Evaluation of Dosimetric Effect of Bone Scatter on Nanoparticle-Enhanced Orthovoltage Radiotherapy: A Monte Carlo Phantom Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Monte Carlo Simulation
2.2. Simulation Model and Geomtry
2.3. Dose Enhancement Ratio (DER)
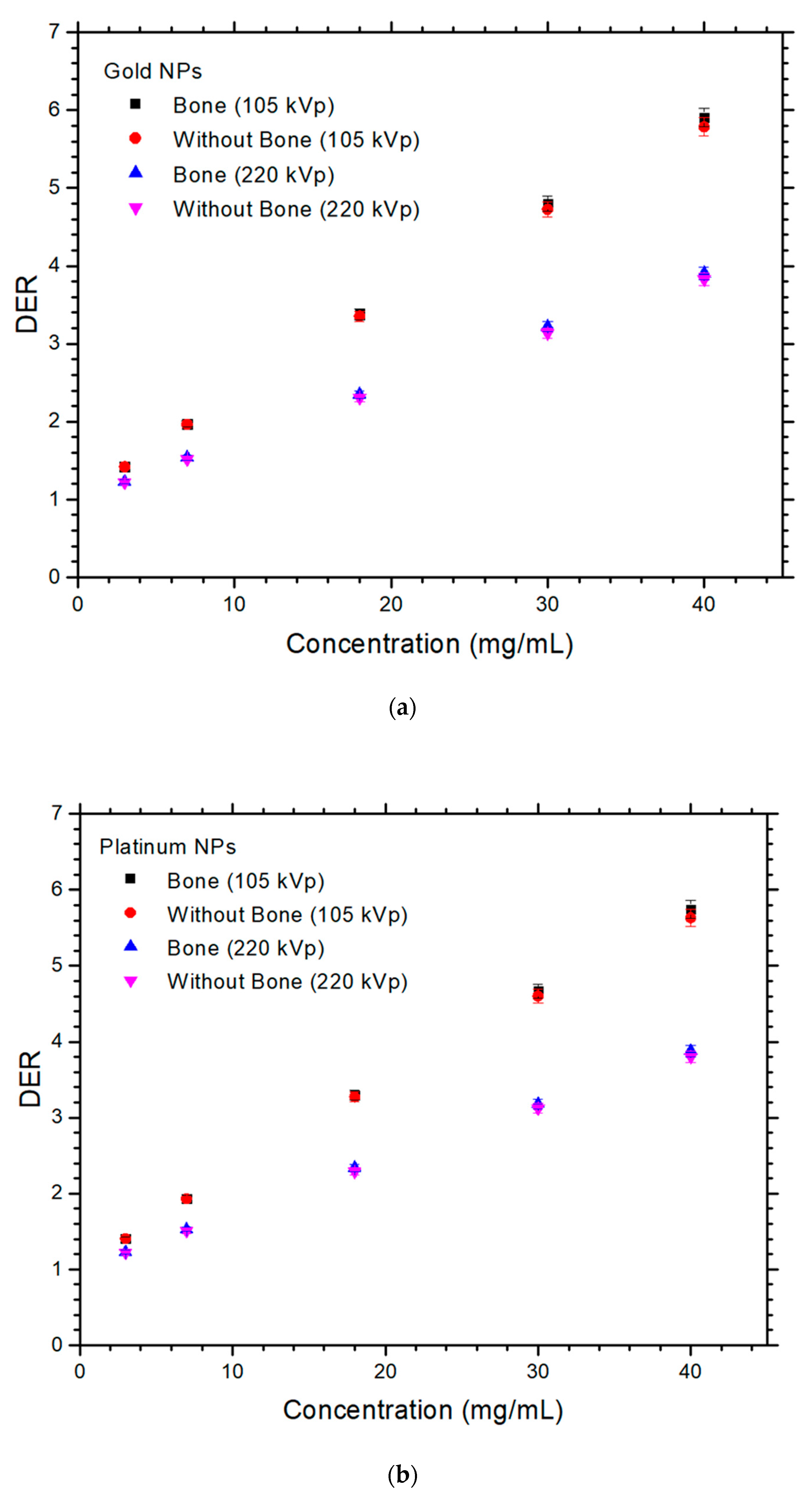
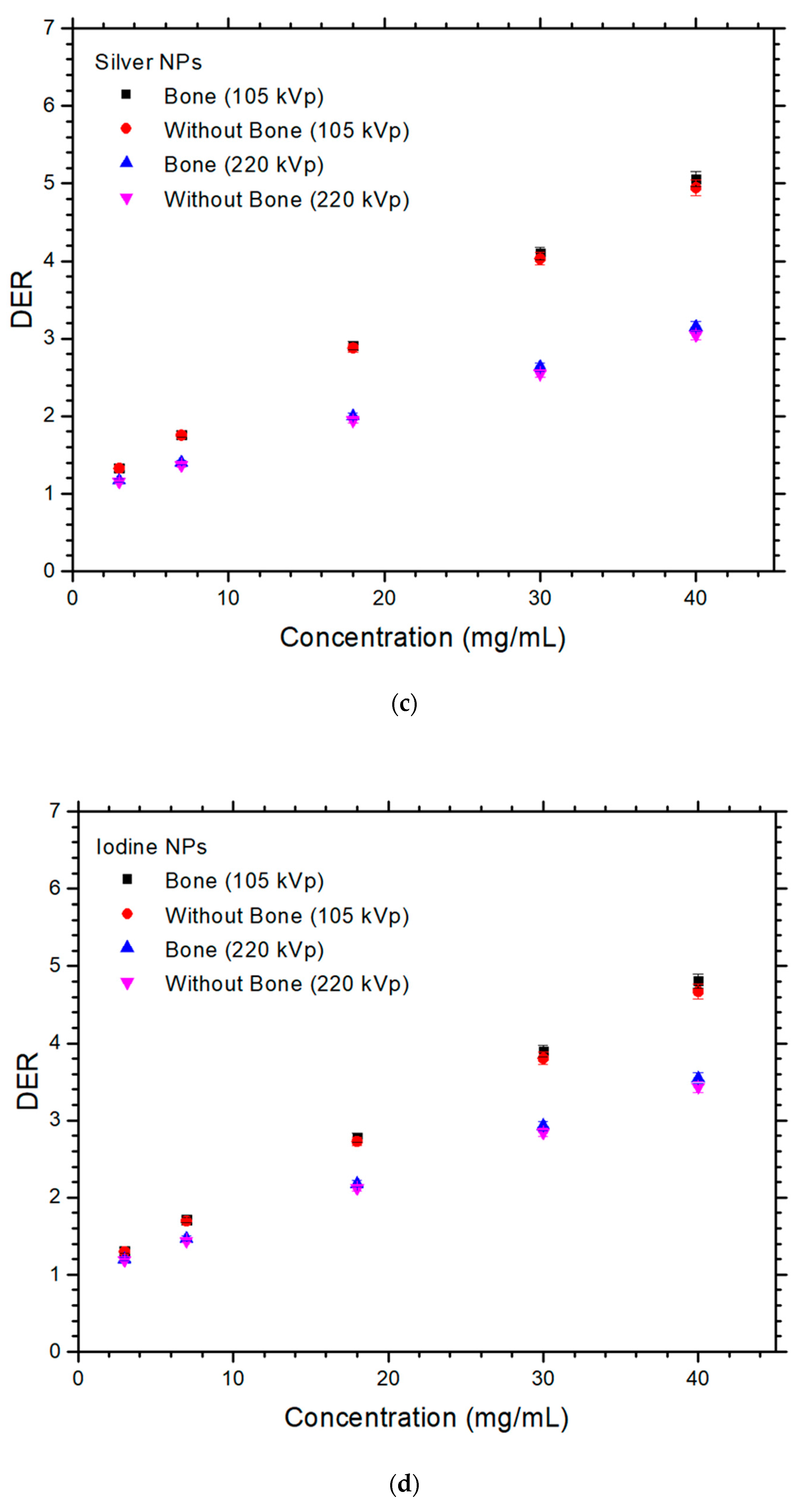
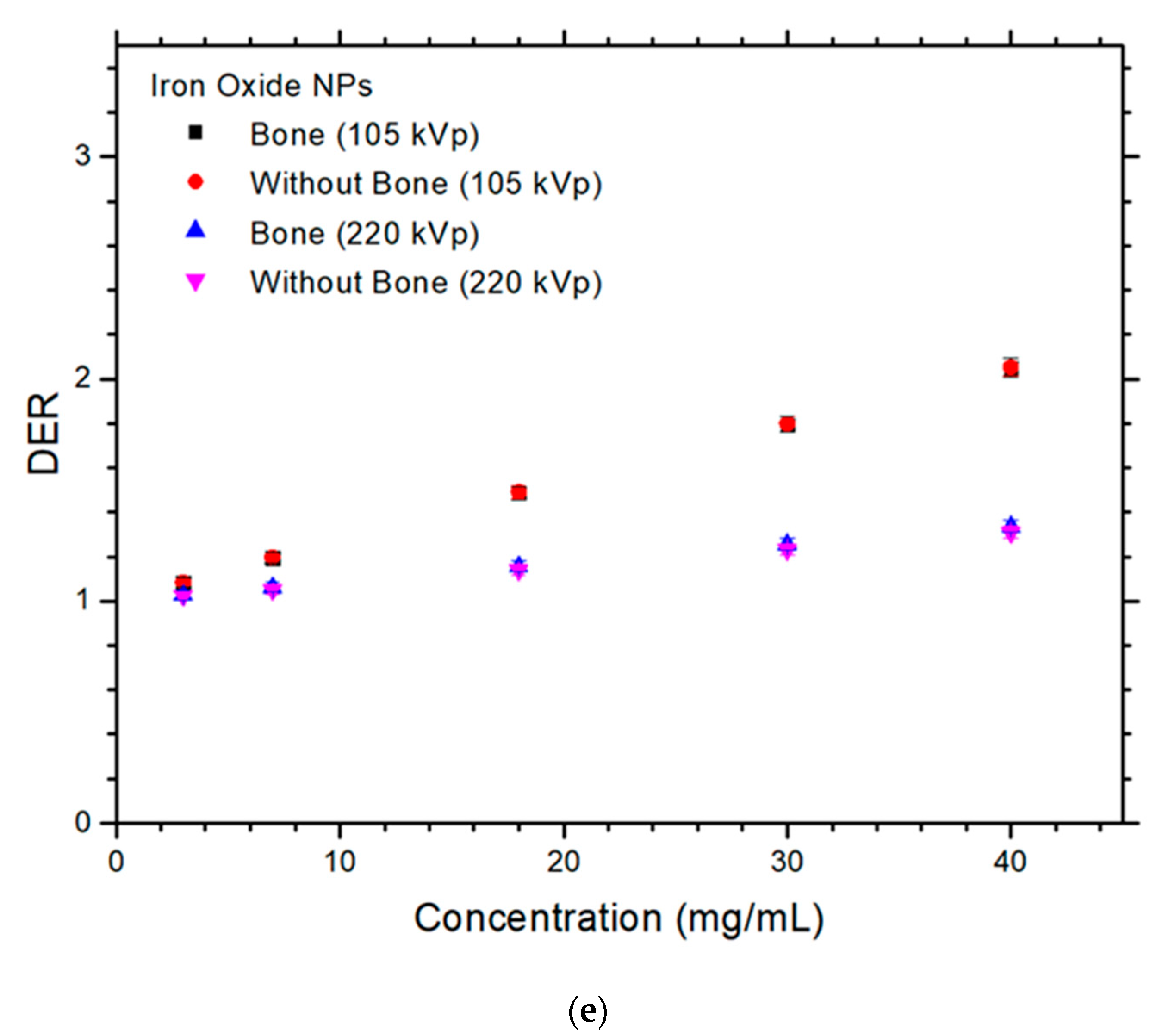
3. Results
4. Discussion
4.1. Dependences of DER on the NP and NP Concentration
4.2. Dependences of DER on the Presence of Bone and Photon Beam Energy
4.3. Difference of DER with and without Presence of Bone
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siddique, S.; Chow, J.C.L. Application of Nanomaterials in Biomedical Imaging and Cancer Therapy. Nanomaterials 2020, 10, 1700. [Google Scholar] [CrossRef]
- Chithrani, D.B.; Jelveh, S.; Jalali, F.; Van Prooijen, M.; Allen, C.; Bristow, R.G.; Hill, R.P.; Jaffray, D.A. Gold nanoparticles as radiation sensitizers in cancer therapy. Radiat. Res. 2010, 173, 719–728. [Google Scholar] [CrossRef]
- Abdulle, A.; Chow, J.C.L. Contrast enhancement for portal imaging in nanoparticle-enhanced radiotherapy: A Monte Carlo phantom evaluation using flattening-filter-free photon beams. Nanomaterials 2019, 9, 920. [Google Scholar] [CrossRef]
- Albayedh, F.; Chow, J.C.L. Monte Carlo simulation on the imaging contrast enhancement in nanoparticle-enhanced radiotherapy. J. Med. Phys. 2018, 43, 195–199. [Google Scholar]
- Chow, J.C.L. Application of Nanoparticle Materials in Radiation Therapy. In Handbook of Ecomaterials; Torres Martinez, L.M., Vasilievna Kharissova, O., Ildusovich Kharisov, B., Eds.; Springer Nature: Cham, Switzerland, 2017; Chapter 150; pp. 3661–3681. [Google Scholar]
- Retif, P.; Pinel, S.; Toussaint, M.; Frochot, C.; Chouikrat, R.; Bastogne, T.; Barberi-Heyob, M. Nanoparticles for radiation therapy enhancement: The key parameters. Theranostics 2015, 5, 1030. [Google Scholar] [CrossRef]
- Regulla, D.F.; Hieber, L.B.; Seidenbusch, M. Physical and biological interface dose effects in tissue due to X-ray-induced release of secondary radiation from metallic gold surfaces. Radiat. Res. 1998, 150, 92–100. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Slatkin, D.N.; Smilowitz, H.M. The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol. 2004, 49, N309. [Google Scholar] [CrossRef]
- Moore, J.; Chow, J.C.L. Recent progress and applications of gold nanotechnology in medical biophysics using artificial intelligence and mathematical modeling. Nano Express 2021, 2, 022001. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C.L. Gold nanoparticles for drug delivery and cancer therapy. Appl. Sci. 2020, 10, 3824. [Google Scholar] [CrossRef]
- Karathanasis, E.; Suryanarayanan, S.; Balusu, S.R.; McNeeley, K.; Sechopoulos, I.; Karellas, A.; Annapragada, A.V.; Bellamkonda, R.V. Imaging nanoprobe for prediction of outcome of nanoparticle chemotherapy by using mammography. Radiology 2009, 250, 398–406. [Google Scholar] [CrossRef]
- Oh, N.; Park, J.H. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. J. Nanomed. 2014, 9 (Suppl. 1), 51. [Google Scholar]
- Hainfeld, J.F.; Dilmanian, F.A.; Zhong, Z.; Slatkin, D.N.; Kalef-Ezra, J.A.; Smilowitz, H.M. Gold nanoparticles enhance the radiation therapy of a murine squamous cell carcinoma. Phys. Med. Biol. 2010, 55, 3045. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H. Estimation of tumour dose enhancement due to gold nanoparticles during typical radiation treatments: A preliminary Monte Carlo study. Phys. Med. Biol. 2005, 50, N163. [Google Scholar] [CrossRef]
- Huynh, N.H.; Chow, J.C.L. DNA dosimetry with gold nanoparticle irradiated by proton beams: A Monte Carlo study on dose enhancement. Appl. Sci. 2021, 11, 10856. [Google Scholar] [CrossRef]
- Jabeen, M.; Chow, J.C.L. Gold Nanoparticle DNA Damage by Photon Beam in a Magnetic Field: A Monte Carlo Study. Nanomaterials 2021, 11, 1751. [Google Scholar] [CrossRef]
- Haume, K.; Rosa, S.; Grellet, S.; Śmiałek, M.A.; Butterworth, K.T.; Solov’yov, A.V.; Prise, K.M.; Golding, J.; Mason, N.J. Gold nanoparticles for cancer radiotherapy: A review. Cancer Nanotechnol. 2016, 7, 8. [Google Scholar] [CrossRef]
- Peukert, D.; Kempson, I.; Douglass, M.; Bezak, E. Metallic nanoparticle radiosensitisation of ion radiotherapy: A review. Phys. Med. 2018, 47, 121–128. [Google Scholar] [CrossRef]
- Zheng, X.J.; Chow, J.C.L. Radiation dose enhancement in skin therapy with nanoparticle addition: A Monte Carlo study on kilovoltage photon and megavoltage electron beams. World J. Radiol. 2017, 9, 63–71. [Google Scholar] [CrossRef]
- Radiation: Ultraviolet (UV) Radiation and Skin Cancer. Available online: https://www.who.int/news-room/questions-and-answers/item/radiation-ultraviolet-(uv)-radiation-and-skin-cancer (accessed on 1 June 2022).
- Fischbach, A.J.; Sause, W.T.; Plenk, H.P. Radiation therapy for skin cancer. West. J. Med. 1980, 133, 379. [Google Scholar]
- Chen, Z.; Chow, J.C.L.; Sun, A.; Nagar, H.; Stevens, K.R.; Knisely, J.P.S. Cancers of the Skin, Including Mycosis Fungoides. In Khan’s Treatment Planning in Radiation Oncology; Khan, F.M., Sperduto, P.W., Gibbons, J.P., Eds.; Wolters Kluwer Health: Philadelphia, PA, USA, 2022; Chapter 13; pp. 284–309. [Google Scholar]
- Pearse, J.; Chow, J.C.L. An Internet of Things App for Monitor Unit Calculation in Superficial and Orthovoltage Skin Therapy. IOP SciNotes 2020, 1, 014002. [Google Scholar] [CrossRef]
- Butson, M.J.; Cheung, T.; Peter, K.N. Measurement of dose reductions for superficial x-rays backscattered from bone interfaces. Phys. Med. Biol. 2008, 53, N329. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.L. Recent progress in Monte Carlo simulation on gold nanoparticle radiosensitization. AIMS Biophys. 2018, 5, 231–244. [Google Scholar] [CrossRef]
- Sheeraz, Z.; Chow, J.C.L. Evaluation of dose enhancement with gold nanoparticles in kilovoltage radiotherapy using the new EGS geometry library in Monte Carlo simulation. AIMS Biophys. 2021, 8, 337–345. [Google Scholar] [CrossRef]
- Sakata, D.; Kyriakou, I.; Tran, H.N.; Bordage, M.C.; Rosenfeld, A.; Ivanchenko, V.; Incerti, S.; Emfietzoglou, D.; Guatelli, S. Electron track structure simulations in a gold nanoparticle using Geant4-DNA. Phys. Med. 2019, 63, 98–104. [Google Scholar] [CrossRef]
- Chun, H.; Chow, J.C.L. Gold nanoparticle DNA damage in radiotherapy: A Monte Carlo study. AIMS Bioeng. 2016, 3, 352–361. [Google Scholar]
- Sharma, M.; Chow, J.C.L. Skin dose enhancement from the application of skin-care creams using FF and FFF photon beams in radiotherapy: A Monte Carlo phantom evaluation. AIMS Bioeng. 2020, 7, 82–90. [Google Scholar] [CrossRef]
- Sotiropoulos, M.; Henthorn, N.T.; Warmenhoven, J.W.; Mackay, R.I.; Kirkby, K.J.; Merchant, M.J. Modelling direct DNA damage for gold nanoparticle enhanced proton therapy. Nanoscale 2017, 9, 18413–18422. [Google Scholar] [CrossRef]
- Chow, J.C.L. A performance evaluation on Monte Carlo simulation for radiation dosimetry using cell processor. J. Comp. Meth. Sci. Eng. 2011, 11, 1–12. [Google Scholar] [CrossRef]
- McMahon, S.J.; Hyland, W.B.; Muir, M.F.; Coulter, J.A.; Jain, S.; Butterworth, K.T.; Schettino, G.; Dickson, G.R.; Hounsell, A.R.; O’sullivan, J.M.; et al. Biological consequences of nanoscale energy deposition near irradiated heavy atom nanoparticles. Sci. Rep. 2011, 1, 18. [Google Scholar] [CrossRef]
- Sung, W.; Ye, S.J.; McNamara, A.L.; McMahon, S.J.; Hainfeld, J.; Shin, J.; Smilowitz, H.M.; Paganetti, H.; Schuemann, J. Dependence of gold nanoparticle radiosensitization on cell geometry. Nanoscale 2017, 9, 5843–5853. [Google Scholar] [CrossRef]
- Rogers, D.W.; Kawrakow, I.; Seuntjens, J.P.; Walters, B.R.; Mainegra-Hing, E. NRC User Codes for EGSnrc; NRCC Report PIRS-702 (Rev. B); NRCC: Ottawa, ON, Canada, 2003. [Google Scholar]
- Rogers, D.W. Fifty years of Monte Carlo simulations for medical physics. Phys. Med. Biol. 2006, 51, R287. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Hill, R.; Kuncic, Z. An evaluation of calculation parameters in the EGSnrc/BEAMnrc Monte Carlo codes and their effect on surface dose calculation. Phys. Med. Biol. 2012, 57, N267. [Google Scholar] [CrossRef] [PubMed]
- Moradi, F.; Saraee, K.R.; Sani, S.A.; Bradley, D.A. Metallic nanoparticle radiosensitization: The role of Monte Carlo simulations towards progress. Rad. Phys. Chem. 2021, 180, 109294. [Google Scholar] [CrossRef]
- Chow, J.C.L.; Owrangi, A.M. Surface dose reduction from bone interface in kilovoltage x-ray radiation therapy: A Monte Carlo study of photon spectra. J. Appl. Clin. Med. Phys. 2012, 13, 215–222. [Google Scholar] [CrossRef]
- Rogers, D.W.; Walters, B.; Kawrakow, I. BEAMnrc Users Manual; NRC Report Pirs.; NRCC: Ottawa, ON, Canada, 2009; Volume 509, p. 12. [Google Scholar]
- Walters, B.R.; Kawrakow, I.; Rogers, D.W. DOSXYZnrc Users Manual; NRC Report Pirs.; NRCC: Ottawa, ON, Canada, 2005; Volume 794, pp. 57–58. [Google Scholar]
- Chow, J.C.L. Dose Enhancement Effect in Radiotherapy: Adding Gold Nanoparticle to Tumour in Cancer Treatment. In Nanostructures for Cancer Therapy; Ficai, A., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Chapter 15; pp. 383–400. [Google Scholar]
- Cooper, D.R.; Bekah, D.; Nadeau, J.L. Gold nanoparticles and their alternatives for radiation therapy enhancement. Front. Chem. 2014, 2, 86. [Google Scholar] [CrossRef]
- Leung, M.K.; Chow, J.C.L.; Chithrani, B.D.; Lee, M.J.; Oms, B.; Jaffray, D.A. Irradiation of gold nanoparticles by x-rays: Monte Carlo simulation of dose enhancements and the spatial properties of the secondary electrons production. Med. Phys. 2011, 38, 624–631. [Google Scholar] [CrossRef]
- Chow, J.C.L. Depth dose enhancement on flattening-filter-free photon beam: A Monte Carlo study in nanoparticle-enhanced radiotherapy. Appl. Sci. 2020, 10, 7052. [Google Scholar] [CrossRef]

| Photon Beam Energy | 105 kVp | 220 kVp | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (mg/mL) | Gold NP | Platinum NP | Silver NP | Iodine NP | Iron Oxide NP | Gold NP | Platinum NP | Silver NP | Iodine NP | Iron Oxide NP |
| 3 | 0.04 | 0.06 | 0.16 | 0.37 | 0.06 | 0.77 | 0.61 | 1.55 | 1.17 | 0.40 |
| 7 | 0.18 | 0.14 | 0.43 | 0.93 | 0.14 | 1.27 | 1.38 | 2.32 | 1.87 | 0.95 |
| 18 | 0.75 | 0.71 | 1.06 | 1.74 | 0.29 | 1.94 | 2.07 | 2.77 | 2.51 | 1.49 |
| 30 | 1.49 | 1.46 | 1.67 | 2.33 | 0.47 | 2.61 | 2.86 | 3.29 | 2.98 | 1.93 |
| 40 | 2.08 | 2.01 | 2.22 | 2.79 | 0.62 | 2.98 | 3.08 | 3.55 | 3.23 | 2.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadiq, A.; Chow, J.C.L. Evaluation of Dosimetric Effect of Bone Scatter on Nanoparticle-Enhanced Orthovoltage Radiotherapy: A Monte Carlo Phantom Study. Nanomaterials 2022, 12, 2991. https://doi.org/10.3390/nano12172991
Sadiq A, Chow JCL. Evaluation of Dosimetric Effect of Bone Scatter on Nanoparticle-Enhanced Orthovoltage Radiotherapy: A Monte Carlo Phantom Study. Nanomaterials. 2022; 12(17):2991. https://doi.org/10.3390/nano12172991
Chicago/Turabian StyleSadiq, Afia, and James C. L. Chow. 2022. "Evaluation of Dosimetric Effect of Bone Scatter on Nanoparticle-Enhanced Orthovoltage Radiotherapy: A Monte Carlo Phantom Study" Nanomaterials 12, no. 17: 2991. https://doi.org/10.3390/nano12172991
APA StyleSadiq, A., & Chow, J. C. L. (2022). Evaluation of Dosimetric Effect of Bone Scatter on Nanoparticle-Enhanced Orthovoltage Radiotherapy: A Monte Carlo Phantom Study. Nanomaterials, 12(17), 2991. https://doi.org/10.3390/nano12172991







