Influence of Nanosized CoTiO3 Synthesized via a Solid-State Method on the Hydrogen Storage Behavior of MgH2
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
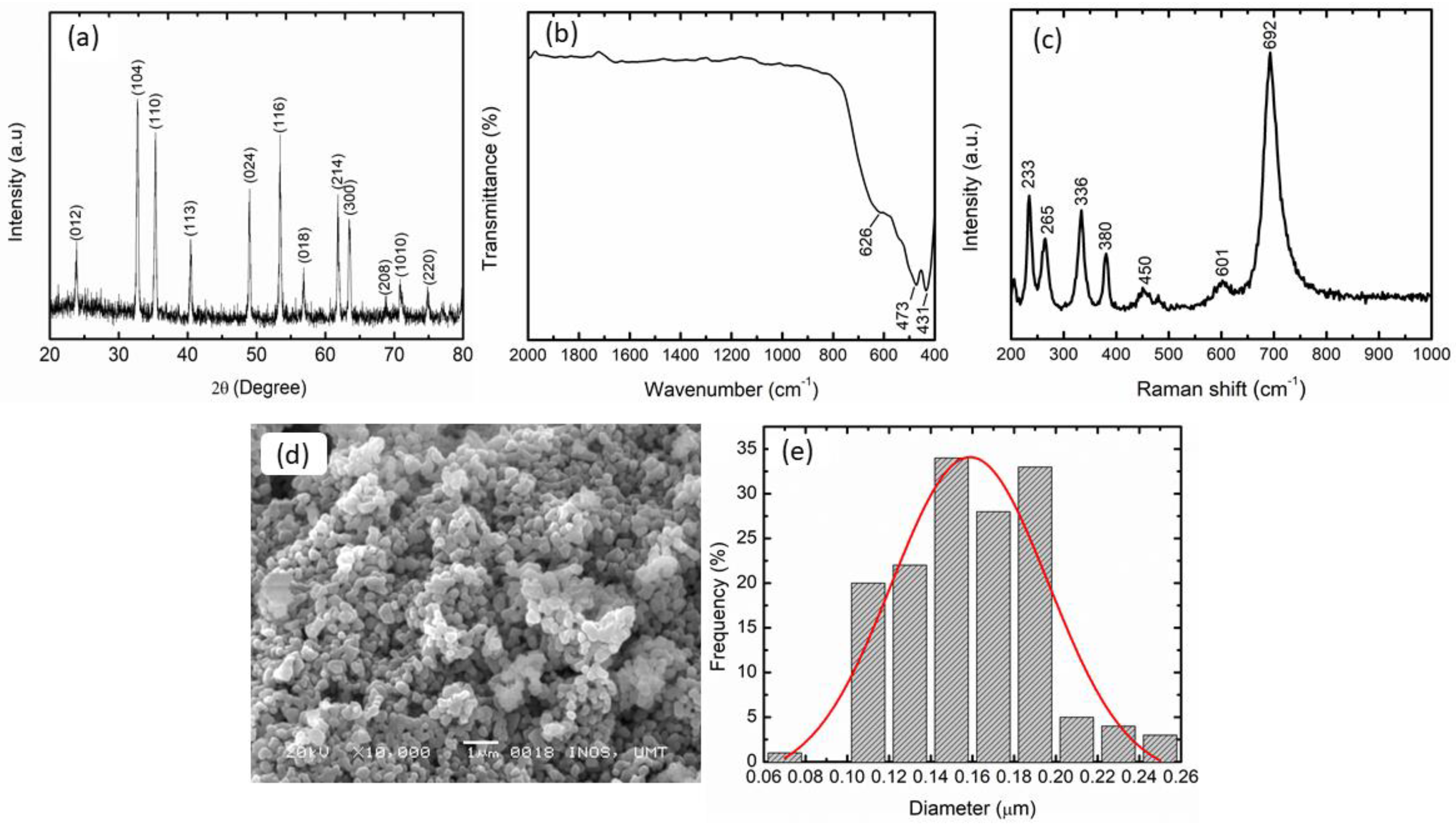
3.1. Synthesis of CoTiO3
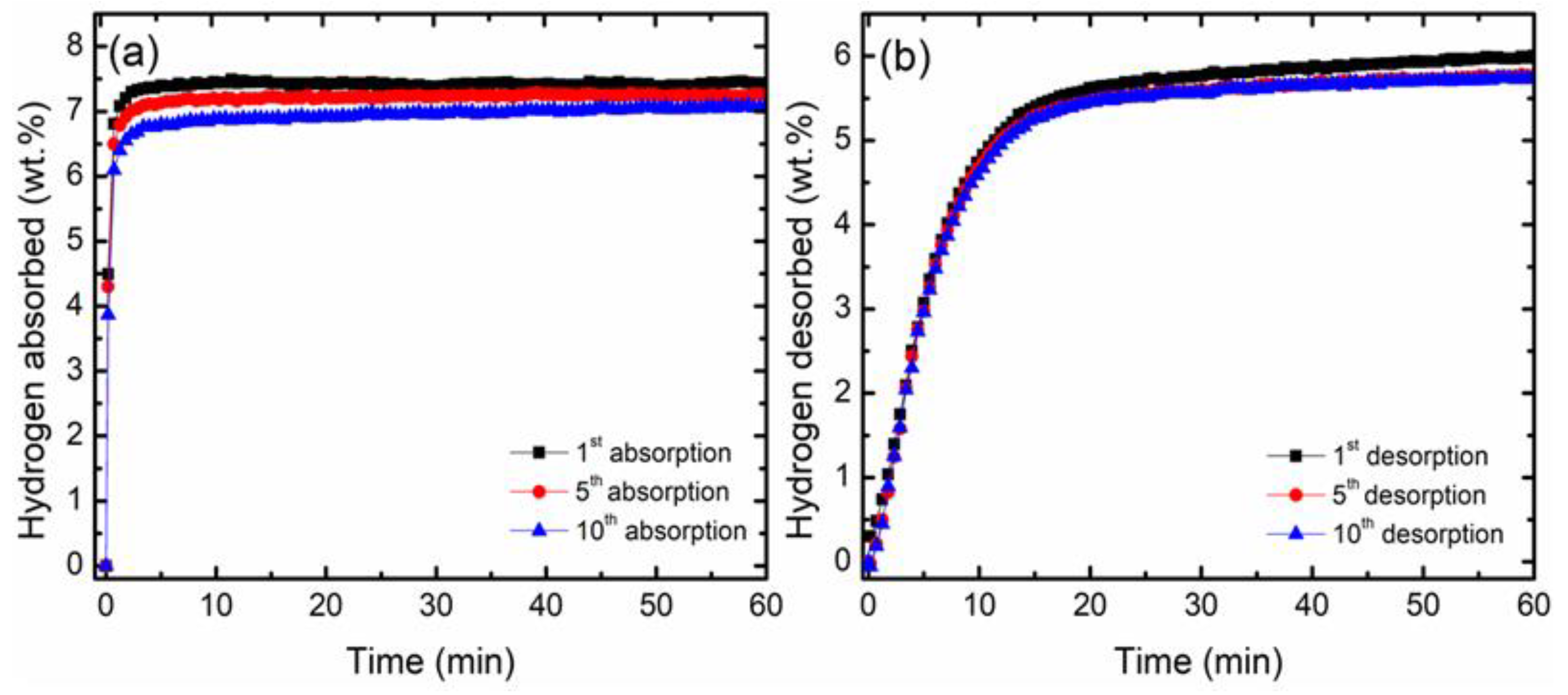
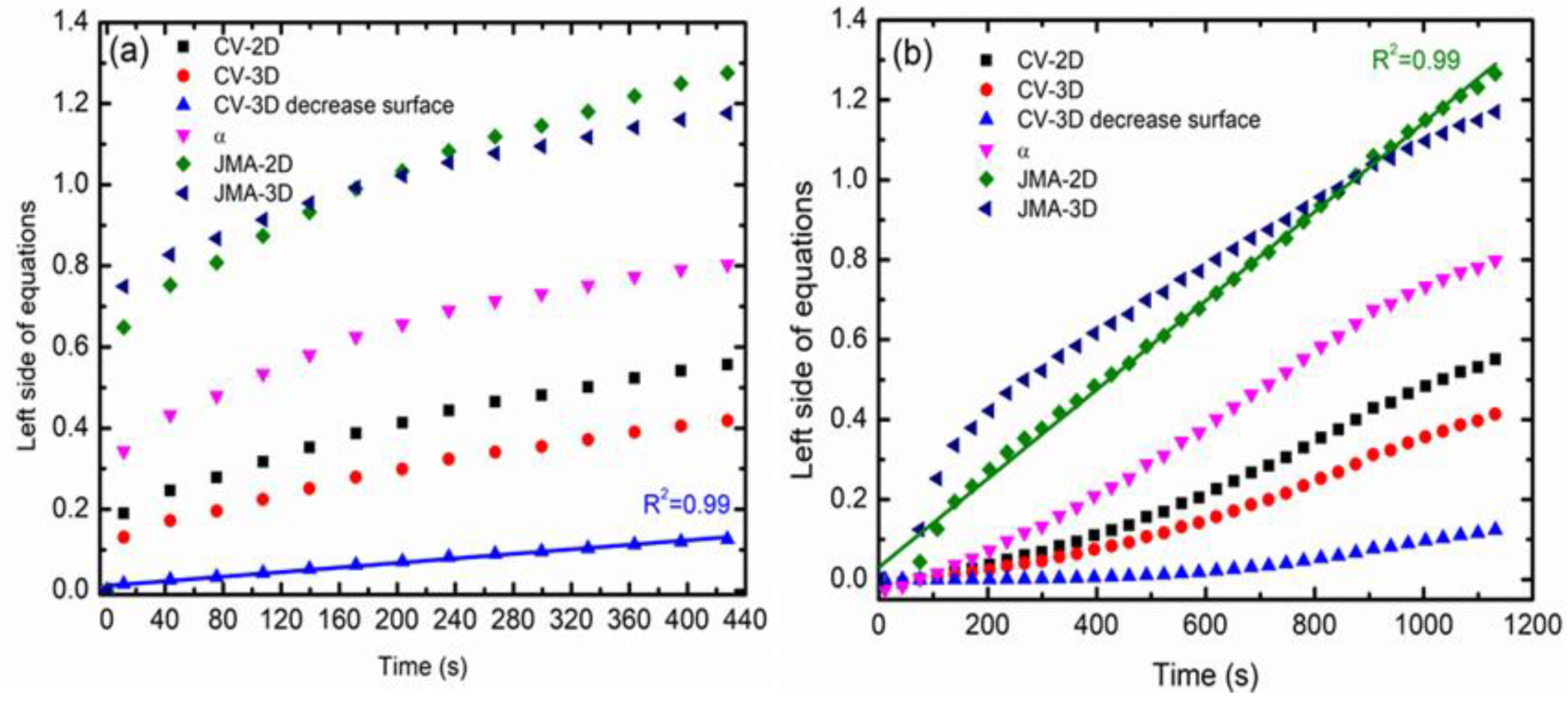
3.2. Hydrogen Storage Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scovell, M.D. Explaining hydrogen energy technology acceptance: A critical review. Int. J. Hydrog. Energy 2022, 47, 10441–10459. [Google Scholar] [CrossRef]
- Endo, N.; Goshome, K.; Tetsuhiko, M.; Segawa, Y.; Shimoda, E.; Nozu, T. Thermal management and power saving operations for improved energy efficiency within a renewable hydrogen energy system utilizing metal hydride hydrogen storage. Int. J. Hydrog. Energy 2021, 46, 262–271. [Google Scholar] [CrossRef]
- Carmo, M.; Stolten, D. Chapter 4—Energy storage using hydrogen produced from excess renewable electricity: Power to hydrogen. In Science and Engineering of Hydrogen-Based Energy Technologies; De Miranda, P.E.V., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 165–199. [Google Scholar]
- Pal, P.; Agarwal, S.; Tiwari, A.; Ichikawa, T.; Jain, A.; Dixit, A. Improved hydrogen desorption properties of exfoliated graphite and graphene nanoballs modified MgH2. Int. J. Hydrog. Energy 2022, in press. [Google Scholar] [CrossRef]
- Tarkowski, R.; Uliasz-Misiak, B. Towards underground hydrogen storage: A review of barriers. Renew Sust. Energy Rev. 2022, 162, 112451. [Google Scholar] [CrossRef]
- Sales of Hyundai Hydrogen Fuel Cells Nexo Pass 1K Unit Mark in 2019. Available online: https://www.greencarcongress.com/2019/05/20190521-nexo.html (accessed on 23 August 2022).
- Thomas, J.M.; Edwards, P.P.; Dobson, P.J.; Owen, G.P. Decarbonising energy: The developing international activity in hydrogen technologies and fuel cells. J. Energy Chem. 2020, 51, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Durbin, D.; Malardier-Jugroot, C. Review of hydrogen storage techniques for on board vehicle applications. Int. J. Hydrog. Energy 2013, 38, 14595–14617. [Google Scholar] [CrossRef]
- Sun, Y.; Shen, C.; Lai, Q.; Liu, W.; Wang, D.-W.; Aguey-Zinsou, K.-F. Tailoring magnesium based materials for hydrogen storage through synthesis: Current state of the art. Energy Storage Mater. 2018, 10, 168–198. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrog. Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Murthy, S.S.; Kumar, E.A. Advanced materials for solid state hydrogen storage:“Thermal engineering issues”. Appl. Therm. Eng. 2014, 72, 176–189. [Google Scholar] [CrossRef]
- Tarhan, C.; Çil, M.A. A study on hydrogen, the clean energy of the future: Hydrogen storage methods. J. Energy Storage 2021, 40, 102676. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Mohan, S. Solid state storage of hydrogen and its isotopes: An engineering overview. Renew Sust. Energy Rev. 2015, 41, 872–883. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, P.; Niu, M.; Maddy, J. The survey of key technologies in hydrogen energy storage. Int. J. Hydrog. Energy 2016, 41, 14535–14552. [Google Scholar] [CrossRef]
- Weng, Z.; Retita, I.; Tseng, Y.-S.; Berry, A.J.; Scott, D.R.; Leung, D.; Wang, Y.; Chan, S. γ-MgH2 induced by high pressure for low temperature dehydrogenation. Int. J. Hydrog. Energy 2021, 46, 5441–5448. [Google Scholar] [CrossRef]
- Wang, J.; Du, Y.; Sun, L.; Li, X. Effects of F and Cl on the stability of MgH2. Int. J. Hydrog. Energy 2014, 39, 877–883. [Google Scholar] [CrossRef]
- Li, Q.; Yan, M.; Xu, Y.; Zhang, X.L.; Lau, K.T.; Sun, C.; Jia, B. Computational investigation of MgH2/NbOx for hydrogen storage. J. Phys. Chem. C 2021, 125, 8862–8868. [Google Scholar] [CrossRef]
- Zhang, X.L.; Liu, Y.F.; Zhang, X.; Hu, J.J.; Gao, M.X.; Pan, H.G. Empowering hydrogen storage performance of MgH2 by nanoengineering and nanocatalysis. Mater. Today Nano 2020, 9, 100064. [Google Scholar] [CrossRef]
- Ma, Z.; Panda, S.; Zhang, Q.; Sun, F.; Khan, D.; Ding, W.; Zou, J. Improving hydrogen sorption performances of MgH2 through nanoconfinement in a mesoporous CoS nano-boxes scaffold. Chem. Eng. 2021, 406, 126790. [Google Scholar] [CrossRef]
- Akbarzadeh, F.Z.; Rajabi, M. Mechanical alloying fabrication of nickel/cerium/MgH2 nanocomposite for hydrogen storage: Molecular dynamics study and experimental verification. J. Alloy. Compd. 2022, 899, 163280. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, L.; Li, Y.; Guo, S.; Yu, H.; Wang, W.; Ren, K.; Zhang, W.; Han, S. Effect of ternary transition metal sulfide FeNi2S4 on hydrogen storage performance of MgH2. J. Magnes. Alloy. 2022, in press. [Google Scholar] [CrossRef]
- Huang, H.; Yuan, J.; Zhang, B.; Zhang, J.; Zhu, Y.; Li, L.; Wu, Y. A noteworthy synergistic catalysis on hydrogen sorption kinetics of MgH2 with bimetallic oxide Sc2O3/TiO2. J. Alloy. Compd. 2020, 839, 155387. [Google Scholar] [CrossRef]
- Dan, L.; Hu, L.; Wang, H.; Zhu, M. Excellent catalysis of MoO3 on the hydrogen sorption of MgH2. Int. J. Hydrog. Energy 2019, 44, 29249–29254. [Google Scholar] [CrossRef]
- Valentoni, A.; Mulas, G.; Enzo, S.; Garroni, S. Remarkable hydrogen storage properties of MgH2 doped with VNbO5. Phys. Chem. Chem. Phys. 2018, 20, 4100–4108. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, M.; Yao, Z.; Xian, K.; Wu, M.; Liu, Y.; Sun, W.; Pan, H. High-loading, ultrafine Ni nanoparticles dispersed on porous hollow carbon nanospheres for fast (de) hydrogenation kinetics of MgH2. J. Magnes. Alloy. 2021, in press. [Google Scholar] [CrossRef]
- Lu, Z.-Y.; Yu, H.-J.; Lu, X.; Song, M.-C.; Wu, F.-Y.; Zheng, J.-G.; Yuan, Z.-F.; Zhang, L.-T. Two-dimensional vanadium nanosheets as a remarkably effective catalyst for hydrogen storage in MgH2. Rare Met. 2021, 40, 3195–3204. [Google Scholar] [CrossRef]
- Youn, J.-S.; Phan, D.-T.; Park, C.-M.; Jeon, K.-J. Enhancement of hydrogen sorption properties of MgH2 with a MgF2 catalyst. Int. J. Hydrog. Energy 2017, 42, 20120–20124. [Google Scholar] [CrossRef]
- Sun, Z.; Lu, X.; Nyahuma, F.M.; Yan, N.; Xiao, J.; Su, S.; Zhang, L. Enhancing hydrogen storage properties of MgH2 by transition metals and carbon materials: A brief review. Front. Chem. 2020, 8, 552. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Zang, L.; Chang, X.; Jiao, L.; Yuan, H. Core-shell Ni3N@ Nitrogen-doped carbon: Synthesis and application in MgH2. J. Alloy. Compd. 2017, 703, 381–388. [Google Scholar] [CrossRef]
- Liao, W.; Jiang, W.; Yang, X.-S.; Wang, H.; Ouyang, L.; Zhu, M. Enhancing (de)hydrogenation kinetics properties of the Mg/MgH2 system by adding ANi5 (A = Ce, Nd, Pr, Sm, and Y) alloys via ball milling. J. Rare Earths 2021, 39, 1010–1016. [Google Scholar] [CrossRef]
- Dong, J.; Panda, S.; Zhu, W.; Zou, J.; Ding, W. Enhanced hydrogen sorption properties of MgH2 when doped with mechanically alloyed amorphous Zr0·67Ni0.33 particles. Int. J. Hydrog. Energy 2020, 45, 28144–28153. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, K.; Liu, Y.; Zhang, X.; Hu, J.; Gao, M.; Pan, H. Highly active multivalent multielement catalysts derived from hierarchical porous TiNb2O7 nanospheres for the reversible hydrogen storage of MgH2. Nano Res. 2020, 14, 148–156. [Google Scholar] [CrossRef]
- Berezovets, V.V.; Denys, R.V.; Zavaliy, I.Y.; Kosarchyn, Y.V. Effect of Ti-based nanosized additives on the hydrogen storage properties of MgH2. Int. J. Hydrog. Energy 2022, 47, 7289–7298. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, S.; Xia, G.; Zhou, X.; Lu, X.; Yu, L.; Yu, X.; Peng, P. Stabilization of low-valence transition metal towards advanced catalytic effects on the hydrogen storage performance of magnesium hydride. J. Magnes. Alloy. 2020, 9, 647–657. [Google Scholar] [CrossRef]
- Huot, J.; Liang, G.; Boily, S.; Van Neste, A.; Schulz, R. Structural study and hydrogen sorption kinetics of ball-milled magnesium hydride. J. Alloy. Compd. 1999, 293–295, 495–500. [Google Scholar] [CrossRef]
- Hanada, N.; Ichikawa, T.; Fujii, H. Catalytic effect of nanoparticle 3d-transition metals on hydrogen storage properties in magnesium hydride MgH2 prepared by mechanical milling. J. Phys. Chem. B 2005, 109, 7188–7194. [Google Scholar] [CrossRef]
- Shang, C.; Bououdina, M.; Song, Y.; Guo, Z. Mechanical alloying and electronic simulations of (MgH2+ M) systems (M= Al, Ti, Fe, Ni, Cu and Nb) for hydrogen storage. Int. J. Hydrog. Energy 2004, 29, 73–80. [Google Scholar] [CrossRef]
- Yan, N.; Lu, X.; Lu, Z.; Yu, H.; Wu, F.; Zheng, J.; Wang, X.; Zhang, L. Enhanced hydrogen storage properties of Mg by the synergistic effect of grain refinement and NiTiO3 nanoparticles. J. Magnes. Alloy. 2021, in press. [Google Scholar] [CrossRef]
- Shan, J.; Li, P.; Wan, Q.; Zhai, F.; Zhang, J.; Li, Z.; Liu, Z.; Volinsky, A.A.; Qu, X. Significantly improved dehydrogenation of ball-milled MgH2 doped with CoFe2O4 nanoparticles. J. Power Sources 2014, 268, 778–786. [Google Scholar] [CrossRef]
- Ismail, M.; Mustafa, N.S.; Ali, N.A.; Sazelee, N.A.; Yahya, M.S. The hydrogen storage properties and catalytic mechanism of the CuFe2O4-doped MgH2 composite system. Int. J. Hydrog. Energy 2019, 44, 318–324. [Google Scholar] [CrossRef]
- Huang, X.; Xiao, X.; Wang, X.; Wang, C.; Fan, X.; Tang, Z.; Wang, C.; Wang, Q.; Chen, L. Synergistic catalytic activity of porous rod-like TMTiO3 (TM= Ni and Co) for reversible hydrogen storage of magnesium hydride. J. Phys. Chem. C 2018, 122, 27973–27982. [Google Scholar] [CrossRef]
- Idris, N.; Mustafa, N.; Ismail, M. MnFe2O4 nanopowder synthesised via a simple hydrothermal method for promoting hydrogen sorption from MgH2. Int. J. Hydrog. Energy 2017, 42, 21114–21120. [Google Scholar] [CrossRef]
- Kitchamsetti, N.; Choudhary, R.J.; Phase, D.M.; Devan, R.S. Structural correlation of a nanoparticle-embedded mesoporous CoTiO3 perovskite for an efficient electrochemical supercapacitor. RSC Adv. 2020, 10, 23446–23456. [Google Scholar] [CrossRef]
- Sharma, Y.K.; Kharkwal, M.; Uma, S.; Nagarajan, R. Synthesis and characterization of titanates of the formula MTiO3 (M=Mn, Fe, Co, Ni and Cd) by co-precipitation of mixed metal oxalates. Polyhedron 2009, 28, 579–585. [Google Scholar] [CrossRef]
- Habibi, M.H.; Shojaee, E. Ilmenite type nano-crystalline Co–Ti–O ternary oxides: Sol–gel thin film on borosilicate glass, characterization and photocatalytic activity in mineralization of reactive red 198. J. Mater. Sci. Mater. Electron. 2017, 28, 8286–8293. [Google Scholar] [CrossRef]
- Maensiri, S.; Laokul, P.; Klinkaewnarong, J. A simple synthesis and room-temperature magnetic behavior of Co-doped anatase TiO2 nanoparticles. J. Magn. Magn. Mater. 2006, 302, 448–453. [Google Scholar] [CrossRef]
- Rashad, M.M.; Elsayed, E.M.; Al-Kotb, M.S.; Shalan, A.E. The structural, optical, magnetic and photocatalytic properties of transition metal ions doped TiO2 nanoparticles. J. Alloy. Compd. 2013, 581, 71–78. [Google Scholar] [CrossRef]
- Acharya, T.; Choudhary, R.N.P. Structural, dielectric and impedance characteristics of CoTiO3. Mater. Chem. Phys. 2016, 177, 131–139. [Google Scholar] [CrossRef]
- Rattan Paul, D.; Sharma, A.; Panchal, P.; Chaudhary, S.; Patidar, D.; Nehra, S.P. Effect of ball milling and iron mixing on structural and morphological properties of magnesium for hydrogen storage application. Mater. Today 2021, 42, 1673–1677. [Google Scholar] [CrossRef]
- Ares-Fernández, J.-R.; Aguey-Zinsou, K.-F. Superior MgH2 kinetics with MgO addition: A tribological effect. Catalysts 2012, 2, 330–343. [Google Scholar] [CrossRef]
- Rahmaninasab, M.A.; Raygan, S.; Abdizadeh, H.; Pourabdoli, M.; Mirghaderi, S.H. Properties of activated MgH2+ mischmetal nanostructured composite produced by ball-milling. Mater. Renew Sustain. Energy 2018, 7, 15. [Google Scholar] [CrossRef]
- Mustafa, N.S.; Sulaiman, N.N.; Ismail, M. Effect of SrFe12O19 nanopowder on the hydrogen sorption properties of MgH2. RSC Adv. 2016, 6, 110004–110010. [Google Scholar] [CrossRef]
- Sulaiman, N.; Mustafa, N.; Ismail, M. Effect of Na3FeF6 catalyst on the hydrogen storage properties of MgH2. Dalton Trans. 2016, 45, 7085–7093. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, A.; Guo, Z.; Yu, X.; Wexler, D.; Calka, A.; Kim, C.; Liu, H. Hydrogen storage properties of MgH2–SiC composites. Mater. Chem. Phys. 2009, 114, 168–172. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Bormann, R. Effect of Nb2O5 content on hydrogen reaction kinetics of Mg. J. Alloy. Compd. 2004, 364, 242–246. [Google Scholar] [CrossRef]
- Yahya, M.S.; Ismail, M. Catalytic effect of SrTiO3 on the hydrogen storage behaviour of MgH2. J. Energy Chem. 2019, 28, 46–53. [Google Scholar] [CrossRef]
- Sulaiman, N.; Ismail, M. Catalytic effect of SrFe12O19 on the hydrogen storage properties of LiAlH4. Int. J. Hydrog. Energy 2017, 42, 19126–19134. [Google Scholar] [CrossRef]
- Shriniwasan, S.; Kar, T.; Neergat, M.; Tatiparti, S.S.V. Mg–C interaction induced hydrogen uptake and enhanced hydrogen release kinetics in MgH2-rGO nanocomposites. J. Phys. Chem. C 2018, 122, 22389–22396. [Google Scholar] [CrossRef]
- Chou, K.-C.; Li, Q.; Lin, Q.; Jiang, L.-J.; Xu, K.-D. Kinetics of absorption and desorption of hydrogen in alloy powder. Int. J. Hydrog. Energy 2005, 30, 301–309. [Google Scholar] [CrossRef]
- Luo, Q.; An, X.-H.; Pan, Y.-B.; Zhang, X.; Zhang, J.-Y.; Li, Q. The hydriding kinetics of Mg–Ni based hydrogen storage alloys: A comparative study on Chou model and Jander model. Int. J. Hydrog. Energy 2010, 35, 7842–7849. [Google Scholar] [CrossRef]
- Pang, Y.; Li, Q. A review on kinetic models and corresponding analysis methods for hydrogen storage materials. Int. J. Hydrog. Energy 2016, 41, 18072–18087. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Bormann, R. Kinetic investigation of the effect of milling time on the hydrogen sorption reaction of magnesium catalyzed with different Nb2O5 contents. J. Alloy. Compd. 2006, 407, 249–255. [Google Scholar] [CrossRef]
- Yonkeu, A.; Swainson, I.; Dufour, J.; Huot, J. Kinetic investigation of the catalytic effect of a body centered cubic-alloy TiV1.1Mn0.9 (BCC) on hydriding/dehydriding properties of magnesium. J. Alloy. Compd. 2008, 460, 559–564. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Gao, J.; Wei, X.; Zhai, T.; Cai, Y. Improved hydrogen storage kinetics of Mg-based alloys by substituting La with Sm. Int. J. Hydrog. Energy 2020, 45, 21588–21599. [Google Scholar] [CrossRef]
- Sadhasivam, T.; Hudson, M.S.L.; Pandey, S.K.; Bhatnagar, A.; Singh, M.K.; Gurunathan, K.; Srivastava, O. Effects of nano size mischmetal and its oxide on improving the hydrogen sorption behaviour of MgH2. Int. J. Hydrog. Energy 2013, 38, 7353–7362. [Google Scholar] [CrossRef]
- Pighin, S.; Capurso, G.; Russo, S.L.; Peretti, H. Hydrogen sorption kinetics of magnesium hydride enhanced by the addition of Zr8Ni21 alloy. J. Alloy. Compd. 2012, 530, 111–115. [Google Scholar] [CrossRef]
- Nachev, S.; de Rango, P.; Skryabina, N.; Skachkov, A.; Aptukov, V.; Fruchart, D.; Marty, P. Mechanical behavior of highly reactive nanostructured MgH2. Int. J. Hydrog. Energy 2015, 40, 17065–17074. [Google Scholar] [CrossRef]
- Anderson, K.P.; Vinci, R.P.; Chan, H.M. Novel metal–ceramic composite microstructures produced through the partial reduction of CoTiO3. J Mater Sci 2018, 53, 8193–8210. [Google Scholar] [CrossRef]
- Gao, S.; Wang, X.; Liu, H.; He, T.; Wang, Y.; Li, S.; Yan, M. CNTs decorated with CoFeB as a dopant to remarkably improve the dehydrogenation/rehydrogenation performance and cyclic stability of MgH2. Int. J. Hydrog. Energy 2020, 45, 28964–28973. [Google Scholar] [CrossRef]
- Yuan, Z.; Sui, Y.; Zhai, T.; Yin, Y.; Luo, L.; Feng, D. Influence of CeO2 nanoparticles on microstructure and hydrogen storage performance of Mg-Ni-Zn alloy. Mater. Charact. 2021, 178, 111248. [Google Scholar] [CrossRef]
- Xie, X.; Hou, C.; Chen, C.; Sun, X.; Pang, Y.; Zhang, Y.; Yu, R.; Wang, B.; Du, W. First-principles studies in Mg-based hydrogen storage Materials: A review. Energy 2020, 211, 118959. [Google Scholar] [CrossRef]
- Ismail, M. Effect of adding different percentages of HfCl4 on the hydrogen storage properties of MgH2. Int. J. Hydrog. Energy 2021, 46, 8621–8628. [Google Scholar] [CrossRef]
- Malka, I.; Pisarek, M.; Czujko, T.; Bystrzycki, J. A study of the ZrF4, NbF5, TaF5, and TiCl3 influences on the MgH2 sorption properties. Int. J. Hydrog. Energy 2011, 36, 12909–12917. [Google Scholar] [CrossRef]
- Wang, P.; Tian, Z.; Wang, Z.; Xia, C.; Yang, T.; Ou, X. Improved hydrogen storage properties of MgH2 using transition metal sulfides as catalyst. Int. J. Hydrog. Energy 2021, 46, 27107–27118. [Google Scholar] [CrossRef]
- Pukazhselvan, D.; Nasani, N.; Yang, T.; Ramasamy, D.; Shaula, A.; Fagg, D.P. Chemically transformed additive phases in Mg2TiO4 and MgTiO3 loaded hydrogen storage system MgH2. Appl. Surf. Sci. 2019, 472, 99–104. [Google Scholar] [CrossRef]
- Aguey-Zinsou, K.F.; Ares Fernandez, J.R.; Klassen, T.; Bormann, R. Using MgO to improve the (de)hydriding properties of magnesium. Mater. Res. Bull. 2006, 41, 1118–1126. [Google Scholar] [CrossRef]
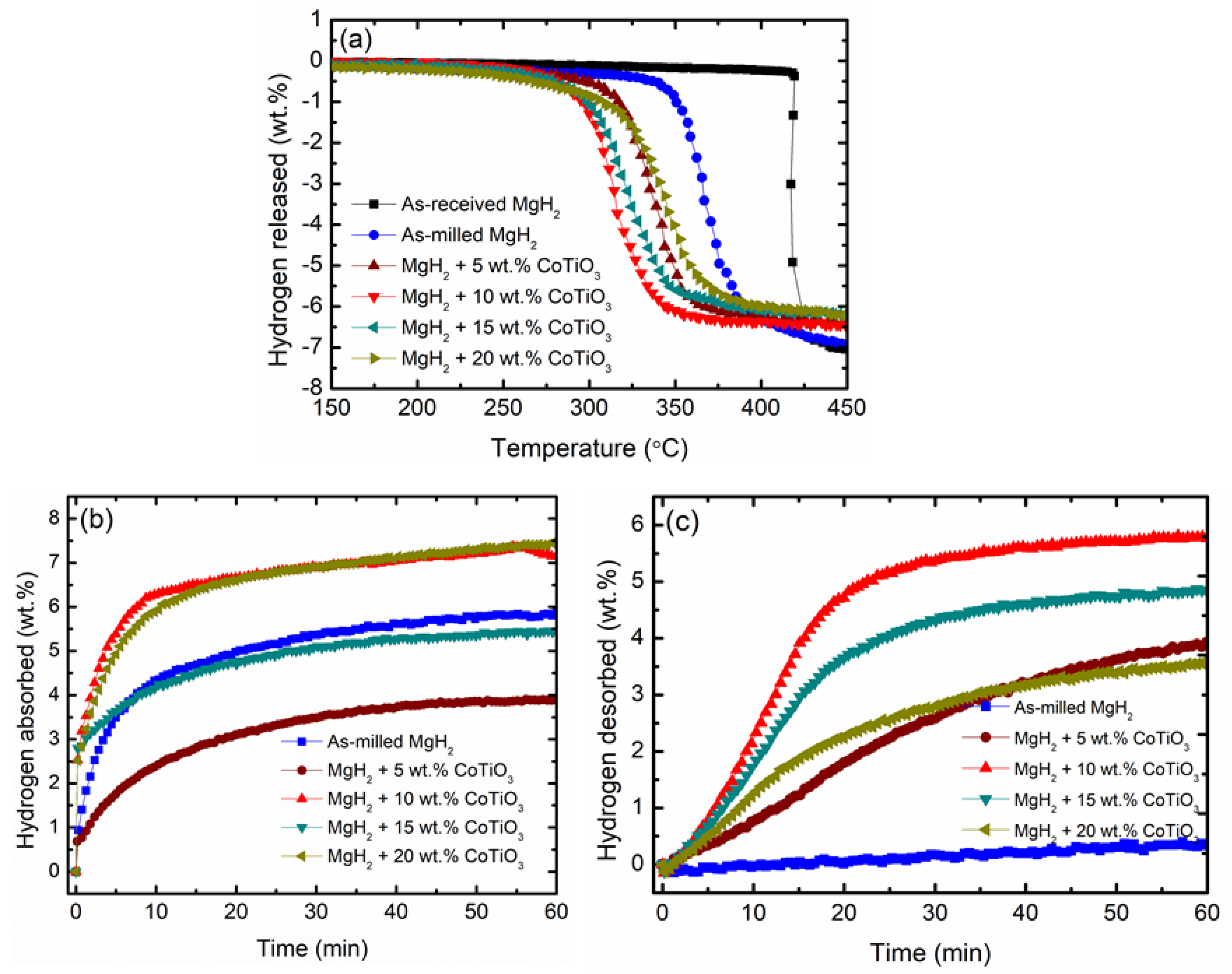
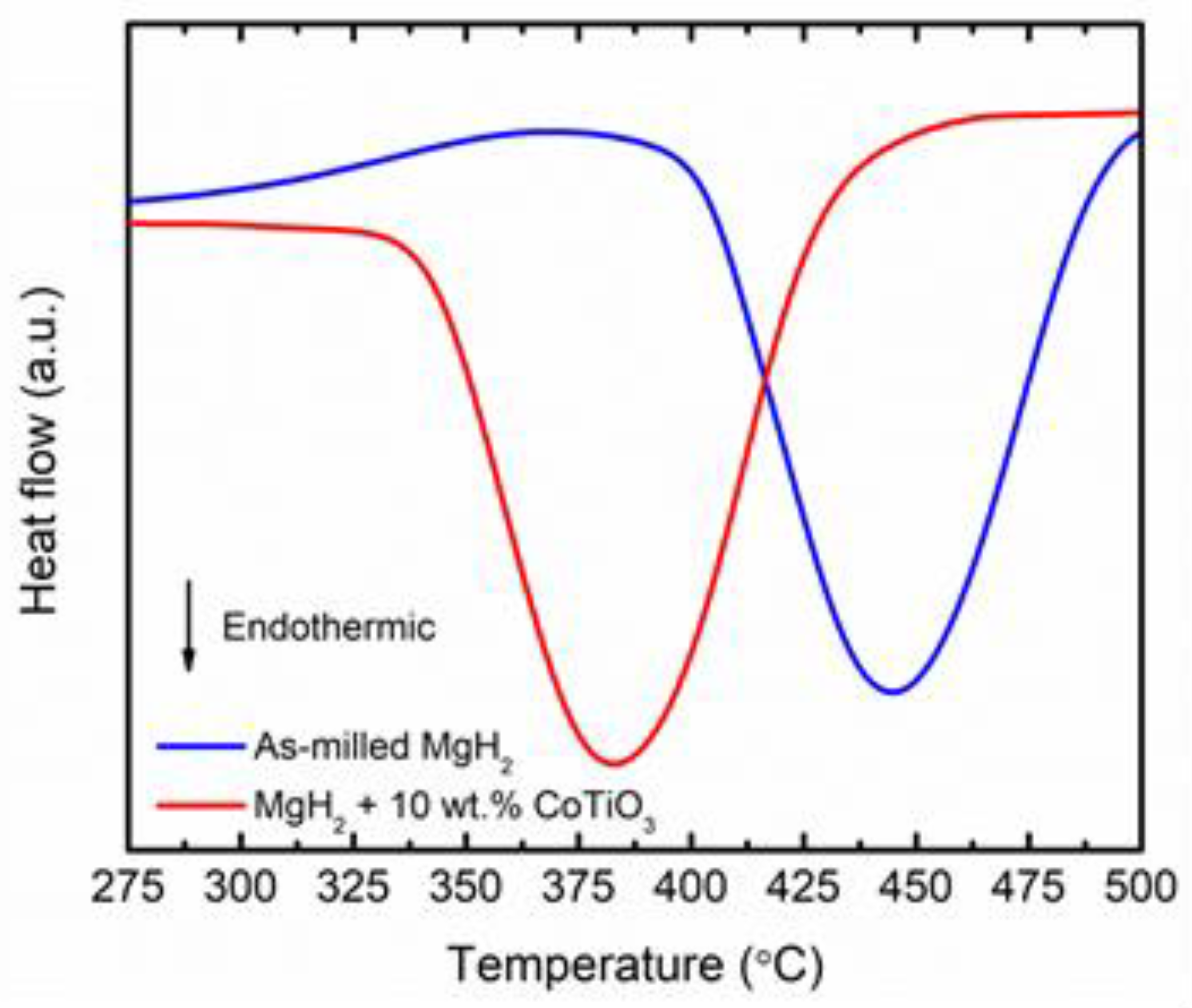





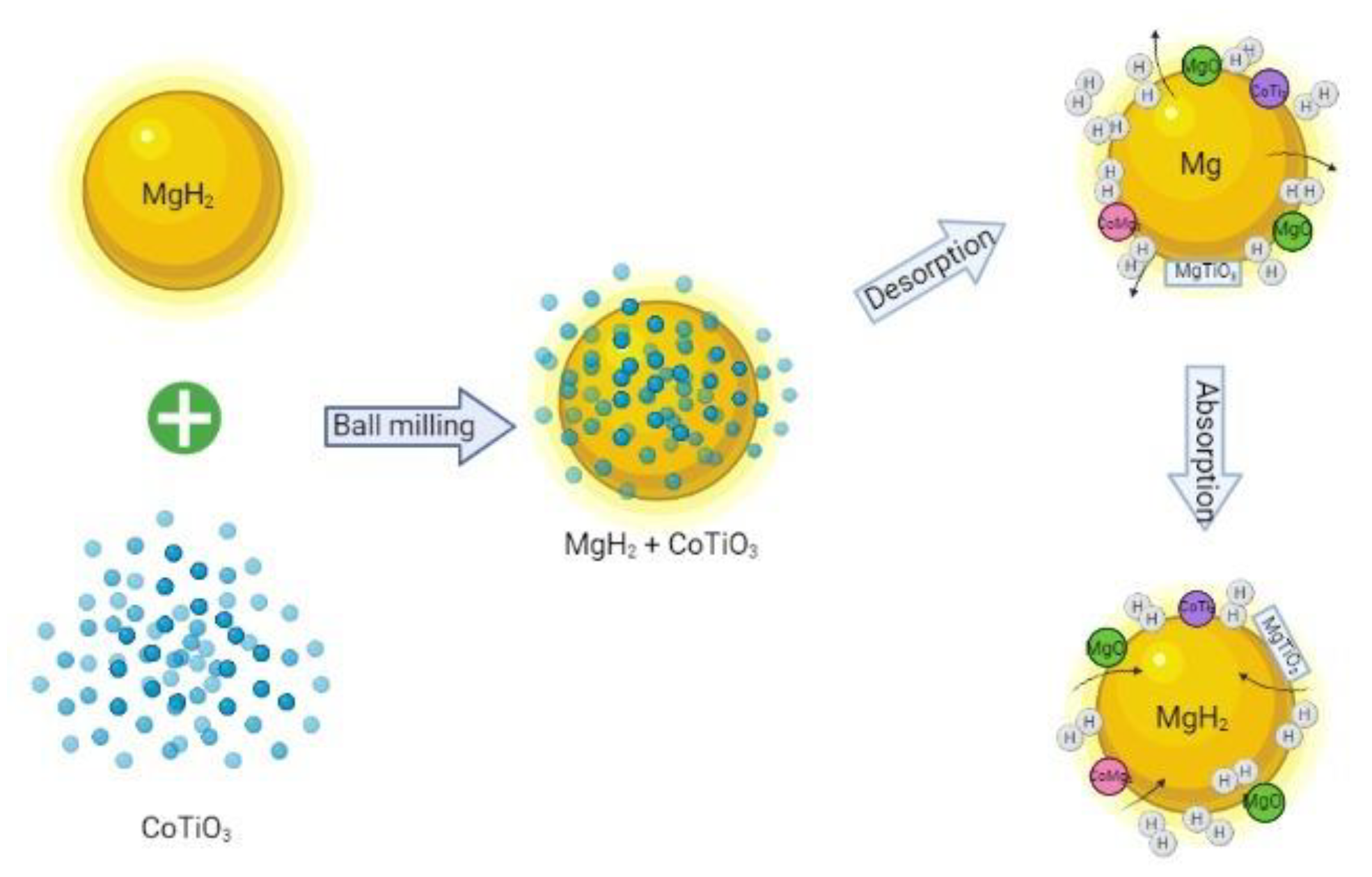
| Sample | Decomposition Temperature (°C) | Absorption Capacity (wt.%) in 10 min | Desorption Capacity (wt.%) in 10 min |
|---|---|---|---|
| Commercial MgH2 | 420 | - | - |
| As-milled MgH2 | 340 | 4.3 | < 0.1 |
| MgH2 + 5 wt.% CoTiO3 | 298 | 2.5 | 0.8 |
| MgH2 + 10 wt.% CoTiO3 | 275 | 6.4 | 2.3 |
| MgH2 + 15 wt.% CoTiO3 | 276 | 4.1 | 1.8 |
| MgH2 + 20 wt.% CoTiO3 | 295 | 6.0 | 1.2 |
| Kinetic Equation | Rate Limiting Step |
|---|---|
| α = kt | Surface controlled. |
| 1-(1-α)1/3 = kt | CV three-dimensional (3D): contracting volume, 3D growth with constant interface velocity. |
| 1-(1-α)1/2 = kt | CV two-dimensional (2D): contracting volume, 2D growth with constant interface velocity. |
| 1-(2α/3)-(1-α)2/3 = kt | CV 3D (variable velocity): contracting volume, 3D growth diffusion controlled with decreasing interface velocity. |
| [-ln(1-α)]1/3 = kt | JMA 3D: 3D growth of existing nuclei with constant interface velocity. |
| [-ln(1-α)]1/2 = kt | JMA 2D: 2D growth of existing nuclei with constant interface velocity. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, N.A.; Yahya, M.S.; Sazelee, N.; Din, M.F.M.; Ismail, M. Influence of Nanosized CoTiO3 Synthesized via a Solid-State Method on the Hydrogen Storage Behavior of MgH2. Nanomaterials 2022, 12, 3043. https://doi.org/10.3390/nano12173043
Ali NA, Yahya MS, Sazelee N, Din MFM, Ismail M. Influence of Nanosized CoTiO3 Synthesized via a Solid-State Method on the Hydrogen Storage Behavior of MgH2. Nanomaterials. 2022; 12(17):3043. https://doi.org/10.3390/nano12173043
Chicago/Turabian StyleAli, Nurul Amirah, Muhammad Syarifuddin Yahya, Noratiqah Sazelee, Muhamad Faiz Md Din, and Mohammad Ismail. 2022. "Influence of Nanosized CoTiO3 Synthesized via a Solid-State Method on the Hydrogen Storage Behavior of MgH2" Nanomaterials 12, no. 17: 3043. https://doi.org/10.3390/nano12173043
APA StyleAli, N. A., Yahya, M. S., Sazelee, N., Din, M. F. M., & Ismail, M. (2022). Influence of Nanosized CoTiO3 Synthesized via a Solid-State Method on the Hydrogen Storage Behavior of MgH2. Nanomaterials, 12(17), 3043. https://doi.org/10.3390/nano12173043










