Intelligent Coatings with Controlled Wettability for Oil–Water Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Modified Sponge
2.3. Oil–Water Mixtures Separation
2.4. Characterization
3. Results and Discussion
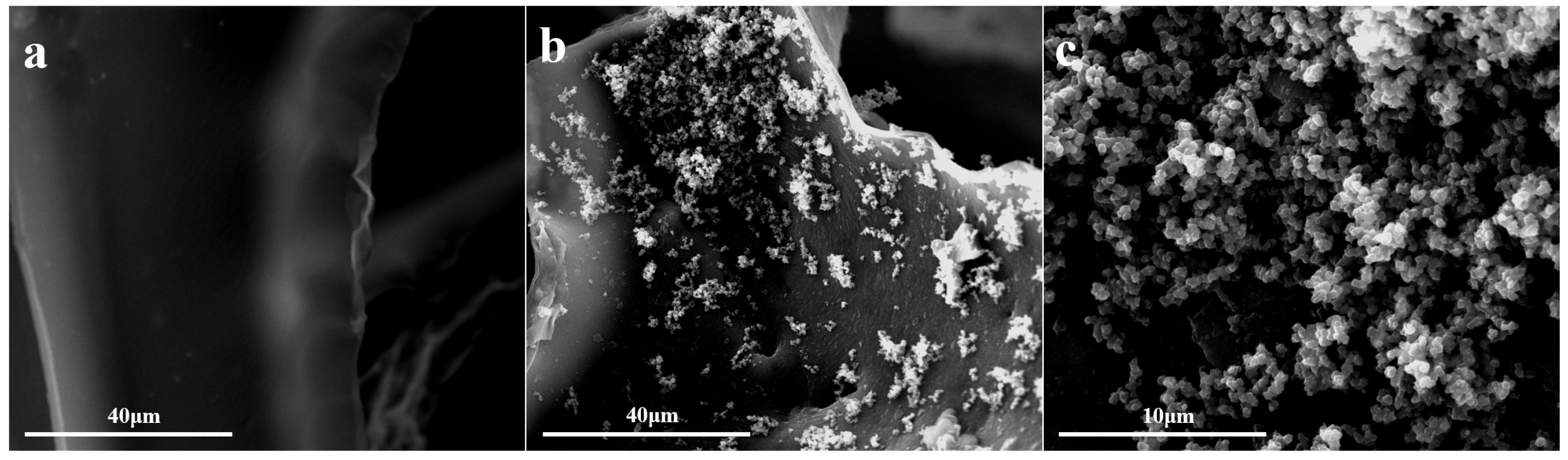
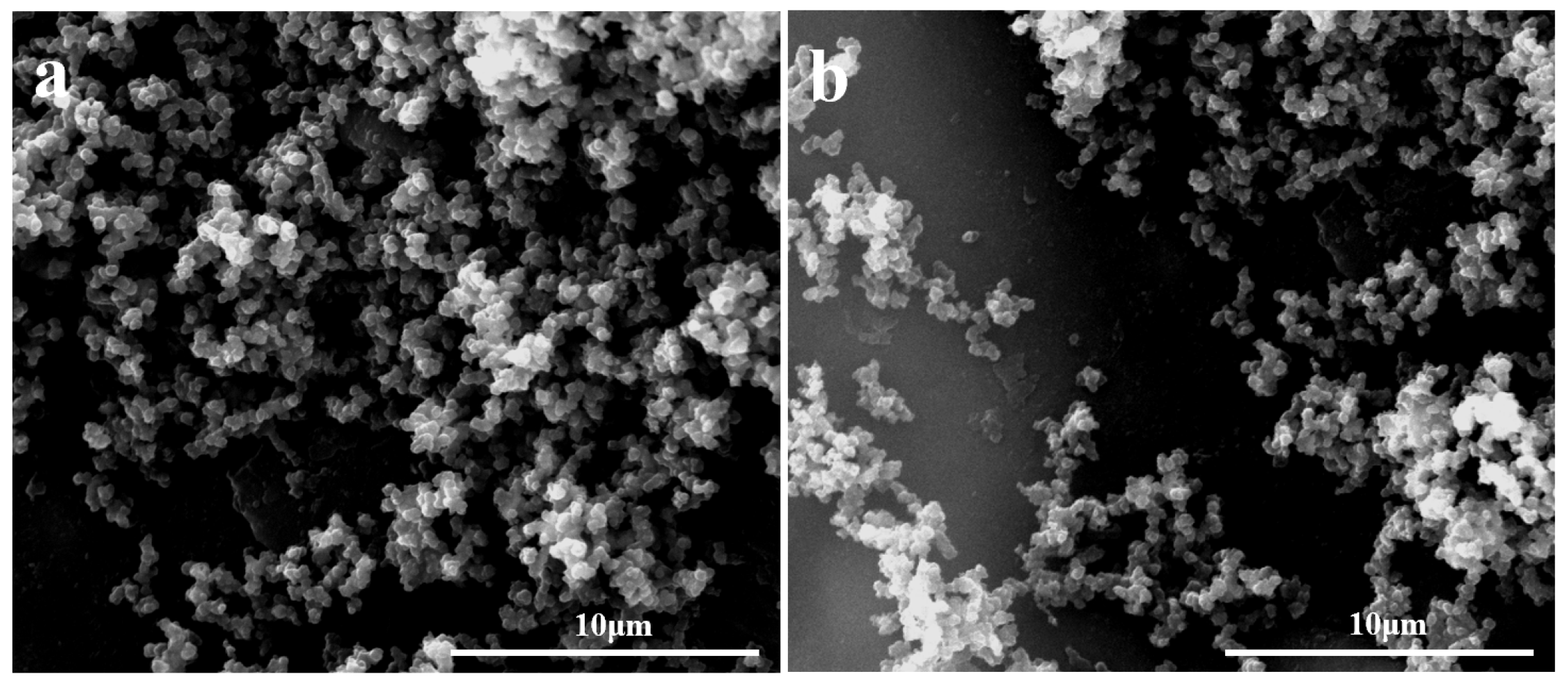
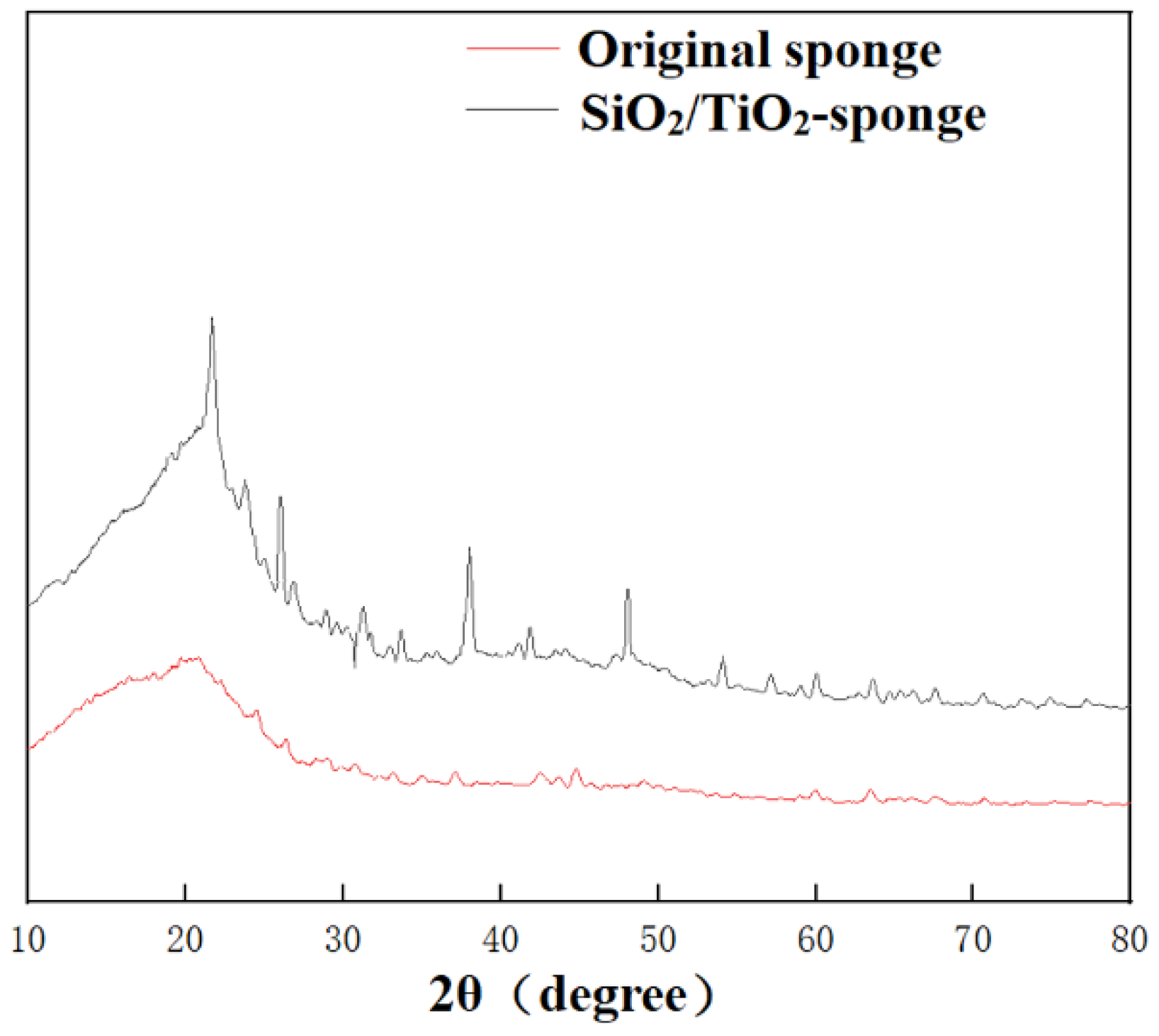
3.1. Characterization of the Modified Surface
3.2. Wettability Transformation Mechanism
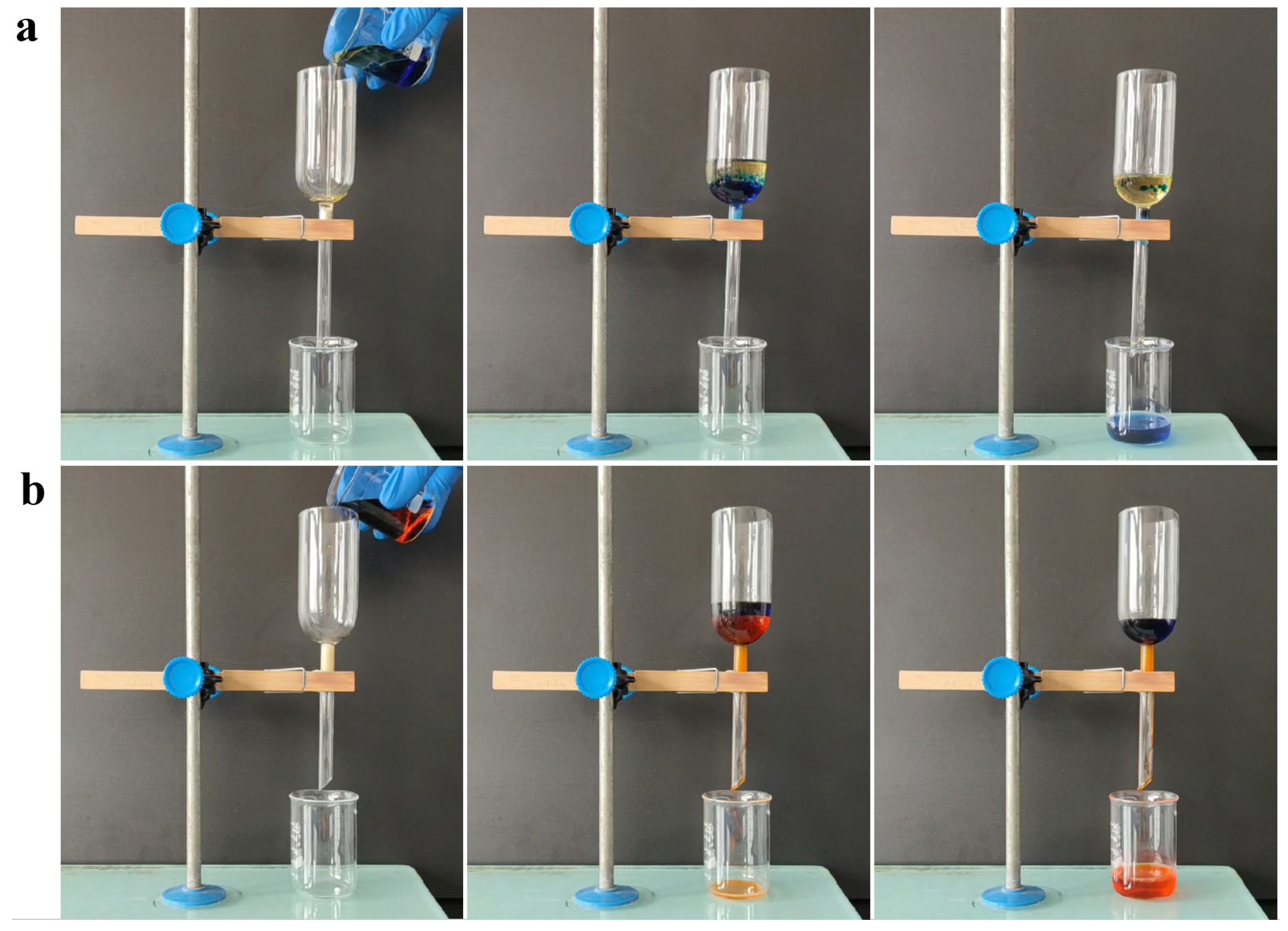
3.3. Oil/Water Separation
3.4. Decomposition of PFOA by Doping of TiO2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, M.; Chen, Y.; Mao, L.B.; Jiang, Y.; Liu, M.F.; Huang, Q.; Yu, Z.; Wang, S.; Yu, S.H.; Lin, C.; et al. Seeded mineralization leads to hierarchical CaCO3 thin coatings on fibers for oil/water separation applications. Langmuir 2018, 34, 2942–2951. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kang, R.; Tang, X.; She, H.; Yang, Y.; Zha, F. Superhydrophobic meshes that can repel hot water and strong corrosive liquids used for efficient gravity-driven oil/water separation. Nanoscale 2016, 8, 7638–7645. [Google Scholar] [CrossRef] [PubMed]
- Lian, Z.; Xu, J.; Wang, Z.; Yu, Z.; Weng, Z.; Yu, H. Nanosecond laser-induced underwater superoleophobic and underoil superhydrophobic mesh for oil/water separation. Langmuir 2018, 34, 2981–2988. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, H.; Tao, Z.; Liu, X.; Wang, Z.; Yue, R.; Cui, Z. 3D superhydrophobic sponge with a novel compression strategy for effective water-in-oil emulsion separation and its separation mechanism. Chem. Eng. J. 2019, 359, 149–158. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, X.; Liu, X.; Yang, Q.; Shang, N.; Zhao, X.; Zang, X.; Wang, C.; Wang, Z.; Shapter, J.G.; et al. Heterointerface optimization in a covalent organic framework-on-MXene for high-performance capacitive deionization of oxygenated saline water. Mater. Horiz. 2022, 9, 1708–1716. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Sathasivam, S.; Song, J.; Chen, F.; Xu, W.; Carmalt, C.J.; Parkin, I.P. Creating superhydrophobic mild steel surfaces for water proofing and oil-water separation. J. Mater. Chem. A 2014, 2, 11628–11634. [Google Scholar] [CrossRef]
- You, Q.; Ran, G.; Wang, C.; Zhao, Y.; Song, Q. A novel superhydrophilic-underwater superoleophobic Zn-ZnO electrodeposited copper mesh for efficient oil/water separation. Sep. Purif. Technol. 2018, 193, 21–28. [Google Scholar] [CrossRef]
- Wang, Z.; Ji, S.; He, F.; Cao, M.; Peng, S.; Li, Y. One-step transformation of highly hydrophobic membranes to superhydrophilic and underwater superoleophobic ones for high-efficiency separation of oil-in-water emulsion. J. Mater. Chem. A 2018, 6, 3391–3396. [Google Scholar] [CrossRef]
- Wu, C.; Huang, X.; Wu, X.; Qian, R.; Jiang, P. Mechanically flexible and multifunctional polymer-based graphene foams for elastic conductors and oil-water separators. Adv. Mater. 2013, 25, 5658–5662. [Google Scholar] [CrossRef]
- Wang, C.F.; Huang, H.C.; Chen, L.T. Protonated melamine sponge for effective oil/water separation. Sci. Rep. 2015, 5, 14294. [Google Scholar] [CrossRef] [Green Version]
- Tao, M.; Xue, L.; Liu, F.; Jiang, L. An intelligent superwetting PVDF membrane showing switchable transport performance for oil/water separation. Adv. Mater. 2014, 26, 2943–2948. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; Li, S.H.; Ge, M.Z.; Wang, L.N.; Xing, T.L.; Chen, G.Q.; Liu, X.F.; Al Deyab, S.S.; Zhang, K.Q.; Chen, T.; et al. Robust superhydrophobic TiO2@fabrics for UV shielding, self-cleaning and oil-water separation. J. Mater. Chem. A 2015, 3, 2825–2832. [Google Scholar] [CrossRef]
- Shang, B.; Wang, Y.; Peng, B.; Deng, Z. Bioinspired polydopamine particles-assisted construction of superhydrophobic surfaces for oil/water separation. J. Colloid Interface Sci. 2016, 482, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shang, B.; Hu, X.; Peng, B.; Deng, Z. Temperature control of mussel-inspired chemistry toward hierarchical superhydrophobic surfaces for oil/water separation. Adv. Mater. Interfaces 2017, 4, 1600727. [Google Scholar] [CrossRef]
- Brown, P.S.; Bhushan, B. Mechanically durable, superoleophobic coatings prepared by layer-by-layer technique for anti-smudge and oil-water separation. Sci. Rep. 2015, 5, 8701. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, N.; Zhang, W.; Feng, L.; Wei, Y. One-step coating toward multifunctional applications: Oil/water mixtures and emulsions separation and contaminants adsorption. ACS Appl. Mater. Interfaces 2016, 8, 3333–3339. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.B.; Song, Y.; Wang, S.; Meng, J.; Yang, G.; Guo, X.; Feng, L.; Jiang, L. Directly coating hydrogel on filter paper for effective oil-water separation in highly acidic, alkaline, and salty environment. Adv. Funct. Mater. 2015, 25, 5368–5375. [Google Scholar] [CrossRef]
- Xue, B.; Gao, L.; Hou, Y.; Liu, Z.; Jiang, L. Temperature controlled water/oil wettability of a surface fabricated by a block copolymer: Application as a dual water/oil on-off switch. Adv. Mater. 2013, 25, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, D.; Jiang, L.; Jin, J. Recent progress in developing advanced membranes for emulsified oil/water separation. NPG Asia Mater. 2014, 6, e101. [Google Scholar] [CrossRef]
- Dang, Z.; Liu, L.; Li, Y.; Xiang, Y.; Guo, G. In situ and ex situ pH-responsive coatings with switchable wettability for controllable oil/water separation. ACS Appl. Mater. Interfaces 2016, 8, 31281–31288. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Wang, P. Smart surfaces with switchable superoleophilicity and superoleophobicity in aqueous media: Toward controllable oil/water separation. NPG Asia Mater. 2012, 4, e8. [Google Scholar] [CrossRef]
- Li, R.; Zhang, G.; Wang, J.; Li, J.; Zhang, C.; Wang, P. Superwetting pH-responsive polyaniline coatings: Toward versatile separation of complex oil-water mixtures. Langmuir 2020, 36, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, C.; Ma, Y.; Du, X.; Shi, Y.; Li, J.; Shi, J. Fabrication of superwetting, antimicrobial and conductive fibrous membranes for removing/collecting oil contaminants. RSC Adv. 2020, 10, 21636. [Google Scholar] [CrossRef]
- Yang, X.; Jin, H.; Tao, X.; Xu, B.; Lin, S. Photo-switchable smart superhydrophobic surface with controllable superwettability. Polym. Chem. 2021, 12, 5303–5309. [Google Scholar] [CrossRef]
- Idriss, H.; Elashnikov, R.; Guselnikova, O.; Postnikov, P.; Kolska, Z.; Lyutakov, O.; Švorčík, V. Reversible wettability switching of piezo-responsive nanostructured polymer fibers by electric field. Chem. Pap. 2020, 75, 191–196. [Google Scholar] [CrossRef]
- Wei, D.; Wang, J.; Li, S.; Liu, Y.; Wang, D.; Wang, H. Novel corrosion-resistant behavior and mechanism of a biomimetic surface with switchable wettability on Mg alloy. Chem. Eng. J. 2021, 425, 130450. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, Y.; Wang, H.; Wang, X.; Lin, T. A superamphiphobic coating with an ammonia-triggered transition to superhydrophilic and superoleophobic for oil−water separation. Angew. Chem. Int. Ed. 2015, 54, 4527–4530. [Google Scholar] [CrossRef]
- Tang, H.; Fu, Y.; Yang, C.; Zhu, D.; Yang, J. A UV-driven superhydrophilic/superoleophobic polyelectrolyte multilayer film on fabric and its application in oil/water separation. RSC Adv. 2016, 6, 91301–91307. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Liang, G.; Zhao, X. Fabrication and properties of amorphous silica particles by fluorination of zircon using ammonium bifluoride. J. Fluor. Chem. 2020, 232, 109467. [Google Scholar] [CrossRef]
- Dutschke, A.; Diegelmann, C.; Löbmann, P. Preparation of TiO2 thin films on polystyrene by liquid phase deposition. J. Mater. Chem. 2013, 13, 1058–1063. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, S.; Li, Y.; Wang, R.; Ma, W.; Shi, Y.; Fan, W.; Zhuo, K.; Xu, G. Intelligent Coatings with Controlled Wettability for Oil–Water Separation. Nanomaterials 2022, 12, 3120. https://doi.org/10.3390/nano12183120
Fan S, Li Y, Wang R, Ma W, Shi Y, Fan W, Zhuo K, Xu G. Intelligent Coatings with Controlled Wettability for Oil–Water Separation. Nanomaterials. 2022; 12(18):3120. https://doi.org/10.3390/nano12183120
Chicago/Turabian StyleFan, Shumin, Yunxiang Li, Rujun Wang, Wenwen Ma, Yipei Shi, Wenxiu Fan, Kelei Zhuo, and Guangri Xu. 2022. "Intelligent Coatings with Controlled Wettability for Oil–Water Separation" Nanomaterials 12, no. 18: 3120. https://doi.org/10.3390/nano12183120
APA StyleFan, S., Li, Y., Wang, R., Ma, W., Shi, Y., Fan, W., Zhuo, K., & Xu, G. (2022). Intelligent Coatings with Controlled Wettability for Oil–Water Separation. Nanomaterials, 12(18), 3120. https://doi.org/10.3390/nano12183120






