (Stearic acid and lecithin) |  | Spherical | 20–80 | Breast cancer | Increased Bax/Bcl-2 ratios. Enhanced biocompatibility. | [140] |
| Micelles (d-hydroxyethyl starch) |  | Spherical | 34.9 | Lung and colorectal cancer | Improved bioavailability, blood circulation, solubility, and stability of the nanocomplex. Enhanced antiproliferation and apoptosis. | [129] |
| Liposomes (l-α-phosphatidyl choline and cholesterol) |  | Irregular spherical | 10–50 | Melanoma and Lung cancer | Greater encapsulation activity. Enhanced antiproliferation and apoptosis. | [137] |
| Polymeric (Poloxamer 188) |  | Spherical | 248.4 | Colon and ovarian carcinoma | Enhanced uptake and cell specificity. Increased cytotoxicity. Improved blood circulation. | [143] |
| Silver (Silver nitrate) |  | Spherical | 15.5 | Breast cancer | Enhanced cellular uptake. Enhanced antiproliferation and apoptosis. | [141] |
| Gold (Chloroauric acid) |  | Spherical | 26–28.2 | Breast, colon, prostate, and renal carcinoma | Improved blood circulation, solubility, and stability of the nanocomplex. Enhanced antiproliferation and apoptosis. | [140,142,144] |
| Albumin (Bovine serum albumin) |  | Spherical | 112–198 | Breast cancer | Enhanced antiproliferation and apoptosis. | [145] |
| Graphene oxide and quantum dots (Graphite powder) |  | Crystal-like | 15.62 | Breast cancer | Enhanced cellular uptake. Increased cytotoxicity. | [146] |
| Cancer | Study Title | Therapeutic | Start and End Dates |
|---|---|---|---|
| Breast | “Window Trial” on Curcumin for Invasive Breast Cancer Primary Tumors | * Curcumin | January 2020– December 2022 |
| Curcumin in Reducing Joint Pain in Breast Cancer Survivors with Aromatase inhibitor-induced Joint Disease | ** Curcumin Nanoemulsion | March 2019– July 2022 | |
| # Curcumin for the Prevention of Radiation-induced Dermatitis in Breast Cancer Patients | * Curcumin c3 | January 2008–April 2011 | |
| # Pilot Study of Curcumin for Women with Obesity and High Risk for Breast Cancer | * Curcumin | June 2013– September 2016 | |
| # Phase II Study of Curcumin vs Placebo for Chemotherapy-Treated Breast Cancer Patients Undergoing Radiotherapy | * Curcumin | May 2015– July 2018 | |
| # Prophylactic Topical Agents in Reducing Radiation-Induced Dermatitis in patients With Non-inflammatory Breast Cancer | * Curcumin | October 2015–September 2016 | |
| # Curcumin in Combination with Chemotherapy in Advanced Breast Cancer | ** Curcumin, Paclitaxel | March 2017– June 2019 | |
| # Disposition of Dietary Polyphenols and Methylxanthines in Mammary Tissues from Breast Cancer Patients | ** Curcumin Polyphenol | June 2017– December 2019 | |
| Colon | Study Investigating the Ability of Plant Exosomes to Deliver Curcumin to Normal and Colon Cancer Tissue | * Curcumin | January 2011– December 2022 |
| # Curcumin Biomarkers | * Curcumin c3 | November 2010–January 2013 | |
| # Combining Curcumin with FOLFOX Chemotherapy in Patients with inoperable Colorectal Cancer | ** Curcumin Chemotherapy | February 2012–May 2017 | |
| # Effect of Curcumin on Dose Limiting Toxicity and Pharmacokinetics of Irinotecan in Patients with Solid Tumors | ** Curcumin, Irinotecan | June 2013– October 2016 | |
| # Avastin/FOLFIRI in Combination with Curcumin in Colorectal Cancer Patients with Unresectable Metastasis | ** Curcumin Avastin/FOLFIRI | August 2015–2019 | |
| Cervical | Curcumin in Advanced Cervical Cancer | * Curcumin | December 2021–2023 |
| # Trial on Safety and Pharmacokinetics of Intravaginal Curcumin | * Curcumin | January 2010–2012 | |
| # Study of Pembrolizumab, Radiation and Immune Modulatory Cocktail in Cervical/Uterine Cancer | ** Curcumin, Pembrolizumab Radiation, Vitamin D Aspirin, Lansoprazole Cyclophosphamide | July 2017– June 2021 | |
| Prostate | Adjuvant Curcumin to Assess Recurrence-Free Survival in Patients Who Have Had a Radical Prostatectomy | * Curcumin | May 2014– June 2023 |
| Trial of Curcumin to Prevent Progression of Low-risk Prostate Cancer Under Active Surveillance | * Curcumin | March 2016– November 2026 | |
| Curcumin and Piperine in Patients on Surveillance for Monoclonal Gammopathy, Smoldering Myeloma or Prostate Cancer | ** Curcumin, Piperine | December 2021–May 2023 | |
| # Comparison of Duration of Treatment Interruption with or Without Curcumin During the off-Treatment Periods in Patients with Prostate Cancer Undergoing Intermittent Androgen Deprivation Therapy | * Curcumin | August 2007–2015 | |
| # Radiosensitizing and Radioprotective Effects of Curcumin in Prostate Cancer | * Curcumin | March 2011– October 2019 | |
| # Multicentre International Study for the Prevention with Ialuril® of Radio-induced Cystitis (MISTIC) | ** Curcumin Radiotherapy | April 2017– May 2019 | |
| # Correlative Analysis of the Genomics of Vitamin D and Omega-3 Fatty Acid Intake in Prostate Cancer | ** Curcumin Vitamin D, Omega-3 | September 2017–December 2019 | |
| Lung | Phase II Trial to Modulate Intermediate Endpoint Biomarkers in Former and Current Smokers | ** Curcumin, Lovaza | June 2019– October 2023 |
| The Thoracic Peri-Operative Integrative Surgical Care Evaluation Trial-Stage II | ** Curcumin, Vitamin D3 Coriolus Versicolor Provitalix Green Tea Extract | April 2022– May 2025 | |
| Head and Neck | # Curcumin Biomarker Trial in Head and Neck Cancer | * Curcumin c3 | June 2010– January 2016 |
| # Curcumin Bioavailability in Glioblastoma Patients | * Curcumin | October 2012–May 2013 | |
| # The Effect of Curcumin on Treatment of Cancer Anorexia-Cachexia Syndrome in Patients with Stage III-IV of Head and Neck Cancer | * Curcumin | February 2020–March 2021 | |
| Leukaemia | Safety and Efficacy of Curcumin in Children with Acute Lymphoblastic Leukemia | * Curcumin | August 2021–September 2022 |
| Oral | # Oral Curcumin for Radiation Dermatitis | * Curcumin | February 2011–January 2015 |
| Pancreatic | Gemcitabine Hydrochloride, Paclitaxel Albumin- Stabilized Nanoparticle Formulation, Metformin Hydrochloride, and a Standardized Dietary Supplement in Treating Patients with Pancreatic Cancer That Cannot Be Removed by Surgery | ** Curcumin Gemcitabine Albumin Metformin | January 2016– December 2022 |
| # Gemcitabine With Curcumin for Pancreatic Cancer | ** Curcumin, Gemcitabine | July 2004– September 2010 | |
| # Trial of Curcumin in Advanced Pancreatic Cancer | * Curcumin | November 2004–April 2014 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkatas, J.; Daniels, A.; Singh, M. The Potential of Curcumin-Capped Nanoparticle Synthesis in Cancer Therapy: A Green Synthesis Approach. Nanomaterials 2022, 12, 3201. https://doi.org/10.3390/nano12183201
Venkatas J, Daniels A, Singh M. The Potential of Curcumin-Capped Nanoparticle Synthesis in Cancer Therapy: A Green Synthesis Approach. Nanomaterials. 2022; 12(18):3201. https://doi.org/10.3390/nano12183201
Chicago/Turabian StyleVenkatas, Jeaneen, Aliscia Daniels, and Moganavelli Singh. 2022. "The Potential of Curcumin-Capped Nanoparticle Synthesis in Cancer Therapy: A Green Synthesis Approach" Nanomaterials 12, no. 18: 3201. https://doi.org/10.3390/nano12183201
APA StyleVenkatas, J., Daniels, A., & Singh, M. (2022). The Potential of Curcumin-Capped Nanoparticle Synthesis in Cancer Therapy: A Green Synthesis Approach. Nanomaterials, 12(18), 3201. https://doi.org/10.3390/nano12183201