Designing Highly Efficient Cu2O-CuO Heterojunction CO Oxidation Catalysts: The Roles of the Support Type and Cu2O-CuO Interface Effect
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of the Supports
2.2. Preparation of the Catalysts
2.2.1. Preparation of Cu2O/S Supported Catalysts
2.2.2. Preparation of Cu2O-CuO Heterojunction Catalysts
2.3. Catalyst Characterizations
2.4. Catalytic Activity Measurements
3. Results and Discussions
3.1. Catalytic Property toward CO Oxidation
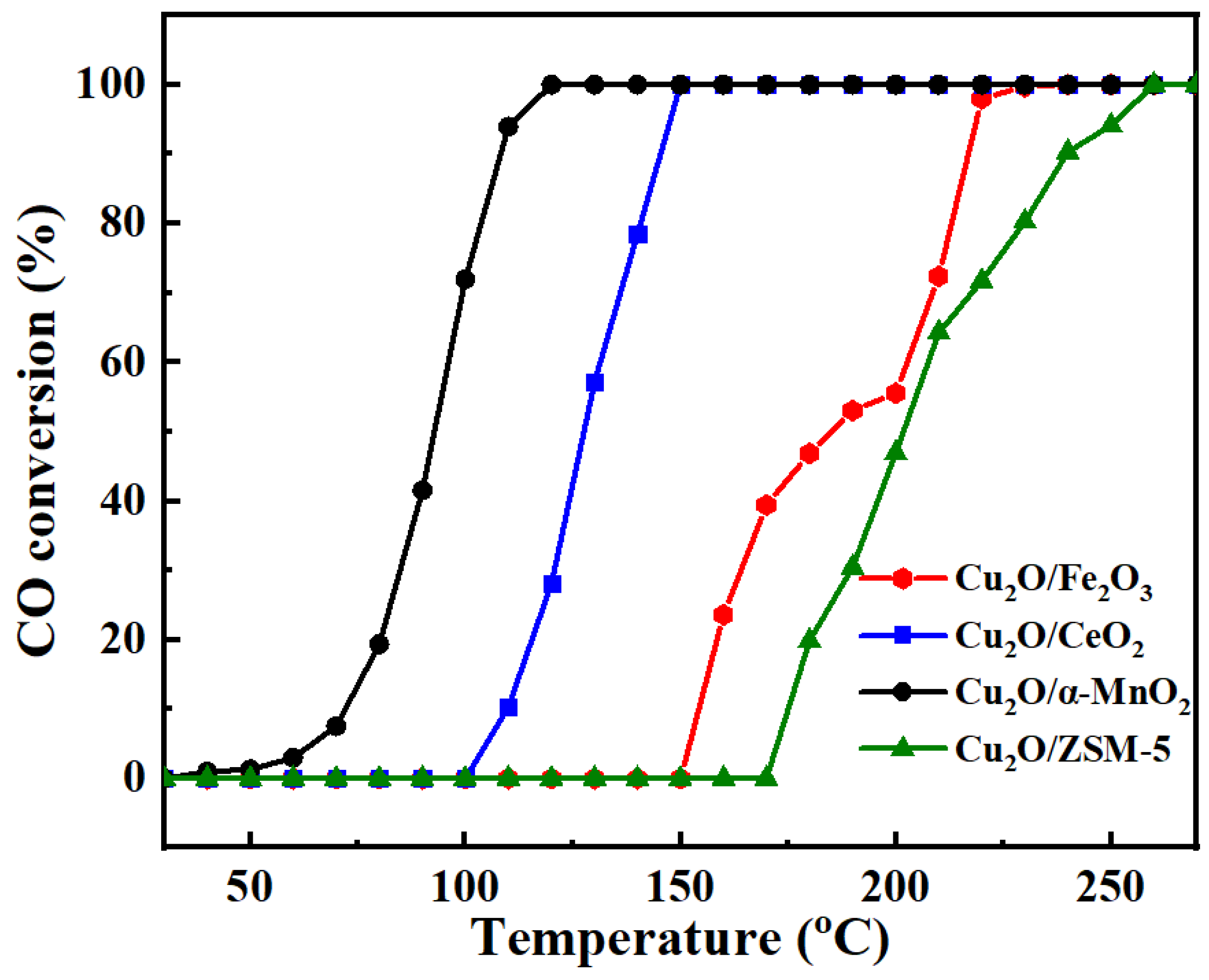
3.1.1. Effect of the Support Type on the Catalytic Activity of CO Oxidation
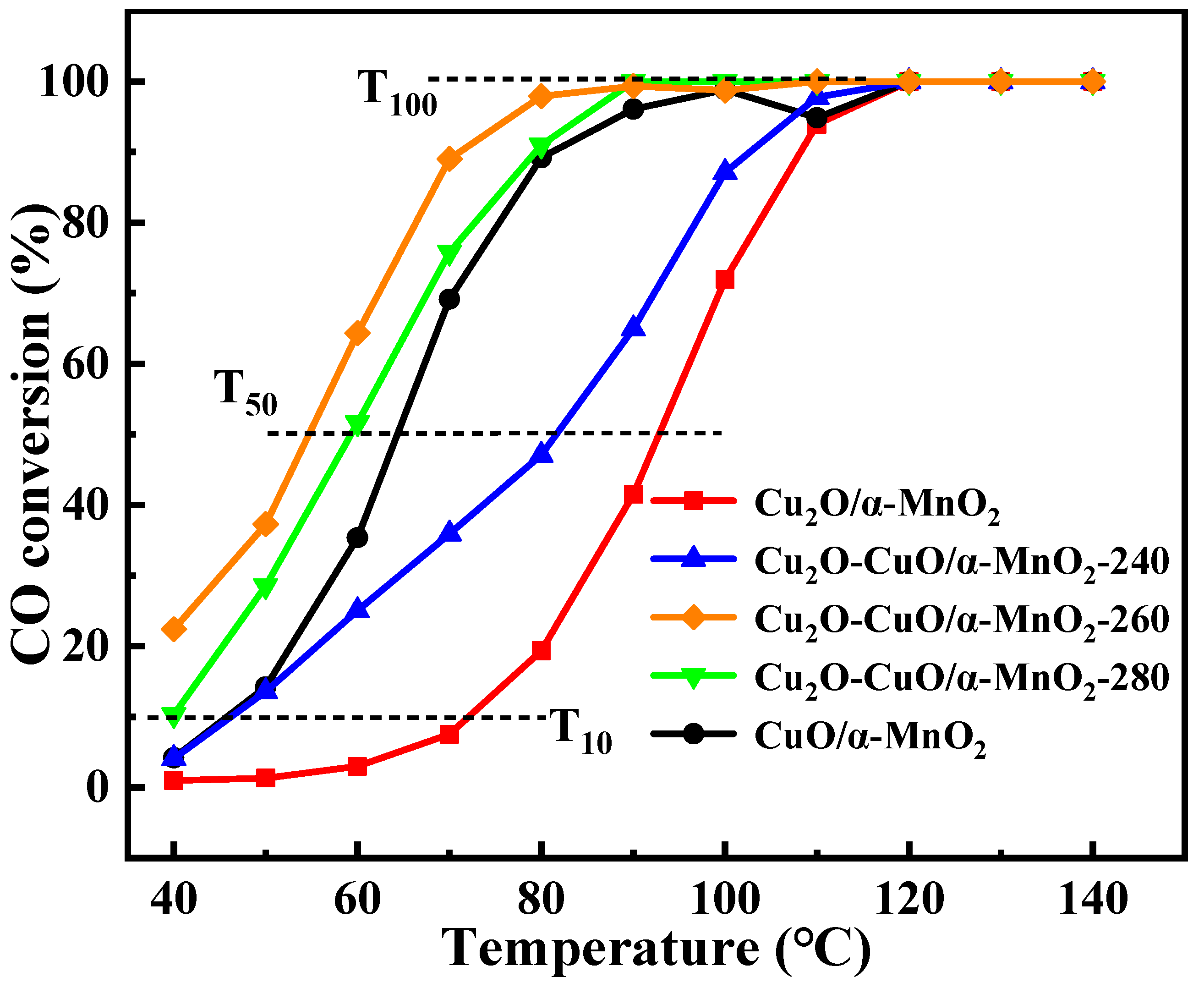
3.1.2. Effect of Cu2O-CuO Heterojunction on the Catalytic Activity of CO Oxidation
3.1.3. Kinetic Study
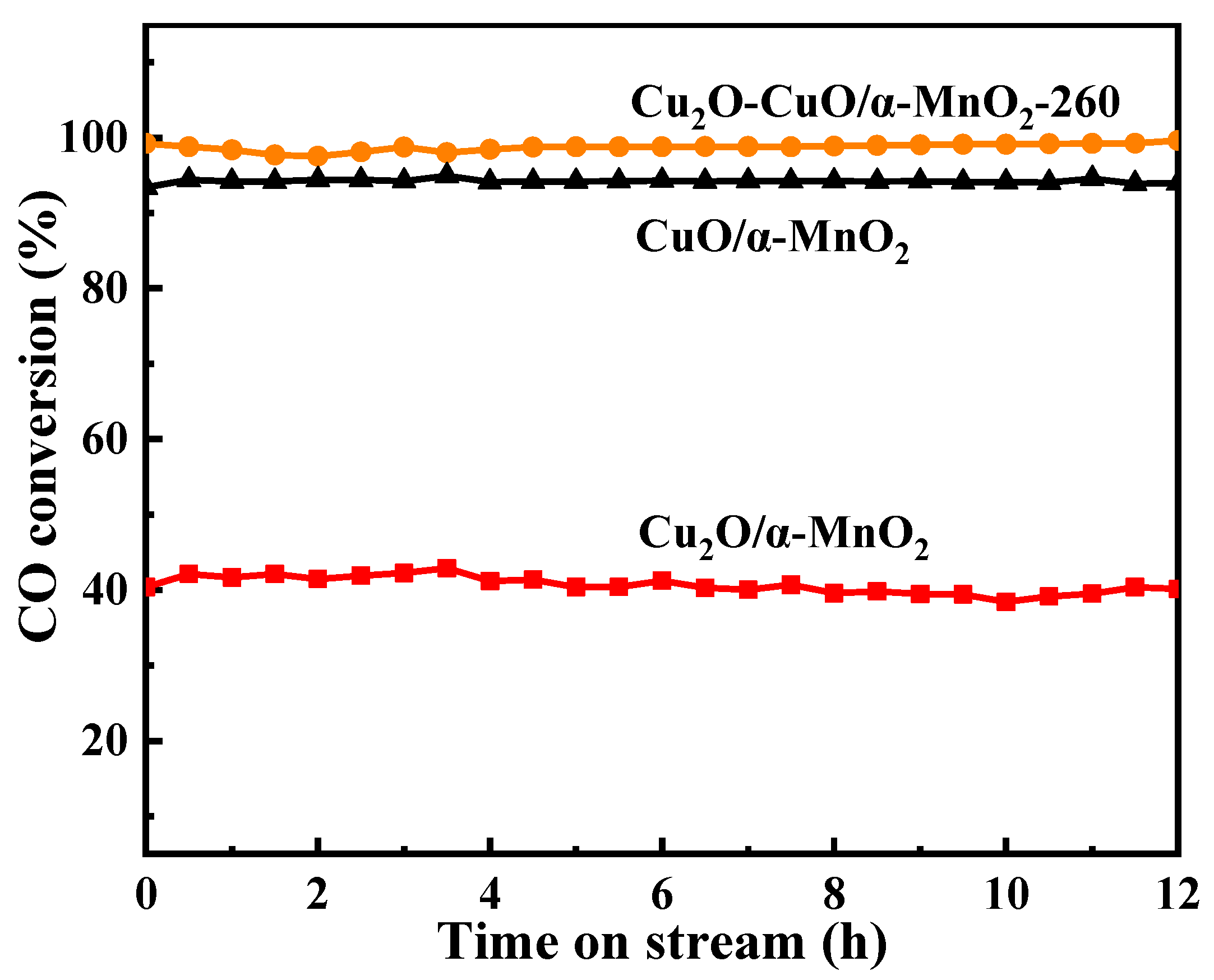
3.1.4. Long-Term Stability Test
3.2. Characterization of the Catalysts
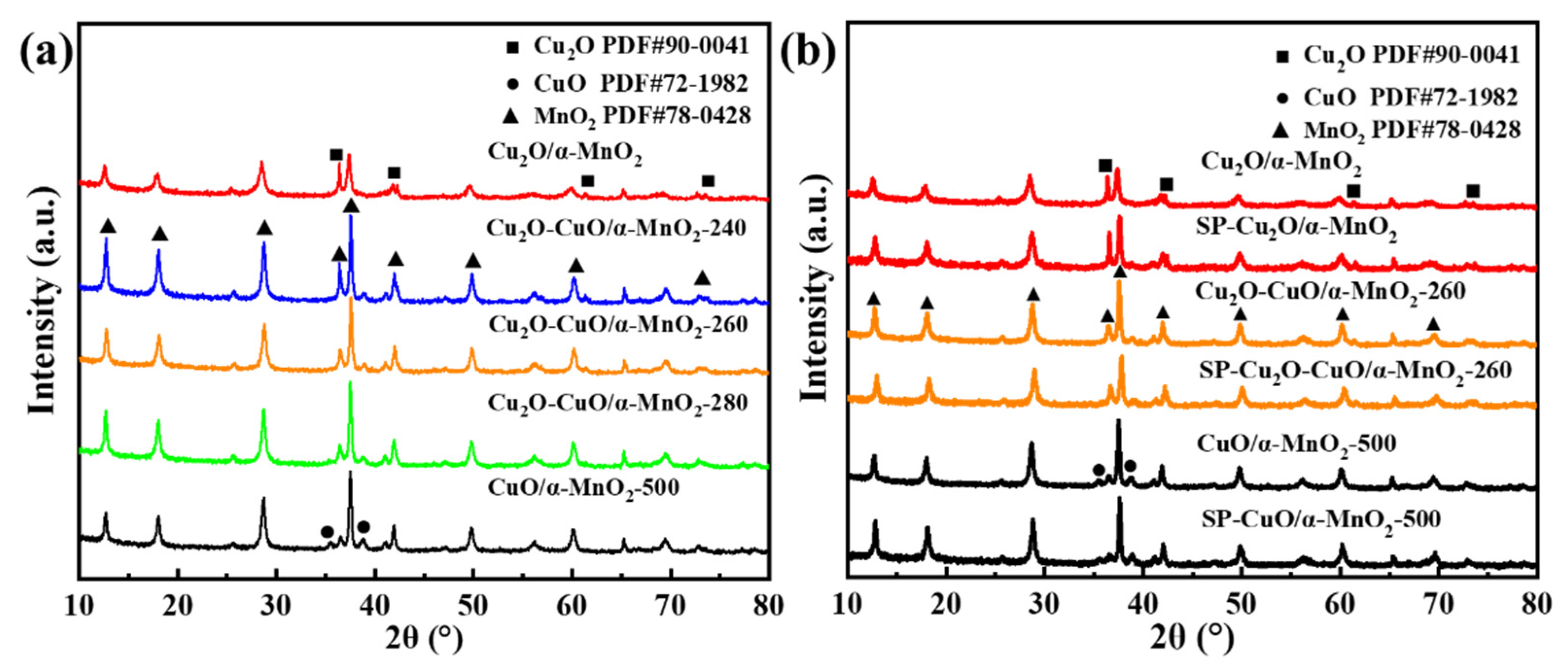
3.2.1. XRD Analysis
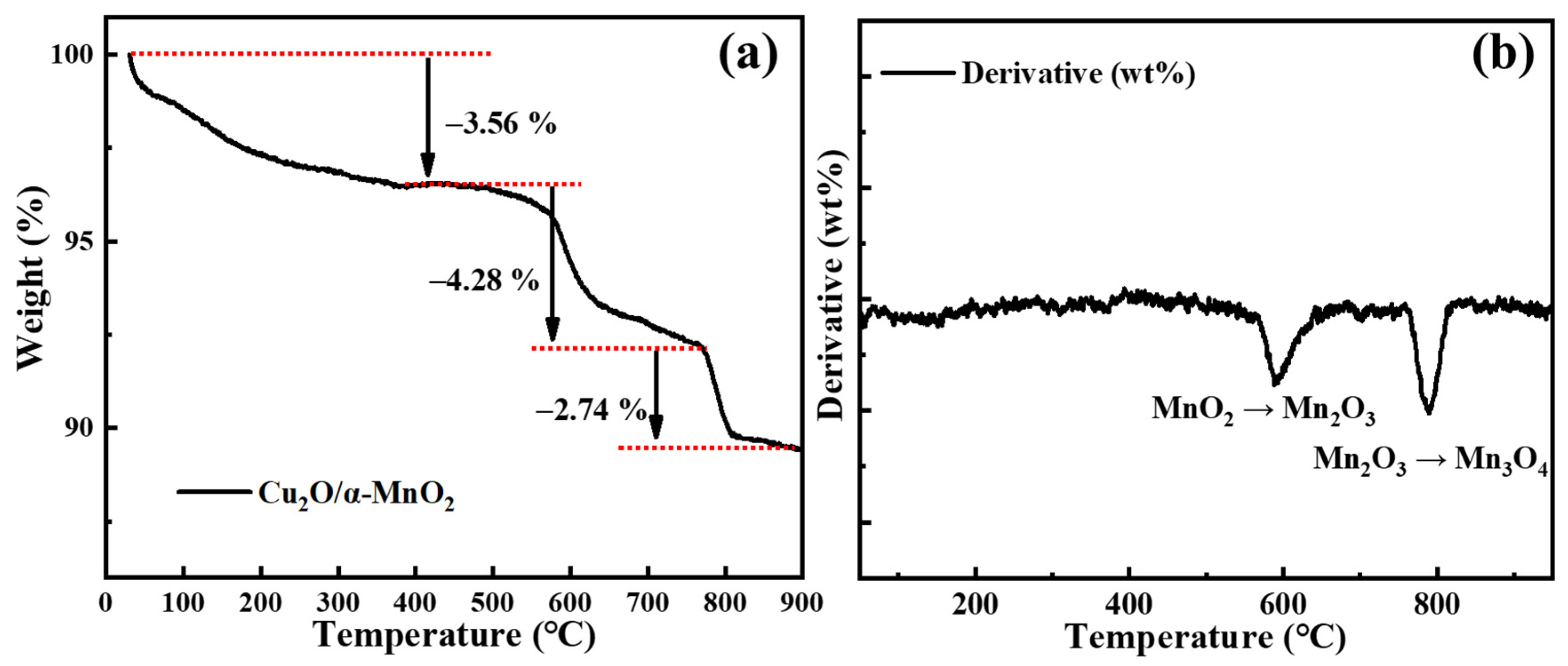
3.2.2. TG Analysis
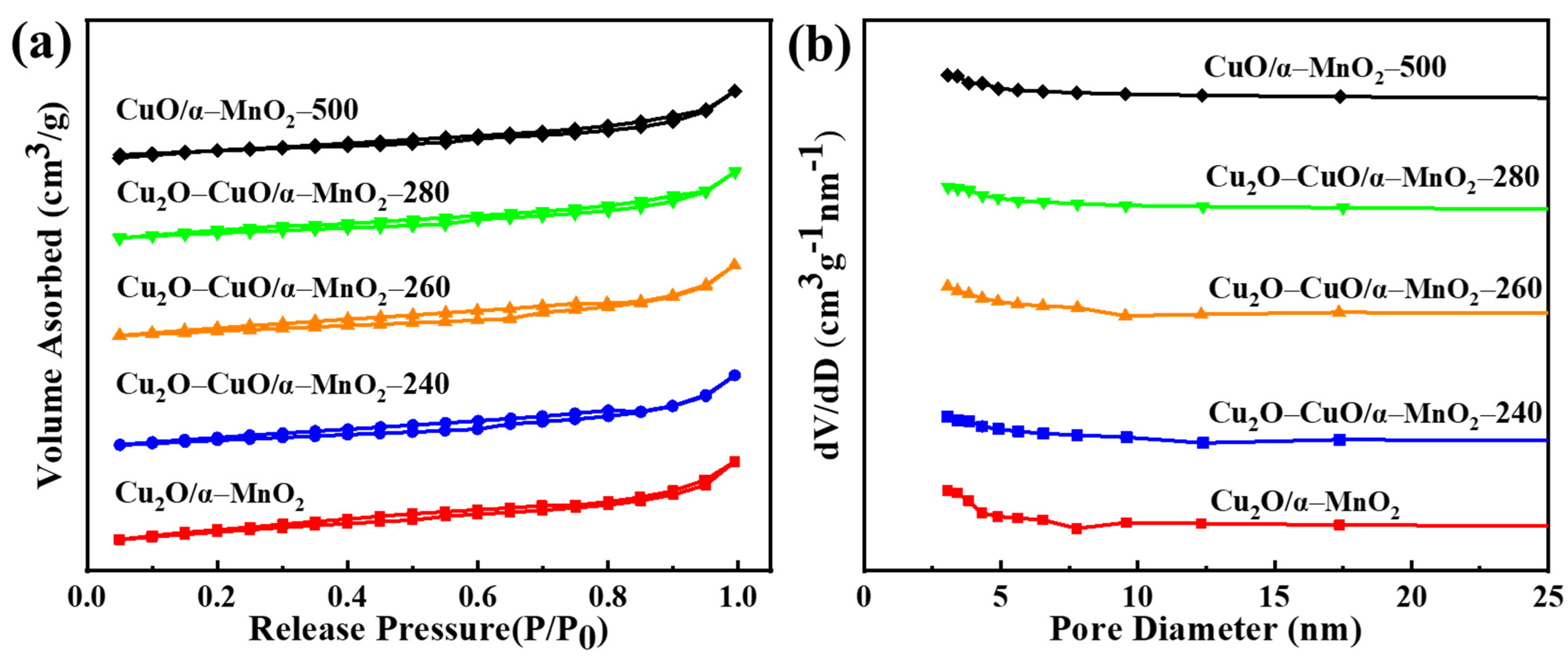
3.2.3. N2 Physisorption Analysis
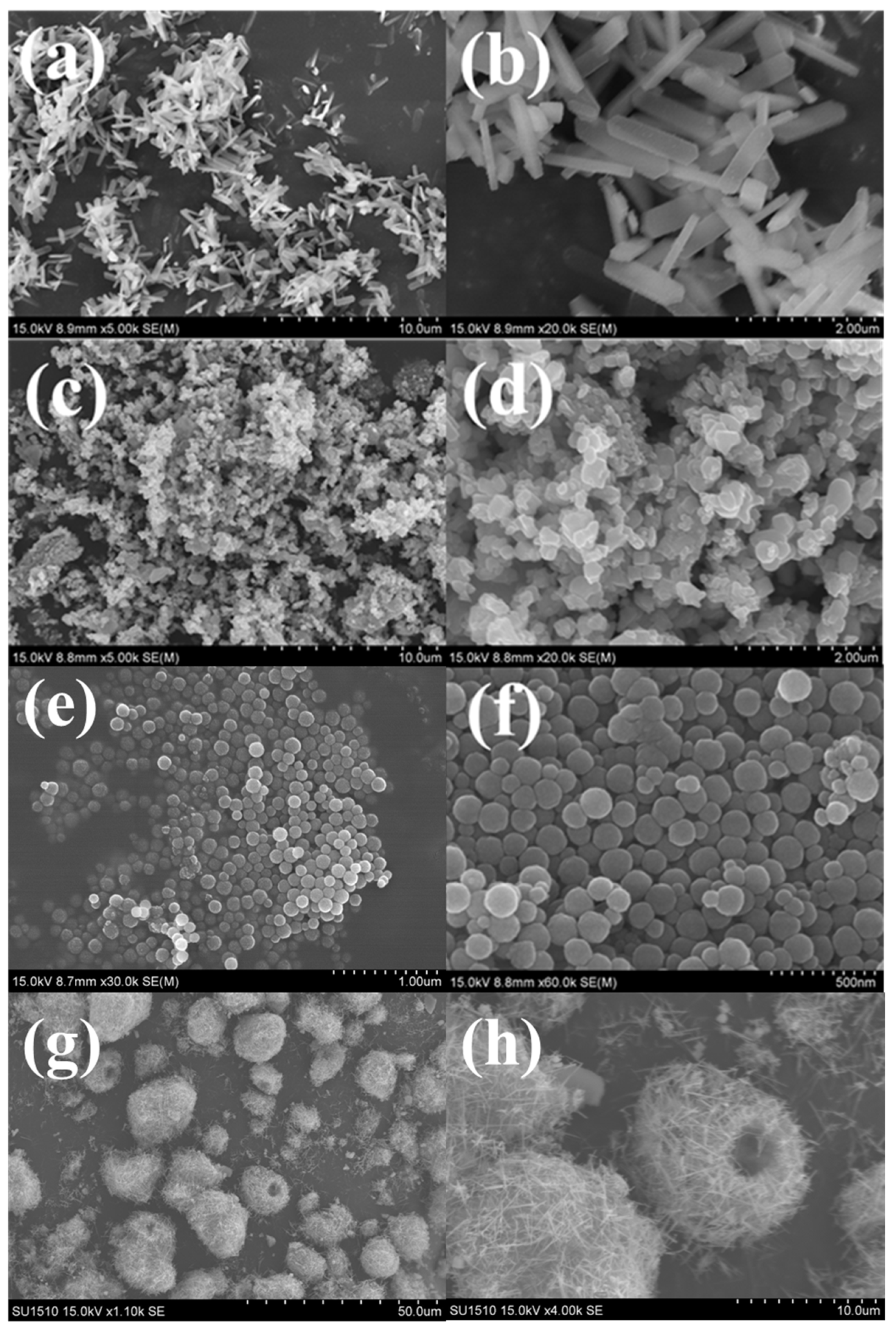
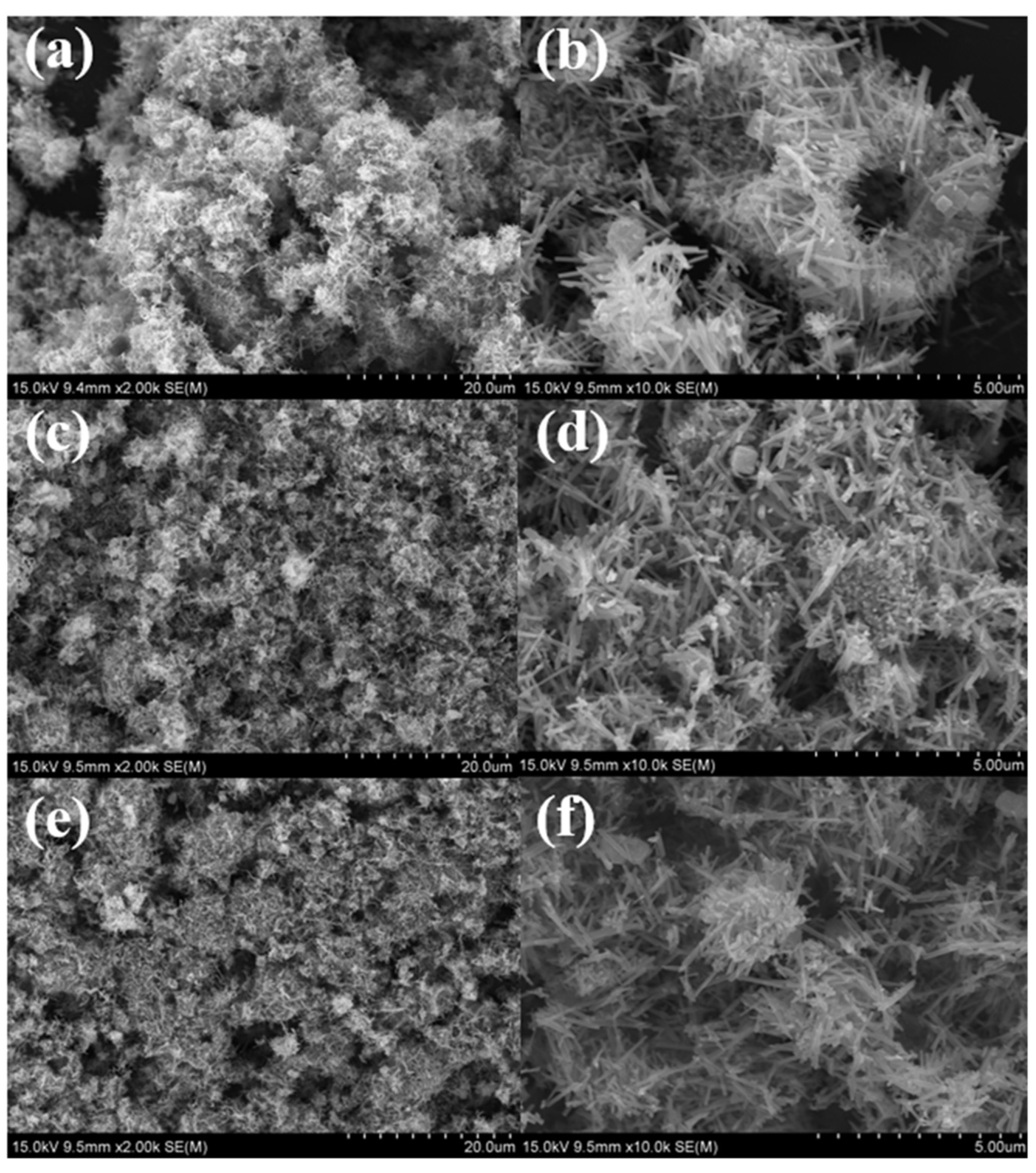
3.2.4. SEM and TEM Analyses
3.2.5. FTIR Analysis
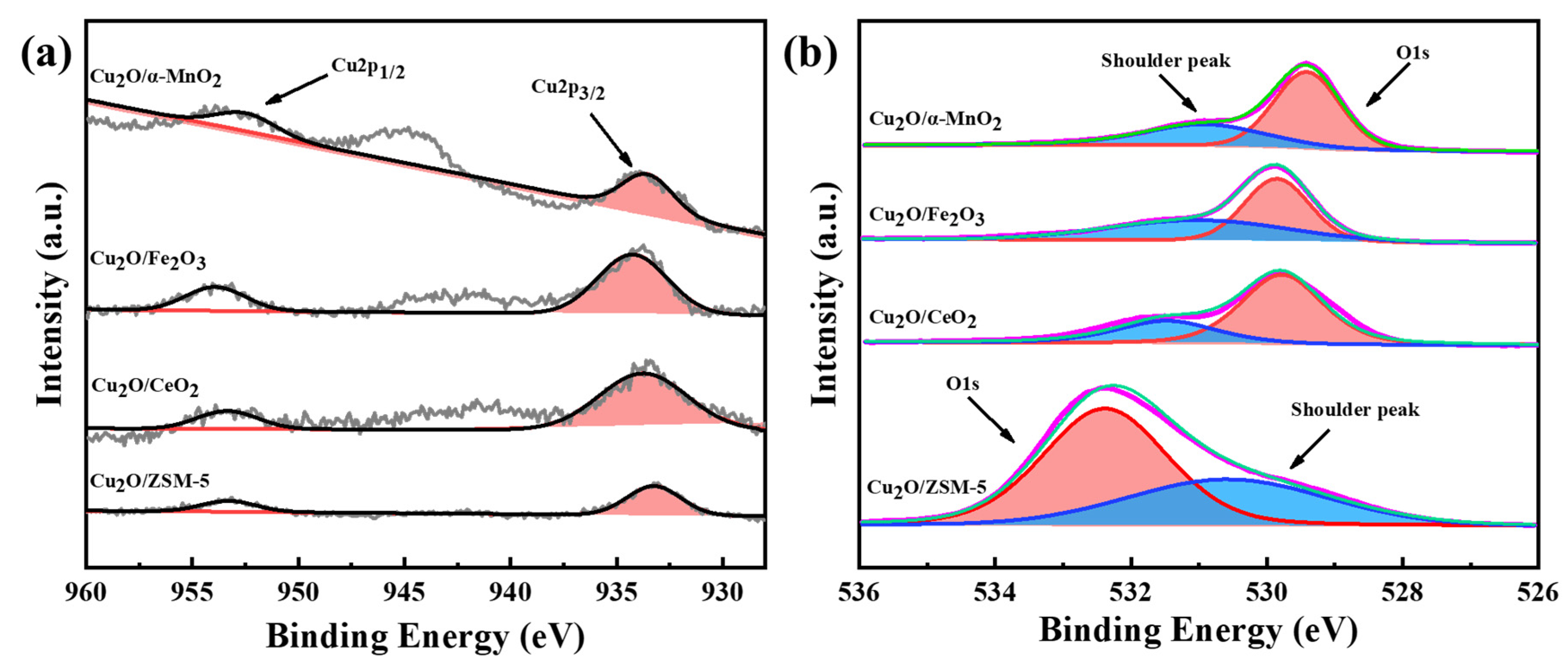
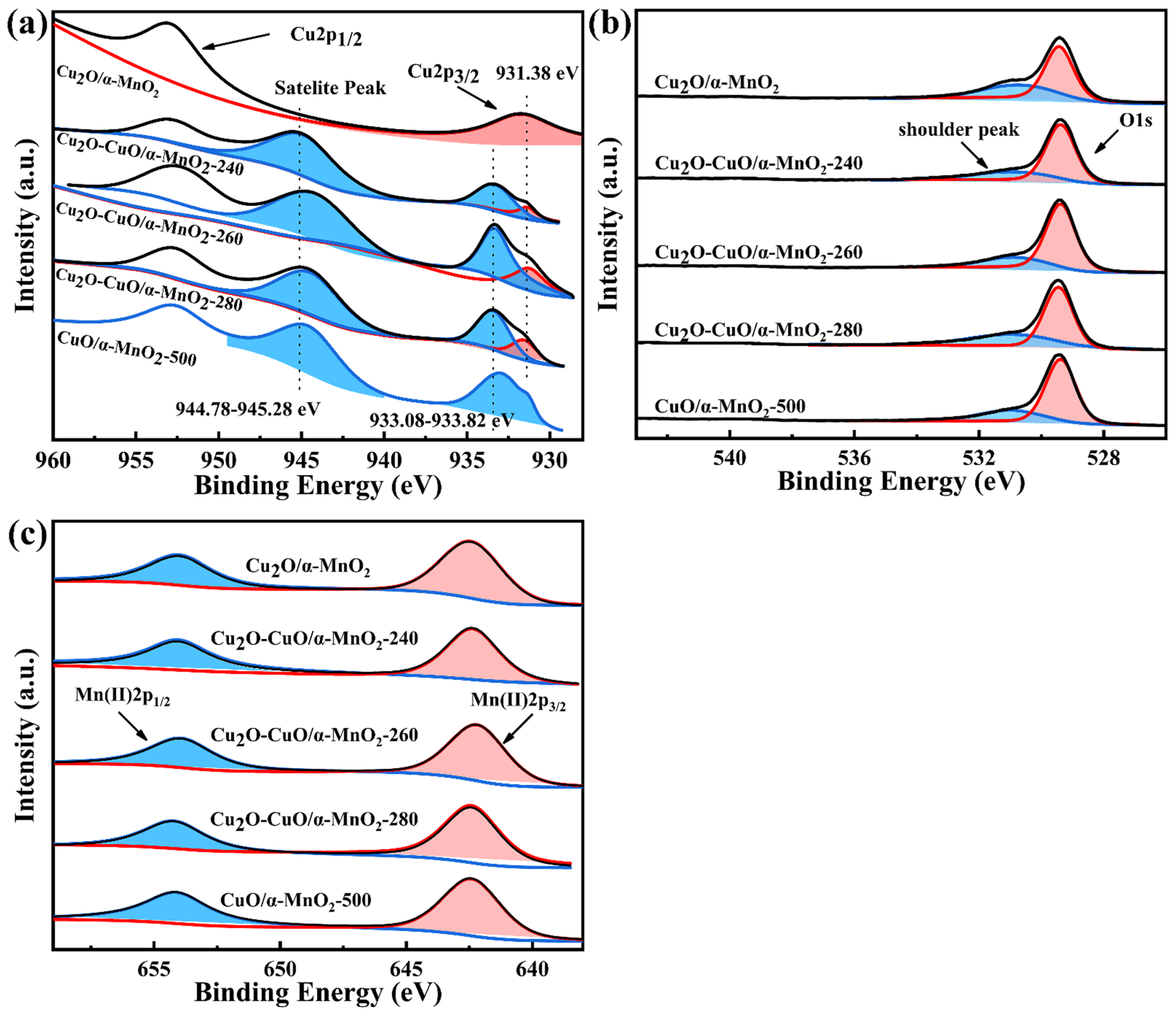
3.2.6. XPS Analysis
3.2.7. H2-TPR Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Boubnov, A.; Dahl, S.; Johnson, E.; Molina, A.P.; Simonsen, S.B.; Cano, F.M.; Helveg, S.; Lemus-Yegres, L.J.; Grunwaldt, J.-D. Structure–activity relationships of Pt/Al2O3 catalysts for CO and NO oxidation at diesel exhaust conditions. Appl. Catal. B Environ. 2012, 126, 315–325. [Google Scholar] [CrossRef]
- Lee, J.; Theis, J.R.; Kyriakidou, E.A. Vehicle emissions trapping materials: Successes, challenges, and the path forward. Appl. Catal. B Environ. 2019, 243, 397–414. [Google Scholar] [CrossRef]
- Sahu, S.; Chakraborty, N.; Sarkar, P. Coal–biomass co-combustion: An overview. Renew. Sustain. Energy Rev. 2014, 39, 575–586. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, L.; Chen, M.; Lian, X.; Wu, C.-E.; Yang, B.; Miao, Z.; Wang, F.; Hu, X. Facilely fabricating mesoporous nanocrystalline Ce–Zr solid solution supported CuO-based catalysts with advanced low-temperature activity toward CO oxidation. Catal. Sci. Technol. 2019, 9, 5605–5625. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, L.; Chen, M.; Lv, C.; Lian, X.; Wu, C.-E.; Yang, B.; Miao, Z.; Wang, F.; Hu, X. CO Oxidation over Metal Oxide (La2O3, Fe2O3, PrO2, Sm2O3, and MnO2) Doped CuO-Based Catalysts Supported on Mesoporous Ce0.8Zr0.2O2 with Intensified Low-Temperature Activity. Catalysts 2019, 9, 724. [Google Scholar] [CrossRef]
- Farrauto, R.J.; Deeba, M.; Alerasool, S. Gasoline automobile catalysis and its historical journey to cleaner air. Nat. Catal. 2019, 2, 603–613. [Google Scholar] [CrossRef]
- Shi, Y.; Xu, L.; Chen, M.; Yang, B.; Cheng, G.; Wu, C.-E.; Miao, Z.; Wang, N.; Hu, X. Fabricating Cu2O-CuO submicron-cubes for efficient catalytic CO oxidation: The significant effect of heterojunction interface. J. Ind. Eng. Chem. 2022, 105, 324–336. [Google Scholar] [CrossRef]
- Song, H.; Xu, L.; Chen, M.; Cui, Y.; Wu, C.-E.; Qiu, J.; Xu, L.; Cheng, G.; Hu, X. Recent progresses in the synthesis of MnO2 nanowire and its application in environmental catalysis. RSC Adv. 2021, 11, 35494–35513. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Y.; Lin, Y.; Zhu, T. A review of the catalysts used in the reduction of NO by CO for gas purification. Environ. Sci. Pollut. Res. 2020, 27, 6723–6748. [Google Scholar] [CrossRef]
- World Health Organization; Regional Office for Europe. Monitoring Ambient Air Quality for Health Impact Assessment; European Series; WHO Regional Publications: Geneva, Switzerland, 1999; p. 196. [Google Scholar]
- Miao, B.; Ma, S.S.K.; Wang, X.; Su, H.; Chan, S.H. Catalysis mechanisms of CO2 and CO methanation. Catal. Sci. Technol. 2016, 6, 4048–4058. [Google Scholar] [CrossRef]
- Śmiechowicz, I.; Kocemba, I.; Rogowski, J.; Czupryn, K. CO oxidation over Pt/SnO2 catalysts. React. Kinet. Mech. Catal. 2018, 124, 633–649. [Google Scholar] [CrossRef]
- Kaneti, Y.V.; Tanaka, S.; Jikihara, Y.; Nakayama, T.; Bando, Y.; Haruta, M.; Hossain, S.A.; Golberg, D.; Yamauchi, Y. Room temperature carbon monoxide oxidation based on two-dimensional gold-loaded mesoporous iron oxide nanoflakes. Chem. Commun. 2018, 54, 8514–8517. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-N.; Li, X.-N.; He, S.-G. Recent research progress in the study of catalytic CO oxidation by gas phase atomic clusters. Sci. China Mater. 2020, 63, 892–902. [Google Scholar] [CrossRef]
- Dehestaniathar, S.; Khajelakzay, M.; Ramezani-Farani, M.; Ijadpanah-Saravi, H. Modified diatomite-supported CuO–TiO2 composite: Preparation, characterization and catalytic CO oxidation. J. Taiwan Inst. Chem. Eng. 2016, 58, 252–258. [Google Scholar] [CrossRef]
- Haruta, M.; Yamada, N.; Kobayashi, T.; Iijima, S. Gold catalysts prepared by coprecipitation for low-temperature oxidation of hydrogen and of carbon monoxide. J. Catal. 1989, 115, 301–309. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.; Zhang, R.; He, D.; Liu, H.; Liao, S. Metal-organic framework as a host for synthesis of nanoscale Co3O4 as an active catalyst for CO oxidation. Catal. Commun. 2011, 12, 875–879. [Google Scholar] [CrossRef]
- El Kasmi, A.; Tian, Z.-Y.; Vieker, H.; Beyer, A.; Chafik, T. Innovative CVD synthesis of Cu2O catalysts for CO oxidation. Appl. Catal. B Environ. 2016, 186, 10–18. [Google Scholar] [CrossRef]
- Ângelo, J.; Magalhães, P.; Andrade, L.; Mendes, A. Characterization of TiO2-based semiconductors for photocatalysis by electrochemical impedance spectroscopy. Appl. Surf. Sci. 2016, 387, 183–189. [Google Scholar] [CrossRef]
- Gerasimov, G.N.; Ikim, M.; Timashev, P.; Gromov, V.F.; Belysheva, T.V.; Spiridonova, E.Y.; Bagratashvili, V.N.; Trakhtenberg, L.I. Small CeO2 clusters on the surface of semiconductor nanoparticles. Russ. J. Phys. Chem. A 2015, 89, 1059–1064. [Google Scholar] [CrossRef]
- Hoseinzadeh, S.; Ghasemiasl, R.; Bahari, A.; Ramezani, A.H. n-type WO3 semiconductor as a cathode electrochromic material for ECD devices. J. Mater. Sci. Mater. Electron. 2017, 28, 14446–14452. [Google Scholar] [CrossRef]
- Malathi, A.; Madhavan, J.; Ashokkumar, M.; Arunachalam, P. A review on BiVO4 photocatalyst: Activity enhancement methods for solar photocatalytic applications. Appl. Catal. A Gen. 2018, 555, 47–74. [Google Scholar] [CrossRef]
- Su, Y.; Lang, J.; Cao, N.; Wang, T.; Zhu, B.; Wang, X. Morphological reconstruction and photocatalytic enhancement of NaTaO3 nanocrystals via Cu2O loading. J. Nanoparticle Res. 2015, 17, 63. [Google Scholar] [CrossRef]
- Kakuta, S.; Abe, T. Photocatalytic activity of Cu2O nanoparticles prepared through novel synthesis method of precursor reduction in the presence of thiosulfate. Solid State Sci. 2009, 11, 1465–1469. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Chen, C.-H.; Huang, M.H. Seed-Mediated Synthesis of Monodispersed Cu2O Nanocubes with Five Different Size Ranges from 40 to 420 nm. Adv. Funct. Mater. 2007, 17, 3773–3780. [Google Scholar] [CrossRef]
- Wei, B.; Yang, N.; Pang, F.; Ge, J. Cu2O–CuO Hollow Nanospheres as a Heterogeneous Catalyst for Synergetic Oxidation of CO. J. Phys. Chem. C 2018, 122, 19524–19531. [Google Scholar] [CrossRef]
- Kong, J.; Xiang, Z.; Li, G.; An, T. Introduce oxygen vacancies into CeO2 catalyst for enhanced coke resistance during photothermocatalytic oxidation of typical VOCs. Appl. Catal. B Environ. 2020, 269, 118755. [Google Scholar] [CrossRef]
- Bahri, S.; Patra, T.; Sonal; Upadhyayula, S. Synergistic effect of bifunctional mesoporous ZSM-5 supported Fe-Co catalyst for selective conversion of syngas with low Ribblet ratio into synthetic fuel. Microporous Mesoporous Mater. 2019, 275, 1–13. [Google Scholar] [CrossRef]
- Shilina, M.; Rostovshchikova, T.; Nikolaev, S.; Udalova, O. Polynuclear Co-oxo cations in the catalytic oxidation of CO on Co-modified ZSM-5 zeolites. Mater. Chem. Phys. 2018, 223, 287–298. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, B.; Zhu, C.; Tian, Y. Preparation of La2NiO4/ZSM-5 catalyst and catalytic performance in CO2/CH4 reforming to syngas. Appl. Catal. A Gen. 2005, 292, 138–143. [Google Scholar] [CrossRef]
- Pang, L.; Fan, C.; Shao, L.; Song, K.; Yi, J.; Cai, X.; Wang, J.; Kang, M.; Li, T. The Ce doping Cu/ZSM-5 as a new superior catalyst to remove NO from diesel engine exhaust. Chem. Eng. J. 2014, 253, 394–401. [Google Scholar] [CrossRef]
- Zhao, K.; Tang, H.; Qiao, B.; Li, L.; Wang, J. High Activity of Au/γ-Fe2O3 for CO Oxidation: Effect of Support Crystal Phase in Catalyst Design. ACS Catal. 2015, 5, 3528–3539. [Google Scholar] [CrossRef]
- Mo, S.; Li, J.; Liao, R.; Peng, P.; Li, J.; Wu, J.; Fu, M.; Liao, L.; Shen, T.; Xie, Q.; et al. Unraveling the decisive role of surface CeO2 nanoparticles in the Pt-CeO2/MnO2 hetero-catalysts for boosting toluene oxidation: Synergistic effect of surface decorated and intrinsic O-vacancies. Chem. Eng. J. 2021, 418, 129399. [Google Scholar] [CrossRef]
- Qian, K.; Qian, Z.; Hua, Q.; Jiang, Z.; Huang, W. Structure–activity relationship of CuO/MnO2 catalysts in CO oxidation. Appl. Surf. Sci. 2013, 273, 357–363. [Google Scholar] [CrossRef]
- Chang, J.-K.; Chen, Y.-L.; Tsai, W.-T. Effect of heat treatment on material characteristics and pseudo-capacitive properties of manganese oxide prepared by anodic deposition. J. Power Sources 2004, 135, 344–353. [Google Scholar] [CrossRef]
- Jeong, Y.U.; Manthiram, A. Nanocrystalline Manganese Oxides for Electrochemical Capacitors with Neutral Electrolytes. J. Electrochem. Soc. 2002, 149, A1419–A1422. [Google Scholar] [CrossRef]
- Reddy, R.N.; Reddy, R.G. Synthesis and electrochemical characterization of amorphous MnO2 electrochemical capacitor electrode material. J. Power Sources 2004, 132, 315–320. [Google Scholar] [CrossRef]
- Zhang, D.-F.; Zhang, H.; Guo, L.; Zheng, K.; Han, X.-D.; Zhang, Z. Delicate control of crystallographic facet-oriented Cu2O nanocrystals and the correlated adsorption ability. J. Mater. Chem. 2009, 19, 5220–5225. [Google Scholar] [CrossRef]
- Golden, D.C.; Chen, C.C.; Dixon, J.B. Synthesis of Todorokite. Science 1986, 231, 717–719. [Google Scholar] [CrossRef]
- Kang, L.; Zhang, M.; Liu, Z.-H.; Ooi, K. IR spectra of manganese oxides with either layered or tunnel structures. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 67, 864–869. [Google Scholar] [CrossRef]
- Sinquin, G.; Petit, C.; Hindermann, J.; Kiennemann, A. Study of the formation of LaMO3 (M = Co, Mn) perovskites by propionates precursors: Application to the catalytic destruction of chlorinated VOCs. Catal. Today 2001, 70, 183–196. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, D.D. Removal of arsenic from water using multifunctional micro-/nano-structured MnO2 spheres and microfiltration. Chem. Eng. J. 2013, 225, 271–279. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Zhang, X.; Qu, F. Template-Free Synthesis of Porous Cu2O Nanospheres at Room Temperature and Investigation on Their Adsorption Property. J. Nanomater. 2013, 2013, 378919. [Google Scholar] [CrossRef]
- Zhou, D.-L.; Feng, J.-J.; Cai, L.-Y.; Fang, Q.-X.; Chen, J.-R.; Wang, A.-J. Facile synthesis of monodisperse porous Cu2O nanospheres on reduced graphene oxide for non-enzymatic amperometric glucose sensing. Electrochimica Acta 2014, 115, 103–108. [Google Scholar] [CrossRef]
- Lu, L.; Xu, X.; Yan, J.; Shi, F.-N.; Huo, Y. Oxygen vacancy rich Cu2O based composite material with nitrogen doped carbon as matrix for photocatalytic H2 production and organic pollutant removal. Dalton Trans. 2018, 47, 2031–2038. [Google Scholar] [CrossRef]
- Amin, I.A.; Yarmo, M.A.; Yusoff, N.I.N.; Yusoff, M.Z.; Ayatillah, A. Mesoporous Silica Sol-Gel as Catalyst for the Synthesis of Alkylpolyglucosides. Adv. Mater. Res. 2013, 620, 446–452. [Google Scholar] [CrossRef]
- Hashemi, A.; Bahari, A. Structural and dielectric characteristic of povidone–silica nanocomposite films on the Si (n) substrate. Appl. Phys. A 2017, 123, 535. [Google Scholar] [CrossRef]
- Avgouropoulos, G.; Ioannides, T. Selective CO oxidation over CuO-CeO2 catalysts prepared via the urea–nitrate combustion method. Appl. Catal. A Gen. 2003, 244, 155–167. [Google Scholar] [CrossRef]
- Chanquía, C.M.; Sapag, K.; Rodríguez-Castellón, E.; Herrero, E.R.; Eimer, G.A. Nature and Location of Copper Nanospecies in Mesoporous Molecular Sieves. J. Phys. Chem. C 2010, 114, 1481–1490. [Google Scholar] [CrossRef]
- Papavasiliou, J.; Avgouropoulos, G.; Ioannides, T. Combined steam reforming of methanol over Cu–Mn spinel oxide catalysts. J. Catal. 2007, 251, 7–20. [Google Scholar] [CrossRef]
- Thompson, T.L.; Yates, J.T.J. Surface Science Studies of the Photoactivation of TiO2—New Photochemical Processes. Chem. Rev. 2006, 106, 4428–4453. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Cai, P.; Zhang, X.; Zhang, Y.; Chen, G.; Dong, C. Cu2O templating strategy for the synthesis of octahedral Cu2O@Mn(OH)2 core–shell hierarchical structures with a superior performance supercapacitor. J. Mater. Chem. A 2018, 6, 13668–13675. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, Y.; Wang, C.-A.; Ran, R. Design and Preparation of MnO2/CeO2–MnO2 Double-Shelled Binary Oxide Hollow Spheres and Their Application in CO Oxidation. ACS Appl. Mater. Interfaces 2016, 8, 8670–8677. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-N.; Tao, L.; You, T.; Li, C.; Shan, H. Effect of sulfation on the performance of Fe2O3/Al2O3 catalyst in catalytic dehydrogenation of propane to propylene. Chem. Eng. J. 2014, 244, 145–151. [Google Scholar] [CrossRef]
- Xie, Y.; Yu, Y.; Gong, X.; Guo, Y.; Guo, Y.; Wang, Y.; Lu, G. Effect of the crystal plane figure on the catalytic performance of MnO2 for the total oxidation of propane. CrystEngComm 2015, 17, 3005–3014. [Google Scholar] [CrossRef]
- Chen, R.; Yu, J.; Xiao, W. Hierarchically porous MnO2 microspheres with enhanced adsorption performance. J. Mater. Chem. A. 2013, 1, 11682–11690. [Google Scholar] [CrossRef]
- Wu, K.; Zhou, L.; Jia, C.-J.; Sun, L.-D.; Yan, C.-H. Pt-embedded-CeO2 hollow spheres for enhancing CO oxidation performance. Mater. Chem. Front. 2017, 1, 1754–1763. [Google Scholar] [CrossRef]
- Li, T.; Krumeich, F.; Ihli, J.; Ma, Z.; Ishikawa, T.; Pinar, A.B.; van Bokhoven, J.A. Heavy atom labeling enables silanol defect visualization in silicalite-1 crystals. ChemComm 2019, 55, 482–485. [Google Scholar] [CrossRef] [Green Version]
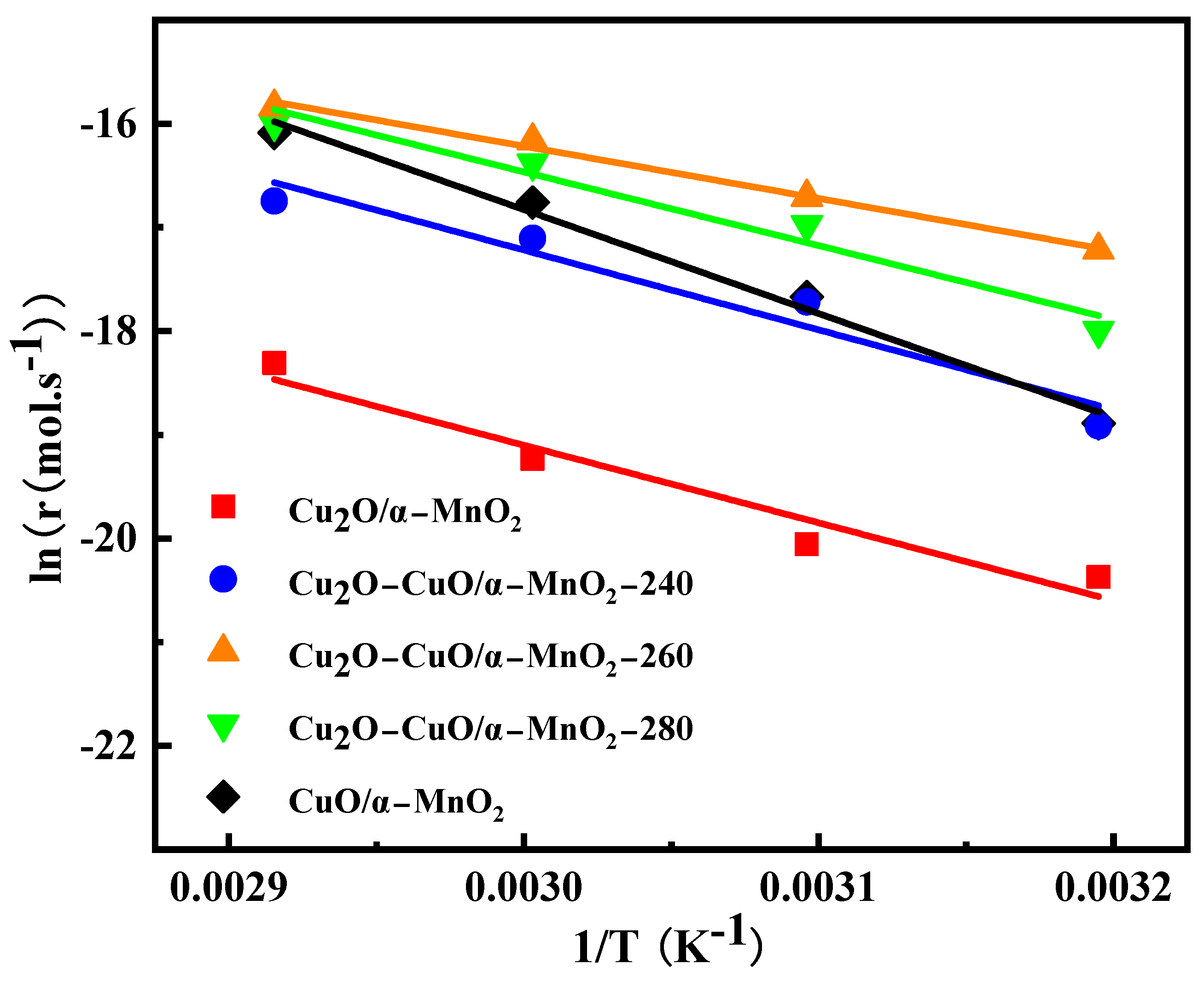


| Samples | Ea (KJ/mol) |
|---|---|
| Cu2O/α-MnO2 | 83.4 |
| Cu2O-CuO/α-MnO2-240 | 62.2 |
| Cu2O-CuO/α-MnO2-260 | 41.9 |
| Cu2O-CuO/α-MnO2-280 | 59.2 |
| CuO/α-MnO2-500 | 64.0 |
| Samples | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) | Isotherm Type |
|---|---|---|---|---|
| Cu2O/α-MnO2 | 43.64 | 0.051 | 3.05 | IV H4 |
| Cu2O-CuO/α-MnO2-240 | 28.33 | 0.048 | 3.06 | IV H4 |
| Cu2O-CuO/α-MnO2-260 | 28.82 | 0.047 | 3.06 | IV H4 |
| Cu2O-CuO/α-MnO2-280 | 26.37 | 0.045 | 3.06 | IV H4 |
| CuO/α-MnO2-500 | 10.49 | 0.045 | 3.06 | IV H4 |
| Samples | O 1s Main Peak Area | O 1s Shoulder Peak Area | O 1s Shoulder Peak Area Ratio a (%) |
|---|---|---|---|
| Cu2O/CeO2 | 283,829.4 | 132,367.2 | 31.80 |
| Cu2O/Fe2O3 | 173,370.0 | 151,701.6 | 46.67 |
| Cu2O/ZSM-5 | 653,388.9 | 391,390.7 | 37.46 |
| Cu2O/α-MnO2 | 223,908.2 | 60,458.9 | 21.26 |
| Cu2O-CuO/α-MnO2-240 | 223,952 | 88,670.38 | 28.36 |
| Cu2O-CuO/α-MnO2-260 | 222,799 | 123,070.98 | 35.58 |
| Cu2O-CuO/α-MnO2-280 | 227,844.3 | 119,566.3 | 34.42 |
| CuO/α-MnO2-500 | 248,099.8 | 98,654.84 | 28.45 |
| Samples | Cu2O Peak Area | CuO Peak Area | Satellite Peak Area | Cu2O Peak Area Percentage (%) | CuO Peak Area Percentage (%) | Cu2+/Cu1+ Relative Ratio |
|---|---|---|---|---|---|---|
| Cu2O/CeO2 | 58,985.93 | 0 | 0 | 100.0 | 0 | 0 |
| Cu2O/Fe2O3 | 23,117.54 | 0 | 0 | 100.0 | 0 | 0 |
| Cu2O/ZSM-5 | 15,803.46 | 0 | 0 | 100.0 | 0 | 0 |
| Cu2O/α-MnO2 | 28,836.83 | 0 | 0 | 100.0 | 0 | 0 |
| Cu2O-CuO/α-MnO2-240 | 8059.77 | 16,647.28 | 33,992.70 | 13.7 | 86.3 | 6.30 |
| Cu2O-CuO/α-MnO2-260 | 8803.58 | 36,178.22 | 49,118.25 | 9.4 | 90.6 | 9.64 |
| Cu2O-CuO/α-MnO2-280 | 7076.77 | 16,647.28 | 54,040.24 | 9.1 | 90.9 | 9.99 |
| CuO/α-MnO2-500 | 0 | 55,346.21 | 96,344.52 | 0.0 | 100 | ∞ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, F.; Shi, Y.; Xu, L.; Chen, M.; Xue, Y.; Wu, C.-E.; Qiu, J.; Cheng, G.; Xu, J.; Hu, X. Designing Highly Efficient Cu2O-CuO Heterojunction CO Oxidation Catalysts: The Roles of the Support Type and Cu2O-CuO Interface Effect. Nanomaterials 2022, 12, 3020. https://doi.org/10.3390/nano12173020
Zhao F, Shi Y, Xu L, Chen M, Xue Y, Wu C-E, Qiu J, Cheng G, Xu J, Hu X. Designing Highly Efficient Cu2O-CuO Heterojunction CO Oxidation Catalysts: The Roles of the Support Type and Cu2O-CuO Interface Effect. Nanomaterials. 2022; 12(17):3020. https://doi.org/10.3390/nano12173020
Chicago/Turabian StyleZhao, Fen, Yiyu Shi, Leilei Xu, Mindong Chen, Yingying Xue, Cai-E Wu, Jian Qiu, Ge Cheng, Jingxin Xu, and Xun Hu. 2022. "Designing Highly Efficient Cu2O-CuO Heterojunction CO Oxidation Catalysts: The Roles of the Support Type and Cu2O-CuO Interface Effect" Nanomaterials 12, no. 17: 3020. https://doi.org/10.3390/nano12173020
APA StyleZhao, F., Shi, Y., Xu, L., Chen, M., Xue, Y., Wu, C.-E., Qiu, J., Cheng, G., Xu, J., & Hu, X. (2022). Designing Highly Efficient Cu2O-CuO Heterojunction CO Oxidation Catalysts: The Roles of the Support Type and Cu2O-CuO Interface Effect. Nanomaterials, 12(17), 3020. https://doi.org/10.3390/nano12173020








