Preparation and Adsorption Properties of Nanostructured Composites Derived from Al/Fe Nanoparticles with Respect to Arsenic
Abstract
:1. Introduction
2. Materials and Methods
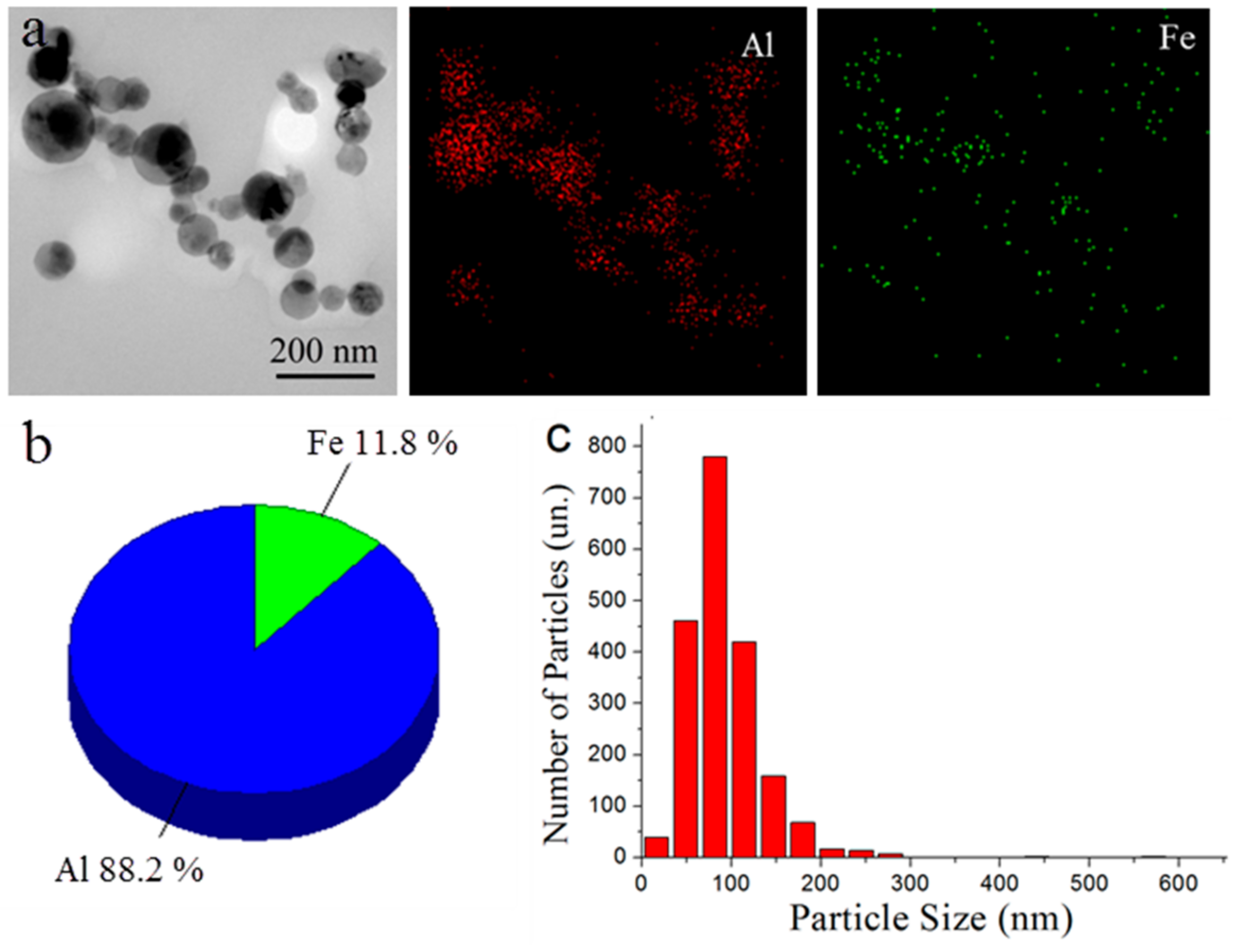
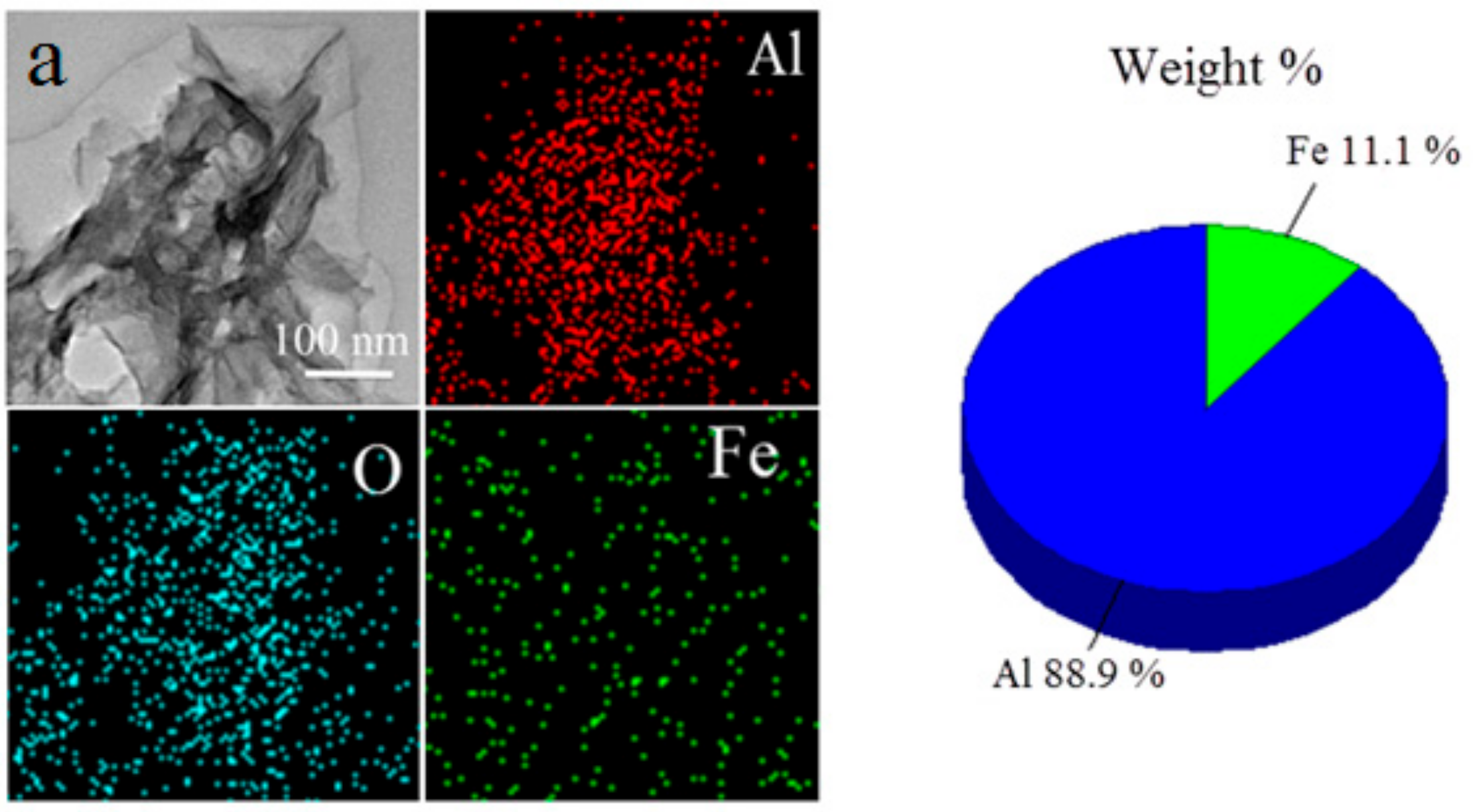
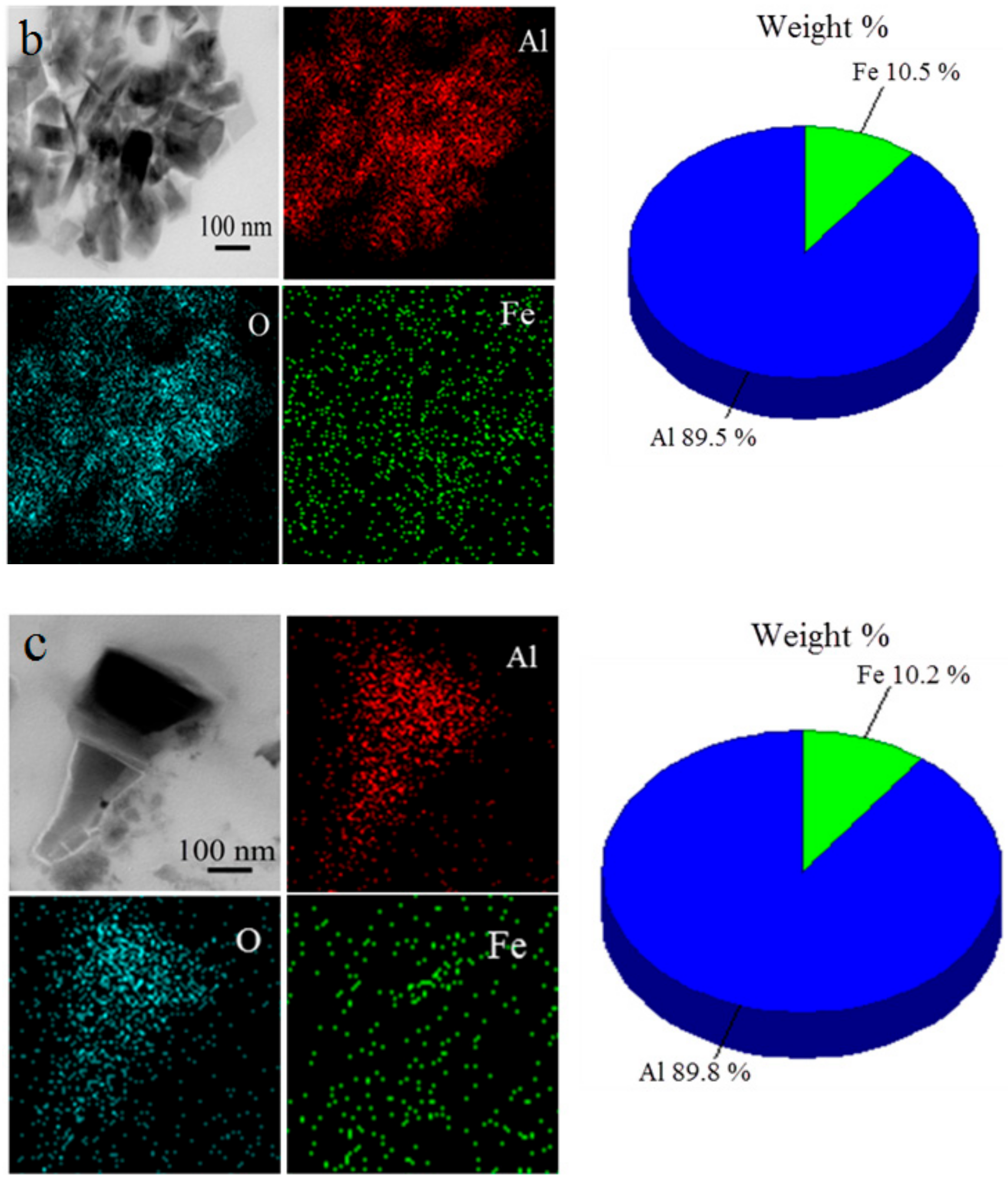
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weerasundara, L.; Ok, Y.-S.; Bundschuh, J. Selective removal of arsenic in water: A critical review. Environ. Pollut. 2021, 268, 115668. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Liu, Z.; Shi, Z. Arsenic removal from aqueous solutions by adsorption onto iron oxide/activated carbon magnetic composite. J. Environ. Health Sci. Eng. 2014, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Pillai, P.; Kakadiya, N.; Timaniya, Z.; Dharaskar, S.; Sillanpaa, M. Removal of arsenic using iron oxide amended with rice husk nanoparticles from aqueous solution. Mater. Today Proc. 2020, 28, 830–835. [Google Scholar] [CrossRef]
- Gallegos-Garciaa, M.; Ramírez-Muñiza, K.; Songa, S. Arsenic removal from water by adsorption using iron oxide minerals as adsorbents: A review. Miner. Process. Extr. Metall. Rev. 2012, 33, 301–315. [Google Scholar] [CrossRef]
- Fadillah, G.; Yudha, S.P.; Sagadevan, S.; Fatimah, I.; Muraza, O. Magnetic iron oxide/clay nanocomposites for adsorption and catalytic oxidation in water treatment applications. Open Chem. 2020, 18, 1148–1166. [Google Scholar] [CrossRef]
- Polowczyk, I.; Cyganowski, P.; Ulatowska, J.; Sawiński, W.; Bastrzyk, A. Synthetic iron oxides for adsorptive removal of arsenic. Water Air Soil Pollut. 2018, 229, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bazrafshan, E.; Faridi, H.; Kord Mostafapour, F.; Mahvi, A.H. Arsenic removal from aqueous environments using Moringa Peregrina seed extract as a natural coagulant. Asian J. Chem. 2013, 25, 3557–3561. [Google Scholar] [CrossRef]
- Koomson, B.; Asiam, E.K. Arsenic Adsorption by Some Iron Oxide Minerals: Influence of Interfacial Chemistry. Ghana Min. J. 2020, 20, 43–48. [Google Scholar] [CrossRef]
- Jeong, Y.; Fan, M.; Singh, S.; Chuang, C.L.; Saha, B.; Van Leeuwen, J.H. Evaluation of iron oxide and aluminum oxide as potential arsenic (V) adsorbents. Chem. Eng. Process.-Process Intensif. 2007, 46, 1030–1039. [Google Scholar] [CrossRef]
- Bae, J.; Kim, S.; Kim, K.S.; Hwang, H.K.; Choi, H. Adsorptive removal of arsenic by mesoporous iron oxide in aquatic systems. Water 2020, 12, 3147. [Google Scholar] [CrossRef]
- Tang, W.; Li, Q.; Gao, S.; Shang, J.K. Arsenic (III, V) removal from aqueous solution by ultrafine α-Fe2O3 nanoparticles synthesized from solvent thermal method. J. Hazard. Mater. 2011, 192, 131–138. [Google Scholar] [CrossRef]
- Meez, E.; Tolkou, A.K.; Giannakoudakis, D.A.; Katsoyiannis, I.A.; Kyzas, G.Z. Activated carbons for arsenic removal from natural waters and wastewaters: A Review. Water 2021, 13, 2982. [Google Scholar] [CrossRef]
- Ha, H.T.; Phong, P.T.; Minh, T.D. Synthesis of iron oxide nanoparticle functionalized activated carbon and its applications in arsenic adsorption. J. Anal. Methods Chem. 2021, 2021, 6668490. [Google Scholar] [CrossRef]
- Sun, Y.; Iris, K.M.; Tsang, D.C.; Cao, X.; Lin, D.; Wang, L.; Graham, N.J.D.; Alessi, D.S.; Komarek, M.; Ok, Y.S.; et al. Multifunctional iron-biochar composites for the removal of potentially toxic elements, inherent cations, and hetero-chloride from hydraulic fracturing wastewater. Environ. Int. 2019, 124, 521–532. [Google Scholar] [CrossRef]
- Alchouron, J.; Navarathna, C.; Chludil, H.D.; Dewage, N.B.; Perez, F.; Pittman, C.U., Jr.; Vega, A.S.; Mlsna, T.E. Assessing South American Guadua chacoensis bamboo biochar and Fe3O4 nanoparticle dispersed analogues for aqueous arsenic (V) remediation. Sci. Total Environ. 2020, 706, 135943. [Google Scholar] [CrossRef]
- Kloster, G.A.; Valiente, M.; Marcovich, N.E.; Mosiewicki, M.A. Adsorption of arsenic onto films based on chitosan and chitosan/nano-iron oxide. Int. J. Biol. Macromol. 2020, 165, 1286–1295. [Google Scholar] [CrossRef]
- Dutta, D.; Borah, J.P.; Puzari, A. Iron oxide coated hollow poly (methylmethacrylate) as an efficient adsorption media for removal of arsenic from water. RSC Adv. 2021, 11, 13376–13385. [Google Scholar] [CrossRef]
- Chai, L.; Wang, Y.; Zhao, N.; Yang, W.; You, X. Sulfate-doped Fe3O4/Al2O3 nanoparticles as a novel adsorbent for fluoride removal from drinking water. Water Res. 2013, 47, 4040–4049. [Google Scholar] [CrossRef]
- Dhawale, V.P.; Khobragade, V.; Kulkarni, S.D. Synthesis and characterization of aluminium oxide (Al2O3) nanoparticles and its application in azodye decolourisation. Chemistry 2018, 27, 31. [Google Scholar]
- Wang, T.; Gowen, A. Adsorption of organic dyes in water by aluminium oxide and a research on the process of adsorbent regeneration. Biosyst. Food Eng. Res. Rev. 2018, 23, 197. [Google Scholar]
- Hami, H.K.; Abbas, R.F.; Eltayef, E.M.; Mahdi, N.I. Applications of aluminum oxide and nano aluminum oxide as adsorbents. Samarra J. Pure Appl. Sci. 2020, 2, 19–32. [Google Scholar] [CrossRef]
- Borthakur, P.; Hussain, N.; Darabdhara, G.; Boruah, P.K.; Sharma, B.; Borthakur, P.; Yadav, A.; Das, M.R. Adhesion of gram-negative bacteria onto α-Al2O3 nanoparticles: A study of surface behaviour and interaction mechanism. J. Environ. Chem. Eng. 2018, 6, 3933–3941. [Google Scholar] [CrossRef]
- El-Taboni, F.; Caseley, E.; Katsikogianni, M.; Swanson, L.; Swift, T.; Romero-González, M.E. Fluorescence spectroscopy analysis of the bacteria–mineral interface: Adsorption of lipopolysaccharides to silica and alumina. Langmuir 2020, 36, 1623–1632. [Google Scholar] [CrossRef]
- Yüzbasi, N.S.; Krawczyk, P.A.; Domagała, K.W.; Englert, A.; Burkhardt, M.; Stuer, M.; Graule, T. Removal of MS2 and fr bacteriophages using MgAl2O4-modified, Al2O3-stabilized porous ceramic granules for drinking water treatment. Membranes 2022, 12, 471. [Google Scholar] [CrossRef]
- Svarovskaya, N.; Bakina, O.; Glazkova, E.; Rodkevich, N.; Lerner, M.; Vornakova, E.; Chzhou, V.; Naumova, L. Synthesis of novel hierarchical micro/nanostructures AlOOH/AlFe and their application for As (V) removal. Environ. Sci. Pollut. Res. 2022, 29, 1246–1258. [Google Scholar] [CrossRef]
- Kazantsev, S.O.; Gorbikov, I.A. Peculiarity of the oxidation by water aluminum nanoparticles in different conditions. Adv. Curr. Nat. Sci. 2015, 10, 27–31. [Google Scholar]
- Kazantsev, S.O.; Lozhkomoev, A.S.; Glazkova, E.A.; Gotman, I.; Gutmanas, E.Y.; Lerner, M.I.; Psakhie, S.G. Preparation of aluminum hydroxide and oxide nanostructures with controllable morphology by wet oxidation of AlN/Al nanoparticles. Mater. Res. Bull. 2018, 104, 97–103. [Google Scholar] [CrossRef]
- Lerner, M.I.; Pervikov, A.V.; Glazkova, E.A.; Svarovskaya, N.V.; Lozhkomoev, A.S.; Psakhie, S.G. Structures of binary metallic nanoparticles produced by electrical explosion of two wires from immiscible elements. Powder Technol. 2016, 288, 371–378. [Google Scholar] [CrossRef]
- Nagy, B.; Mânzatu, C.; Măicăneanu, A.; Indolean, C.; Barbu-Tudoran, L.; Majdik, C. Linear and nonlinear regression analysis for heavy metals removal using Agaricus bisporus macrofungus. Arab. J. Chem. 2017, 10, S3569–S3579. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and interpretation of adsorption isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Amrhar, O.; Nassali, H.; Elyoubi, M.S. Application of nonlinear regression analysis to select the optimum absorption isotherm for Methylene Blue adsorption onto Natural Illitic Clay. Bull. Soc. R. Sci. Liège 2015, 84, 116–130. [Google Scholar]
- Elmorsi, T.M. Equilibrium isotherms and kinetic studies of removal of methylene blue dye by adsorption onto miswak leaves as a natural adsorbent. J. Environ. Prot. 2011, 2, 817. [Google Scholar] [CrossRef]
- Ho, Y.S.; Chiu, W.T.; Wang, C.C. Regression analysis for the sorption isotherms of basic dyes on sugarcane dust. Bioresour. Technol. 2005, 96, 1285–1291. [Google Scholar] [CrossRef]
- Erdogan, F.O. Freundlich, Langmuir, Temkin and Harkins-Jura isotherms studies of H2 adsorption on porous adsorbents. Chemistry 2019, 13, 129–135. [Google Scholar] [CrossRef]




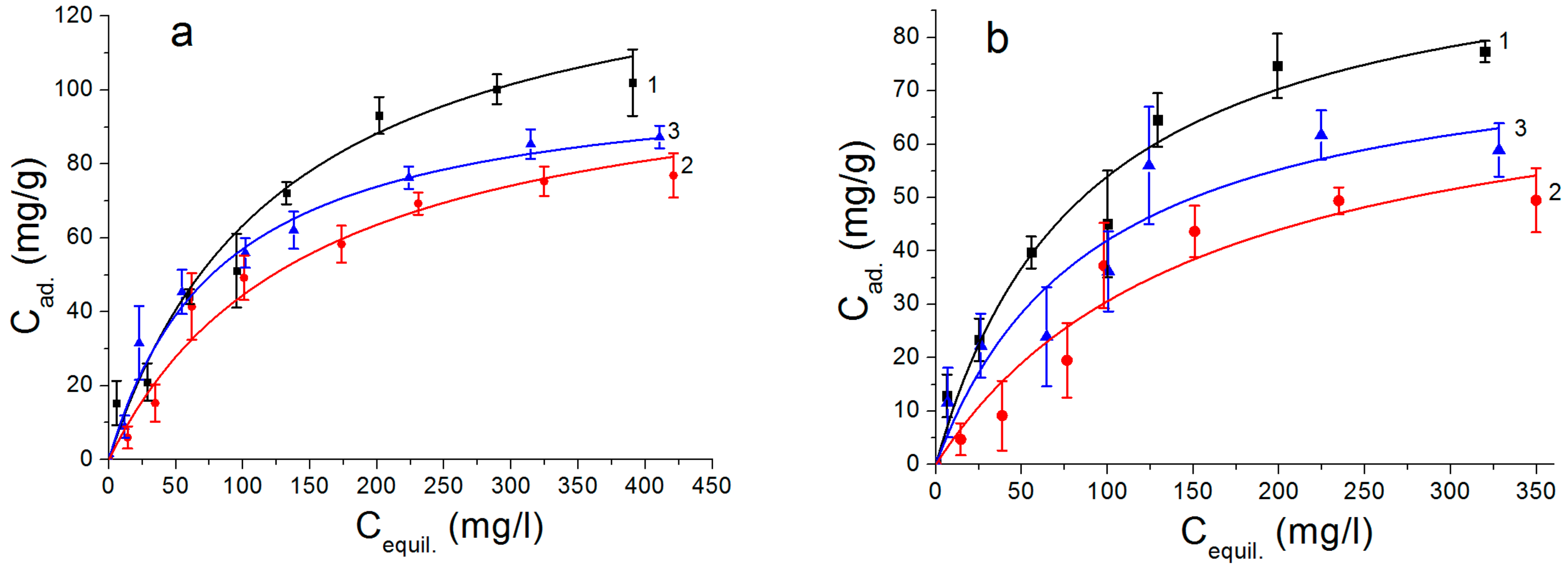

| Samples | BET Surface Areas (m2/g) | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|---|
| qmax (mg/g) | Ka | R2 | Kf | 1/n | R2 | ||
| AlOOH nanosheets | 252 | 80.5 | 0.0201 | 0.9371 | 5.17 | 0.48 | 0.9731 |
| AlOOH nanoplates | 72 | 117.3 | 0.0027 | 0.9717 | 0.58 | 0.79 | 0.9085 |
| Al(OH)3 nanorods | 60 | 54.7 | 0.0259 | 0.8881 | 4.68 | 0.45 | 0.9061 |
| AlOOH/FeAl2 nanosheets | 330 | 135.2 | 0.0080 | 0.7585 | 4.57 | 0.53 | 0.9816 |
| AlOOH/Fe2O3/FeAl2 nanoplates | 75 | 141.1 | 0.0037 | 0.9802 | 1.01 | 0.75 | 0.9893 |
| Al(OH)3/FeAl2 nanorods | 43 | 116.0 | 0.0089 | 0.8948 | 2.53 | 0.62 | 0.9923 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazantsev, S.O.; Lozhkomoev, A.S.; Rodkevich, N.G. Preparation and Adsorption Properties of Nanostructured Composites Derived from Al/Fe Nanoparticles with Respect to Arsenic. Nanomaterials 2022, 12, 3177. https://doi.org/10.3390/nano12183177
Kazantsev SO, Lozhkomoev AS, Rodkevich NG. Preparation and Adsorption Properties of Nanostructured Composites Derived from Al/Fe Nanoparticles with Respect to Arsenic. Nanomaterials. 2022; 12(18):3177. https://doi.org/10.3390/nano12183177
Chicago/Turabian StyleKazantsev, Sergey O., Aleksandr S. Lozhkomoev, and Nikolay G. Rodkevich. 2022. "Preparation and Adsorption Properties of Nanostructured Composites Derived from Al/Fe Nanoparticles with Respect to Arsenic" Nanomaterials 12, no. 18: 3177. https://doi.org/10.3390/nano12183177
APA StyleKazantsev, S. O., Lozhkomoev, A. S., & Rodkevich, N. G. (2022). Preparation and Adsorption Properties of Nanostructured Composites Derived from Al/Fe Nanoparticles with Respect to Arsenic. Nanomaterials, 12(18), 3177. https://doi.org/10.3390/nano12183177





