Multifaceted Elevation of ROS Generation for Effective Cancer Suppression
Abstract
:1. Introduction
2. Results and Discussion
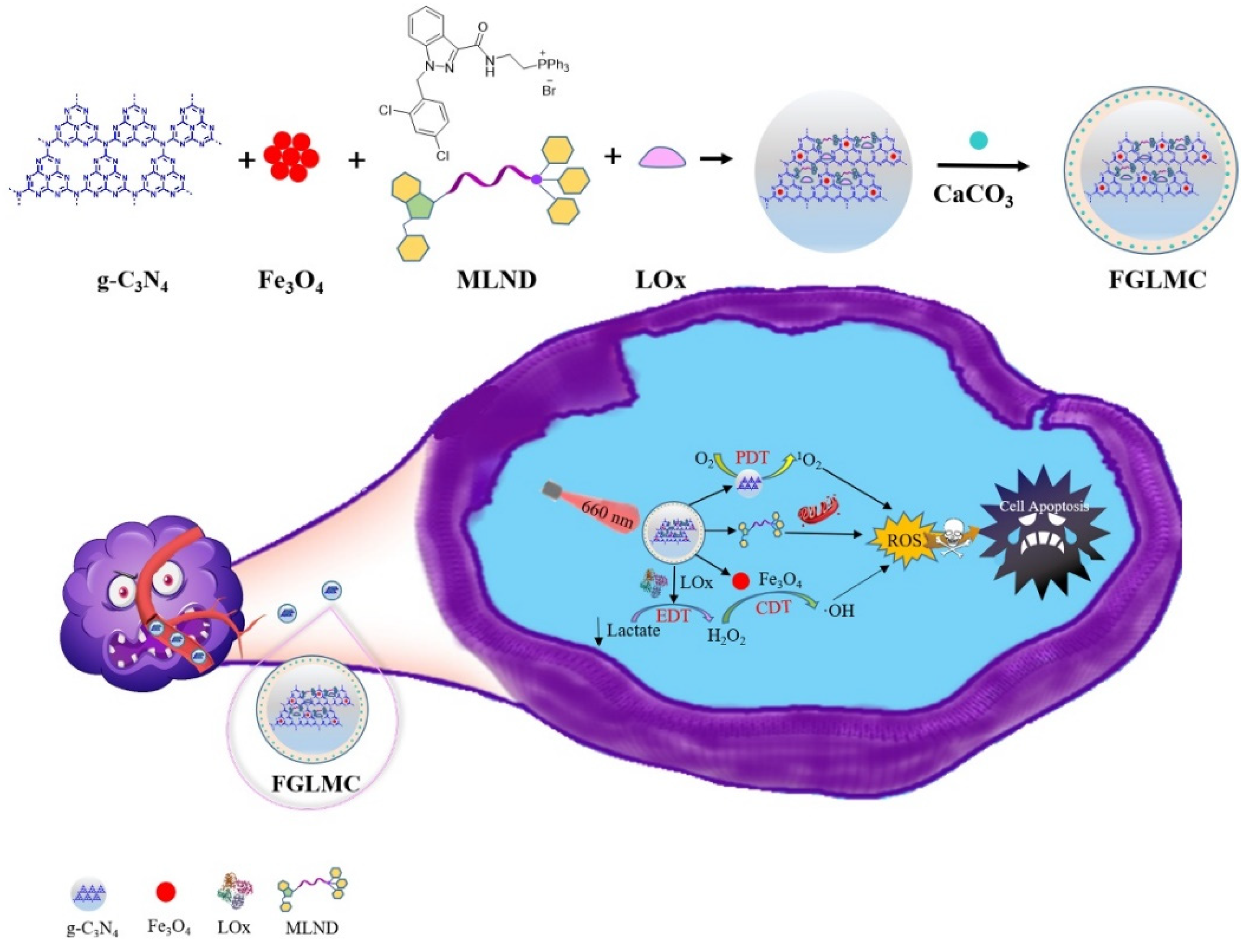
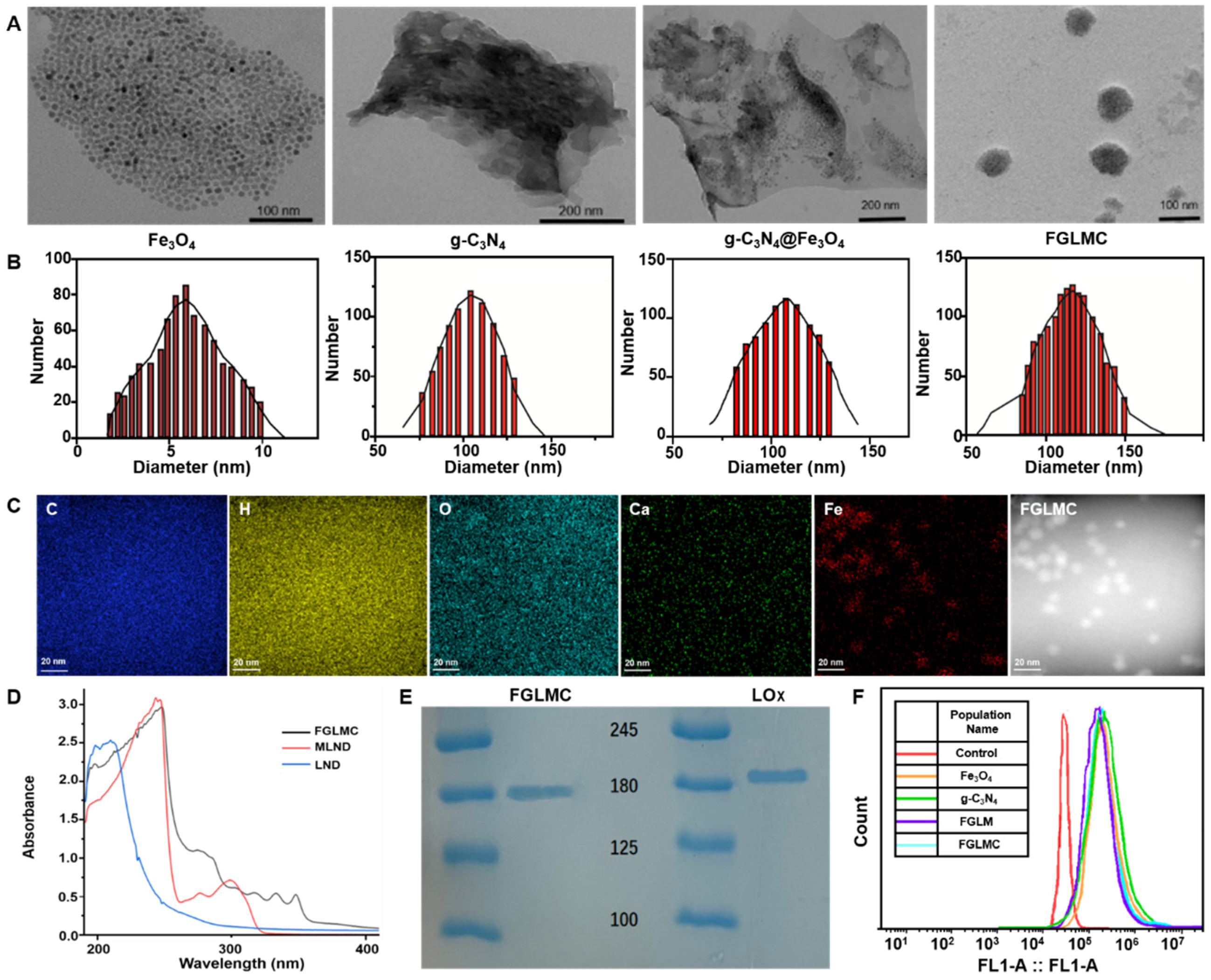
2.1. Synthesis and Characterization of FGLMC
2.1.1. LOx Loading
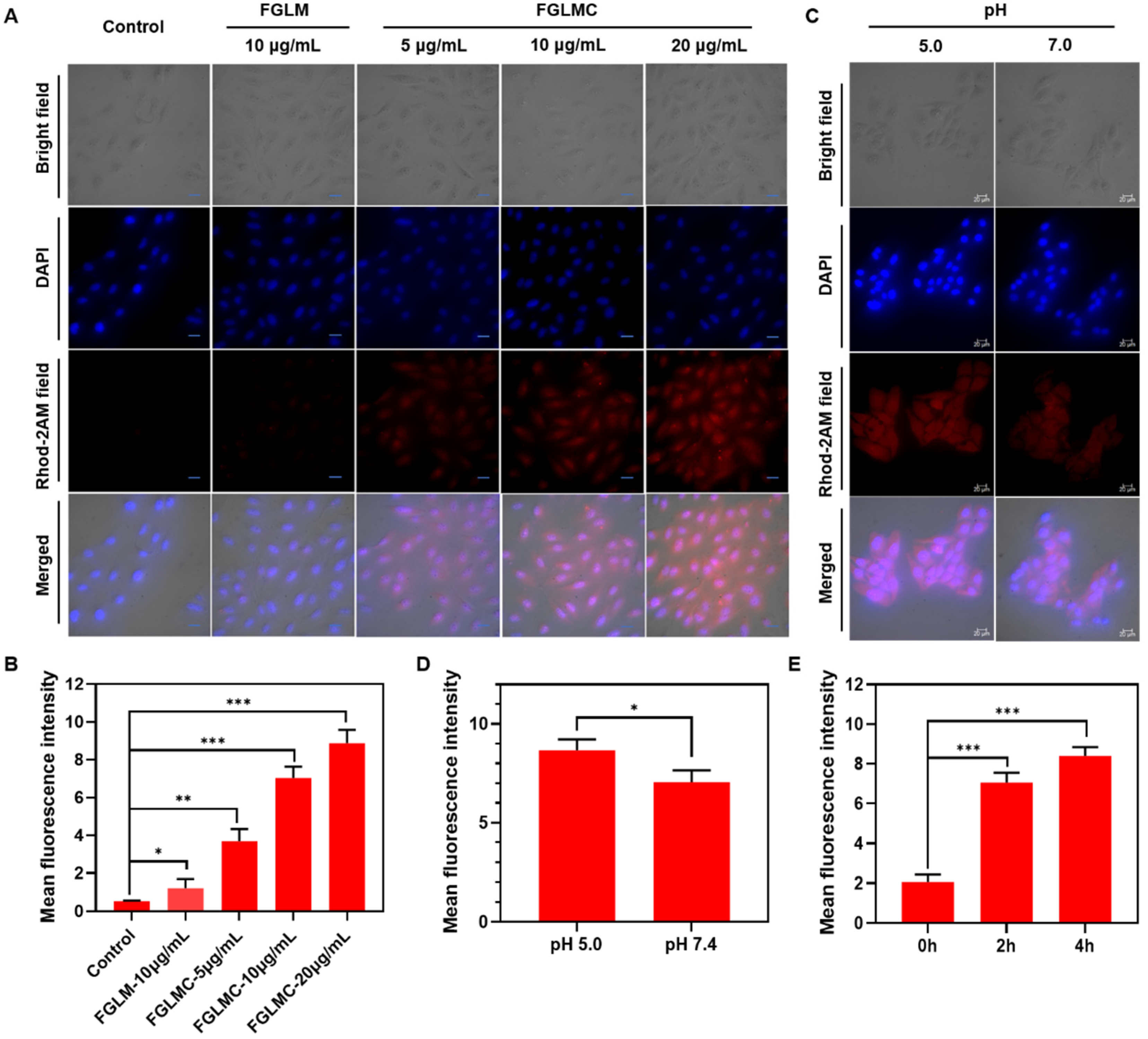
2.1.2. Mitochondrial Targeting
2.1.3. Ca2+ Releasing
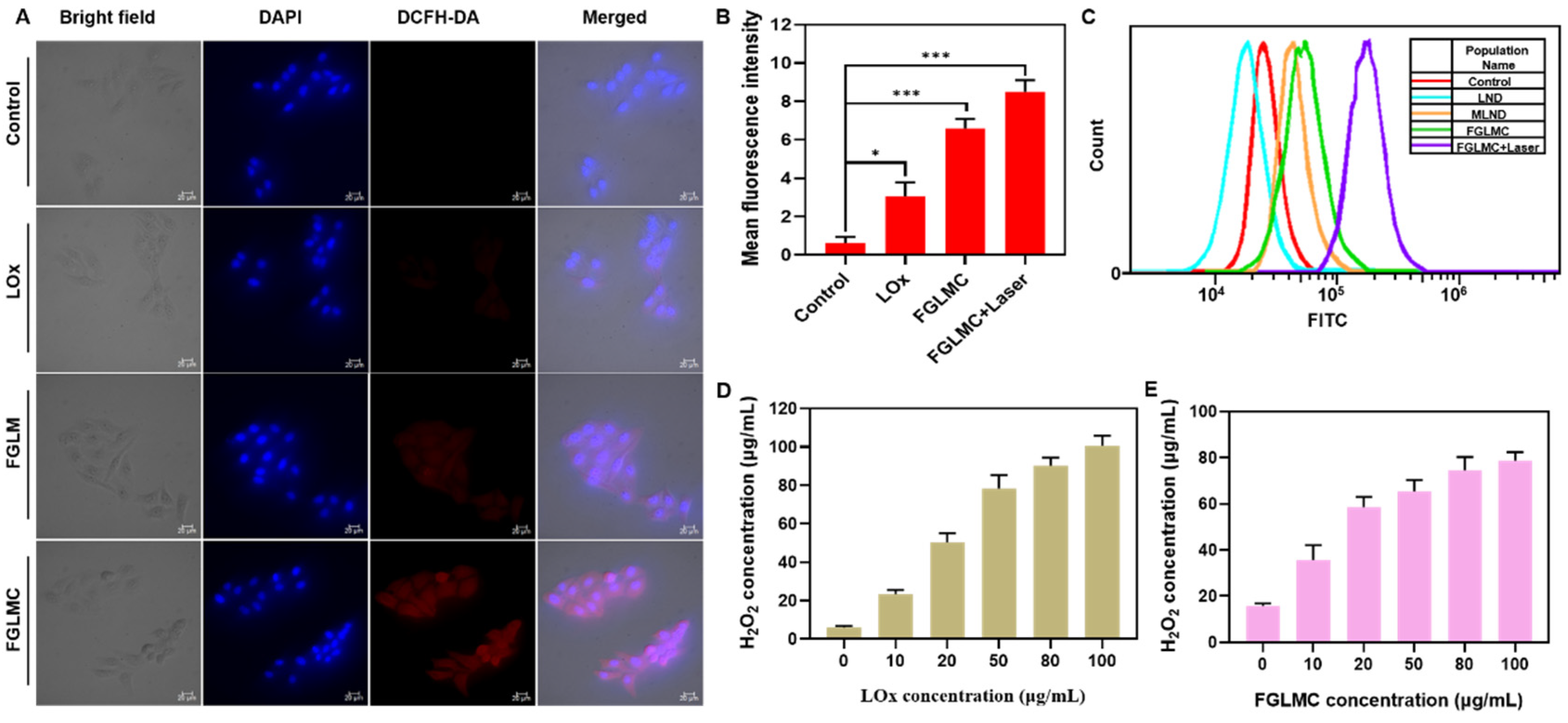
2.2. ROS Generation
2.3. H2O2 Detection
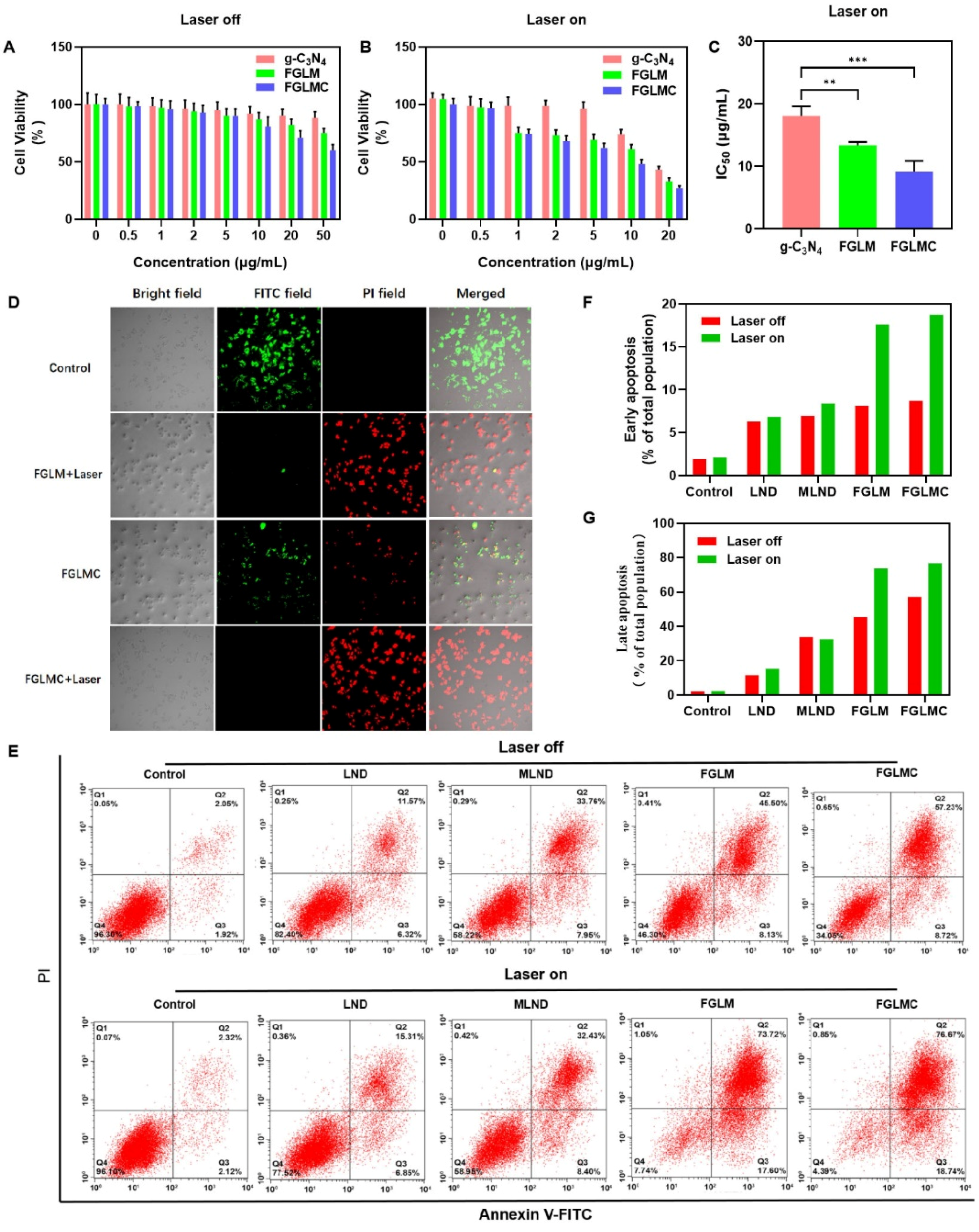
2.4. In Vitro Cytotoxicity
2.5. FGLMC Suppress Cancer Cell Migration and Invasion
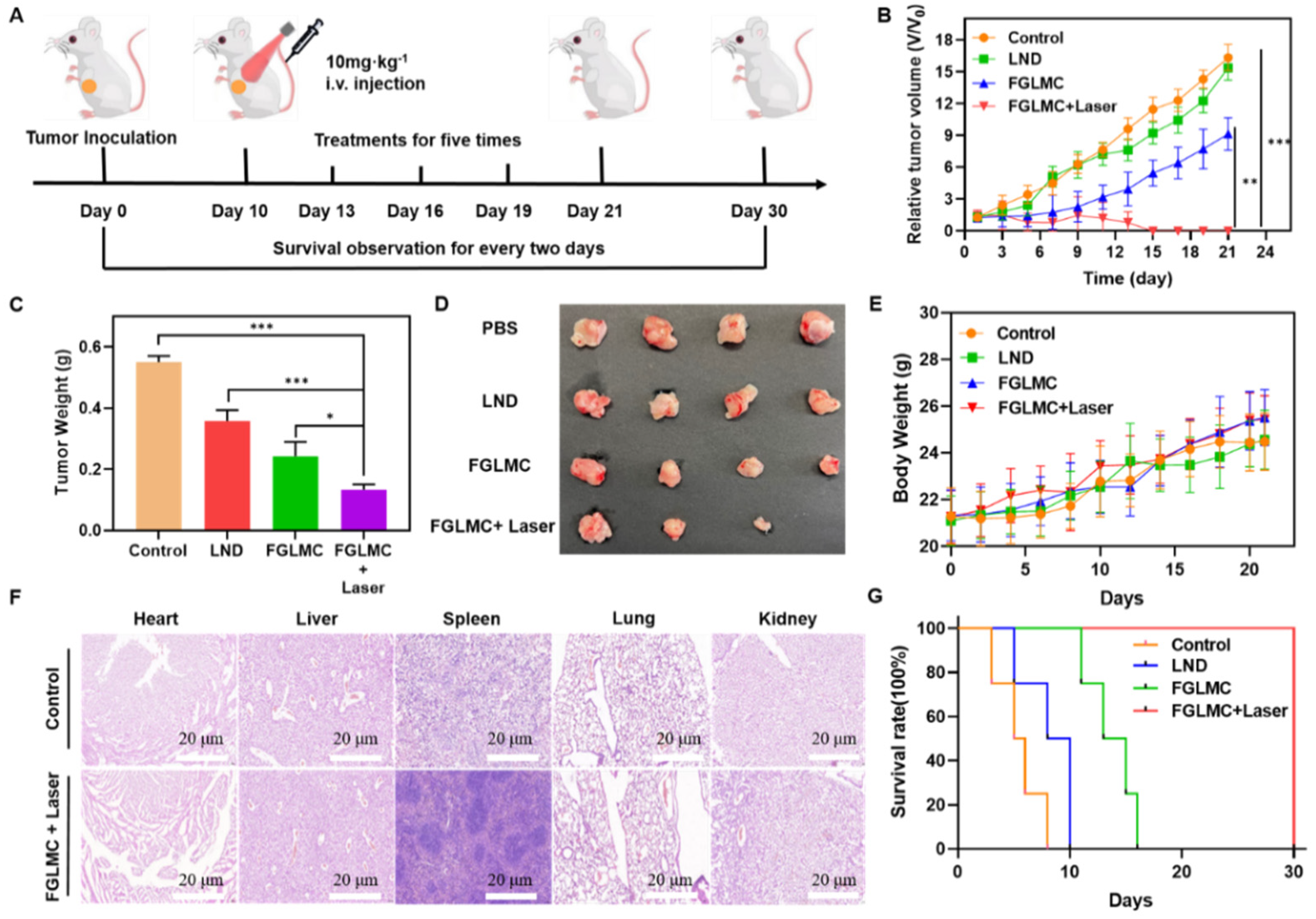
2.6. Antitumor Effect In Vivo
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Harris, I.S.; DeNicola, G.M. The Complex Interplay between Antioxidants and ROS in Cancer. Trends Cell Biol. 2020, 30, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Chio, I.I.C.; Tuveson, D.A. ROS in cancer: The burning question. Trends Mol. Med. 2017, 23, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Pelicano, H.; Carney, D.; Huang, P. ROS stress in cancer cells and therapeutic implications. Drug Resist. Updates 2004, 7, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yi, J. Background: Two paradoxical ROS-manipulation strategies in cancer treatment. Cancer Biol. Ther. 2008, 7, 1875–1884. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Panieri, E.; Santoro, M.M. ROS homeostasis and metabolism: A dangerous liason in cancer cells. Cell Death Dis. 2016, 7, e2253. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, X.; Liu, Z.; Cheng, L. Recent progress of chemodynamic therapy-induced combination cancer therapy. Nano Today 2020, 35, 100946. [Google Scholar] [CrossRef]
- Li, S.L.; Jiang, P.; Jiang, F.L.; Liu, Y. Recent Advances in Nanomaterial-Based Nanoplatforms for Chemo-dynamic Cancer Therapy. Adv. Funct. Mater. 2021, 31, 2100243. [Google Scholar] [CrossRef]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; He, Z.; Wang, X.; Zhang, Q.; Wei, Q.; Ma, S.; Ma, C.; Li, J.; Wang, Q. Cascade enzymes within self-assembled hybrid nanogel mimicked neutrophil lysosomes for singlet oxygen elevated cancer therapy. Nat. Commun. 2019, 10, 240. [Google Scholar] [CrossRef] [PubMed]
- Schaue, D.; McBride, W.H. Opportunities and challenges of radiotherapy for treating cancer. Nat. Rev. Clin. Oncol. 2015, 12, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, D.; Zhang, S.; Cheng, Y.; Yang, F.; Xing, Y.; Xu, T.; Dong, H.; Zhang, X. Biodegradable bio-mimic copper/manganese silicate nanospheres for chemodynamic/photodynamic synergistic therapy with simultaneous glutathione depletion and hypoxia relief. ACS Nano 2019, 13, 4267–4277. [Google Scholar] [CrossRef]
- Shi, L.; Wang, Y.; Zhang, C.; Zhao, Y.; Lu, C.; Yin, B.; Yang, Y.; Gong, X.; Teng, L.; Liu, Y.; et al. An Acidity-Unlocked Magnetic Nanoplatform Enables Self-Boosting ROS Generation through Upregulation of Lactate for Imaging-Guided Highly Specific Chemodynamic Therapy. Angew. Chem. Int. Ed. 2021, 60, 9562–9572. [Google Scholar] [CrossRef]
- Li, L.; Zang, Z.; Fan, W.; He, L.; Cui, C.; Zou, J.; Tang, W.; Jacobson, O.; Wang, Z.; Niu, G.; et al. In situ polymerized hollow mesoporous organosilica biocatalysis nanoreactor for enhancing ROS-mediated anti-cancer therapy. Adv. Funct. Mater. 2020, 30, 1907716. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, J.; Wan, Y.; Fang, F.; Chen, R.; Shen, D.; Huang, Z.; Tian, S.; Xiao, Y.; Li, X.; et al. Dual Fenton Catalytic Nanoreactor for Integrative Type-I and Type-II Photodynamic Therapy Against Hypoxic Cancer Cells. ACS Appl. Bio Mater. 2019, 2, 3854–3860. [Google Scholar] [CrossRef]
- Won, M.; Koo, S.; Li, H.; Sessler, J.L.; Lee, J.Y.; Sharma, A.; Kim, J.S. An ethacrynic acid-brominated BODIPY photosensitizer (EA-BPS) construct enhances the lethality of reactive oxygen species in hypoxic tumor-targeted photodynamic therapy. Angew. Chem. Int. Ed. 2021, 60, 3196–3204. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Y.; Gao, Y.; Wang, P.; He, G.; Blum, N.T.; Lin, J.; Liu, Q.; Wang, X.; Huang, P. Six Birds with One Stone: Versatile Nanoporphyrin for Single-Laser-Triggered Synergistic Phototheranostics and Robust Immune Activation. Adv. Mater. 2020, 32, e2004481. [Google Scholar] [CrossRef]
- Fu, L.-H.; Wan, Y.; Li, C.; Qi, C.; He, T.; Yang, C.; Zhang, Y.; Lin, J.; Huang, P. Biodegradable calcium phosphate nanotheranostics with tumor-specific activatable cascade catalytic reactions-augmented photodynamic therapy. Adv. Funct. Mater. 2021, 31, 2009848. [Google Scholar] [CrossRef]
- Sun, J.; Cai, X.; Wang, C.; Du, K.; Chen, W.; Feng, F.; Wang, S. Cascade reactions by nitric oxide and hydrogen radical for anti-hypoxia photodynamic therapy using an activatable photosensitizer. J. Am. Chem. Soc. 2021, 143, 868–878. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Wu, C.; Niu, D.; Qin, L.; Wang, X.; Wang, Q.; Li, Y. Peroxisome inspired hybrid enzyme nanogels for chemodynamic and photodynamic therapy. Nat. Commun. 2021, 12, 5243. [Google Scholar] [CrossRef] [PubMed]
- Hui, S.; Ghergurovich, J.M.; Morscher, R.J.; Jang, C.; Teng, X.; Lu, W.; Esparza, L.A.; Reya, T.; Zhan, L.; Guo, J.Y.; et al. Glucose feeds the TCA cycle via circulating lactate. Nature 2017, 551, 115–118. [Google Scholar] [CrossRef]
- Tang, Y.; Jia, C.; Wang, Y.; Wan, W.; Li, H.; Huang, G.; Zhang, X. Lactate Consumption via Cascaded Enzymes Combined VEGF siRNA for Synergistic Anti-Proliferation and Anti-Angiogenesis Therapy of Tumors. Adv. Healthcare Mater. 2021, 10, 2100799. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhao, W.; Wang, M.; Zhang, S.; Li, Y.; Hu, W.; Ren, L.; Luo, S.; Chen, Z. Dual-Modal Therapeutic Role of the Lactate Oxidase-Embedded Hierarchical Porous Zeolitic Imidazolate Framework as a Nanocatalyst for Effective Tumor Suppression. ACS Appl. Mater. Interfaces 2020, 12, 32278–32288. [Google Scholar] [CrossRef] [PubMed]
- Faubert, B.; Li, K.Y.; Cai, L.; Hensley, C.T.; Kim, J.; Zacharias, L.G.; Yang, C.; Do, Q.N.; Doucette, S.; Burguete, D.; et al. Lactate Metabolism in Human Lung Tumors. Cell 2017, 171, 358–371.e9. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.-X.; Kempson, I.M.; Hsieh, C.-C.; Tseng, S.J.; Yang, P.-C. Potential therapeutics using tumor-secreted lactate in nonsmall cell lung cancer. Drug Discov. Today 2021, 26, 2508–2514. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Tang, Y.; Liu, W.L.; Zou, M.Z.; Huang, C.; Liu, C.J.; Zhang, X.Z. Intra/extracellular lactic acid exhaustion for synergistic metabolic therapy and immunotherapy of tumors. Adv. Mater. 2019, 31, 1904639. [Google Scholar] [CrossRef]
- Liao, Z.-X.; Ou, D.-L.; Hsieh, M.-J.; Hsieh, C.-C. Synergistic effect of repolarization of m2 to m1 macro-phages induced by iron oxide nanoparticles combined with lactate oxidase. Int. J. Mol. Sci. 2021, 22, 13346. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, H.; Wei, J.; Zhang, H.X.; Wu, X.; Li, Y.; Li, C.; Zhang, J.; Ye, J. Integrating the g-C3N4 nanosheet with B–H bonding decorated metal–organic framework for CO2 activation and photoreduction. ACS Nano 2018, 12, 5333–5340. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Cui, M.; Xu, Y.; Liu, T.; Gu, Y.; Wang, P.; Tang, H. Multifaceted Elevation of ROS Generation for Effective Cancer Suppression. Nanomaterials 2022, 12, 3150. https://doi.org/10.3390/nano12183150
Wang H, Cui M, Xu Y, Liu T, Gu Y, Wang P, Tang H. Multifaceted Elevation of ROS Generation for Effective Cancer Suppression. Nanomaterials. 2022; 12(18):3150. https://doi.org/10.3390/nano12183150
Chicago/Turabian StyleWang, Huizhe, Mengyuan Cui, Yanqi Xu, Tianguang Liu, Yueqing Gu, Peng Wang, and Hui Tang. 2022. "Multifaceted Elevation of ROS Generation for Effective Cancer Suppression" Nanomaterials 12, no. 18: 3150. https://doi.org/10.3390/nano12183150
APA StyleWang, H., Cui, M., Xu, Y., Liu, T., Gu, Y., Wang, P., & Tang, H. (2022). Multifaceted Elevation of ROS Generation for Effective Cancer Suppression. Nanomaterials, 12(18), 3150. https://doi.org/10.3390/nano12183150





