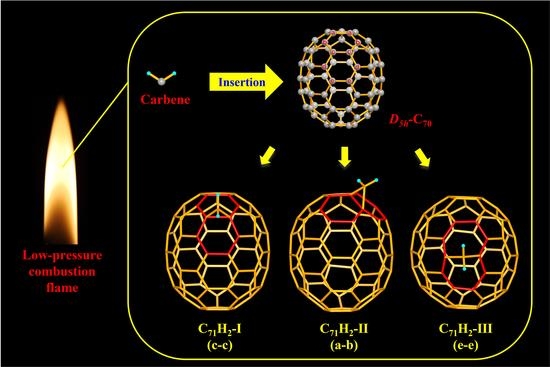
Carbene Addition Isomers of C70 formed in the Flame of Low-Pressure Combustion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Separation
2.3. Characterization
3. Results and Discussion
3.1. Mass Spectrometric Analysis of C71H2-I, C71H2-II, and C71H2-III
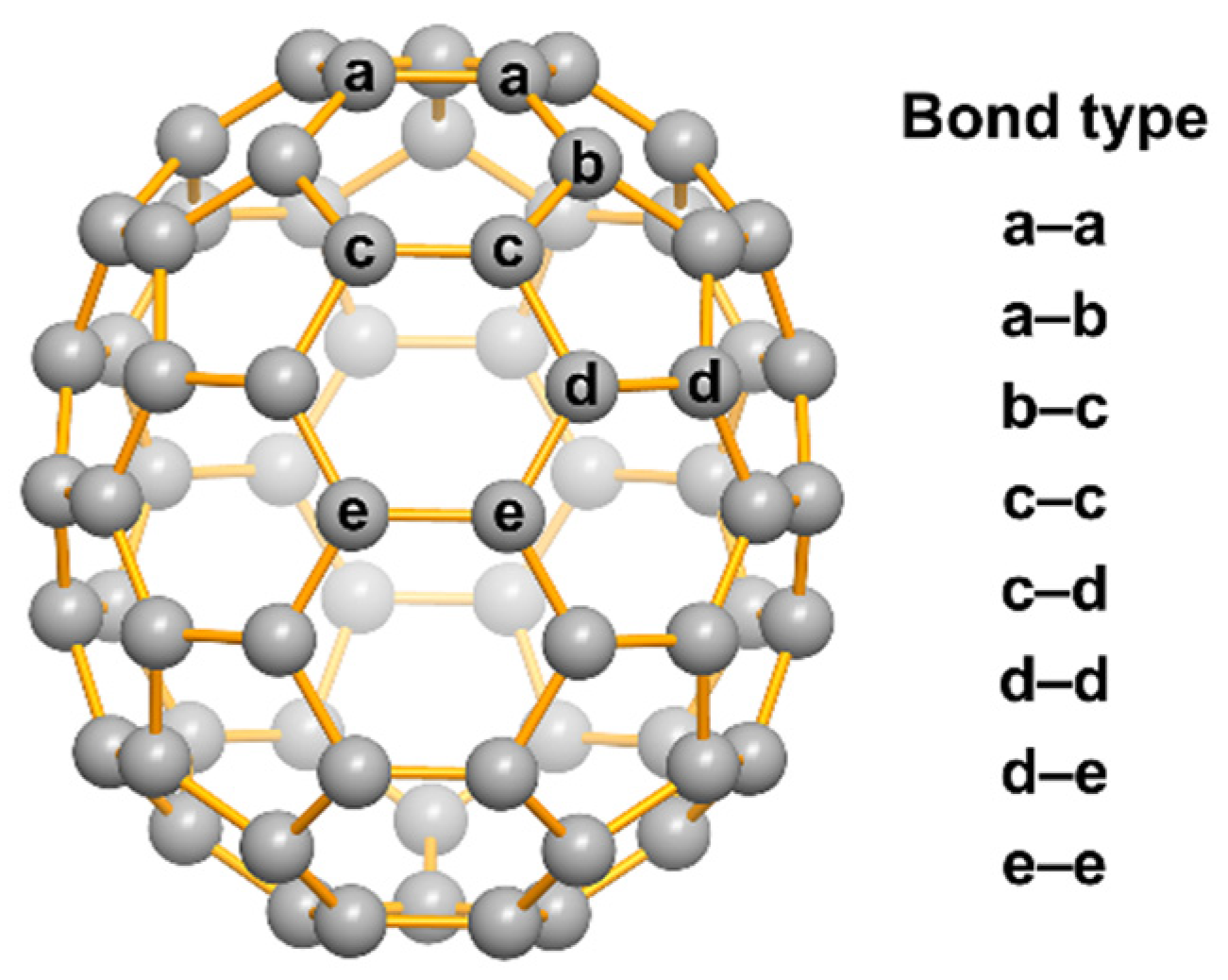
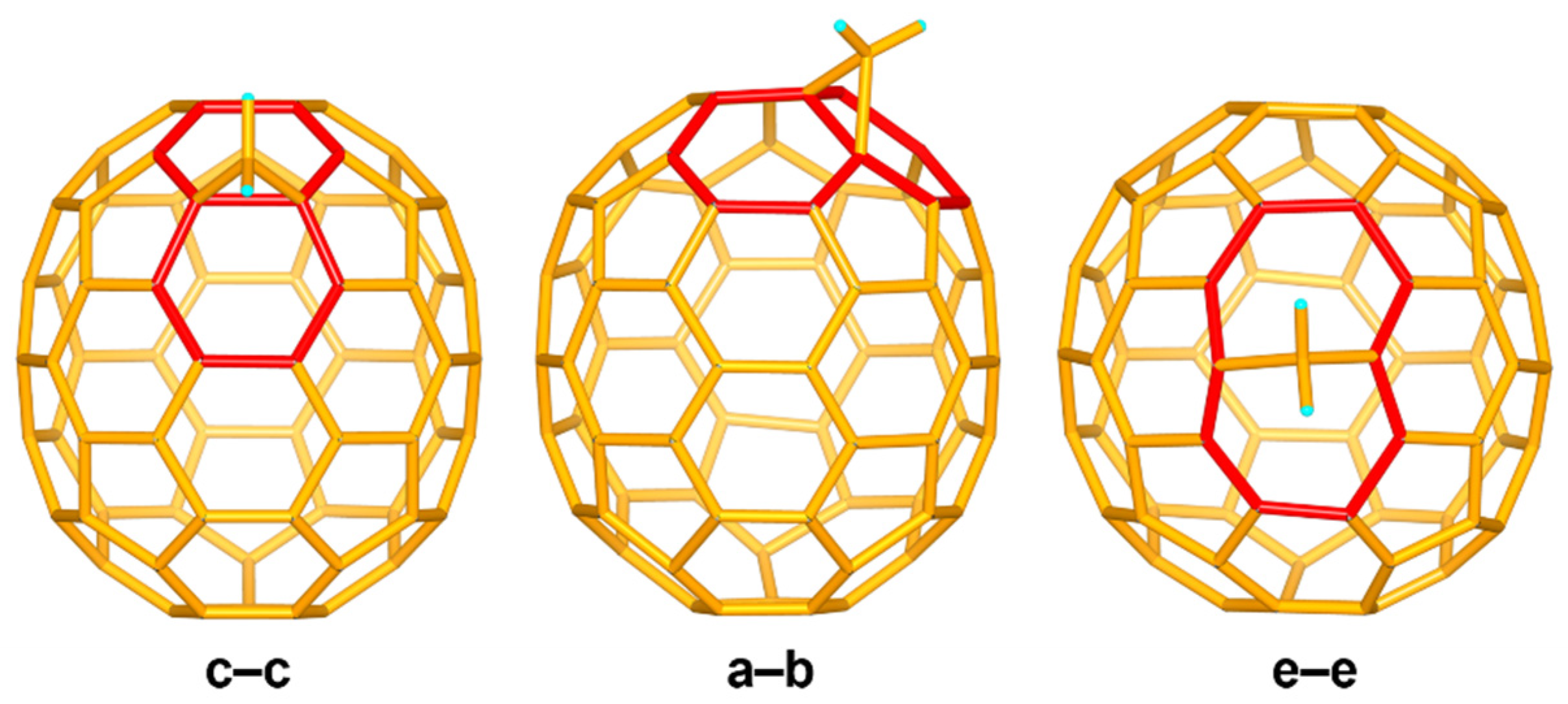
3.2. Crystallographic Identification of C71H2-I, C71H2-II, and C71H2-III
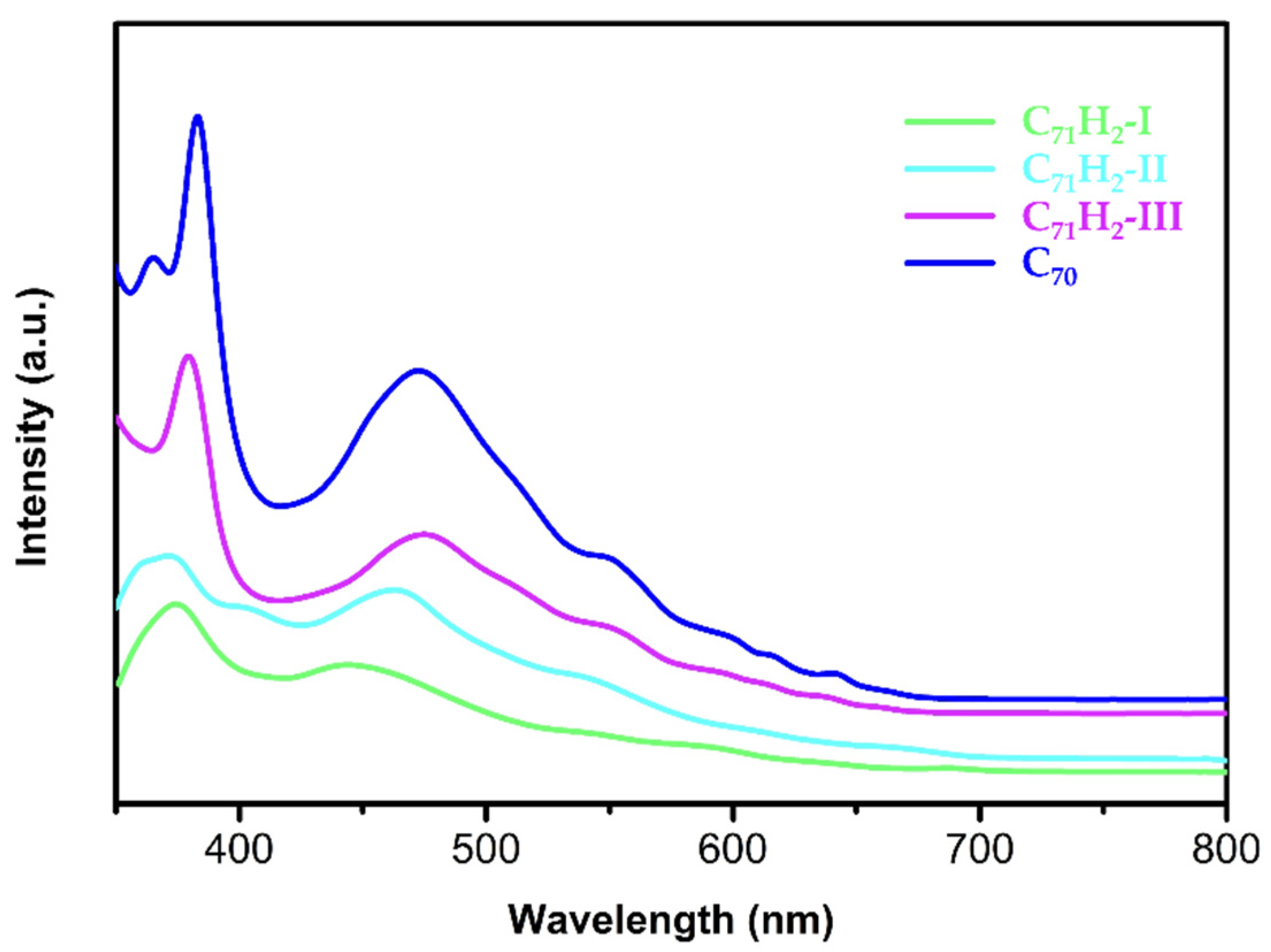
3.3. UV-Vis Spectra of C71H2-I, C71H2-II, and C71H2-III
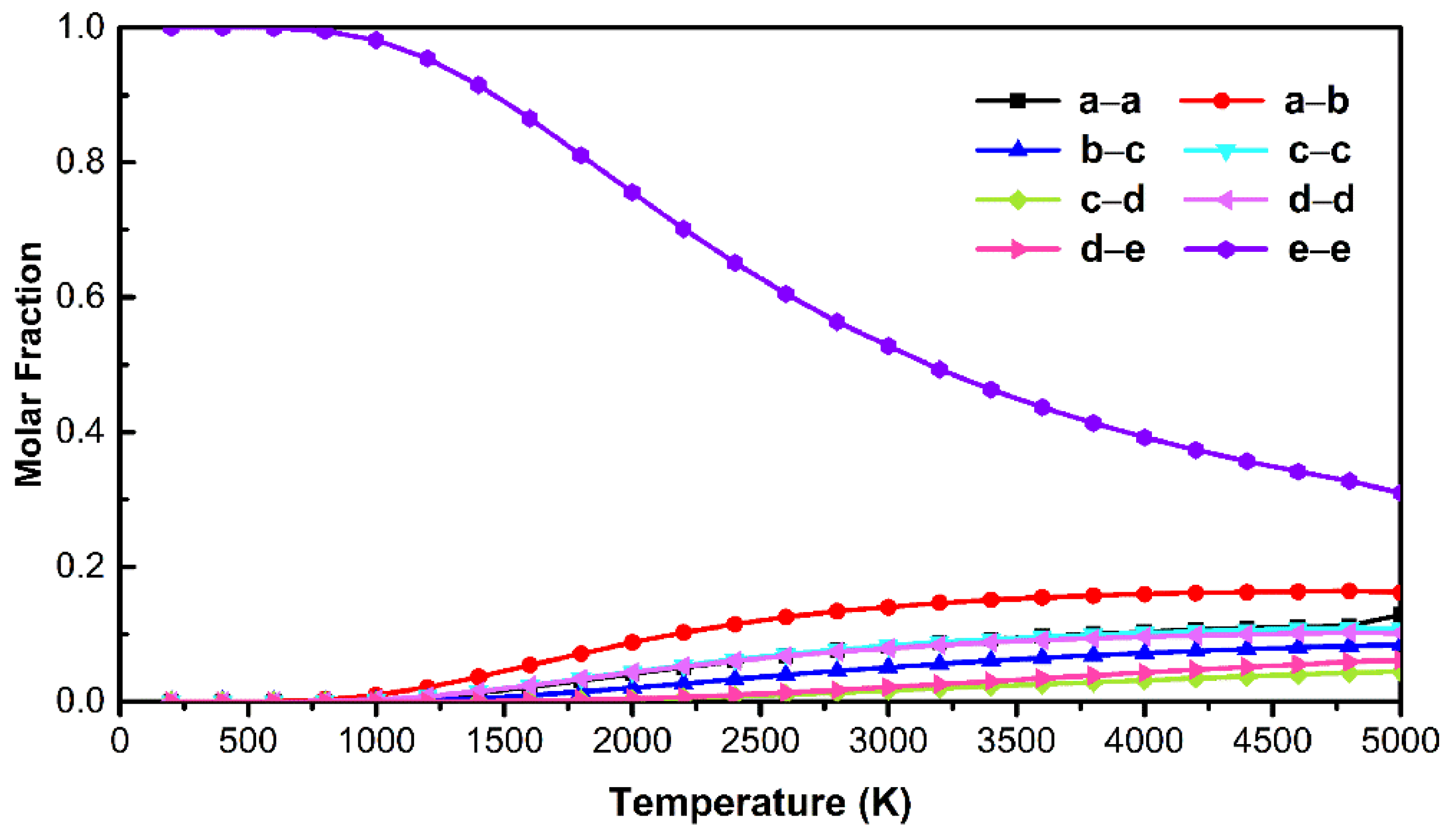
3.4. Theoretical Calculation Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kroto, H.W. The Stability of the Fullerenes Cn, with n = 24, 28, 32, 36, 50, 60 and 70. Nature 1987, 329, 529–531. [Google Scholar] [CrossRef]
- Weng, Q.H.; He, Q.; Sun, D.; Huang, H.Y.; Xie, S.Y.; Lu, X.; Huang, R.B.; Zheng, L.S. Separation and Characterization of C70(C14H10) and C70(C5H6) from an Acetylene-Benzene-Oxygen Flame. J. Phys. Chem. C 2011, 115, 11016–11022. [Google Scholar] [CrossRef]
- Wu, X.Z.; Yao, Y.R.; Chen, M.M.; Tian, H.R.; Xiao, J.; Xu, Y.Y.; Lin, M.S.; Abella, L.; Tian, C.B.; Gao, C.L.; et al. Formation of Curvature Subunit of Carbon in Combustion. J. Am. Chem. Soc. 2016, 138, 9629–9633. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Gao, Z.Y.; Weng, Q.H.; Jiang, W.S.; He, Q.; Liang, H.; Deng, L.L.; Xie, S.L.; Huang, H.Y.; Lu, X.; et al. Combustion Synthesis and Electrochemical Properties of the Small Hydrofullerene C50H10. Chem. Eur. J. 2012, 18, 3408–3415. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.Y.; Jiang, W.S.; Sun, D.; Xie, S.Y.; Huang, R.B.; Zheng, L.S. Synthesis of C3v-#1911C64H4 Using a Low-Pressure Benzene/Oxygen Diffusion Flame: Another Pathway toward Non-IPR Fullerenes. Combust. Flame 2010, 157, 966–969. [Google Scholar] [CrossRef]
- Weng, Q.H.; He, Q.; Liu, T.; Huang, H.Y.; Chen, J.H.; Gao, Z.Y.; Xie, S.Y.; Lu, X.; Huang, R.B.; Zheng, L.S. Simple Combustion Production and Characterization of Octahydro[60]Fullerene with a Non-IPR C60 Cage. J. Am. Chem. Soc. 2010, 132, 15093–15095. [Google Scholar] [CrossRef]
- Tian, H.R.; Chen, M.M.; Wang, K.; Chen, Z.C.; Fu, C.Y.; Zhang, Q.; Li, S.H.; Deng, S.L.; Yao, Y.R.; Xie, S.Y.; et al. An Unconventional Hydrofullerene C66H4 with Symmetric Heptagons Retrieved in Low-Pressure Combustion. J. Am. Chem. Soc. 2019, 141, 6651–6657. [Google Scholar] [CrossRef]
- Hirsch, A.; Brettreich, M.B. Fullerenes: Chemistry and Reactions; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; ISBN 3527308202. [Google Scholar]
- Krusic, P.J.; Wasserman, E.; Keizer, P.N.; Morton, J.R.; Preston, K.F. Radical Reactions of C60. Science 1991, 254, 1183–1185. [Google Scholar] [CrossRef]
- Zeynalov, E.B.; Allen, N.S.; Salmanova, N.I. Radical Scavenging Efficiency of Different Fullerenes C60-C70 and Fullerene Soot. Polym. Degrad. Stab. 2009, 94, 1183–1189. [Google Scholar] [CrossRef]
- Rotello, V.M.; Howard, J.B.; Yadav, T.; Morgan Conn, M.; Viani, E.; Giovane, L.M.; Lafleur, A.L. Isolation of Fullerene Products from Flames: Structure and Synthesis of the C60-Cyclopentadiene Adduct. Tetrahedron Lett. 1993, 34, 1561–1562. [Google Scholar] [CrossRef]
- Gerhardt, P.; Löffler, S.; Homann, K.H. Polyhedral Carbon Ions in Hydrocarbon Flames. Chem. Phys. Lett. 1987, 137, 306–310. [Google Scholar] [CrossRef]
- Howard, J.B.; Mckinnon, J.T.; Makarovsky, Y.; Lafleur, A.L.; Elaine Johnson, M. Fullerenes C60 and C70 in Flames. Nature 1991, 352, 139. [Google Scholar] [CrossRef]
- Howard, J.B.; McKinnon, J.T.; Elaine Johnson, M.; Makarovsky, Y.; Lafleur, A.L. Production of C60 and C70 Fullerenes in Benzene-Oxygen Flames. J. Phys. Chem. 1992, 96, 6657–6662. [Google Scholar] [CrossRef]
- Richter, H.; Labrocca, A.J.; Grieco, W.J.; Taghizadeh, K.; Lafleur, A.L.; Howard, J.B. Generation of Higher Fullerenes in Flames. J. Phys. Chem. B 1997, 101, 1556–1560. [Google Scholar] [CrossRef]
- Goel, A.; Hebgen, P.; Vander Sande, J.B.; Howard, J.B. Combustion Synthesis of Fullerenes and Fullerenic Nanostructures. Carbon 2002, 40, 177–182. [Google Scholar] [CrossRef]
- Takehara, H.; Fujiwara, M.; Arikawa, M.; Diener, M.D.; Alford, J.M. Experimental Study of Industrial Scale Fullerene Production by Combustion Synthesis. Carbon 2005, 43, 311–319. [Google Scholar] [CrossRef]
- Bellavia-Lund, C.; Wudl, F. Synthesis of [70]Azafulleroids: Investigations of Azide Addition to C70. J. Am. Chem. Soc. 1997, 119, 943–946. [Google Scholar] [CrossRef]
- Smith, A.B.; Strongin, R.M.; Brard, L.; Furst, G.T.; Romanow, W.J.; Owens, K.G.; Goldschmidt, R.J. C71H2 Cyclopropanes and Annulenes: Synthesis and Characterization. J. Chem. Soc. Chem. Commun. 1994, 18, 2187. [Google Scholar] [CrossRef]
- Smith, A.B.; Strongin, R.M.; Brard, L.; Furst, G.T.; Romanow, W.J.; Owens, K.G.; Goldschmidt, R.J.; King, R.C. Synthesis of Prototypical Fullerene Cyclopropanes and Annulenes. Isomer Differentiation via NMR and UV Spectroscopy. J. Am. Chem. Soc. 1995, 117, 5492–5502. [Google Scholar] [CrossRef]
- Kiely, A.F.; Haddon, R.C.; Meier, M.S.; Selegue, J.P.; Brock, C.P.; Patrick, B.O.; Wang, G.W.; Chen, Y.S. The First Structurally Characterized Homofullerene (Fulleroid). J. Am. Chem. Soc. 1999, 121, 7971–7972. [Google Scholar] [CrossRef]
- Bühl, M.; Hirsch, A. Spherical Aromaticity of Fullerenes. Chem. Rev. 2001, 101, 1153–1184. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.F.; King, R.B. Spherical Aromaticity: Recent Work on Fullerenes, Polyhedral Boranes, and Related Structures. Chem. Rev. 2005, 105, 3613–3642. [Google Scholar] [CrossRef] [PubMed]
- Bühl, M. The Relation Between Endohedral Chemical Shifts and Local Aromaticities in Fullerenes. Chem. Eur. J. 1998, 4, 734–739. [Google Scholar] [CrossRef]
- Li, B.; Shu, C.Y.; Lu, X.; Dunsch, L.; Chen, Z.F.; Dennis, T.J.S.; Shi, Z.Q.; Jiang, L.S.; Wang, T.; Xu, W.; et al. Addition of Carbene to the Equator of C70 To Produce the Most Stable C71H2 Isomer: 2 AH-2(12)a-Homo(C70-D5h(6))[5,6]Fullerene. Angew. Chem. Int. Ed. 2010, 49, 962–966. [Google Scholar] [CrossRef]
- Teng, Q.W.; Zhao, X.Z.; Cai, Z.S.; Tang, A.C. Theoretical Studies on the Structures and Electronic Spectra of C70CH2. Int. J. Quantum Chem. 1997, 61, 815–822. [Google Scholar] [CrossRef]
- Akasaka, T.; Mitsuhida, E.; Ando, W.; Kobayashi, K.; Nagase, S. Adduct of C70 at the Equatorial Belt: Photochemical Cycloaddition with Disilirane. J. Am. Chem. Soc. 1994, 116, 2627–2628. [Google Scholar] [CrossRef]
- Krätschmer, W.; Lamb, L.D.; Fostiropoulos, K.; Huffman, D.R. Solid C60: A New Form of Carbon. Nature 1990, 347, 354–358. [Google Scholar] [CrossRef]
- Yao, Y.R.; Shi, X.M.; Zheng, S.Y.; Chen, Z.C.; Xie, S.Y.; Huang, R.B.; Zheng, L.S. Atomically Precise Insights into Metal–Metal Bonds Using Comparable Endo-Units of Sc2 and Sc2C2. CCS Chem. 2021, 3, 294–302. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. Structure Validation in Chemical Crystallography. Acta Cryst. D Biol. Cryst. 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Y.; Tian, H.R.; Li, S.H.; Chen, Z.C.; Yao, Y.R.; Wang, S.S.; Zhang, X.; Zhu, Z.Z.; Deng, S.L.; Zhang, Q.Y.; et al. Flexible Decapyrrylcorannulene Hosts. Nat. Commun. 2019, 10, 485. [Google Scholar] [CrossRef] [PubMed]
- Slanina, Z.; Lee, S.L.; Uhlík, F.; Adamowicz, L.; Nagase, S. Computing Relative Stabilities of Metallofullerenes by Gibbs Energy Treatments. Theor. Chem. Acc. 2007, 117, 315–322. [Google Scholar] [CrossRef]
- Baum, T.; Löffler, S.; Löffler, P.; Weilmünster, P.; Homann, K.H. Fullerene Ions and Their Relation to PAH and Soot in Low-pressure Hydrocarbon Flames. Ber. Bunsenges. Phys. Chem. 1992, 96, 841–857. [Google Scholar] [CrossRef]
- Lafleur, A.L.; Howard, J.B.; Marr, J.A.; Yadav, T. Proposed Fullerene Precursor Corannulene Identified in Flames Both in the Presence and Absence of Fullerene Production. J. Phys. Chem. 1993, 97, 13539–13543. [Google Scholar] [CrossRef]
- Ahrens, J.; Bachmann, M.; Baum, T.; Griesheimer, J.; Kovacs, R.; Weilmünster, P.; Homann, K.H. Fullerenes and Their Ions in Hydrocarbon Flames. Int. J. Mass Spectrom. Ion Processes 1994, 138, 133–148. [Google Scholar] [CrossRef]
- Lafleur, A.L.; Howard, J.B.; Taghizadeh, K.; Plummer, E.F.; Scott, L.T.; Necula, A.; Swallow, K.C. Identification of C20H10 Dicyclopentapyrenes in Flames: Correlation with Corannulene and Fullerene Formation. J. Phys. Chem. 1996, 100, 17421–17428. [Google Scholar] [CrossRef]
- Homann, K.H. Fullerenes and Soot Formation—New Pathways to Large Particles in Flames. Angew. Chem. Int. Ed. 1998, 37, 2434–2451. [Google Scholar] [CrossRef]
- Poater, J.; Fradera, X.; Duran, M.; Sola, M. An Insight into the Local Aromaticities of Polycyclic Aromatic Hydrocarbons and Fullerenes. Chem. Eur. J. 2003, 9, 1113–1122. [Google Scholar] [CrossRef]
| HOMO | LUMO | H-L gap | RC–C (Å) | ∆E (kcal/mol) | |
|---|---|---|---|---|---|
| D5h-C70 | −5.92 | −3.24 | 2.69 | - | - |
| e–e | −5.83 | −3.16 | 2.67 | 2.315 a (1.471 b) | 0.00 |
| a–b | −5.66 | −3.10 | 2.55 | 1.647 a (1.397 b) | 9.88 |
| d–d | −5.72 | −3.19 | 2.52 | 2.154 a (1.434 b) | 11.62 |
| c–c | −5.64 | −3.12 | 2.52 | 1.612 a (1.389 b) | 11.77 |
| a–a | −5.78 | −3.15 | 2.63 | 2.183 a (1.452 b) | 12.59 |
| b–c | −5.78 | −3.14 | 2.65 | 2.181 a (1.448 b) | 15.30 |
| c–d | −5.71 | −3.16 | 2.55 | 2.178 a (1.449 b) | 22.81 |
| d–e | −5.74 | −3.14 | 2.60 | 2.131 a (1.421 b) | 23.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, F.-F.; Chen, Z.-C.; Wu, Y.-H.; Tian, H.-R.; Deng, S.-L.; Xie, S.-Y.; Zheng, L.-S. Carbene Addition Isomers of C70 formed in the Flame of Low-Pressure Combustion. Nanomaterials 2022, 12, 3087. https://doi.org/10.3390/nano12183087
Xie F-F, Chen Z-C, Wu Y-H, Tian H-R, Deng S-L, Xie S-Y, Zheng L-S. Carbene Addition Isomers of C70 formed in the Flame of Low-Pressure Combustion. Nanomaterials. 2022; 12(18):3087. https://doi.org/10.3390/nano12183087
Chicago/Turabian StyleXie, Fang-Fang, Zuo-Chang Chen, You-Hui Wu, Han-Rui Tian, Shun-Liu Deng, Su-Yuan Xie, and Lan-Sun Zheng. 2022. "Carbene Addition Isomers of C70 formed in the Flame of Low-Pressure Combustion" Nanomaterials 12, no. 18: 3087. https://doi.org/10.3390/nano12183087
APA StyleXie, F.-F., Chen, Z.-C., Wu, Y.-H., Tian, H.-R., Deng, S.-L., Xie, S.-Y., & Zheng, L.-S. (2022). Carbene Addition Isomers of C70 formed in the Flame of Low-Pressure Combustion. Nanomaterials, 12(18), 3087. https://doi.org/10.3390/nano12183087