Assembly of Hydrophobic ZIF-8 on CeO2 Nanorods as High-Efficiency Catalyst for Electrocatalytic Nitrogen Reduction Reaction
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of CeO2
2.2. Synthesis of CeO2-ZIF-8
2.3. Synthesis of CeO2-ZIF-8 on Carbon Paper (CPs)
3. Results and Discussion
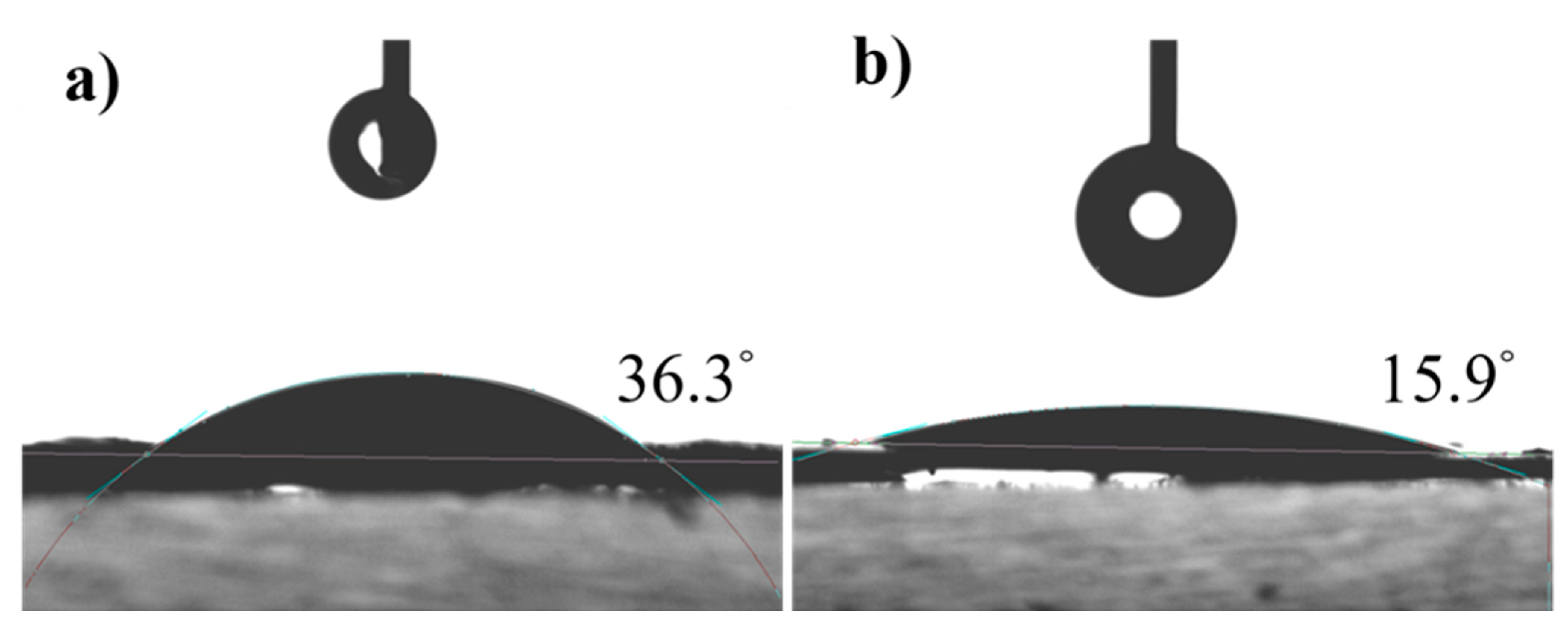
3.1. Investigation of Morphology and Structure of CeO2-ZIF-8
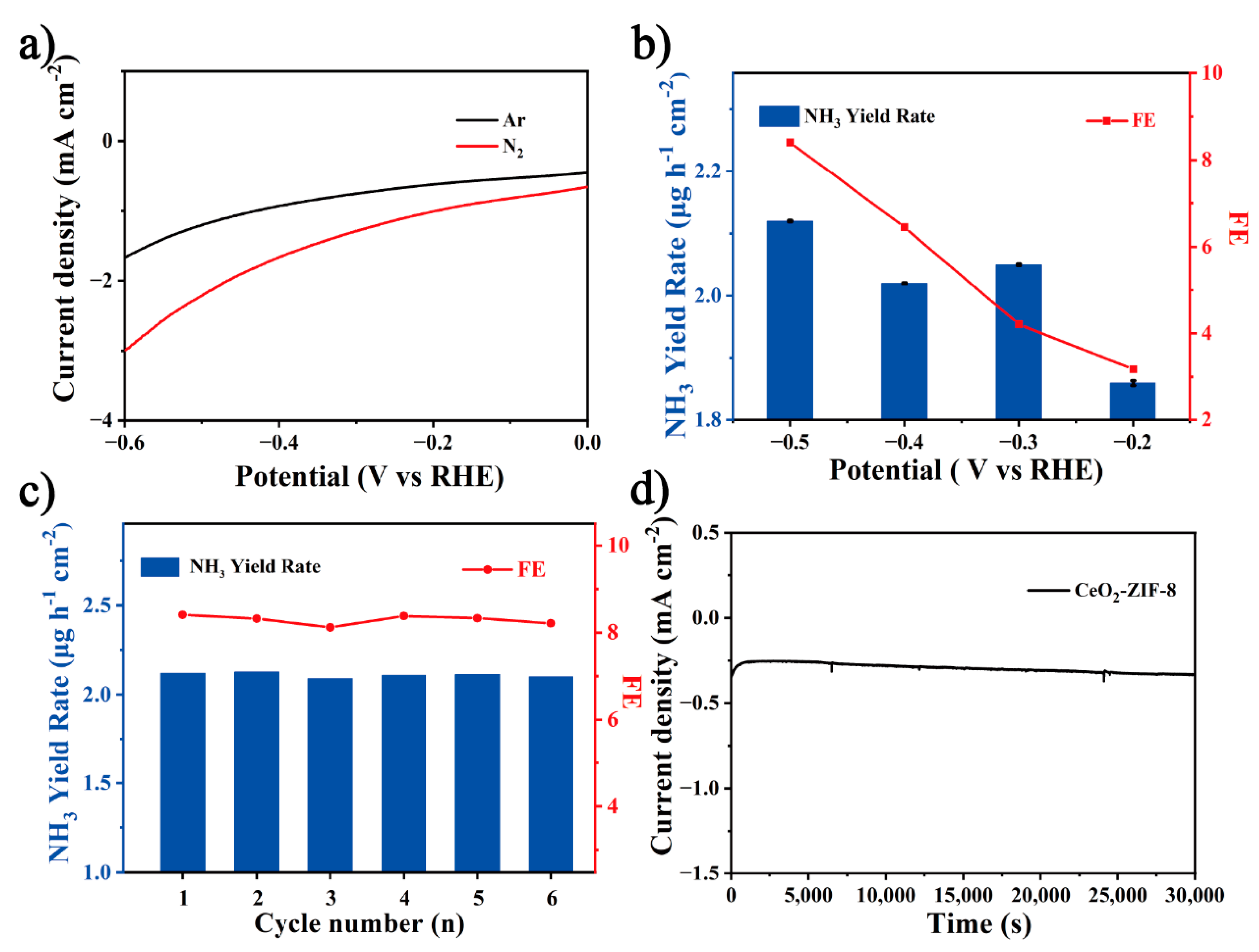
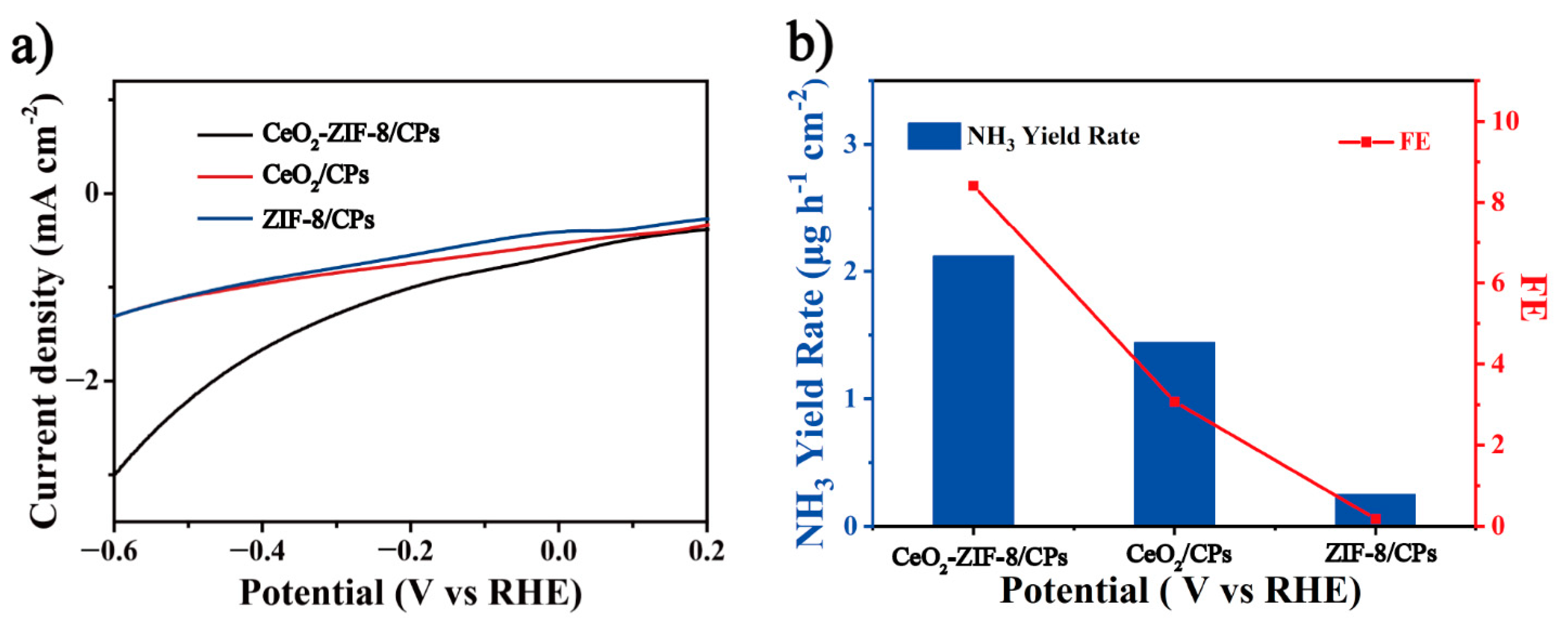
3.2. Electrocatalytic Nitrogen Reduction Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suryanto, B.H.R.; Du, H.-L.; Wang, D.; Chen, J.; Simonov, A.N.; MacFarlane, D.R. Challenges and prospects in the catalysis of electroreduction of nitrogen to ammonia. Nat. Catal. 2019, 2, 290–296. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Antonaropoulos, G.; Antonatos, N.; Rosado, M.; Storozhuk, L.; Takahashi, M.; Maenosono, S.; Luxa, J.; Sofer, Z.; Ballesteros, B.; et al. Heat-up colloidal synthesis of shape-controlled Cu-Se-S nanostructures-role of precursor and surfactant reactivity and performance in N2 electroreduction. Nanomaterials 2021, 11, 3369. [Google Scholar] [CrossRef]
- Luo, H.; Wang, X.; Wan, C.; Xie, L.; Song, M.; Qian, P. A theoretical study of Fe adsorbed on pure and nonmetal (N, F, P, S, Cl)-doped Ti3C2O2 for electrocatalytic nitrogen reduction. Nanomaterials 2022, 12, 1081. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, T.; Ma, Y.; Wei, Q.; Qiu, W.; Guo, H.; Shi, X.; Zhang, P.; Asiri, A.M.; Chen, L.; et al. Boosted electrocatalytic N2 reduction to NH3 by defect-rich MoS2 nanoflower. Adv. Energy Mater. 2018, 8, 1801357. [Google Scholar] [CrossRef]
- Zhao, L.; Xiong, Y.; Wang, X.; Zhao, R.; Chi, X.; Zhou, Y.; Wang, H.; Yang, Z.; Yan, Y.-M. Shearing sulfur edges of VS2 electrocatalyst enhances its nitrogen reduction performance. Small 2022, 18, 2106939. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Chi, X.; Wang, X.; Zhao, L.; Zhou, Y.; Xiong, Y.; Yao, S.; Wang, S.; Wang, D.; Fu, Z.; et al. In operando identification of the V4+-site-dependent nitrogen reduction reaction of VSx. J. Mater. Chem. A 2022, 10, 10219. [Google Scholar] [CrossRef]
- Vanderham, C.M.; Koper, M.M.; Hetterscheid, D.H. Challenges in reduction of dinitrogen by proton and electron transfer. Chem. Soc. Rev. 2014, 43, 5183–5191. [Google Scholar] [CrossRef]
- Xu, B.; Xia, L.; Zhou, F.; Zhao, R.; Chen, H.; Wang, T.; Zhou, Q.; Liu, Q.; Cui, G.; Xiong, X.; et al. Engineering, enhancing electrocatalytic N2 reduction to NH3 by CeO2 nanorod with oxygen vacancies. ACS Sustain. Chem. Eng. 2019, 7, 2889–2893. [Google Scholar] [CrossRef]
- Shipman, M.A.; Symes, M.D. Recent progress towards the electrosynthesis of ammonia from sustainable resources. Catal. Today 2016, 286, 57–68. [Google Scholar] [CrossRef]
- Cui, B.; Zhang, J.; Liu, S.; Liu, X.; Xiang, W.; Liu, L.; Xin, H.; Lefler, M.J.; Stuart, L. Electrochemical synthesis of ammonia directly from N2 and water over iron-based catalysts supported on activated carbon. Green Chem. 2017, 19, 298–304. [Google Scholar] [CrossRef]
- Brito-Ravicini, A.; Calle-Vallejo, F. Interplaying coordination and ligand effects to break or make adsorption-energy scaling relations. Exploration 2022, 2, 20210062. [Google Scholar] [CrossRef]
- Singh, A.R.; Rohr, B.A.; Schwalbe, J.A.; Cargnello, M.; Chan, K.; Jaramillo, T.F.; Chorkendorff, I.; Nørskov, J.K. Electrochemical ammonia synthesis-the selectivity challenge. ACS Catal. 2016, 7, 706–709. [Google Scholar] [CrossRef]
- Renner, J.N.; Greenlee, L.F.; Herring, A.M.; Ayers, K.E. Electrochemical synthesis of ammonia: A low pressure, low temperature approach. J. Electrochem. Soc. 2015, 24, 51–57. [Google Scholar] [CrossRef]
- Kyriakou, V.; Garagounis, I.; Vasileiou, E.; Vourros, A.; Stoukides, M. Progress in the electrochemical synthesis of ammonia. Catal. Today 2017, 286, 2–13. [Google Scholar] [CrossRef]
- Chen, S.; Perathoner, S.; Ampelli, C.; Mebrahtu, C.; Su, D.; Centi, G. Electrocatalytic synthesis of ammonia at room temperature and atmospheric pressure from water and nitrogen on a carbon-nanotube-based electrocatalyst. Angew. Chem. Int. Ed. 2017, 56, 2699–2703. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Li, Y. Advances on theory and experiments of the energy applications in graphdiyne. CCS Chem. 2022. [Google Scholar] [CrossRef]
- He, H.; Zhu, Q.-Q.; Yan, Y.; Zhang, H.-W.; Han, Z.-Y.; Sun, H.; Chen, J.; Li, C.-P.; Zhang, Z.; Du, M. Metal-organic framework supported Au nanoparticles with organosilicone coating for high-efficiency electrocatalytic N2 reduction to NH3. Appl. Catal. B 2022, 302, 120840. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, B.; Liang, W.; Zhou, G.; Liang, Z.; Wang, Y.; Qu, J.; Sun, Y.; Jiang, L. Three-phase electrolysis by gold nanoparticle on hydrophobic interface for enhanced electrochemical nitrogen reduction reaction. Adv. Sci. 2020, 7, 2002630. [Google Scholar] [CrossRef]
- Lu, Z.; Xu, W.; Ma, J.; Li, Y.; Sun, X.; Jiang, L. Superaerophilic carbon-nanotube-array electrode for high-performance oxygen reduction reaction. Adv. Mater. 2016, 28, 7155–7161. [Google Scholar] [CrossRef]
- Li, A.; Cao, Q.; Zhou, G.; Schmidt, B.; Zhu, W.; Yuan, X.; Huo, H.; Gong, J.; Antonietti, M. Three-phase photocatalysis for the enhanced selectivity and activity of CO2 reduction on a hydrophobic surface. Angew. Chem. Int. Ed. 2019, 58, 14549–14555. [Google Scholar] [CrossRef]
- Xu, W.; Lu, Z.; Sun, X.; Jiang, L.; Duan, X. Superwetting electrodes for gas-involving electrocatalysis. Acc. Chem. Res. 2018, 51, 1590–1598. [Google Scholar] [CrossRef] [PubMed]
- Bidault, F.; Brett, D.J.L.; Middleton, P.H.; Brandon, N.P. Review of gas diffusion cathodes for alkaline fuel cells. J. Power Sources 2009, 187, 39–48. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, L.; Luo, Q.; Cao, Y.; Dai, Y.; Li, Z.; Li, H.; Zheng, X.; Yan, W.; Yang, J.; et al. Molecular-level insight into how hydroxyl groups boost catalytic activity in CO2 hydrogenation into methanol. Chem 2018, 4, 613–625. [Google Scholar] [CrossRef]
- Lee, H.K.; Koh, C.S.L.; Lee, Y.H.; Liu, C.; Phang, I.Y.; Han, X.; Tsung, C.K.; Ling, X.Y. Favoring the unfavored: Selective electrochemical nitrogen fixation using a reticular chemistry approach. Sci. Adv. 2018, 4, eaar3208. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, S.Q.; Wen, H.; Ye, T.; Du, M. Nanoporous gold embedded ZIF composite for enhanced electrochemical nitrogen fixation. Angew. Chem. Int. Ed. 2019, 58, 15362–15366. [Google Scholar] [CrossRef] [PubMed]
- Sim, H.Y.F.; Chen, J.R.T.; Koh, C.S.L.; Lee, H.K.; Han, X.; Phan-Quang, G.C.; Pang, J.Y.; Lay, C.L.; Pedireddy, S.; Phang, I.Y.; et al. ZIF-induced d-band modification in a bimetallic nanocatalyst: Achieving over 44% efficiency in the ambient nitrogen reduction reaction. Angew. Chem. Int. Ed. 2020, 59, 16997–17003. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, C.; Guan, L.; Li, K.; Lin, Y. Oxygen vacancy regulation strategy promotes electrocatalytic nitrogen fixation by doping Bi into Ce-MOF-derived CeO2 nanorods. J. Phys. Chem. C 2020, 124, 18003–18009. [Google Scholar] [CrossRef]
- Qi, J.; Zhou, S.; Xie, K.; Lin, S. Catalytic role of assembled Ce Lewis acid sites over ceria for electrocatalytic conversion of dinitrogen to ammonia. J. Energy Chem. 2021, 60, 249–258. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, C.; Liu, Y.; Li, W.; Wang, J.; Wang, G.; Zhang, Y.; Zhang, H.; Zhao, H. Cu doping in CeO2 to form multiple oxygen vacancies for dramatically enhanced ambient N2 reduction performance. Chem. Commun. 2019, 55, 2952–2955. [Google Scholar] [CrossRef]
- Tran, U.P.N.; Le, K.K.A.; Phan, N.T.S. Expanding applications of metal-organic frameworks: Zeolite imidazolate framework ZIF-8 as an efficient heterogeneous catalyst for the knoevenagel reaction. ACS Catal. 2011, 1, 120–127. [Google Scholar] [CrossRef]
- Yang, F.; Xie, J.; Liu, X.; Wang, G.; Lu, X. Linker defects triggering boosted oxygen reduction activity of Co/Zn-ZIF nanosheet arrays for rechargeable Zn-Air batteries. Small 2020, 17, 2007085. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Jiang, Q.; Yu, P.; Yang, L.; Mao, L. Zeolitic imidazolate framework-based electrochemical biosensor for in vivo electrochemical measurements. Anal. Chem. 2013, 85, 7550–7557. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.-C.; Guo, Y.; Chen, L.-W.; Shu, M.; Wang, X.-Y.; Bu, T.-A.; Gao, W.-Y.; Zhang, N.; Su, X.; Feng, X.; et al. Promoting nitrogen electroreduction to ammonia with bismuth nanocrystals and potassium cations in water. Nat. Catal. 2019, 2, 448–456. [Google Scholar] [CrossRef]
- Chen, G.; Guo, Z.; Zhao, W.; Gao, D.; Li, C.; Ye, C.; Sun, G. Design of porous/hollow structured ceria by partial thermal decomposition of Ce-MOF and selective etching. ACS Appl. Mater. Interfaces 2017, 9, 39594–39601. [Google Scholar] [CrossRef]
- Wang, W.; Chen, X.; Liu, G.; Shen, Z.; Xia, D.; Wong, P.K.; Yu, J.C. Monoclinic dibismuth tetraoxide: A new visible-light-driven photocatalyst for environmental remediation. Appl. Catal. B 2015, 176, 444–453. [Google Scholar] [CrossRef]
- Liu, B.; Wang, Q.; Yu, S.; Zhao, T.J.; Han, J.; Jing, P.; Hu, W.; Liu, L.; Zhang, J.; Sun, L.-D.; et al. Double shelled hollow nanospheres with dual noble metal nanoparticle encapsulation for enhanced catalytic application. Nanoscale 2013, 5, 9747–9757. [Google Scholar] [CrossRef]
- Han, J.; Meeprasert, J.; Maitarad, P.; Nammuangruk, S.; Shi, L.; Zhang, D. Investigation of the facet-dependent catalytic performance of Fe2O3/CeO2 for the selective catalytic reduction of NO with NH3. J. Phys. Chem. C 2016, 120, 1523–1533. [Google Scholar] [CrossRef]
- Liu, Y.; Han, M.; Xiong, Q.; Zhang, S.; Zhao, C.; Gong, W.; Wang, G.; Zhang, H.; Zhao, H. Dramatically enhanced ambient ammonia electrosynthesis performance by in-operando created Li-S interactions on MoS2 electrocatalyst. Adv. Energy Mater. 2019, 9, 1803935. [Google Scholar] [CrossRef]
- Chen, S.; Perathoner, S.; Ampelli, C.; Mebrahtu, C.; Su, D.; Centi, G. Room-temperature electrocatalytic synthesis of NH3 from H2O and N2 in a gas-liquid-solid three-phase reactor. ACS Sustain. Chem. Eng. 2017, 5, 7393–7400. [Google Scholar]
- Li, S.; Bao, D.; Shi, M.; Wu, B.; Yan, J.; Jiang, Q. Amorphizing of Au nanoparticles by CeOx-RGO hybrid support towards highly efficient electrocatalyst for N2 reduction under ambient conditions. Adv. Mater. 2017, 29, 1700001. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, X.; Zhang, B.; Luo, Y.; Cui, G.; Xie, F.; Asiri, A.M.; Sun, X. Ambient N2 fixation to NH3 electrocatalyzed by a spinel Fe3O4 nanorod. Nanoscale 2018, 10, 14389–14396. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Cao, X.; Wu, S.; Zeng, X.; Ding, L.; Zhu, M.; Wang, H. Ammonia electrosynthesis with high selectivity under ambient conditions via a Li+ incorporation strategy. J. Am. Chem. Soc. 2017, 139, 9771–9774. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Asim, K.; Jun, W.; Gang, C.; Kaden, W.E.; Xiao, F. Ambient electrochemical ammonia synthesis with high selectivity on Fe/Fe-oxide catalyst. ACS Catal. 2018, 8, 9312–9319. [Google Scholar]
- Huang, H.; Xia, L.; Shi, X.; Asiri, A.M.; Sun, X. Ag nanosheets for efficient electrocatalytic N2 fixation to NH3 under ambient conditions. Chem. Commun. 2018, 54, 11427–11430. [Google Scholar] [CrossRef] [PubMed]
- Bao, D.; Zhang, Q.; Meng, F.; Zhong, H.; Shi, M.; Zhang, Y.; Yan, J.; Jiang, Q.; Zhang, X. Electrochemical reduction of N2 under ambient conditions for artificial N2 fixation and renewable energy storage using N2/NH3 cycle. Adv. Mater. 2017, 29, 1604799. [Google Scholar] [CrossRef] [PubMed]
- Watt, G.W.; Chrisp, J.D. Spectrophotometric method for determination of hydrazine. Anal. Chem. 1952, 24, 2006–2008. [Google Scholar] [CrossRef]


| Catalyst | Electrolyte | NH3 Yield | FE (%) | Ref. |
|---|---|---|---|---|
| 30%-Fe2O3-CNT | 0.50 M KOH | 0.11 μg h−1 cm−2 | 0.59 | [39] |
| Fe/Fe Oxide | 0.10 M PBS | 0.19 μg h−1 cm−2 | 8.29 | [40] |
| Fe3O4/Ti | 0.10 M Na2SO4 | 3.43 μg h−1 cm−2 | 2.60 | [41] |
| PEBCD/C | 0.50 M Li2SO4 | 1.58 μg h−1 cm−2 | 2.85 | [42] |
| Fe/Fe Oxide | 0.10 M PBS | 0.19 μg h−1 cm−2 | 8.29 | [43] |
| Ag nanosheets | 0.10 M HCl | 2.80 μg h−1 cm−2 | 4.80 | [44] |
| Au nanorods This work | 0.10 M KOH 0.50 M K2SO4 | 1.65 μg h−1 cm−2 2.21 μg h−1 cm−2 | 4.00 8.41 | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Meng, X.; Zhao, Z.; Li, K.; Lin, Y. Assembly of Hydrophobic ZIF-8 on CeO2 Nanorods as High-Efficiency Catalyst for Electrocatalytic Nitrogen Reduction Reaction. Nanomaterials 2022, 12, 2964. https://doi.org/10.3390/nano12172964
Liu Y, Meng X, Zhao Z, Li K, Lin Y. Assembly of Hydrophobic ZIF-8 on CeO2 Nanorods as High-Efficiency Catalyst for Electrocatalytic Nitrogen Reduction Reaction. Nanomaterials. 2022; 12(17):2964. https://doi.org/10.3390/nano12172964
Chicago/Turabian StyleLiu, Yiwen, Xianbin Meng, Zhiqiang Zhao, Kai Li, and Yuqing Lin. 2022. "Assembly of Hydrophobic ZIF-8 on CeO2 Nanorods as High-Efficiency Catalyst for Electrocatalytic Nitrogen Reduction Reaction" Nanomaterials 12, no. 17: 2964. https://doi.org/10.3390/nano12172964
APA StyleLiu, Y., Meng, X., Zhao, Z., Li, K., & Lin, Y. (2022). Assembly of Hydrophobic ZIF-8 on CeO2 Nanorods as High-Efficiency Catalyst for Electrocatalytic Nitrogen Reduction Reaction. Nanomaterials, 12(17), 2964. https://doi.org/10.3390/nano12172964





