QD:Puf Nanohybrids Are Compatible with Studies in Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Quantum Dots and Proteins
2.2. Cell Culture and QD:Puf Treatment
2.3. Endocytosis Inhibition
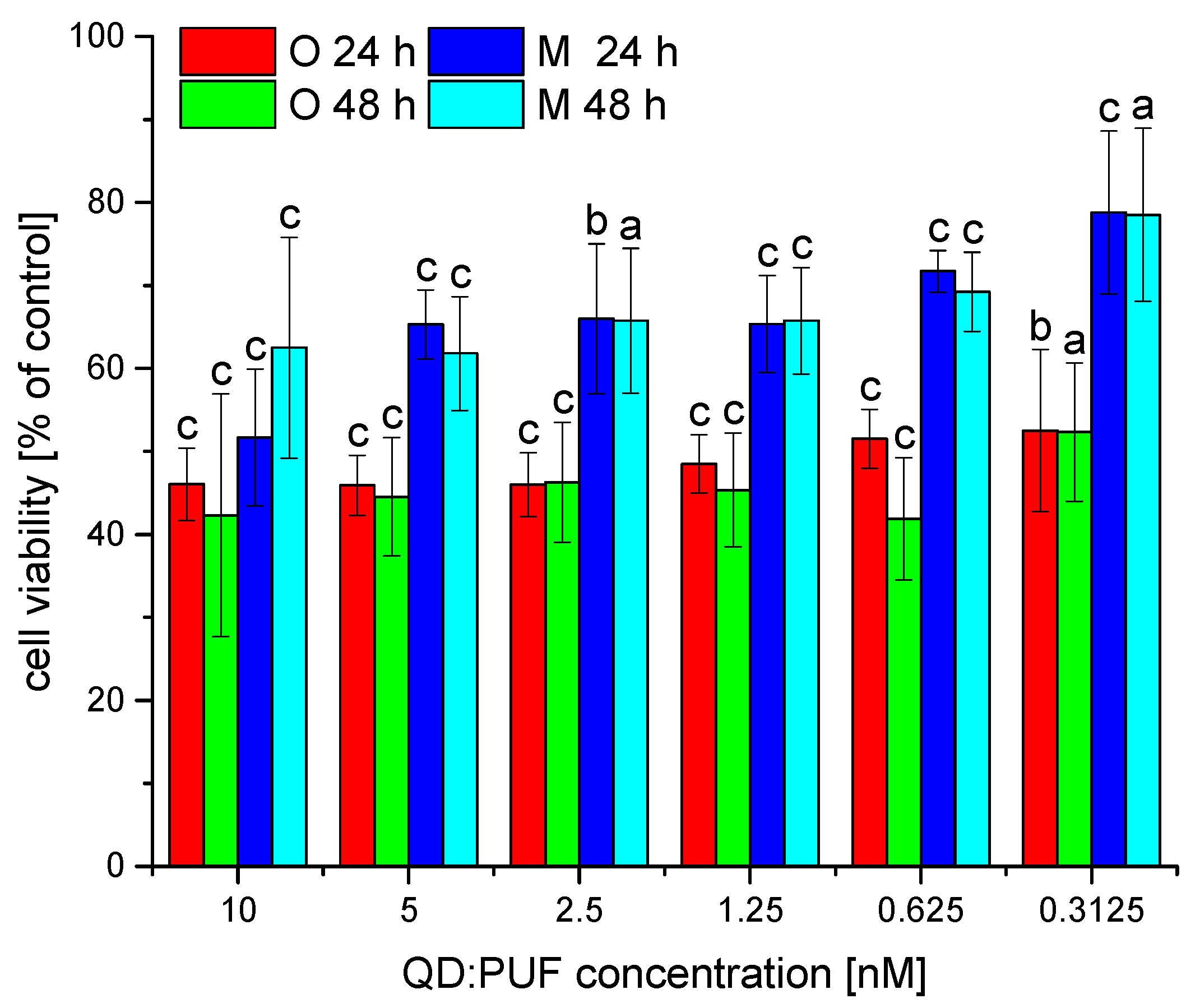
2.4. Toxicity Tests
2.5. Spectrophotometry and Spectrofluorometry
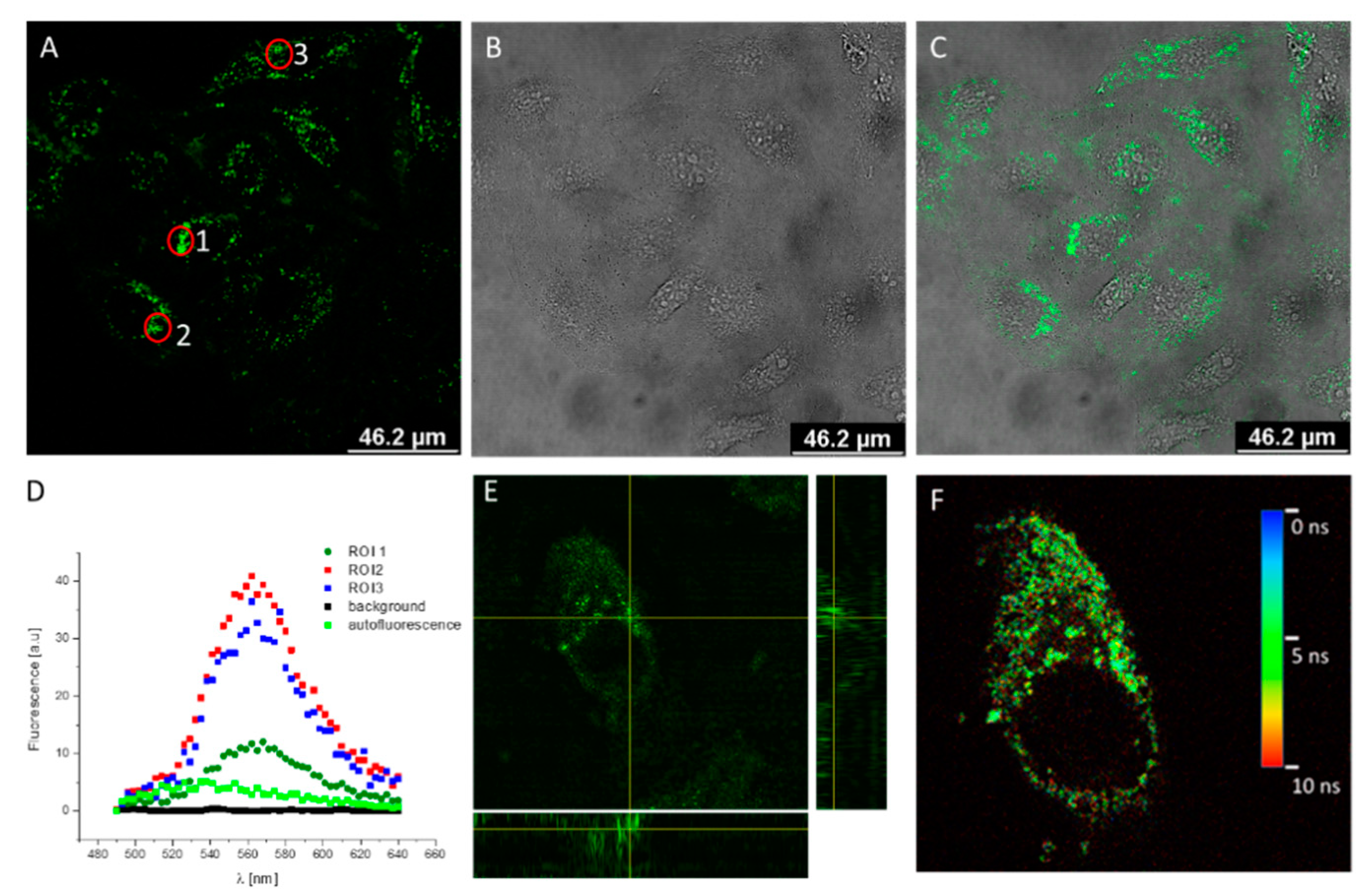
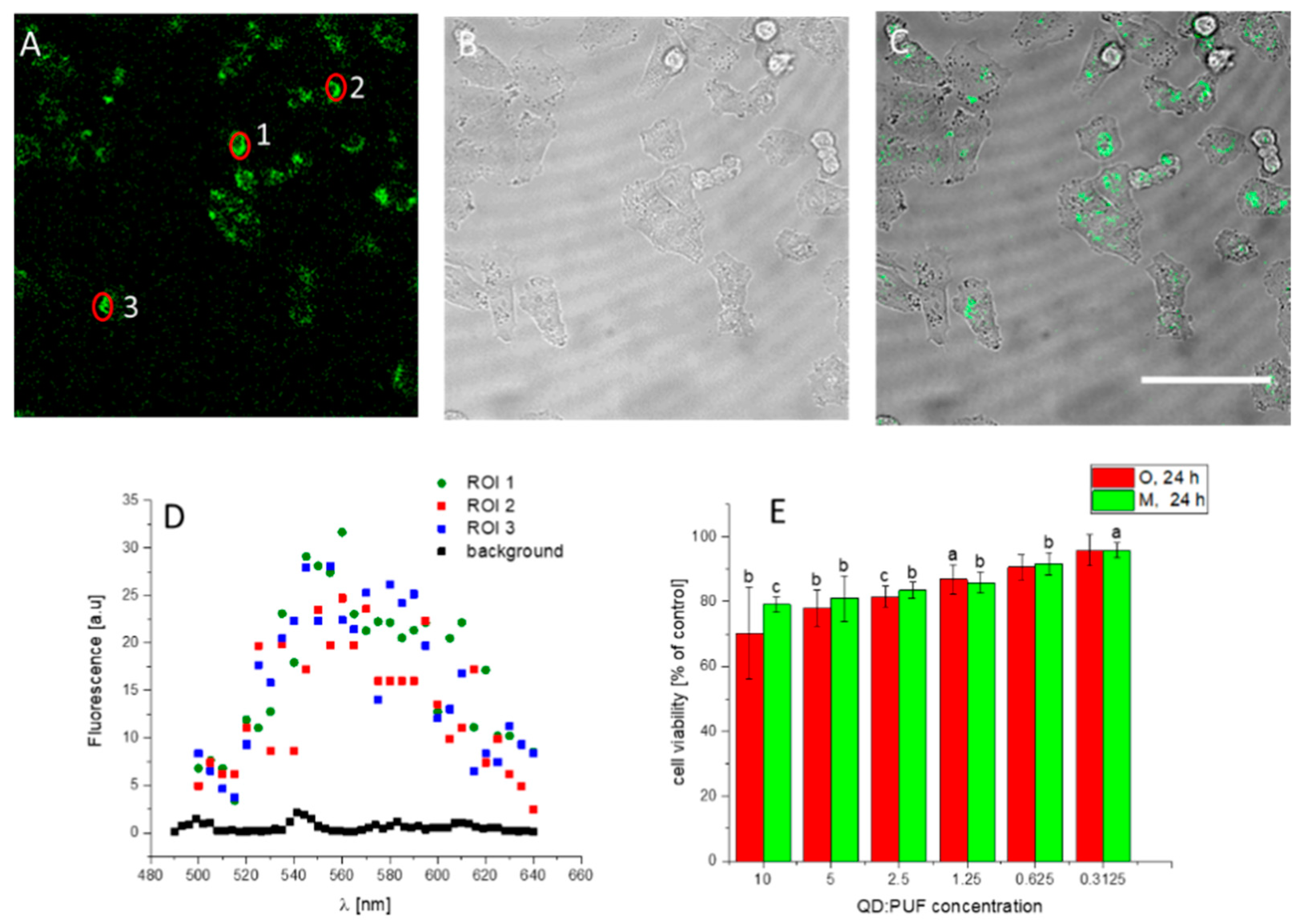
2.6. Confocal Laser Scanning Microscopy (CLSM)
2.7. Cell Staining
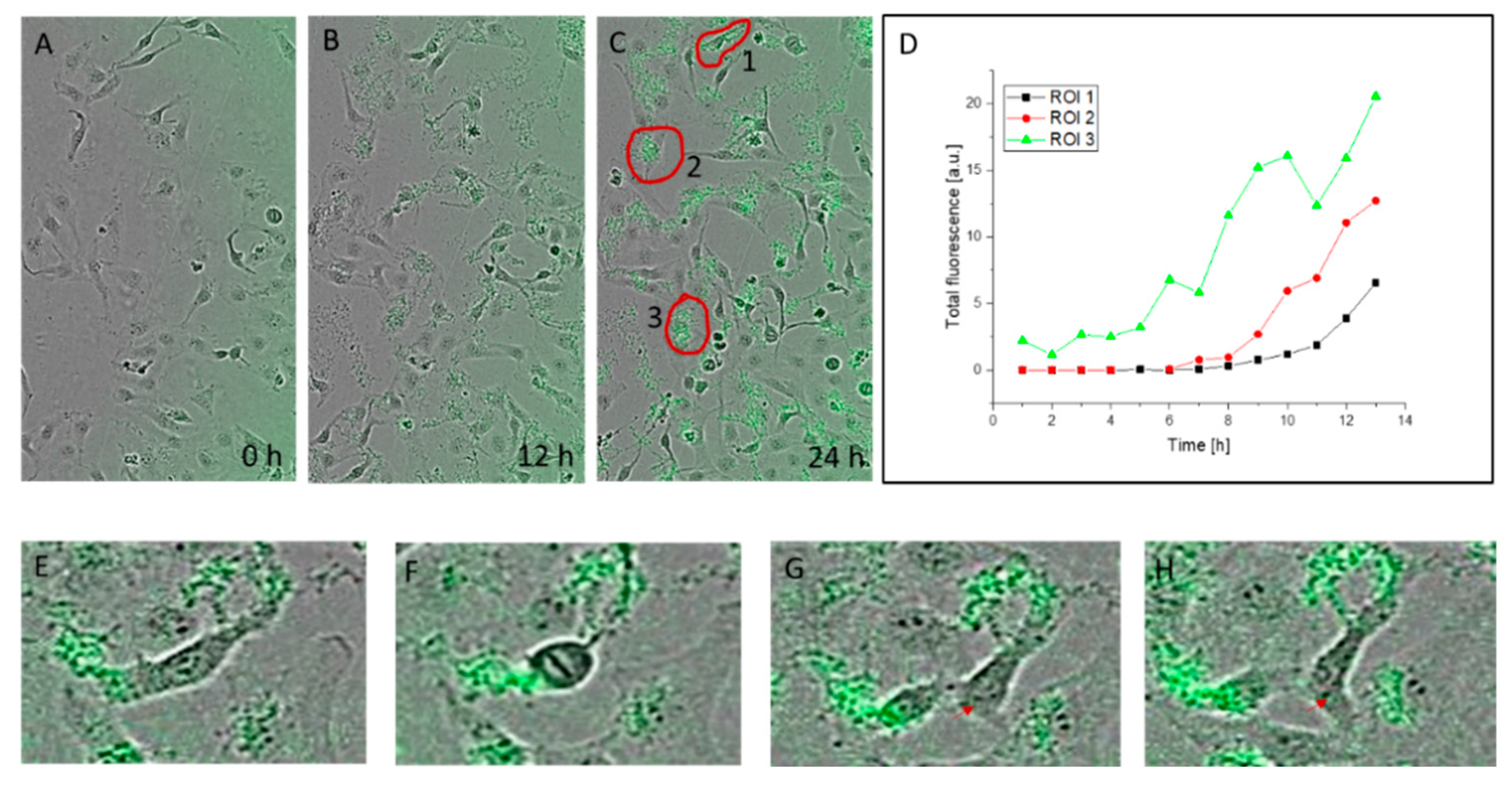
2.8. IncuCyte Imaging
2.9. Statistical Analysis
3. Results and Discussion
3.1. Optimization of QD:Puf Concentration for In-Cell Delivery
3.2. In Cell Localization of Nanohybrids
3.3. Kinetics of Nanohybrid Absorption by Cells
3.4. Toxicity of Nanohybrids
3.5. Other Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, H.; Lin, Y.; Tian, Z.Q.; Zhu, D.L.; Zhang, Z.L.; Pang, D.W. Ultrasmall Pb: Ag2S Quantum Dots with Uniform Particle Size and Bright Tunable Fluorescence in the NIR-II Window. Small 2018, 14, 1703296. [Google Scholar] [CrossRef]
- Jin, T.; Imamura, Y. Applications of highly bright PbS quantum dots to non-invasive near-infrared fluorescence imaging in the second optical window. ECS J. Solid State Sci. Technol. 2015, 5, R3138. [Google Scholar] [CrossRef]
- Speranskaya, E.S.; Beloglazova, N.V.; Lenain, P.; De Saeger, S.; Wang, Z.; Zhang, S.; Hens, Z.; Knopp, D.; Niessner, R.; Potapkin, D.V. Polymer-coated fluorescent CdSe-based quantum dots for application in immunoassay. Biosens. Bioelectron. 2014, 53, 225–231. [Google Scholar] [CrossRef]
- Bagheri, E.; Ansari, L.; Abnous, K.; Taghdisi, S.M.; Ramezani, P.; Ramezani, M.; Alibolandi, M. Silica–Quantum Dot Nanomaterials as a Versatile Sensing Platform. Crit. Rev. Anal. Chem. 2020, 51, 687–708. [Google Scholar] [CrossRef]
- Kopeć, K.; Pędziwiatr, M.; Gront, D.; Sztatelman, O.; Sławski, J.; Łazicka, M.; Worch, R.; Zawada, K.; Makarova, K.; Nyk, M. Comparison of α-Helix and β-Sheet Structure Adaptation to a Quantum Dot Geometry: Toward the Identification of an Optimal Motif for a Protein Nanoparticle Cover. ACS Omega 2019, 4, 13086–13099. [Google Scholar] [CrossRef]
- Dąbrowska, A.; Nyk, M.; Worch, R.; Grzyb, J. Hydrophilic colloidal quantum dots with long peptide chain coats. Colloid Surf. B 2016, 145, 662–670. [Google Scholar] [CrossRef]
- Ding, S.; Zhang, N.; Lyu, Z.; Zhu, W.; Chang, Y.-C.; Hu, X.; Du, D.; Lin, Y. Protein-based nanomaterials and nanosystems for biomedical applications: A review. Mater. Today 2021, 43, 166–184. [Google Scholar] [CrossRef]
- Sztatelman, O.; Kopec, K.; Pedziwiatr, M.; Trojnar, M.; Worch, R.; Wielgus-Kutrowska, B.; Jemiola-Rzeminska, M.; Bzowska, A.; Grzyb, J. Heterodimerizing helices as tools for nanoscale control of the organization of protein-protein and protein-quantum dots. Biochimie 2019, 167, 93–105. [Google Scholar] [CrossRef]
- Grzyb, J.; Walczewska-Szewc, K.; Slawski, J.; Trojnar, M. Quantum dot clusters as self-assembled antennae with phycocyanine and phycobilisomes as energy acceptors. Phys. Chem. Chem. Phys. 2021, 23, 24505–24517. [Google Scholar] [CrossRef]
- Xu, W.; Wu, Y.; Jiao, L.; Gu, W.; Du, D.; Lin, Y.; Zhu, C. Engineering Metal-Organic Framework-based Nanozymes for Enhanced Biosensing. Curr. Anal. Chem. 2022, 18, 739–752. [Google Scholar] [CrossRef]
- Lyu, Z.; Ding, S.; Du, D.; Qiu, K.; Liu, J.; Hayashi, K.; Zhang, X.; Lin, Y. Recent advances in biomedical applications of 2D nanomaterials with peroxidase-like properties. Adv. Drug Deliv. Rev. 2022, 185, 114269. [Google Scholar] [CrossRef]
- Sławski, J.; Grzyb, J. Nanoparticles as energy donors and acceptors in bionanohybrid systems. Acta Biochim. Pol. 2019, 66, 469–481. [Google Scholar] [CrossRef]
- Antoniak, M.A.; Grzyb, J.; Nyk, M. Preserved two-photon optical properties of hydrophilic proteins-conjugated quantum dots. J. Lumin. 2019, 209, 57–60. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Clift, M.J.; Rothen-Rutishauser, B.; Brown, D.M.; Duffin, R.; Donaldson, K.; Proudfoot, L.; Guy, K.; Stone, V. The impact of different nanoparticle surface chemistry and size on uptake and toxicity in a murine macrophage cell line. Toxicol. Appl. Pharmacol. 2008, 232, 418–427. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, P.; Wang, Y.; Zhu, Z.; Lao, F.; Liu, X.; Cong, W.; Chen, C.; Gao, Y.; Liu, Y. The Influence on Cell Cycle and Cell Division by Various Cadmium-Containing Quantum Dots. Small 2013, 9, 2440–2451. [Google Scholar] [CrossRef]
- Jin, T.; Fujii, F.; Yamada, E.; Nodasaka, Y.; Kinjo, M. Control of the optical properties of quantum dots by surface coating with calix [n] arene carboxylic acids. J. Am. Chem. Soc. 2006, 128, 9288–9289. [Google Scholar] [CrossRef]
- Szczepaniak, K.; Worch, R.; Grzyb, J. Ferredoxin:NADP+ oxidoreductase in junction with CdSe/ZnS quantum dots: Characteristics of an enzymatically active nanohybrid. J. Phys. Condens. Matter. 2013, 25, 194102. [Google Scholar] [CrossRef]
- Lesiak, A.; Banski, M.; Halicka, K.; Cabaj, J.; Żak, A.; Podhorodecki, A. pH-dependent fluorescence of thiol-coated CdSe/CdS quantum dots in an aqueous phase. Nanotechnology 2020, 32, 075705. [Google Scholar]
- Schwabacher, J.C.; Kodaimati, M.S.; Weiss, E.A. Origin of the pH Dependence of Emission of Aqueous Dihydrolipoic Acid-Capped PbS Quantum Dots. J. Phys. Chem. C 2019, 123, 17574–17579. [Google Scholar]
- Westmoreland, D.E.; Nap, R.J.; Arcudi, F.; Szleifer, I.; Weiss, E.A. pH-Dependent structure of water-exposed surfaces of CdSe quantum dots. Chem. Commun. 2019, 55, 5435–5438. [Google Scholar] [CrossRef]
- Javier, A.; Magana, D.; Jennings, T.; Strouse, G.F. Nanosecond exciton recombination dynamics in colloidal CdSe quantum dots under ambient conditions. Appl. Phys. Lett. 2003, 83, 1423–1425. [Google Scholar] [CrossRef]
- Darżynkiewicz, Z.M.; Pędziwiatr, M.; Grzyb, J. Quantum dots use both LUMO and surface trap electrons in photoreduction process. J. Lumin. 2017, 183, 401–409. [Google Scholar] [CrossRef]
- Nawrot, K.C.; Sharma, M.; Cichy, B.; Sharma, A.; Delikanli, S.; Samoć, M.; Demir, H.V.; Nyk, M. Spectrally Resolved Nonlinear Optical Properties of Doped Versus Undoped Quasi-2D Semiconductor Nanocrystals: Copper and Silver Doping Provokes Strong Nonlinearity in Colloidal CdSe Nanoplatelets. ACS Photonics 2022, 9, 256–267. [Google Scholar] [CrossRef]
- Corazzari, I.; Gilardino, A.; Dalmazzo, S.; Fubini, B.; Lovisolo, D. Localization of CdSe/ZnS quantum dots in the lysosomal acidic compartment of cultured neurons and its impact on viability: Potential role of ion release. Toxicol. In Vitro 2013, 27, 752–759. [Google Scholar] [CrossRef]
- Li, H.; Dou, S.-X.; Liu, Y.-R.; Li, W.; Xie, P.; Wang, W.-C.; Wang, P.-Y. Mapping intracellular diffusion distribution using single quantum dot tracking: Compartmentalized diffusion defined by endoplasmic reticulum. J. Am. Chem. Soc. 2015, 137, 436–444. [Google Scholar] [CrossRef]
- Yuan, M.; Guo, Y.; Wei, J.; Li, J.; Long, T.; Liu, Z. Optically active blue-emitting carbon dots to specifically target the Golgi apparatus. RSC Adv. 2017, 7, 49931–49936. [Google Scholar] [CrossRef]
- Wang, Z.-G.; Liu, S.-L.; Hu, Y.-J.; Tian, Z.-Q.; Hu, B.; Zhang, Z.-L.; Pang, D.-W. Dissecting the factors affecting the fluorescence stability of quantum dots in live cells. ACS Appl. Mater. Interfaces 2016, 8, 8401–8408. [Google Scholar] [CrossRef]
- Hoshino, A.; Fujioka, K.; Oku, T.; Nakamura, S.; Suga, M.; Yamaguchi, Y.; Suzuki, K.; Yasuhara, M.; Yamamoto, K. Quantum dots targeted to the assigned organelle in living cells. Microbiol. Immunol. 2004, 48, 985–994. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Chang, Q.; Sun, Z.-X.; Liu, J.; Deng, X.; Liu, Y.; Cao, A.; Wang, H. Fate of CdSe/ZnS quantum dots in cells: Endocytosis, translocation and exocytosis. Colloid Surf. B 2021, 208, 112140. [Google Scholar] [CrossRef]
- Sun, Y.; Gong, J.; Cao, Y. Multi-walled carbon nanotubes (MWCNTs) activate apoptotic pathway through ER stress: Does surface chemistry matter? Int. J. Nanomed. 2019, 14, 9285. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Z.; Qin, H.; Liu, F.; Cheng, K.; Wu, R.a.; Zou, H. Cell nucleus targeting for living cell extraction of nucleic acid associated proteins with intracellular nanoprobes of magnetic carbon nanotubes. Anal. Chem. 2013, 85, 7038–7043. [Google Scholar] [CrossRef]
- Jung, Y.K.; Shin, E.; Kim, B.-S. Cell nucleus-targeting zwitterionic carbon dots. Sci. Rep. 2015, 5, 18807. [Google Scholar] [CrossRef]
- Koga, D.; Ushiki, T. Three-dimensional ultrastructure of the Golgi apparatus in different cells: High-resolution scanning electron microscopy of osmium-macerated tissues. Arch. Histol. Cytol. 2006, 69, 357–374. [Google Scholar] [CrossRef]
- Pelassa, I.; Zhao, C.; Pasche, M.; Odermatt, B.; Lagnado, L. Synaptic vesicles are “primed” for fast clathrin-mediated endocytosis at the ribbon synapse. Front. Mol. Neurosci. 2014, 7, 91. [Google Scholar] [CrossRef]
- Oh, N.; Park, J.-H. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. J. Nanomed. 2014, 9, 51. [Google Scholar]
- Rennick, J.J.; Johnston, A.P.; Parton, R.G. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat. Nanotechnol. 2021, 16, 266–276. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, W.; Zhang, J.; Liu, J.; Chen, G.; Pope, C. In vitro and in vivo toxicity of CdTe nanoparticles. J. Nanosci. Nanotechnol. 2007, 7, 497–503. [Google Scholar] [CrossRef]
- Lovric, J.; Cho, S.J.; Winnik, F.M.; Maysinger, D. Unmodified cadmium telluride quantum dots induce reactive oxygen species formation leading to multiple organelle damage and cell death. Chem. Biol. 2005, 12, 1227–1234. [Google Scholar] [CrossRef]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS generation and antioxidant defense systems in normal and malignant cells. Oxidative Med. Cell. Longev. 2019, 2019, 6175804. [Google Scholar]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Masters, J.R. HeLa cells 50 years on: The good, the bad and the ugly. Nat. Rev. Cancer 2002, 2, 315–319. [Google Scholar] [CrossRef]
- Benga, G. Basic studies on gene therapy of human malignant melanoma by use of the human interferon β gene entrapped in cationic multilamellar liposomes: 1. Morphology and growth rate of six melanoma cell lines used in transfection experiments with the human interferon β gene. J. Cell. Mol. Med. 2001, 5, 402–408. [Google Scholar]
- Kuzu, O.F.; Noory, M.A.; Robertson, G.P. The role of cholesterol in cancer. Cancer Res. 2016, 76, 2063–2070. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójtowicz, K.; Antoniak, M.A.; Trojnar, M.; Nyk, M.; Trombik, T.; Grzyb, J. QD:Puf Nanohybrids Are Compatible with Studies in Cells. Nanomaterials 2022, 12, 3174. https://doi.org/10.3390/nano12183174
Wójtowicz K, Antoniak MA, Trojnar M, Nyk M, Trombik T, Grzyb J. QD:Puf Nanohybrids Are Compatible with Studies in Cells. Nanomaterials. 2022; 12(18):3174. https://doi.org/10.3390/nano12183174
Chicago/Turabian StyleWójtowicz, Karolina, Magda A. Antoniak, Martyna Trojnar, Marcin Nyk, Tomasz Trombik, and Joanna Grzyb. 2022. "QD:Puf Nanohybrids Are Compatible with Studies in Cells" Nanomaterials 12, no. 18: 3174. https://doi.org/10.3390/nano12183174
APA StyleWójtowicz, K., Antoniak, M. A., Trojnar, M., Nyk, M., Trombik, T., & Grzyb, J. (2022). QD:Puf Nanohybrids Are Compatible with Studies in Cells. Nanomaterials, 12(18), 3174. https://doi.org/10.3390/nano12183174








