Bioactive Coatings Based on Nanostructured TiO2 Modified with Noble Metal Nanoparticles and Lysozyme for Ti Dental Implants
Abstract
:1. Introduction
2. Materials and Methods
Material Synthesis
3. Results
3.1. SEM
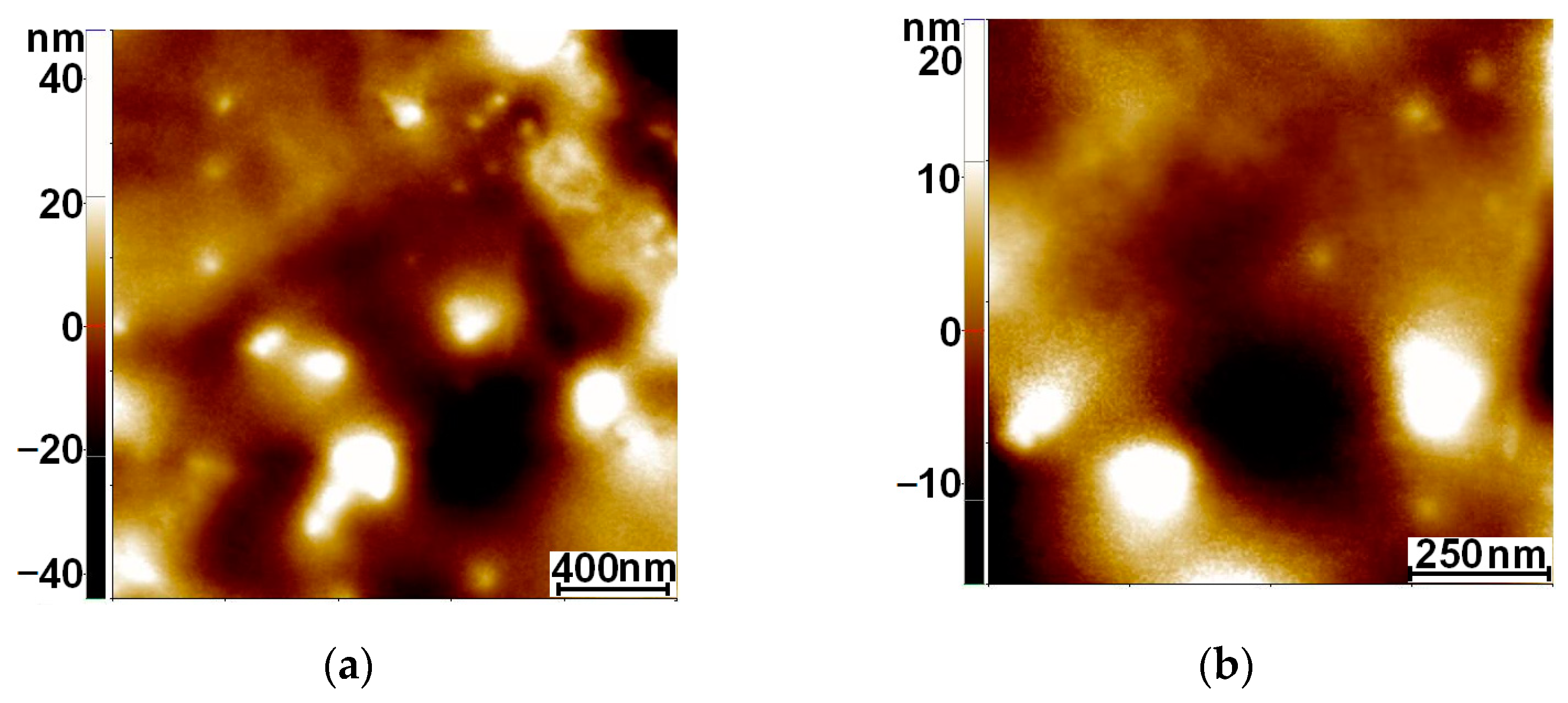
3.2. AFM
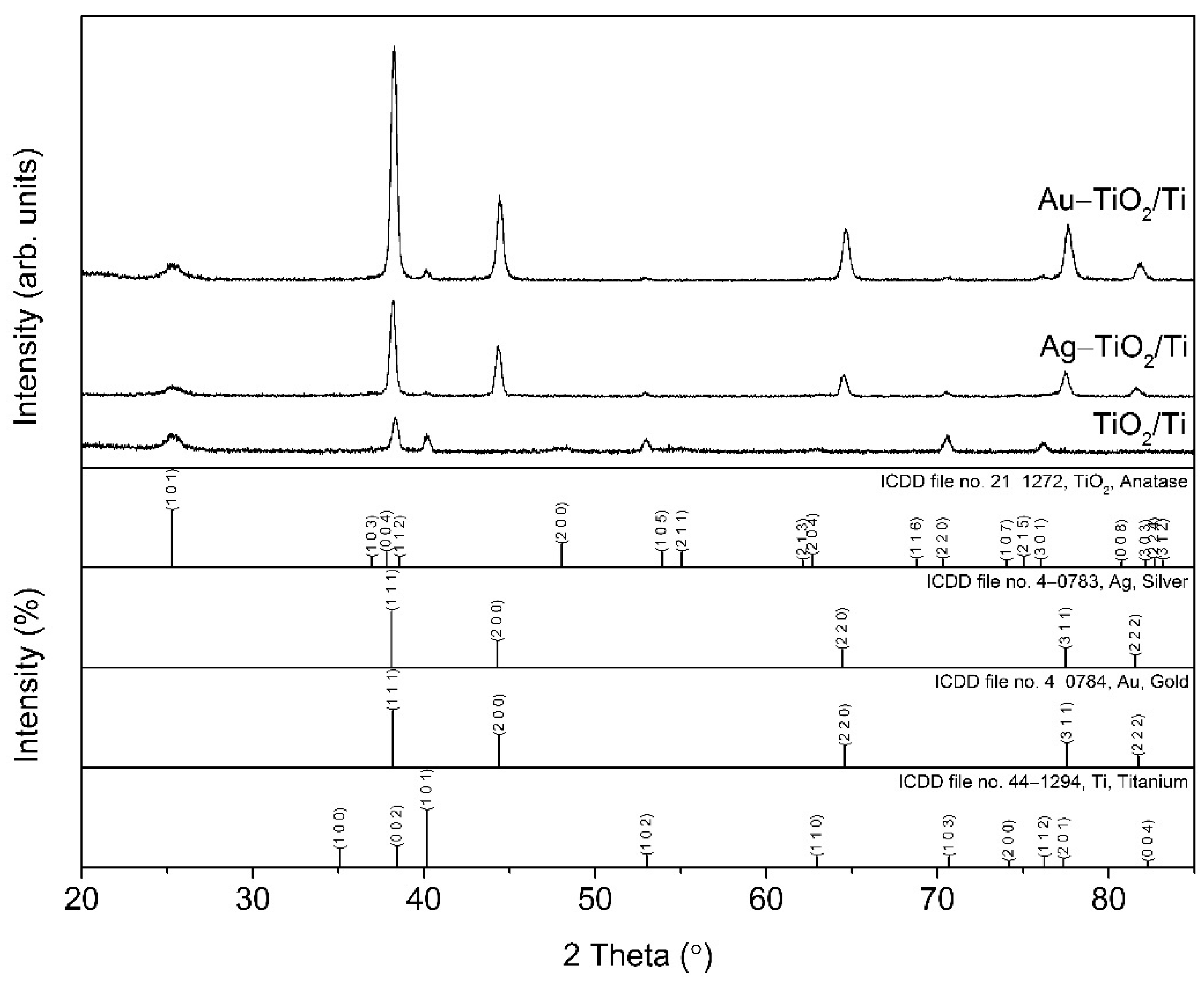
3.3. XRD
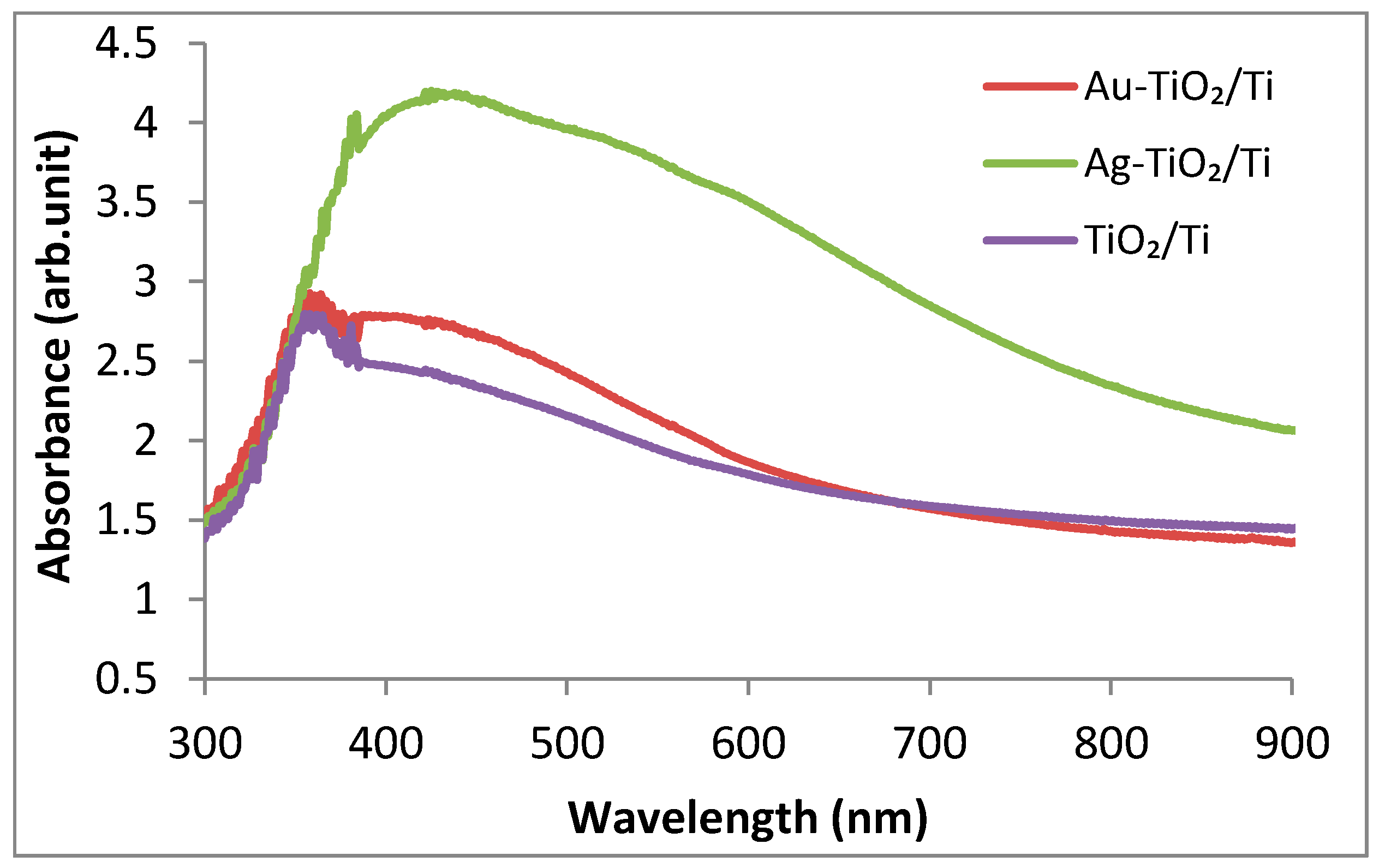
3.4. UV–Vis Spectroscopy
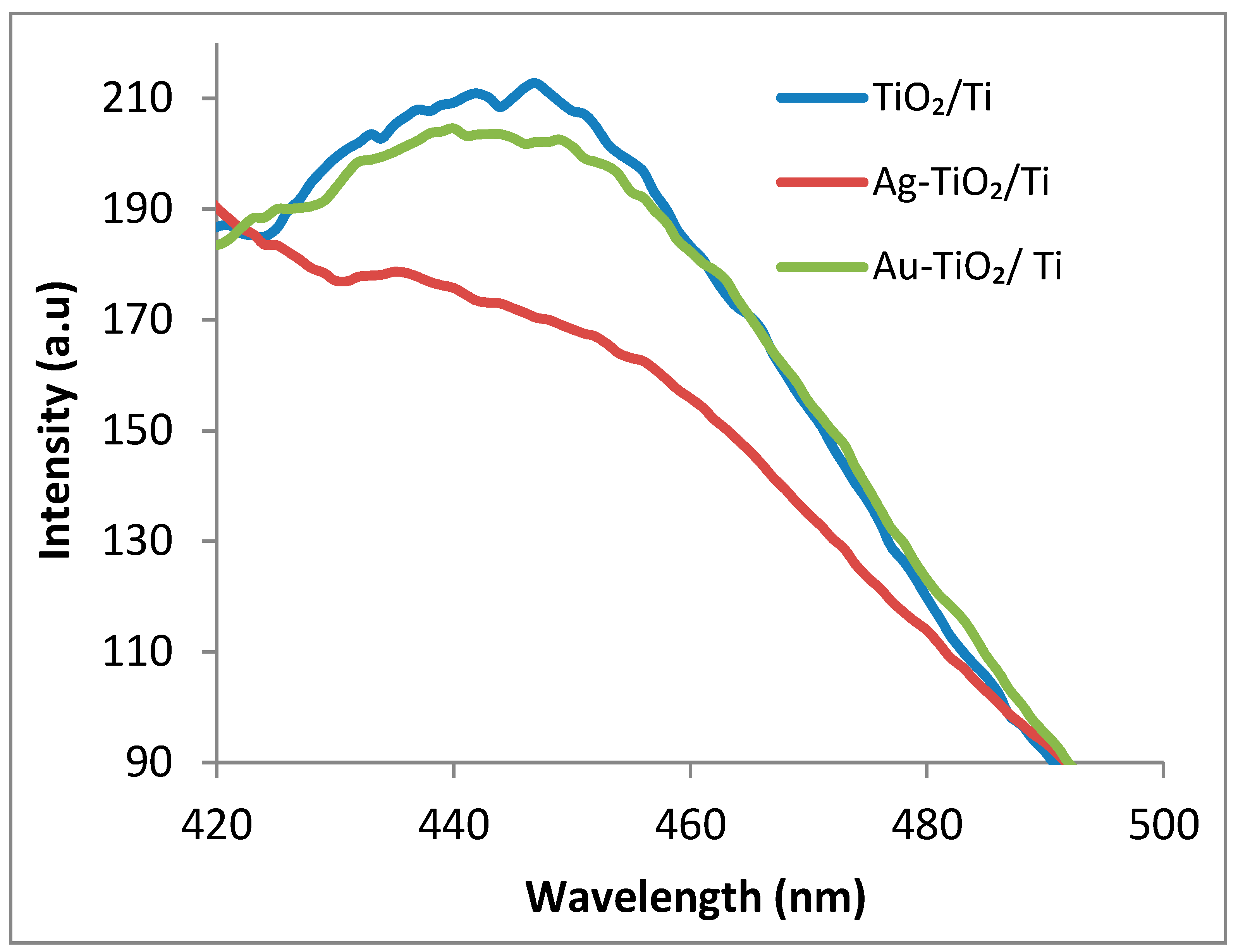
3.5. Photoluminescence Measurements
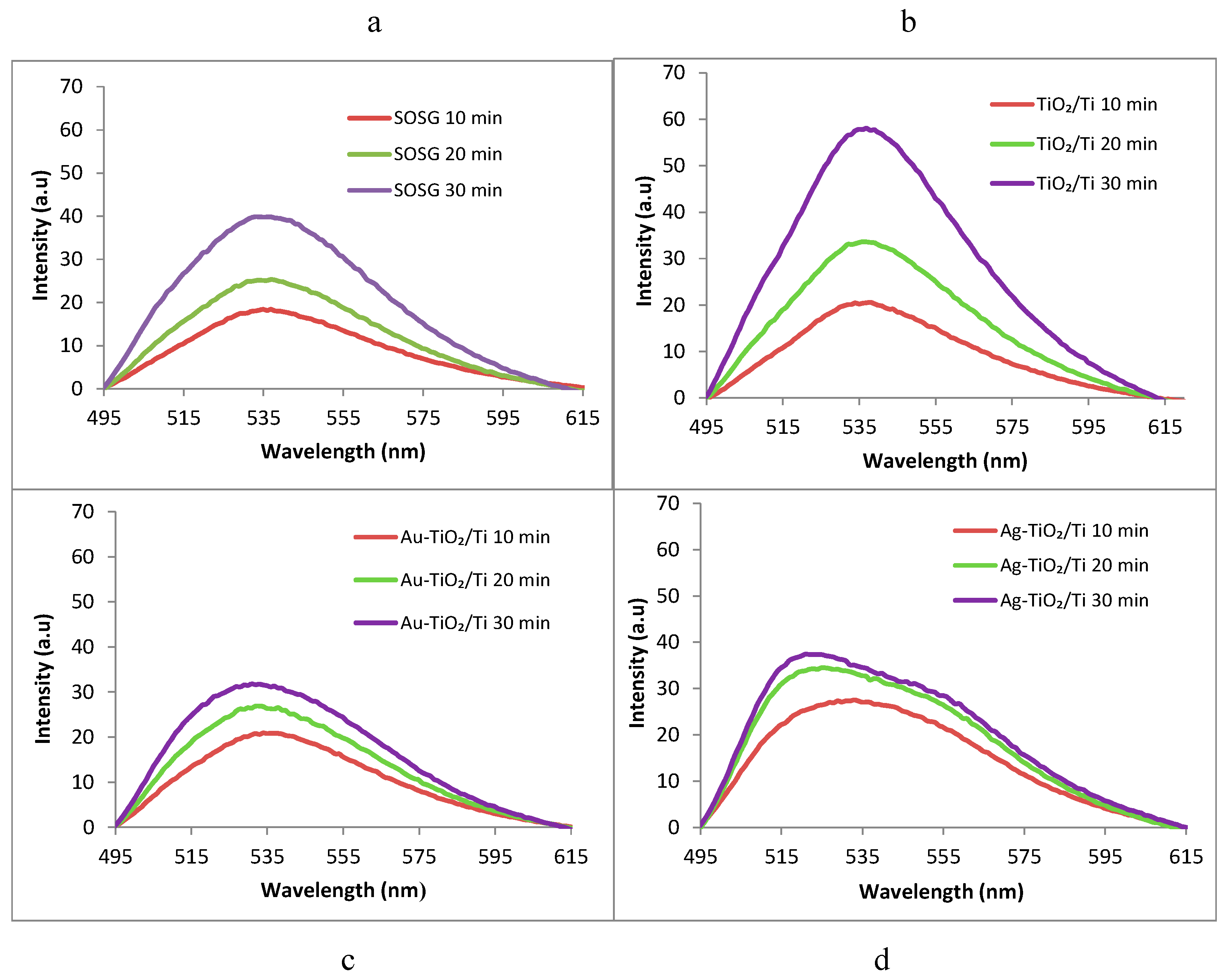
3.6. ROS (Singlet Oxygen 1O2) Identification
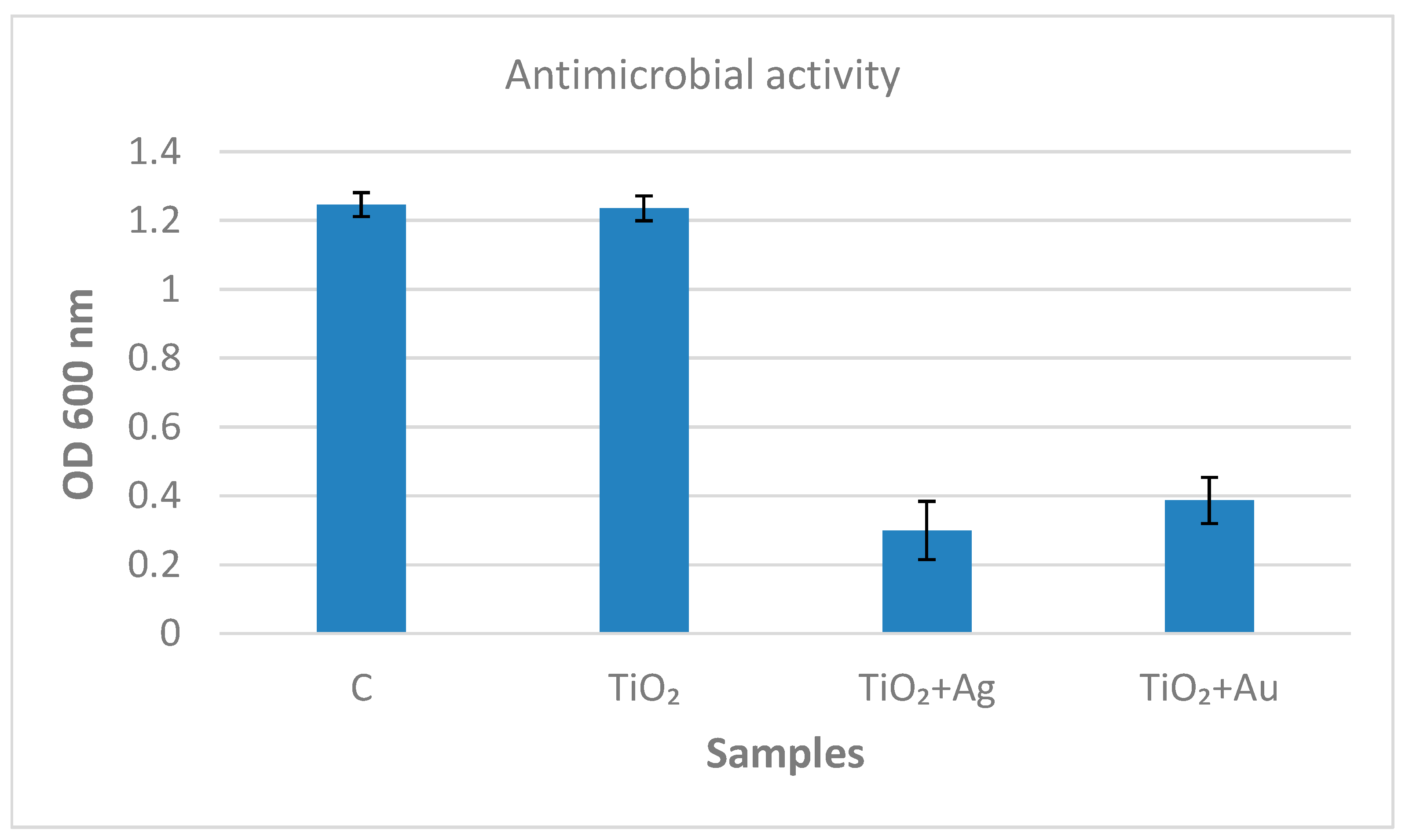
3.7. Antibacterial Activity Assays of Inorganic Coatings TiO2/Ti, Ag–TiO2/Ti, and Au–TiO2/Ti against M. lysodeicticus
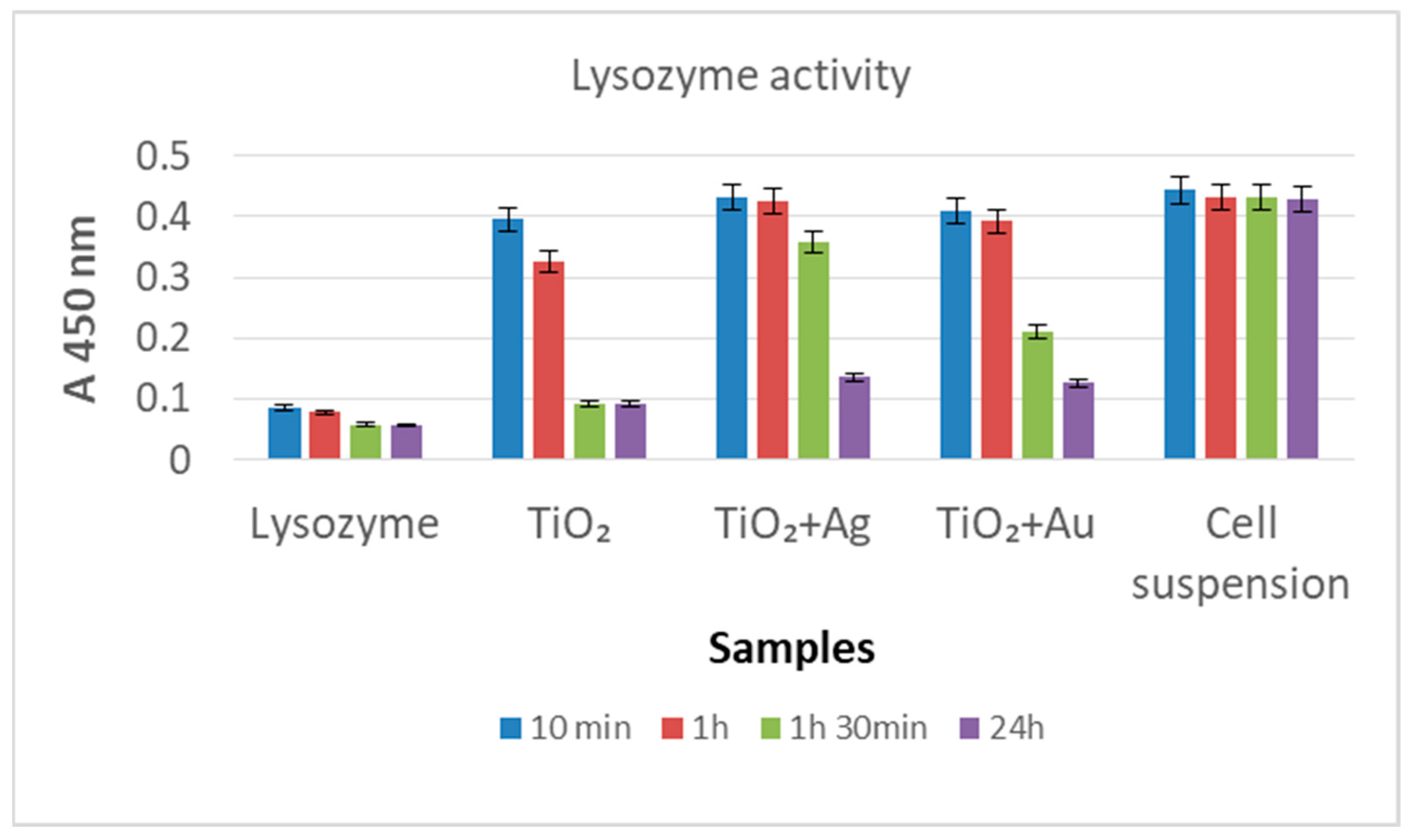
3.8. Lysozyme (Lys/TiO2/Ti, Lys/Ag–TiO2/Ti, Lys/Au–TiO2/Ti) Activity Assays on Microbial Substrate (Micrococcus lysodeicticus)
3.9. Lysozyme (Lys/TiO2/Ti, Lys/Ag–TiO2/Ti, Lys/Au–TiO2/Ti) Activity Assays on Synthetic Substrate [4–MU–β– (GlcNAc)3]
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pałka, K.; Pokrowiecki, R. Porous Titanium Implants: A Review. Adv. Eng. Mater. 2018, 20, 1700648. [Google Scholar] [CrossRef]
- Babíka, O.; Czána, A.; Holubjaka, J.; Kameníka, R.; Pilca, J. Identification of surface characteristics created by miniature machining of dental implants made of titanium based materials. Procedia Eng. 2017, 192, 1016–1021. [Google Scholar] [CrossRef]
- Banerjee, D.; Shivapriya, P.M.; Kumar, P.; Gautam, P.K.; Misra, K.; Sahoo, A.K.; Samanta, S.K. A review on basic biology of bacterial biofilm infections and their treatments by nanotechnology-based approaches. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2019, 90, 243–259. [Google Scholar] [CrossRef]
- Yang, W.E.; Hsu, M.L.; Lin, M.C.; Chen, Z.H.; Chen, L.K.; Huang, H.H. Nano/submicron-scale TiO2 network on titanium surface for dental implant application. J. Alloys Compd. 2009, 479, 642–647. [Google Scholar] [CrossRef]
- Cho, S.-A.; Park, K.-T. The removal torque of titanium screw inserted in rabbit tibia treated by dual acid etching. Biomaterials 2003, 24, 3611–3617. [Google Scholar] [CrossRef]
- Iwaya, Y.; Machigashira, M.; Kanbara, K.; Miyamoto, M.; Nouguchi, K.; Izumi, Y.; Ban, S. Surface properties and biocompatibility of acid-etched titanium. Dent. Mater. J. 2008, 27, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Shemtov-Yona, K.; Rittel, D.; Dorogoy, A. Mechanical assessment of grit blasting surface treatments of dental implants. J. Mech. Behav. Biomed. Mater. 2014, 39, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.A.; Ramírez-Fernandez, M.P.; Granero Marín, J.M.; Barbosa Salles, M.; Del Fabbro, M.; Calvo Guirado, J.L. A comparative evaluation between aluminium and titanium dioxide microparticles for blasting the surface titanium dental implants: An experimental study in rabbits. Clin. Oral Implants Res. 2018, 29, 802–807. [Google Scholar] [CrossRef]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface modifications and their effects on titanium dental implants. BioMed Res. Int. 2015, 2015, 791725. [Google Scholar] [CrossRef]
- Visentin, F.; El Habra, N.; Fabrizio, M.; Brianese, N.; Gerbasi, R.; Nodari, L.; Zin, V. TiO2-HA bi-layer coatings for improving the bioactivity and service-life of Ti dental implants. Surf. Coat. Technol. 2019, 378, 125049. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, H.; Huo, K.; Cui, L.; Zhang, W.; Ni, H.; Zhang, Y.; Wu, Z.; Chu, P.K. Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials 2011, 32, 5706–5716. [Google Scholar] [CrossRef]
- Romero-Gavilan, F.; Araújo-Gomes, N.; Sánchez-Pérez, A.M.; García-Arnáez, I.; Elortza, F.; Azkargorta, M.; Martín de Llano, J.J.; Carda, C.; Gurruchaga, M.; Suay, J.; et al. Bioactive potential of silica coatings and its effect on the adhesion of proteins to titanium implants. Colloids Surf. B Biointerfaces 2011, 162, 316–325. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Lin, T.; Yang, F.; Zhang, J.; Cai, X.; Yang, Y.; Zhang, P.; Tan, S. Fabrication of a TiO2@Cu Core–Shell Nanorod Array as Coating for Titanium Substrate with Mechanical and Chemical Dual Antibacterial Property. ACS Appl. Bio Mater. 2022, 5, 3349–3359. [Google Scholar] [CrossRef]
- Huang, H.; Chang, Y.Y.; Weng, J.C.; Chen, Y.C.; Lai, C.H.; Shiehe, T.M. Anti-bacterial performance of Zirconia coatings on Titanium implants. Thin Solid Films 2013, 528, 151–156. [Google Scholar] [CrossRef]
- Roknian, M.; Fattah-Alhosseini, A.; Gasht, S.O.; Keshavarz, M.K. Study of the effect of ZnO nanoparticles addition to PEO coatings on pure titanium substrate: Microstructural analysis, antibacterial effect and corrosion behavior of coatings in Ringer’s physiological solution. J. Alloys Compd. 2018, 740, 330–345. [Google Scholar] [CrossRef]
- Shibli, S.M.A.; Mathai, S. Development and bio-electrochemical characterization of a novel TiO2–SiO2 mixed oxide coating for titanium implants. J. Mater. Sci. Mater. Med. 2018, 19, 2971–2981. [Google Scholar] [CrossRef]
- Kumaravel, V.; Nair, K.M.; Mathew, S.; Bartlett, J.; Kennedy, J.E.; Manning, H.G.; Whelan, B.J.; Leyland, N.S.; Pillai, S.C. Antimicrobial TiO2 nanocomposite coatings for surfaces, dental and orthopaedic implants. Chem. Eng. J. 2021, 416, 129071. [Google Scholar] [CrossRef]
- Negrete, O.; Bradfute, S.; Larson, S.R.; Sinha, A.; Coombes, K.R.; Goeke, R.S.; Keenan, L.A.; Duay, J.; Van Heukelom, M.; Meserole, S. Photocatalytic Material Surfaces for SARS-CoV-2 Virus Inactivation; No. SAND-2020-9861; Sandia National Laboratories: Livermore, CA, USA; Sandia National Laboratories: Albuquerque, NM, USA, 2020.
- Fürst, M.M.; Salvi, G.E.; Lang, N.P.; Persson, G.R. Bacterial colonization immediately after installation on oral titanium implants. Clin. Oral Implants Res. 2017, 18, 501–508. [Google Scholar] [CrossRef]
- Vu, T.V.; Nguyen, V.T.; Nguyen-Tri, P.; Nguyen, T.H.; Nguyen, T.V.; Nguyen, T.A. Chapter 17—Antibacterial nanocomposite coatings. In Nanotoxicity: Prevention and Antibacterial Applications of Nanomaterials (Micro and Nano Technologies), 1st ed.; Rajendran, S., Mukherjee, A., Nguyen, T., Godugu, C., Shukla, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 355–364. ISBN 978-0-12-819943-5. [Google Scholar]
- Anastasescu, C.; Gifu, I.C.; Negrila, C.; Socoteanu, R.; Atkinson, I.; Calderon-Moreno, J.M.; Munteanu, C.; Plavan, G.; Strungaru, S.A.; Cheatham, B.; et al. Morpho-structural properties of ZnSe, TiO2-ZnSe materials and enzymatic activity of their bioinorganic hybrids with lysozyme. Mater. Sci. Eng. B 2021, 272, 115350. [Google Scholar] [CrossRef]
- Kao, X.K.-C.; Lin, T.-S.; Mou, C.-Y. Enhanced activity and stability of Lysozyme by immobilization in the matching nanochannels of mesoporous silica nanoparticles. J. Phys. Chem. C 2014, 118, 6734–6743. [Google Scholar] [CrossRef]
- Soares, R.V.; Lin, T.; Siqueira, C.C.; Bruno, L.S.; Li, X.; Oppenheim, F.G.; Offner, G.; Troxler, R.F. Salivary micelles: Identification of complexes containing MG2, sIgA, lactoferrin, amylase, glycosylated proline-rich protein and lysozyme. Arch. Oral Biol. 2004, 49, 337–343. [Google Scholar] [CrossRef]
- Silletti, E.; Vingerhoeds, M.H.; Norde, W.; van Aken, G.A. Complex formation in mixtures of lysozyme-stabilized emulsions and human saliva. J. Colloid Interface Sci. 2007, 313, 485–493. [Google Scholar] [CrossRef]
- Franken, C.; Meijer, C.J.; Dijkman, J.H. Tissue distribution of antileukoprotease and lysozyme in humans. J. Histochem. Cytochem. 1989, 37, 493–498. [Google Scholar] [CrossRef]
- Larsericsdotter, H.; Oscarsson, S.; Buijs, J. Thermodynamic analysis of proteins adsorbed on silica particles: Electrostatic effects. J. Colloid Interface Sci. 2001, 237, 98–103. [Google Scholar] [CrossRef]
- Perevedentseva, E.; Cai, P.J.; Chiu, Y.C.; Cheng, C.L. Characterizing protein activities on the Lysozyme and nanodiamond complex prepared for bio applications. Langmuir 2011, 27, 1085–1091. [Google Scholar] [CrossRef]
- Anastasescu, C.; Spataru, N.; Culita, D.; Atkinson, I.; Spataru, T.; Bratan, V.; Munteanu, C.; Anastasescu, M.; Negrila, C.; Balint, I. Chemically assembled light harvesting CuOx-TiO2 p–n heterostructures. Chem. Eng. J. 2015, 281, 303–311. [Google Scholar] [CrossRef]
- Takeuchi, M.; Abe, Y.; Yoshida, Y.; Nakayama, Y.; Okazaki, M.; Akagawa, Y. Acid pretreatment of titanium implants. Biomaterials 2003, 24, 1821–1827. [Google Scholar] [CrossRef]
- Xiong, Z.; Ma, J.; Ng, W.J.; Waite, T.D.; Zao, X.S. Silver-modified mesoporous TiO2 photocatalyst for water purification. Water Res. 2011, 45, 2095–2103. [Google Scholar] [CrossRef] [PubMed]
- Liqiang, J.; Yichun, Q.; Baiqi, W.; Shudan, L.; Baojiang, J.; Libin, Y.; Wei, F.; Honggang, F.; Jiazhong, S. Review of photoluminescence performance of nano-sized semiconductor materials and its relationships with photocatalytic activity. Sol. Energ. Mat. Sol. Cells 2006, 90, 1773–1787. [Google Scholar] [CrossRef]
- Bumajdad, A.; Madkou, M. Understanding the superior photocatalytic activity of noble metals modified titania under UV and visible light irradiation. Phys. Chem. Chem. Phys. 2014, 16, 7146–7158. [Google Scholar] [CrossRef]
- Chen, S.; Li, J.; Qian, K.; Xu, W.; Lu, Y.; Huang, W.; Yu, S. Large scale photochemical synthesis of M@TiO2 nanocomposites (M = Ag, Pd, Au, Pt) and their optical properties, CO oxidation performance, and antibacterial effect. Nano Res. 2010, 3, 244–255. [Google Scholar] [CrossRef]
- Zampini, G.; Planas, O.; Marmottini, F.; Gulias, O.; Agut, M.; Nonell, S.; Latterin, L. Morphology effects on singlet oxygen production and bacterial photoinactivation efficiency by different silica-protoporphyrin IX nanocomposites. RSC Adv. 2017, 7, 14422–14429. [Google Scholar] [CrossRef]
- Maisch, T.; Baier, J.; Franz, B.; Maier, M.; Landthaler, M.; Szeimies, R.-M.; Baümler, W. The role of singlet oxygen and oxygen concentration in photodynamic inactivation of bacteria. Proc. Natl. Acad. Sci. USA 2007, 104, 7223–7228. [Google Scholar] [CrossRef]
- Anastasescu, C.; Negrila, C.; Angelescu, D.G.; Atkinson, I.; Anastasescu, M.; Spataru, N.; Zaharescu, M.; Balint, I. Particularities of photocatalysis and formation of reactive oxygen species on insulators and semiconductors: Cases of SiO2, TiO2 and their composite. SiO2-TiO2. Catal. Sci. Technol. 2018, 8, 5657–5668. [Google Scholar] [CrossRef]
- Ràgas, X.; Jiménez Garcia, A.; Batlori, X.; Nonell, S. Singlet oxygen photosensitisation by the fluorescent probe Singlet Oxygen Sensor Green. Chem. Commun. 2009, 20, 2920–2922. [Google Scholar] [CrossRef]
- Kim, S.; Fujitsuka, M.; Majima, T. Photochemistry of Singlet Oxygen Sensor Green. J. Phys. Chem. B. 2013, 117, 13985–13992. [Google Scholar] [CrossRef]
- Zeng, Y.; Wan, Y.; Zhang, D. Lysozyme as sensitive reporter for fluorometric and PCR based detection of E. coli and S. aureus using magnetic microbeads. Microchim. Acta 2016, 183, 741–748. [Google Scholar] [CrossRef]
- Kwon, K.; Lee, K.Y.; Lee, Y.W.; Kim, M.; Heo, J.; Ahn, S.J.; Han, S.W. Controlled Synthesis of Icosahedral Gold Nanoparticles and Their Surface-Enhanced Raman Scattering Property. J. Phys. Chem. C 2007, 111, 1161–1165. [Google Scholar] [CrossRef]
- Sancho-Parramon, J. Surface plasmon resonance broadening of metallic particles in the quasi-static approximation: A numerical study of size confinement and interparticle interaction effects. Nanotechnology 2009, 20, 235706. [Google Scholar] [CrossRef]
- Sarbu, I.; Vassu, T.; Chifiriuc, M.C.; Bucur, M.; Stoica, I.; Petrut, S.; Rusu, E.; Moldovan, H.; Pelinescu, D. Assessment the Activity of Some Enzymes and Antibiotic Substances Sensitivity on Pathogenic Bacteria Species. Rev. De Chim. 2017, 68, 3015–3021. [Google Scholar] [CrossRef]
- Ibrahim, H.R.; Matsuzaki, T.; Aoki, T. Genetic evidence that antibacterial activity of lysozyme is independent of its catalytic function. FEBS Lett. 2001, 506, 27–32. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chifor, E.; Bordeianu, I.; Anastasescu, C.; Calderon-Moreno, J.M.; Bratan, V.; Eftemie, D.-I.; Anastasescu, M.; Preda, S.; Plavan, G.; Pelinescu, D.; et al. Bioactive Coatings Based on Nanostructured TiO2 Modified with Noble Metal Nanoparticles and Lysozyme for Ti Dental Implants. Nanomaterials 2022, 12, 3186. https://doi.org/10.3390/nano12183186
Chifor E, Bordeianu I, Anastasescu C, Calderon-Moreno JM, Bratan V, Eftemie D-I, Anastasescu M, Preda S, Plavan G, Pelinescu D, et al. Bioactive Coatings Based on Nanostructured TiO2 Modified with Noble Metal Nanoparticles and Lysozyme for Ti Dental Implants. Nanomaterials. 2022; 12(18):3186. https://doi.org/10.3390/nano12183186
Chicago/Turabian StyleChifor, Emilian, Ion Bordeianu, Crina Anastasescu, Jose Maria Calderon-Moreno, Veronica Bratan, Diana-Ioana Eftemie, Mihai Anastasescu, Silviu Preda, Gabriel Plavan, Diana Pelinescu, and et al. 2022. "Bioactive Coatings Based on Nanostructured TiO2 Modified with Noble Metal Nanoparticles and Lysozyme for Ti Dental Implants" Nanomaterials 12, no. 18: 3186. https://doi.org/10.3390/nano12183186
APA StyleChifor, E., Bordeianu, I., Anastasescu, C., Calderon-Moreno, J. M., Bratan, V., Eftemie, D.-I., Anastasescu, M., Preda, S., Plavan, G., Pelinescu, D., Ionescu, R., Stoica, I., Zaharescu, M., & Balint, I. (2022). Bioactive Coatings Based on Nanostructured TiO2 Modified with Noble Metal Nanoparticles and Lysozyme for Ti Dental Implants. Nanomaterials, 12(18), 3186. https://doi.org/10.3390/nano12183186








