Growth and Characterization of Ultrathin Vanadium Oxide Films on HOPG
Abstract
:1. Introduction
2. Experimental Section
2.1. Thin Film Deposition
2.2. Post-Annealing Treatment
2.3. Characterization
3. Results and Discussions
3.1. RHEED Analysis
3.2. Surface Morphology
3.3. XPS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.M.; Dimitrakopoulos, C.; Jenkins, K.A.; Farmer, D.B.; Chiu, H.Y.; Grill, A.; Avouris, P. 100-GHz Transistors from Wafer-Scale Epitaxial Graphene. Science 2010, 327, 662–662. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Chang, Y.C.; Norris, T.B.; Zhong, Z. Graphene photodetectors with ultra-broadband and high responsivity at room temperature. Nat. Nanotechnol. 2014, 9, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Dhinakaran, V.; Lavanya, M.; Vigneswari, K.; Ravichandran, M.; Vijayakumar, M.D. Review on exploration of graphene in diverse applications and its future horizon. Mater. Today Proc. 2020, 27, 824–828. [Google Scholar] [CrossRef]
- Iannaccone, G.; Bonaccorso, F.; Colombo, L.; Fiori, G. Quantum engineering of transistors based on 2D materials heterostructures. Nat. Nanotechnol. 2018, 13, 183–191. [Google Scholar] [CrossRef]
- Geim, A.K.; Grigorieva, I.V. Van der Waals heterostructures. Nature 2013, 499, 419–425. [Google Scholar] [CrossRef]
- Quang, H.T.; Bachmatiuk, A.; Dianat, A.; Ortmann, F.; Zhao, J.; Warner, J.H.; Eckert, J.; Cunniberti, G.; Rümmeli, M.H. In Situ Observations of Free-Standing Graphene-like Mono- and Bilayer ZnO Membranes. ACS Nano 2015, 9, 11408–11413. [Google Scholar] [CrossRef]
- Yang, T.; Song, T.T.; Callsen, M.; Zhou, J.; Chai, J.W.; Feng, Y.P.; Wang, S.J.; Yang, M. Atomically Thin 2D Transition Metal Oxides: Structural Reconstruction, Interaction with Substrates, and Potential Applications. Adv. Mater. Interfaces 2018, 6, 1801160. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Abidi, N.; Cabrales, L. Wettability and Surface Free Energy of Graphene Films. Langmuir 2009, 25, 11078–11081. [Google Scholar] [CrossRef]
- Wang, W.H.; Han, W.; Pi, K.; McCreary, K.M.; Miao, F.; Bao, W.; Lau, C.N.; Kawakami, R.K. Growth of atomically smooth MgO films on graphene by molecular beam epitaxy. Appl. Phys. Lett. 2008, 93, 183107. [Google Scholar] [CrossRef]
- Jandhyala, S.; Mordi, G.; Lee, B.; Lee, G.; Floresca, C.; Cha, P.R.; Ahn, J.; Wallace, R.M.; Chabal, Y.J.; Kim, M.J.; et al. Atomic Layer Deposition of Dielectrics on Graphene Using Reversibly Physisorbed Ozone. ACS Nano 2012, 6, 2722–2730. [Google Scholar] [CrossRef]
- Addou, R.; Dahal, A.; Batzill, M. Growth of a two-dimensional dielectric monolayer on quasi-freestanding graphene. Nat. Nanotechnol. 2012, 8, 41–45. [Google Scholar] [CrossRef]
- Liu, X.J.; Wang, C.Z.; Hupalo, M.; Lin, H.Q.; Ho, K.M.; Tringides, M.C. Metals on Graphene: Interactions, Growth Morphology, and Thermal Stability. Crystals 2013, 3, 79–111. [Google Scholar] [CrossRef]
- Monfort, O.; Petrisková, P. Binary and Ternary Vanadium Oxides: General Overview, Physical Properties, and Photochemical Processes for Environmental Applications. Processes 2021, 9, 214. [Google Scholar] [CrossRef]
- Amiri, V.; Roshan, H.; Mirzaei, A.; Sheikhi, M.H. A Review of Nanostructured Resistive-Based Vanadium Oxide Gas Sensors. Chemosensors 2020, 8, 105. [Google Scholar] [CrossRef]
- Fu, H.T.; Zhang, Z.K.; Yang, X.H.; An, X.Z. Two-Step Synthesis of V2O5 Nanosheets with High Sensing Properties toward Acetone. In Proceedings of the 3rd Annual International Conference on Advanced Material Engineering (AME 2017), Shanghai, China, 14–16 April 2017. [Google Scholar]
- Hua, L.; Ma, Z.Y.; Shi, P.P.; Li, L.; Rui, K.; Zhou, J.Y.; Huang, X.; Liu, X.; Zhu, J.X.; Sun, G.Z.; et al. Ultrathin and large-sized vanadium oxide nanosheets mildly prepared at room temperature for high performance fiber-based supercapacitors. J. Mater. Chem. A 2017, 5, 2483–2487. [Google Scholar] [CrossRef]
- Zeng, J.; Huang, J.D.; Liu, J.; Xie, T.; Peng, C.Q.; Lu, Y.K.; Lu, P.J.; Zhang, R.Z.; Min, J. Self-assembly of single layer V2O5 nanoribbon/graphene heterostructures as ultrahigh-performance cathode materials for lithium-ion batteries. Carbon 2019, 154, 24–32. [Google Scholar] [CrossRef]
- Wang, C.; Yi, Y.K.; Li, H.P.; Wu, P.W.; Li, M.T.; Jiang, W.; Chen, Z.G.; Li, H.M.; Zhu, W.S.; Dai, S. Rapid gas-assisted exfoliation promises V2O5 nanosheets for high performance lithium-sulfur batteries. Nano Energy 2020, 67, 104253. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.; Kim, K.S.; Jeong, H.Y.; Jang, A.R.; Han, S.H.; Yoon, D.H.; Suh, K.S.; Shin, H.S.; Kim, T.Y.; et al. Flexible Thermochromic Window Based on Hybridized VO2/Graphene. ACS Nano 2013, 7, 5769–5776. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.; Kim, T.Y.; Jang, A.R.; Jeong, H.Y.; Han, S.H.; Yoon, D.H.; Shin, H.S.; Bae, D.J.; Kim, K.S.; et al. Enhanced optical response of hybridized VO2/graphene films. Nanoscale 2013, 5, 2632. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.F.; Liu, Y.; Li, H.B.; Li, L.L.; Deng, D.H.; Yang, F.; Bao, X.H. Towards the atomic-scale characterization of isolated iron sites confined in a nitrogen-doped graphene matrix. Appl. Surf. Sci. 2017, 410, 111–116. [Google Scholar] [CrossRef]
- Cai, J.Y.; Han, X.X.; Wang, X.; Meng, X.B. Atomic Layer Deposition of Two-Dimensional Layered Materials: Processes, Growth Mechanisms, and Characteristics. Matter 2020, 2, 587–630. [Google Scholar] [CrossRef]
- Cai, Z.Y.; Liu, B.L.; Zou, X.L.; Cheng, H.M. Chemical Vapor Deposition Growth and Applications of Two-Dimensional Materials and Their Heterostructures. Chem. Rev. 2018, 118, 6091–6133. [Google Scholar] [CrossRef]
- Van Bilzen, B.; Homm, P.; Dillemans, L.; Su, C.Y.; Menghini, M.; Sousa, M.; Marchiori, C.; Zhang, L.M.; Seo, J.W.; Locquet, J.P. Production of VO2 thin films through post-deposition annealing of V2O3 and VOx films. Thin Solid Films 2015, 591, 143–148. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Cent. Eur. J. Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef]
- Casa Software Ltd. Version 2.3.23PR1.0. Casaxps, Casa Software Ltd: Teignmouth, UK, 2005. Available online: http://www.casaxps.com/(accessed on 3 January 2021).
- Mendialdua, J.; Casanova, R.; Barbaux, Y. XPS studies of V2O5, V6O13, VO2 and V2O3. J. Electron Spectrosc. Relat. Phenom. 1995, 71, 249–261. [Google Scholar] [CrossRef]
- Hryha, E.; Rutqvist, E.; Nyborg, L. Stoichiometric vanadium oxides studies by XPS. Surf. Interface Anal. 2011, 44, 1022–1025. [Google Scholar] [CrossRef]
- Silversmit, G.; Depla, D.; Poelman, H.; Marin, G.B.; De Gryse, R. Determination of the V2p XPS binding energies for different vanadium oxidation states (V5+ to V0+). J. Electron Spectrosc. Relat. Phenom. 2004, 135, 167–175. [Google Scholar] [CrossRef]
- Appy, D.; Lei, H.P.; Wang, C.Z.; Tringides, M.C.; Liu, D.J.; Evans, J.W.; Thiel, P.A. Transition metals on the (0 0 0 1) surface of graphite: Fundamental aspects of adsorption, diffusion, and morphology. Prog. Surf. Sci. 2014, 89, 219–238. [Google Scholar] [CrossRef] [Green Version]
- Guimond, S.; Göbke, D.; Romanyshyn, Y.; Sturm, J.M.; Naschitzki, M.; Kuhlenbeck, H.; Freund, H.J. Growth and Characterization of Ultrathin V2Oy (y ≈ 5) Films on Au(111). J. Phys. Chem. C 2008, 112, 12363–12373. [Google Scholar] [CrossRef]
- Guimond, S.; Sturm, J.M.; Göbke, D.; Romanyshyn, Y.; Naschitzki, M.; Kuhlenbeck, H.; Freund, H.J. Well-Ordered V2O5(001) Thin Films on Au(111): Growth and Thermal Stability. J. Phys. Chem. C 2008, 112, 11835–11846. [Google Scholar] [CrossRef]
- Enjalbert, R.; Galy, J. A refinement of the structure of V2O5. Acta Cryst. 1986, C42, 1467–1469. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.; Gerson, A.R.; Smart, R.S. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Zimmermann, R.; Claessen, R.; Reinert, F.; Steiner, P.; Hüfner, S. Strong hybridization in vanadium oxides: Evidence from photoemission and absorption spectroscopy. J. Phys. Condens. Matter 1998, 10, 5697–5716. [Google Scholar] [CrossRef]
- Wu, Q.H.; Thissen, A.; Jaegermann, W.; Liu, M.L. Photoelectron spectroscopy study of oxygen vacancy on vanadium oxides surface. Appl. Surf. Sci. 2004, 236, 473–478. [Google Scholar] [CrossRef]
- Dahal, A.; Addou, R.; Coy-Diaz, H.; Lallo, J.; Batzill, M. Charge doping of graphene in metal/graphene/dielectric sandwich structures evaluated by C-1s core level photoemission spectroscopy. APL Mater. 2013, 1, 042107. [Google Scholar] [CrossRef]
- Choi, J.G. The surface properties of vanadium compounds by X-ray photoelectron spectroscopy. Appl. Surf. Sci. 1999, 148, 64–72. [Google Scholar] [CrossRef]
- Demeter, M.; Neumann, M.; Reichelt, W. Mixed-valence vanadium oxides studied by XPS. Surf. Sci. 2000, 454–456, 41–44. [Google Scholar] [CrossRef]
- Szymanski, N.J.; Liu, Z.T.Y.; Alderson, T.; Podraza, N.J.; Sarin, P.; Khare, S.V. Electronic and optical properties of vanadium oxides from first principles. Comput. Mater. Sci. 2018, 146, 310–318. [Google Scholar] [CrossRef]
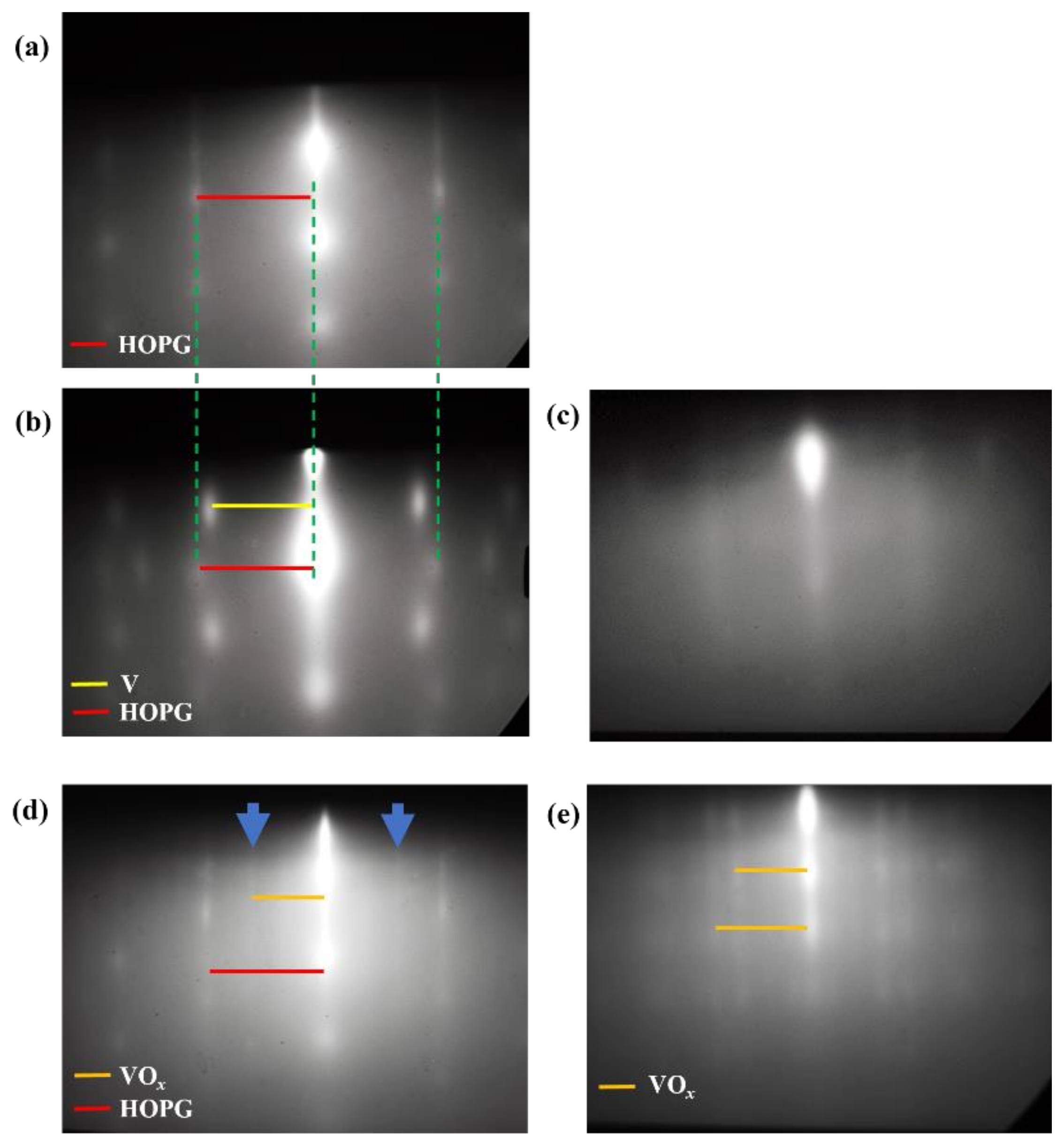
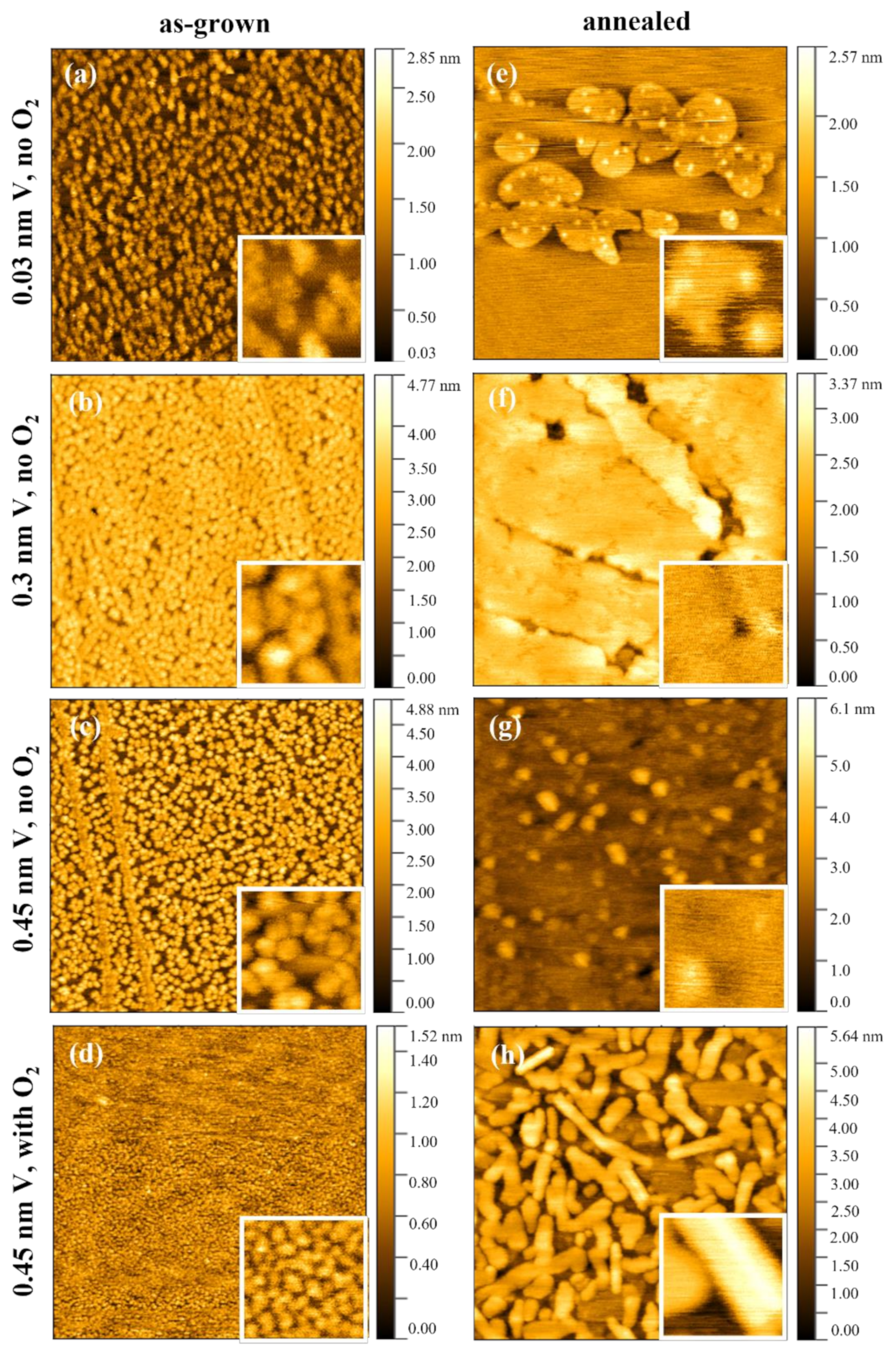


| Sample | a1 | b1 | c1 | d1 |
|---|---|---|---|---|
| V-thickness (nm) | 0.03 | 0.3 | 0.45 | 0.45 |
| O2 partial pressure | No O2 | No O2 | No O2 | 1 × 10−6 mbar |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Schouteden, K.; Recaman Payo, M.; Locquet, J.-P.; Seo, J.W. Growth and Characterization of Ultrathin Vanadium Oxide Films on HOPG. Nanomaterials 2022, 12, 3134. https://doi.org/10.3390/nano12183134
Sun Y, Schouteden K, Recaman Payo M, Locquet J-P, Seo JW. Growth and Characterization of Ultrathin Vanadium Oxide Films on HOPG. Nanomaterials. 2022; 12(18):3134. https://doi.org/10.3390/nano12183134
Chicago/Turabian StyleSun, Yue, Koen Schouteden, María Recaman Payo, Jean-Pierre Locquet, and Jin Won Seo. 2022. "Growth and Characterization of Ultrathin Vanadium Oxide Films on HOPG" Nanomaterials 12, no. 18: 3134. https://doi.org/10.3390/nano12183134
APA StyleSun, Y., Schouteden, K., Recaman Payo, M., Locquet, J.-P., & Seo, J. W. (2022). Growth and Characterization of Ultrathin Vanadium Oxide Films on HOPG. Nanomaterials, 12(18), 3134. https://doi.org/10.3390/nano12183134








